Introduction
Osteoarthritis (OA) is a degenerative disease
characterized by cartilage degeneration, osteophyte formation, and
narrowing of the joint space. This disease has become the fourth
most disabling cause in the world (1). A major focus in bone research has
been the understanding of the etiology and pathogenesis of OA.
Recent studies have revealed that long non-coding RNAs (lncRNAs)
are associated with a variety of diseases, including OA (2–4). The
lncRNA growth arrest-specific transcript 5 (GAS5) is one of the
most important lncRNAs noted in human T lymphocytes and in
non-transformed lymphocytes, and its current functional annotation
is that of a tumor suppressor gene currently (5). LncRNAs can exert their biological
functions by targeting miRNAs. Previous studies that investigated
GAS5 and its target miRNAs have mainly focused on cancer (6,7). A
limited number of studies have explored the role of GAS5 in the
development of OA (8). In the
present study, GAS5 expression was investigated with regard to
chondrocyte proliferation, apoptosis, extracellular matrix (ECM)
metabolism and inflammatory response by silencing its expression in
osteoarthritic chondrocytes (OACs).
Materials and methods
Source of specimens
A total of 30 patients who underwent knee
arthroplasty from January 2016 to June 2018 at the Second
Affiliated Hospital of Harbin Medical University were enrolled (OA
group). In addition, 30 patients with artificial hip arthroplasty
due to femoral neck fracture were selected as the control group.
Cartilage tissue samples of OA and control patients were obtained
during surgery. The cartilage tissue was stored in a sample bottle
of phosphate-buffered saline (PBS) solution containing 20% medium
and 5% calf serum for chondrocyte culture. The present study was
approved by the Second Affiliated Hospital of Harbin Medical
University Ethics Committee and all subjects signed the relevant
informed consent.
Isolation and culture of
chondrocytes
The cartilage tissue was cut into small pieces of
approximately 1 mm3 in diameter using a pair of
ophthalmic scissors. The tissue fragments were digested and
centrifuged (250 × g for 15 min at room temperature), the
supernatant was discarded and the primary chondrocytes were
isolated from the precipitate. The collected primary chondrocytes
were resuspended in RPMI-1640 complete medium containing 10% fetal
bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin. The
cells were cultured at 37°C, in the presence of 5% CO2.
The culture medium was replaced every two days. The chondrocytes
were identified by toluidine blue staining and immunocytochemical
analysis. The subculture was carried out when the cells were grown
to 80% confluence. The samples from the second-generation
chondrocytes were obtained for subsequent experiments.
Toluidine blue staining
Following routine digestion, the chondrocytes were
inoculated in 24-well culture plates and pre-plated with glass
slides. The chondrocytes were collected and the culture solution
was discarded. The cells were placed in 4% paraformaldehyde and
fixed at 4°C for 1 h. The slides were washed with tap water for 15
min and placed in toluidine blue dye solution for 2 h. Excess dye
solution was removed and the slides were placed under an inverted
microscope for visualization.
Transfection
The second generation OACs were obtained for further
experiments. The following groups were included: Control, si-NC and
si-GAS5. The cells were transfected with GAS5 siRNA and the
corresponding GAS5 siRNA negative sequences using Lipofectamine
2000 transfection reagent. The molecular mechanism of GAS5 that
affected chondrocyte apoptosis in OA was examined. miR-34a
overexpression or inhibitory cell lines (miR-34a mimic and its
negative control mimic-NC, as well as miR-34a inhibitor and its
negative control inhibitor-NC) were used for further experiments.
In addition, a GAS5 overexpression or knockout cell line
(transfection of OA articular chondrocytes with GAS5 and sh-GAS5
plasmids to overexpress or knockdown intracellular GAS5) and a
co-transfected cell model that overexpressed GAS5 and miR-34a
(miR-34a mimic and its negative control mimic-NC, as well as GAS5
and its negative control sh-GAS5) were established. All protocols
were carried out in accordance with the manufacturer's
instructions. Following transfection and incubation for 24 h, the
transfection efficiency was verified by reverse
transcription-quantitative PCR (RT-qPCR) detection of GAS5.
RT-qPCR
Total RNA was extracted following cell transfection.
RNA extraction was conducted using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) in an RNAase-free environment. The total
RNA concentration and purity were detected by the ultra-micro
nuclear protein assay. A quantitative PCR amplification instrument
was used to detect the expression levels of the objective gene. The
primers sequences of GAS5,miR-34a and Bcl-2 were designed and
synthesized by Shanghai Sangon Biotech (Table I). The reaction conditions were as
follows: preheating at 50°C for 2 min and initial denaturation at
95°C for 10 min. A total of 35 cycles were performed that included
the following steps: Denaturation at 95°C for 10 sec, annealing at
60°C for 1 min and extension at 72°C for 55 sec. The analysis was
carried out by the 2−ΔΔCq (9) method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Sequences |
|---|
| GAS5 | (F)
5′-TCGGCTTGACTACACTGTGT-3′ |
|
| (R)
5′-GGAGGCTGAGGATCACTTGA-3′ |
| Bcl-2 | (F)
5′-GAGCATCTCACACTCGTTG-3′ |
|
| (R)
5′-GAAAGAGGGATGCTGTCTCG-3′ |
| β-actin | (F)
5′-CATGGAATCTGTGGCATGG-3′ |
|
| (R)
5′-TGATCTTCATGGTGCTGGGA-3′ |
| miR-34a | Stem loop:
5′-AGCTCAGAAGCTGCCACAAT-3′ |
|
| (F)
5′-TTCAAGAACACCTGCACAGC |
|
| (R)
5′-GGAAAGTACGCAGCCAAGTC |
MTT assay for the detection of
chondrocyte growth
The cells were cultured for 48 h following
transfection. The cell density was adjusted at 2×104
cells/ml and the cells were transferred into 96-well plates. A
total of 5 replicate wells were set in each group and cultured at
37°C in the presence of 5% CO2. A total of 20 µl MTT
solution (5 mg/ml) was added to each well. Following continuous
culture for 4 h, 150 µl of dimethyl sulfoxide (DMSO) was added to
each well, shaken and mixed for 10 min at room temperature. The
absorbance value (OD) of each group of cells was measured at 490
nm. The measurements were repeated three times per well.
Flow cytometry (FCM) for apoptosis
detection and cell cycle analysis
The cells were cultured for 48 h following
transfection. The samples were centrifuged at 1,000 × g for 5 min
at room temperature and 500 µl of buffer was added to adjust the
cell suspension concentration to 1×106 cells/ml. A total
of 5 µl of Annexin V-FITC was added and mixed with the samples. The
samples were incubated at 4°C for 15 min in the dark. A total of 5
µl of PI staining solution was added and the samples were incubated
at 4°C for 5 min in the dark. Apoptosis was detected by FCM. The
cell suspension was transferred to a 1.5-ml centrifuge tube, and 95
µl pre-cooled 75% ethanol was added. The final samples were
incubated at 4°C for 24 h. Following centrifugation at 1,000 × g
for 5 min at room temperature, the supernatant was discarded and
the cells were resuspended in PBS. A total of 500 µl PI staining
solution (0.5%) was added and the cells were resuspended and
incubated in the dark. The cell cycle was detected by FCM.
Western blot analysis
Following transfection, each group of cells was
collected. The cells were lysed with RIPA cell lysate (Beyotime
Institute of Biotechnology) and centrifuged at 12,000 × g for 10
min at 4°C. The protein concentration was determined by BCA.
Protein (40 µg) was separated by 10% SDS-PAGE and transferred into
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked by TBST (0.25%Tween) with 5% skimmed milk for 2 h at room
temperature, and incubated with the corresponding primary
antibodies (Invitrogen; Thermo Fisher Scientific, Inc.): Anti-Bcl2
(1:1,000, cat. no. 138800), Aanti-Bax (1:1,000, cat. no. BMS163),
anti-MMP3 (1:5,000, cat. no. MA514247), anti-Collagen II (1:5,000,
cat. no. MA512789), anti-Aggrecan (1:1,000, cat. no. MA316888),
anti-Ki-67 (1:200, MA514520), anti-PCNA (1:100, MA511358),
anti-CDK2 (1:2,000, cat. no. MA532017), Anti-p53 (1:1,000, cat. no.
MA512557), at 4°C overnight. The secondary antibodies Invitrogen;
Thermo Fisher Scientific, Inc.): Goat anti-mouse HRP-IgG (1:10,000,
cat. no. G21040) or Goat anti-rabbit HRP-IgG (1:10,000, cat. no.
G21234) were incubated for 2 h at room temperature. The ECL
luminescent agent (Tiangen Biotech Co., Ltd.) was added for film
development in a dark room. β-actin was used as an internal
reference. The analysis was performed by Quantity-One software
(v4.6.6; Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The expression levels of the inflammatory factors
IL-6 and TNF-α were assessed by ELISA in chondrocytes derived from
osteoarthritis patients. The experiments were performed step by
step in strict accordance with the kit instructions.
Luciferase reporter assay
The binding site of GAS5 on miR-34a was predicted by
the Starbase v2 software (http://starbase.sysu.edu.cn/index.php), and the
binding site of miR-34a and Bcl-2 was predicted by TargetScan
(http://www.targetscan.org/vert_72/).
PCR amplified a fragment of the miR-34a binding site in GAS5 and
Bcl-2, respectively. The corresponding fragments were inserted into
the pcDNA-Report vector, respectively. The cells were
co-transfected with the miR-34a mimic or the mimic-NC using
GAS5-MUT (or GAS5-WT) and Bcl-2-MUT (or Bcl-2-WT) plasmids,
respectively. Fluorescence intensity was determined according to
the Dual Luciferase reporter kit (Invitrogen; Thermo Fisher
Scientific, Inc.). All experiments were carried out in strict
accordance with the manufacturer's instructions.
Statistical analysis
All data in this study were processed using SPSS
20.0 statistical software (IBM Corp.). All data are expressed as
the mean ± standard deviation (SD). Comparison among multi-groups
were conducted by ANOVA and pairwise comparisons were performed
using the LSD t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
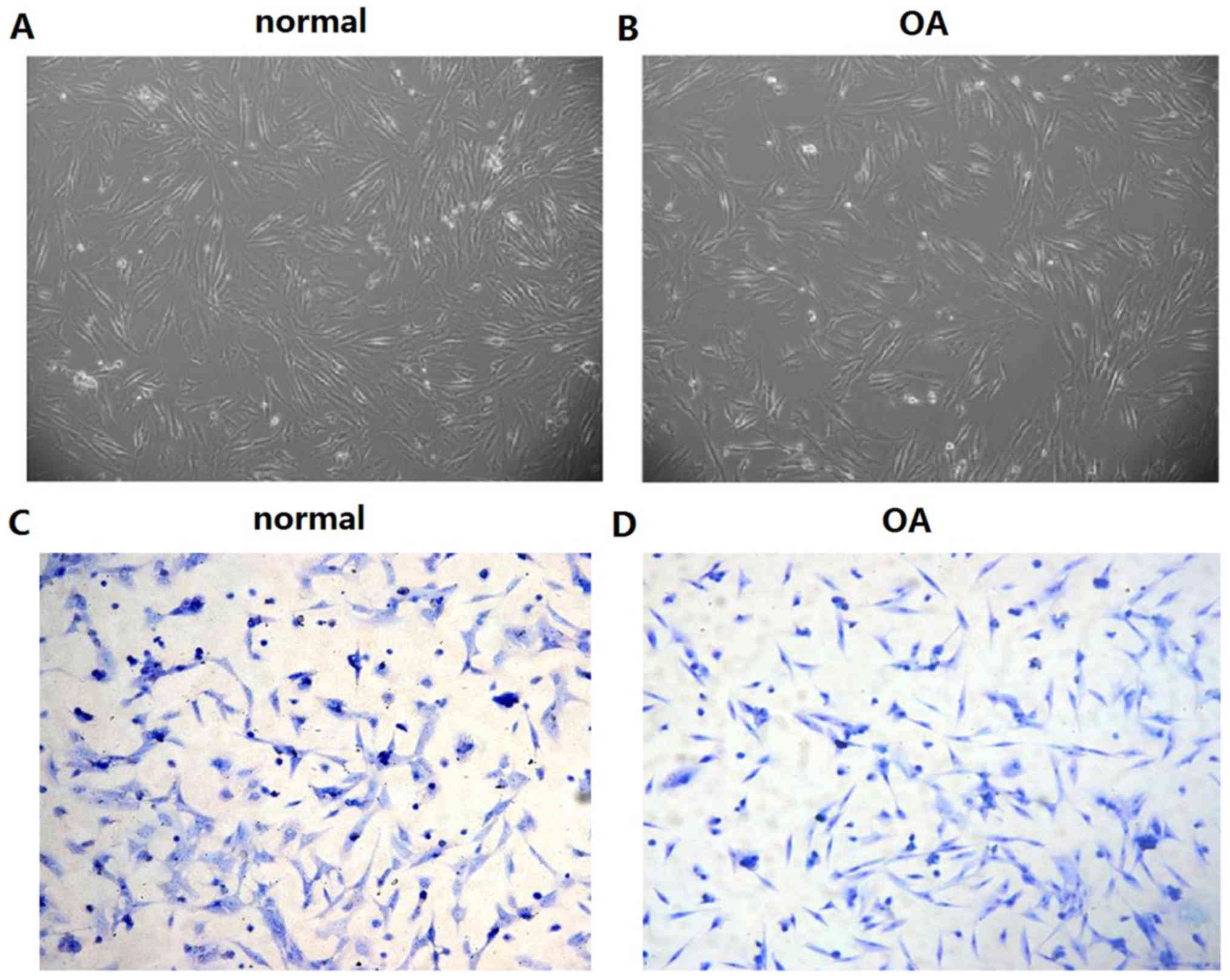
Primary culture and identification of
chondrocytes
The morphology of articular chondrocytes in the two
groups and in the second-generation chondrocytes were stained with
toluidine blue and observed using microscopy. The chondrocytes in
the two groups were normal in morphology and exhibited a long
fusiform structure (Fig. 1A).
Following staining with toluidine blue, the nuclei of the cells
were stained dark blue and the nucleolus was visible (Fig. 1B). The data indicated that the
chondrocytes in the normal group exhibited higher density than
those of the OA group.
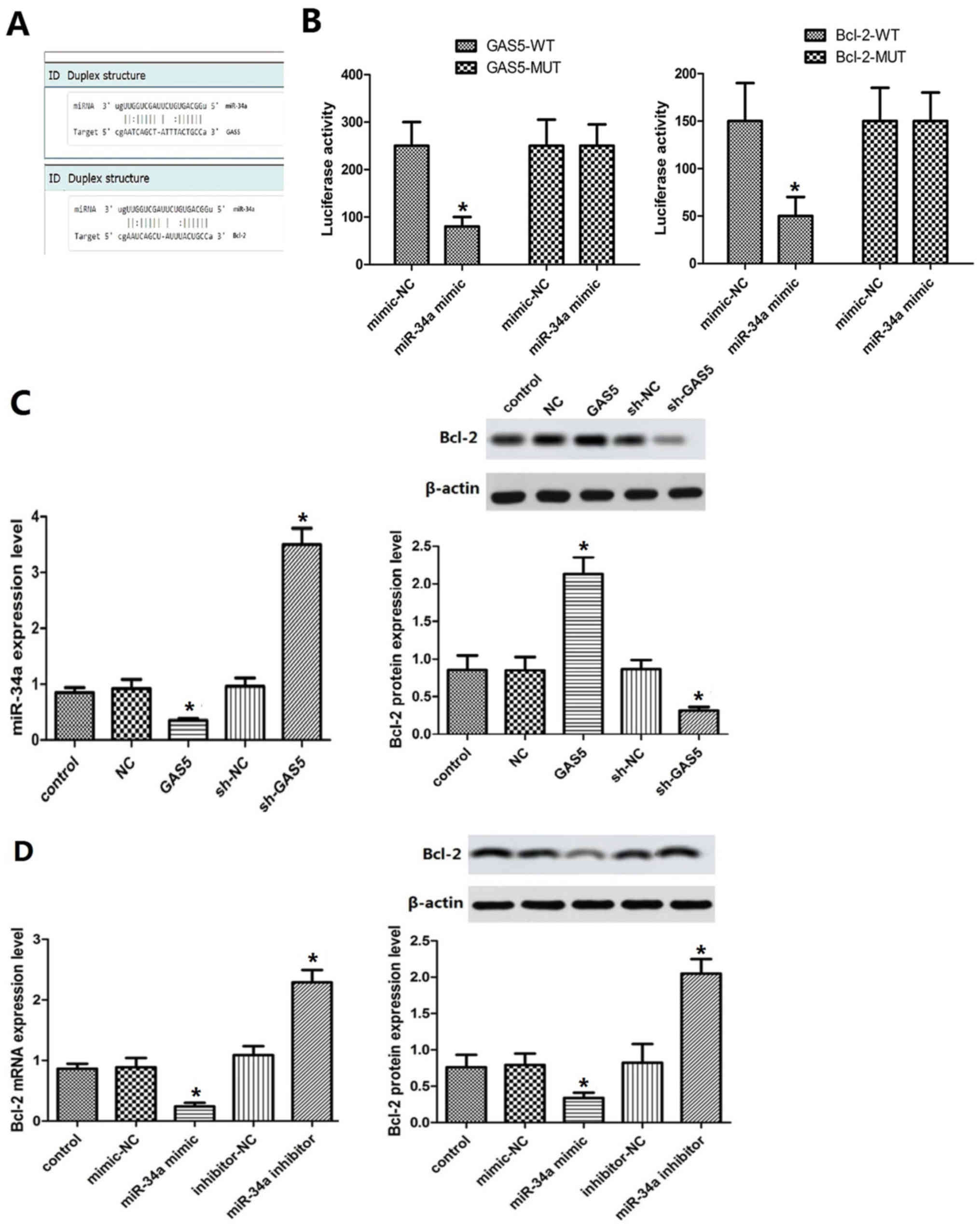
Comparison of lncRNA GAS5 expression
in normal chondrocytes and OACs
The expression of GAS5 in primary cultured
chondrocytes was detected by RT-qPCR. GAS5 was expressed in both
articular and normal chondrocytes and its expression levels in the
osteoarthritic chondrocytes was significantly higher than those
noted in normal chondrocytes (2.37±1.12 vs. 1.07±0.22) (P<0.05,
data not shown).
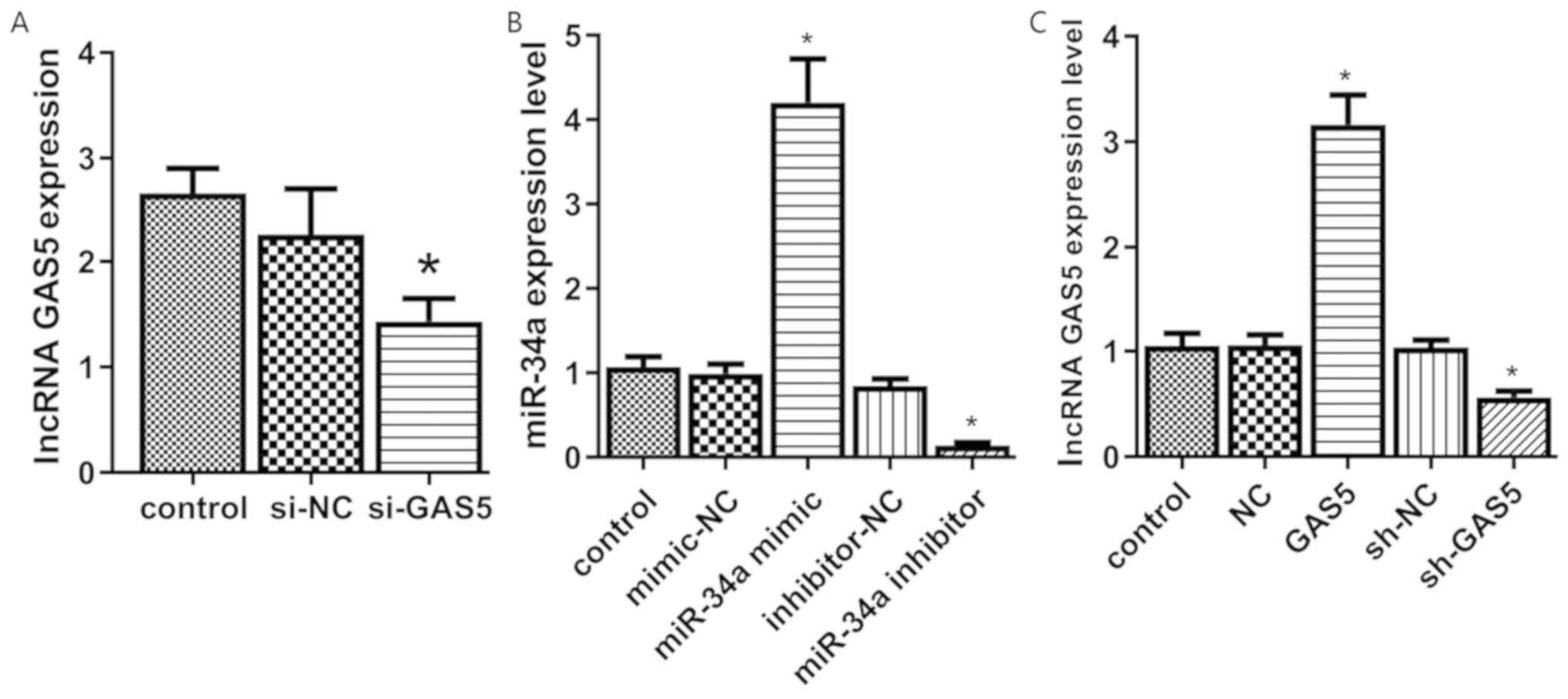
Transfection efficacy
The transfection efficacy was verified by RT-qPCR.
The expression levels of GAS5 in the si-GAS5 group were
significantly lower compared with those of the control group
(P<0.05). The expression levels in the si-NC and control groups
exhibited no significant difference (P>0.05, Fig. 2A).
The expression levels of miR-34a were significantly
higher in the miR-34a mimic group, while the miR-34a inhibitor
group exhibited significantly lower miR-34a levels than those of
the control group (Fig. 2B). No
significant differences were noted in the expression levels of
miR-34a between the mimic-NC, the inhibitor-NC and the control
groups (P>0.05). GAS5 overexpression or knockout in transfected
cell lines (GAS5 or sh-GAS5) was successfully established in
chondrocytes (Fig. 2C).
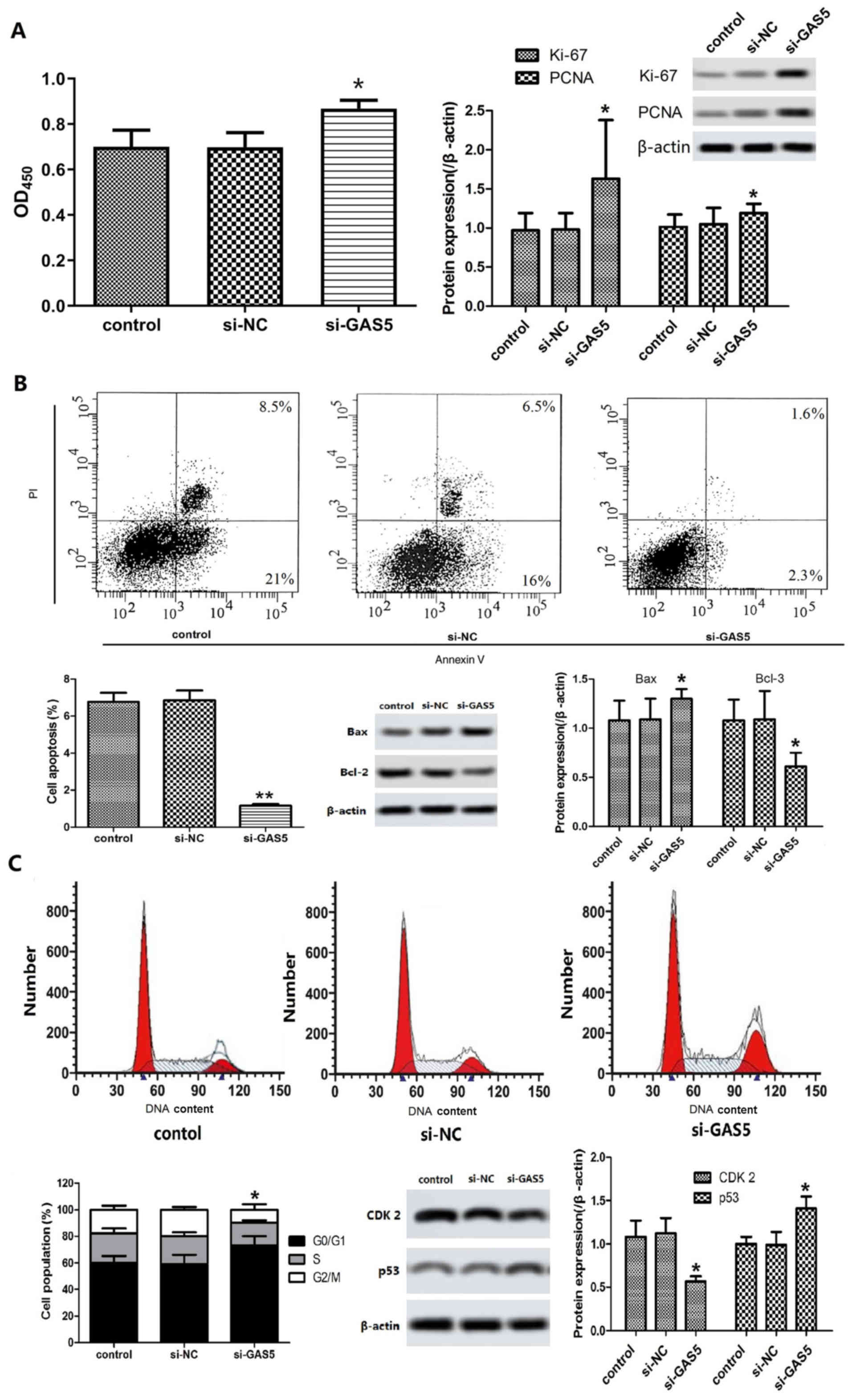
Effects of GAS5 silencing on
chondrocyte proliferation, cell cycle and apoptosis induction
The damage caused in articular cartilage is
considered the pathological basis of OA. During that process,
chondrocyte proliferation and apoptosis play an important role
(10). MTT assay demonstrated that
the proliferation of cells in the si-GAS5 group was significantly
increased compared with that of the control group (P<0.05).
Western blot analysis indicated that the expression levels of
proliferating cell nuclear antigen (PCNA) and Ki-67 in the si-GAS5
group were significantly higher than those of the control group
(P<0.05, Fig. 3A). The results
suggested that GAS5 silencing could promote the proliferation of
OACs.
FCM analysis indicated that the apoptosis rate of
OACs in the si-GAS5 group was significantly lower than that of the
control group following transfection and cell culture for 48 h
(Fig. 3B, P<0.05). The
expression levels of Bax in the si-GAS5 group were significantly
increased, while the expression levels of Bcl-2 were significantly
decreased (P<0.05, Fig. 3B).
The results of the FCM analysis further demonstrated that the
percentage of G0/G1 phase cells in the si-GAS5 group was increased,
while the number of cells in the G2/M phase decreased significantly
compared with that of the control group (Fig. 3C, P<0.05). Western blot analysis
indicated that the expression levels of CDK2 in the si-GAS5 group
was significantly lower than that in the control group, while the
expression of p53 was higher than that in the control group
(Fig. 3C, P<0.05). These
results indicated that silencing of GAS5 could cause G1 arrest,
promote proliferation of OACs and inhibit the induction of
apoptosis.
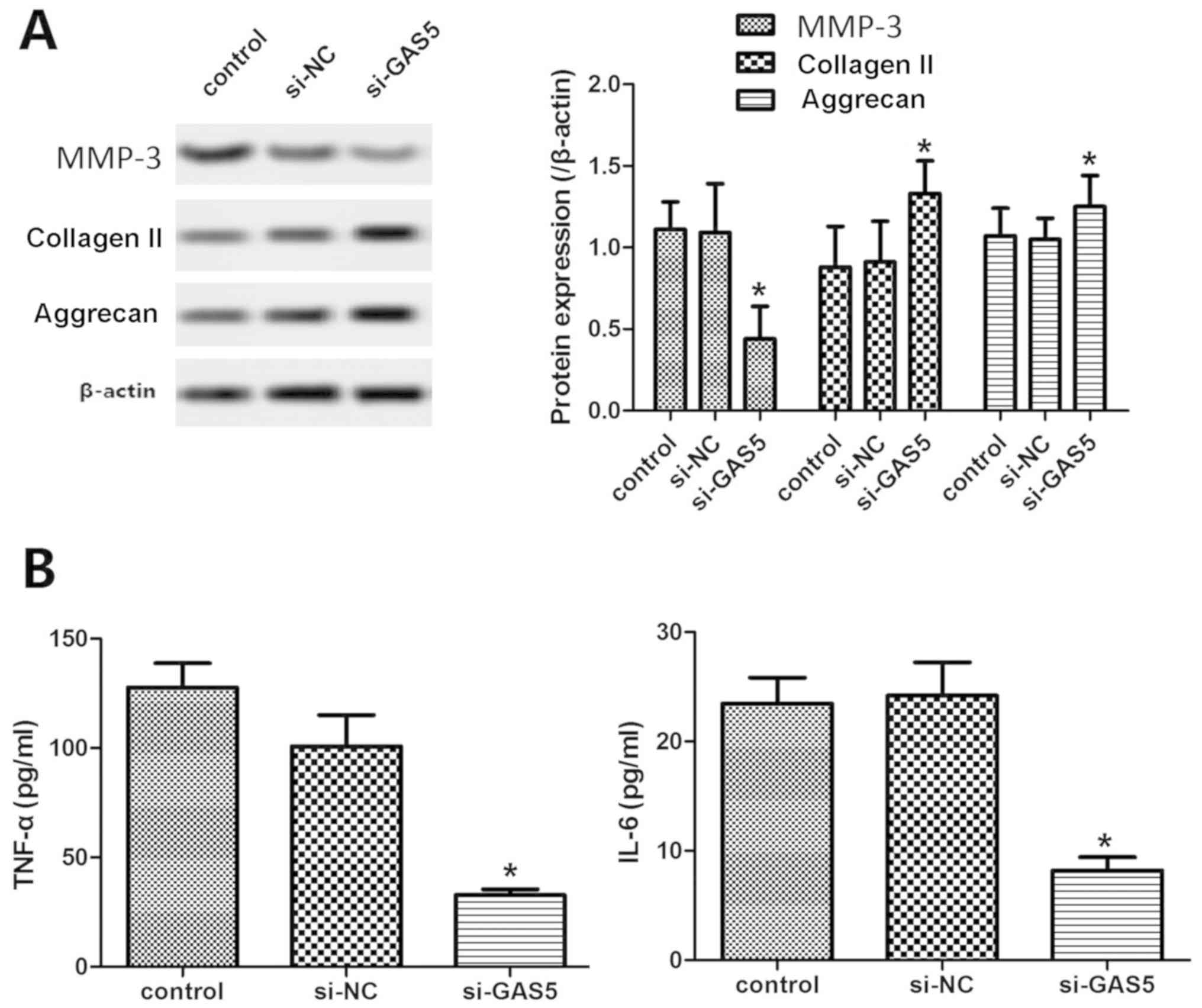
Effects of GAS5 silencing on ECM
metabolism and inflammatory response in OACs
Articular cartilage ECM protects chondrocytes from
mechanical stress. The most important component is collagen II,
followed by proteoglycans. Excessive degradation and loss of ECM is
one of the important signs of the onset of OA (11). The results indicated that the
expression levels of type II collagen and aggrecan were
significantly increased in the si-GAS5 group compared with those of
the control group, while the expression levels of MMP-13 were
significantly decreased (P<0.05, Fig. 4A), indicating that GAS5 silencing
inhibited the degradation of the cartilage matrix.
The inflammatory response plays an important role in
the development of OA (12). ELISA
indicated that the levels of TNF-α and IL-6 in the si-GAS5 group
were significantly lower than those of the control group
(P<0.05, Fig. 4B), indicating
that GAS5 silencing could reduce the severity of the inflammatory
response of OACs.
GAS5 targets miR-34a and regulates the
expression of Bcl-2
It was predicted by TargetScan that miR-34a may be a
target of GAS5 and Bcl-2 a candidate target gene of miR-34a
(Fig. 5A). The results of the dual
luciferase reporter assay demonstrated that luciferase activity was
significantly decreased in cells co-transfected with GAS5-WT and
miR-34a mimic (P<0.05, Fig.
5B). Bcl-2-WT and miR-34a mimic co-transfection resulted in a
significant decrease in luciferase activity (P<0.05). GAS5
overexpression or knockout cell lines were established. Moreover,
miR-34a overexpression was achieved by transfection of miR-34a to
the cells, and miR-34a inhibition by addition of a miR-34a
inhibitor. The results indicated that miR-34a was downregulated in
the GAS5 group (Fig. 5C), while
the expression levels of the Bcl-2 protein were increased compared
with those of the control group (P<0.05, Fig. 5C). The effect noted in the sh-GAS5
group was contradictory to these findings. The expression levels of
Bcl-2 in the miR-34a mimic and miR-34a inhibitor groups were lower
and higher than those of the control group, respectively
(P<0.05, Fig. 5D). These
results indicated that GAS5 could indirectly regulate the
expression levels of Bcl-2 by targeting miR-34a.
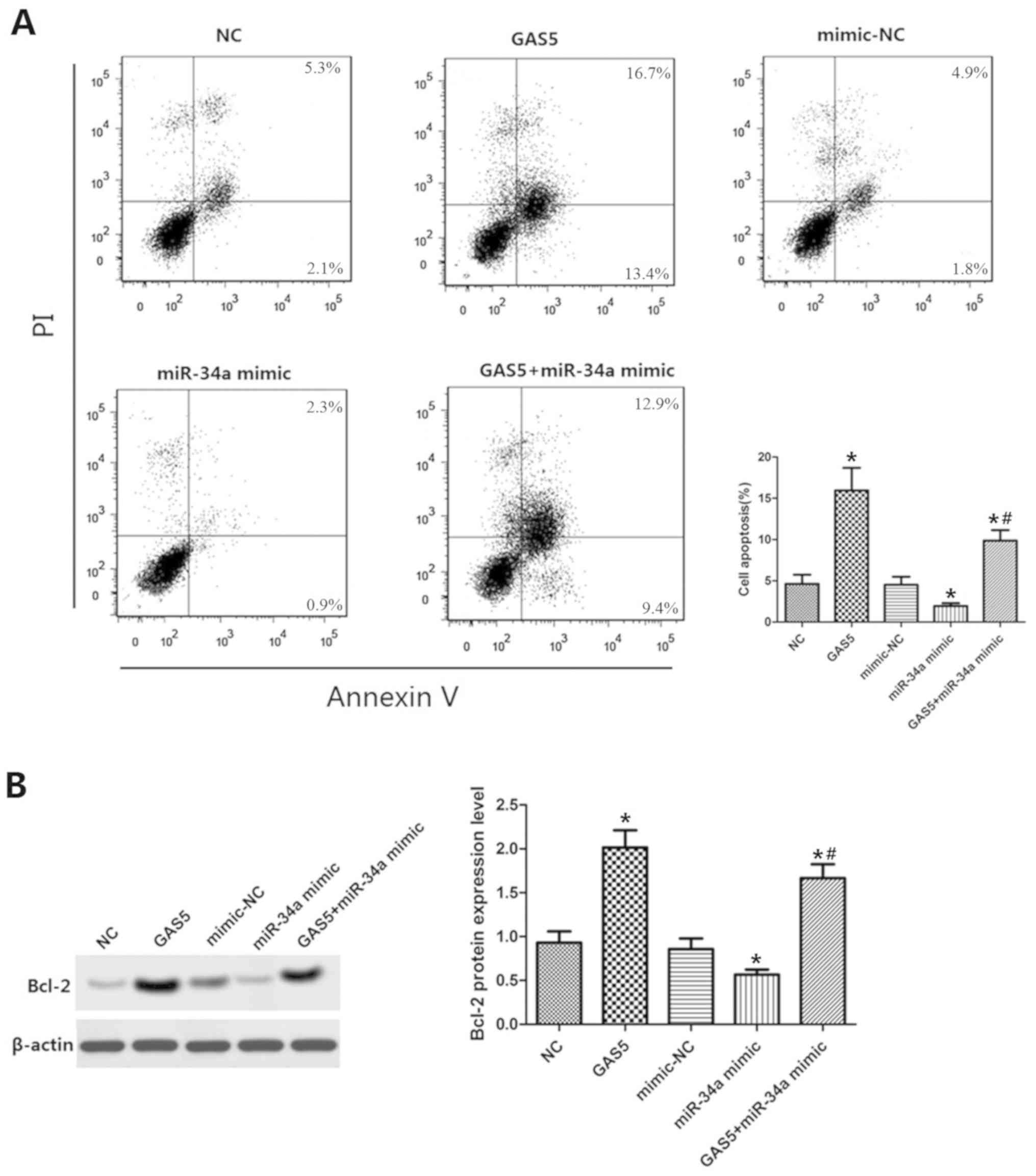
GAS5 targets miR-34a/Bcl-2 to regulate
apoptosis
Cell lines that were co-transfected with GAS5 and
miR-34a were established. The results of the FCM analysis indicated
that the apoptotic rate of the GAS5 group was significantly
increased compared with that of the control group (P<0.05,
Fig. 6A), suggesting that GAS5
overexpression promoted the induction of apoptosis. The apoptotic
rate in the miR-34a mimic with GAS5 overexpression group was lower
than that of the GAS5 group alone and higher than that of the
miR-34a mimic group alone (P<0.05). Consequently, the expression
levels of Bcl-2 were significantly increased and significantly
decreased in the GAS5 and in the miR-34a mimic groups, respectively
(P<0.05, Fig. 6B). These
findings indicated that the induction of OAC apoptosis by GAS5 was
reduced by miR-34a and that the Bcl-2-mediated inhibition of
miR-34a expression was reduced by GAS5. Collectively, the data
indicated that GAS5 regulated the induction of OAC apoptosis by
targeting the miR-34a/Bcl-2 axis.
Discussion
The main process involved in the pathogenesis of OA
is the degeneration of articular cartilage, in which chondrocyte
proliferation and apoptosis play an important role (13,14).
Under normal circumstances, the processes of proliferation and
apoptosis of chondrocytes are coordinated in an orderly manner in
cartilage tissues. During excessive induction of chondrocyte
apoptosis, internal cartilage disorders may occur, leading to
abnormal cartilage function (15).
Blanco et al (16)
demonstrated that the proportion of apoptotic chondrocytes in the
osteoarthritic cartilage was significantly higher than that noted
in normal tissues (11% vs. 5.1%, P<0.01), which confirmed that
chondrocyte apoptosis was associated with OA. Several lncRNAs have
been revealed to be closely associated with the proliferation and
apoptosis of chondrocytes. Li et al (17) revealed that PVT1 expression in OACs
was significantly increased, while inhibition of PVIT1 could
inhibit cell apoptosis. Zhang et al (18) indicated that the expression of UFC
in OACs was downregulated and that this process could inhibit
chondrocyte proliferation and promote apoptosis by targeting
miR-34a. A previous study revealed that GAS5 overexpression played
an important role in cell proliferation, apoptosis and growth
(19). In radiation-induced thymic
lymphoma, upregulation of GAS5 expression was involved in the
regulation of cell proliferation and colony formation by
participating in the chromosomal rearrangement of Notch1 (20). In non-small cell lung cancer, GAS5
could inhibit cell development by regulation of cell cycle
progression via the p53/E2F1 signaling pathway, which acted as a
tumor suppressor (21). Moreover,
previous studies have revealed that GAS5 blockers accelerate cell
proliferation and reduce apoptosis by promoting cell cycle
progression (22). However, the
role of GAS5 on OACs is not very clear. In the present study,
differential expression of GAS5 in OACs and normal chondrocytes was
observed. Silencing of GAS5 promoted cell proliferation and
inhibited apoptosis, which revealed that GAS5 may be involved in
the progression of OA by regulating OAC cell cycle progression.
The metabolic balance of the cartilage matrix
guarantees the normal function of the cartilage tissue. Degradation
of type II collagen in the ECM of chondrocytes can lead to abnormal
cartilage morphology, as well as subchondral bone and trabecular
bone structure (23). The direct
cause of cartilage matrix degradation is mainly caused by increased
protease activity of matrix metalloproteinases (MMPs) (24). Recent studies have revealed that
lncRNAs play an important role in the ECM balance of chondrocytes,
including lncRNA CIR (4,25) and MSR (26). In the present study, GAS5 silencing
significantly inhibited the expression levels of MMP-13 in OACs. In
addition, it increased the content of type II collagen and aggrecan
in OACs, indicating that GAS5 may be involved in the development of
OA by the regulation of chondrocyte ECM metabolism. OA is
considered a degenerative and an inflammatory disease (12). Proinflammatory cytokines act on
chondrocytes in order to cause chondrocyte metabolism and secretion
disorders as well as chondrocyte proliferation and apoptosis
abnormalities (27). Previous
studies have suggested that lncRNAs may be considered a bridge
between inflammatory factors and cartilage destruction (2,28,29).
Therefore, lncRNAs can be used as anti-inflammatory drugs for OA
and can replace the use of glucocorticoid drugs, which exhibit
several adverse reactions. The present study demonstrated that
silencing of GAS5 reduced the levels of IL-6 and TNF-α in OACs,
indicating that GAS5 may be associated with the regulation of
several inflammatory factors in the osteoarthritic cartilage.
miR-34a expression is downregulated in various
tumors, such as prostate cancer (30) and breast cancer (31), indicating tumor suppressor
functions of this RNA. Previous studies have revealed that miR-34a
can inhibit cell proliferation and promote cell apoptosis (32). Bcl-2 is a target of miR-34a
and are both involved in the regulation of apoptosis (33). In a previous study, bioinformatics
analysis revealed binding of GAS5 and miR-34a and of miR-34a and
Bcl-2, indicating that these molecules may be involved in
the induction of OAC apoptosis. Moreover, luciferase reporter
assays were used to test this hypothesis and it was revealed that
GAS5 overexpression in OACs downregulated the expression of
miR-34a, while it upregulated Bcl-2 levels in order to promote
apoptosis induction. GAS5 and miR-34a were co-transfected in OACs
to further validate the association between GAS5 and the
miR-34a/Bcl-2 pathway. The results indicated that GAS5
overexpression could reverse the effects of miR-34a overexpression
caused on the induction of apoptosis and on the expression of
Bcl-2. Collectively, the results demonstrated that GAS5 could
regulate the induction of OAC apoptosis by targeting the
miR-34a/Bcl-2 axis.
In summary, the present study indicated that the
expression levels of GAS5 were upregulated in OACs. GAS5 may
participate in the development of OA by regulating apoptosis,
cartilage ECM metabolism and chondrocyte inflammatory response. The
mechanism of these processes may be associated with the regulation
of the miR-34a/Bcl-2 pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY contributed to the conception and design of the
present study, QJ contributed to the acquisition, analysis and
interpretation of data, and drafted the manuscript. XQ contributed
to the case collection, statistical analysis of clinical data and
revised the manuscript critically for important intellectual
content. YL and DW contributed to data collection and the
performance of basic experiments. QJ contributed to the final
manuscript revision and all authors agreed to be accountable for
all aspects of the present study in ensuring that questions related
to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by The Second
Affiliated Hospital of Harbin Medical University Ethics Committee
and all subjects signed the relevant informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mandl LA: Osteoarthritis year in review
2018: Clinical. Osteoarthritis Cartilage. 27:359–364. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang C, Wang P, Jiang P, Lv Y, Dong C,
Dai X, Tan L and Wang Z: Upregulation of lncRNA HOTAIR contributes
to IL-1β-induced MMP overexpression and chondrocytes apoptosis in
temporomandibular joint osteoarthritis. Gene. 586:248–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing D, Liang JQ, Li Y, Lu J, Jia HB, Xu
LY and Ma XL: Identification of long noncoding RNA associated with
osteoarthritis in humans. Orthop Surg. 6:288–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L,
Zhou C and Ao Y: Long noncoding RNA related to cartilage injury
promotes chondrocyte extracellular matrix degradation in
osteoarthritis. Arthritis Rheumatol. 66:969–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y,
Yang X, Shen J, Liu Q and Zhang J: Long noncoding RNA GAS5
suppresses the migration and invasion of hepatocellular carcinoma
cells via miR-21. Tumour Biol. 37:2691–2702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao T, Lu R, Zhang J, Fang X, Fan L, Huang
C, Lin R and Lin Z: Growth arrest-specific 5 attenuates
cisplatin-induced apoptosis in cervical cancer by regulating STAT3
signaling via miR-21. J Cell Physiol. 234:9605–9615. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YY, Chen Y, Wang WC, Tang Q, Wu R,
Zhu WH, Li D and Liao LL: Cyclin D1 regulates osteoarthritis
chondrocyte apoptosis via WNT3/β-catenin signalling. Artif Cells
Nanomed Biotechnol. 47:1971–1977. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vedicherla S and Buckley CT: In vitro
extracellular matrix accumulation of nasal and articular
chondrocytes for intervertebral disc repair. Tissue Cell.
49:503–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vila S: Inflammation in Osteoarthritis. P
R Health Sci J. 36:123–129. 2017.PubMed/NCBI
|
|
13
|
Niu J, Clancy M, Aliabadi P, Vasan R and
Felson DT: Metabolic syndrome, its components, and knee
osteoarthritis: The framingham osteoarthritis study. Arthritis
Rheumatol. 69:1194–1203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi S, Nishiyama T, Miura Y, Fujishiro
T, Kanzaki N, Hashimoto S, Matsumoto T, Kurosaka M and Kuroda R:
DcR3 induces cell proliferation through MAPK signaling in
chondrocytes of osteoarthritis. Osteoarthritis Cartilage.
19:903–910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almonte-Becerril M, Navarro-Garcia F,
Gonzalez-Robles A, Vega-Lopez MA, Lavalle C and Kouri JB: Cell
death of chondrocytes is a combination between apoptosis and
autophagy during the pathogenesis of Osteoarthritis within an
experimental model. Apoptosis. 15:631–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blanco FJ, Guitian R, Vazquez-Martul E, de
Toro FJ and Galdo F: Osteoarthritis chondrocytes die by apoptosis.
A possible pathway for osteoarthritis pathology. Arthritis Rheum.
41:284–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Li S, Luo Y, Liu Y and Yu N: LncRNA
PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as
a sponge for miR-488-3p. DNA Cell Biol. 36:571–580. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang G, Wu Y, Xu D and Yan X: Long
noncoding RNA UFC1 promotes proliferation of chondrocyte in
osteoarthritis by acting as a sponge for miR-34a. DNa Cell Biol.
35:691–695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Yang W, Guo Y, Chen W, Zheng P,
Zeng J and Tong W: Exosomal lncRNA GAS5 regulates the apoptosis of
macrophages and vascular endothelial cells in atherosclerosis. PLoS
One. 12:e1854062017.
|
|
20
|
Mourtada-Maarabouni M and Williams GT:
Role of GAS5 noncoding RNA in mediating the effects of rapamycin
and its analogues on mantle cell lymphoma cells. Clin Lymphoma
Myeloma Leuk. 14:468–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi X, Sun M, Liu H, Yao Y, Kong R, Chen F
and Song Y: A critical role for the long non-coding RNA GAS5 in
proliferation and apoptosis in non-small-cell lung cancer. Mol
Carcinog. 54 (Suppl 1):E1–E12. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Udayakumar T, Shareef MM, Diaz DA, Ahmed
MM and Pollack A: The E2F1/Rb and p53/MDM2 pathways in DNA repair
and apoptosis: Understanding the crosstalk to develop novel
strategies for prostate cancer radiotherapy. Semin Radiat Oncol.
20:258–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Onuora S: Osteoarthritis: Cartilage matrix
stiffness regulates chondrocyte metabolism and OA pathogenesis. Nat
Rev Rheumatol. 11:5042015. View Article : Google Scholar
|
|
24
|
Tang LP, Ding JB, Liu ZH and Zhou GJ:
LncRNA TUG1 promotes osteoarthritis-induced degradation of
chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev
Med Pharmacol Sci. 22:8574–8581. 2018.PubMed/NCBI
|
|
25
|
Li YF, Li SH, Liu Y and Luo YT: Long
noncoding RNA CIR promotes chondrocyte extracellular matrix
degradation in osteoarthritis by acting as a sponge for Mir-27b.
Cell Physiol Biochem. 43:602–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Q, Hu X, Zhang X, Dai L, Duan X, Zhou
C and Ao Y: The TMSB4 pseudogene lncRNA functions as a competing
endogenous RNA to promote cartilage degradation in human
osteoarthritis. Mol Ther. 24:1726–1733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nixon AJ, Grol MW, Lang HM, Ruan MZC,
Stone A, Begum L, Chen Y, Dawson B, Gannon F, Plutizki S, et al:
Disease-modifying osteoarthritis treatment with interleukin-1
receptor antagonist gene therapy in small and large animal models.
Arthritis Rheumatol. 70:1757–1768. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar MM and Goyal R: LncRNA as a
therapeutic target for angiogenesis. Curr Top Med Chem.
17:1750–1757. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong X, Gu PC, Xu SZ and Lin XJ: Long
non-coding RNA-DANCR in human circulating monocytes: A potential
biomarker associated with postmenopausal osteoporosis. Biosci
Biotechnol Biochem. 79:732–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan X, Tang B, Chen B, Shan Y, Yang H;
Reproducibility Project: Cancer Biology, ; Iorns E, Tsui R, Denis
A, Perfito N and Errington TM: Replication Study: The microRNA
miR-34a inhibits prostate cancer stem cells and metastasis by
directly repressing CD44. Elife. 8(pii): e435112019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He R, Liu P, Xie X, Zhou Y, Liao Q, Xiong
W, Li X, Li G, Zeng Z and Tang H: circGFRA1 and GFRA1 act as ceRNAs
in triple negative breast cancer by regulating miR-34a. J Exp Clin
Cancer Res. 36:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun TY, Xie HJ, Li Z, Kong LF, Gou XN, Li
DJ, Shi YJ and Ding YZ: miR-34a regulates HDAC1 expression to
affect the proliferation and apoptosis of hepatocellular carcinoma.
Am J Transl Res. 9:103–114. 2017.PubMed/NCBI
|
|
33
|
Huang Q, Zheng Y, Ou Y, Xiong H, Yang H,
Zhang Z, Chen S and Ye Y: miR-34a/Bcl-2 signaling pathway
contributes to age-related hearing loss by modulating hair cell
apoptosis. Neurosci Lett. 661:51–56. 2017. View Article : Google Scholar : PubMed/NCBI
|