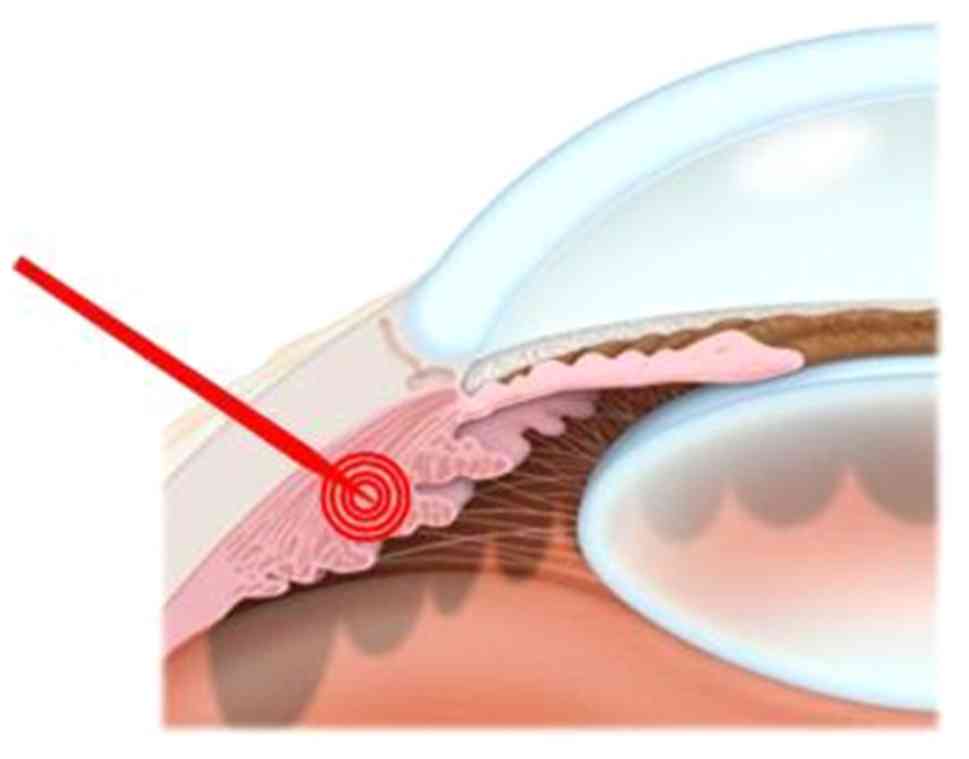
Introduction
Glaucoma is a progressive optic nerve disorder that
is characterized by structural changes of the optic nerve head, and
commonly accompanied by the development of visual functional
defects (1). The World Health
Organization has listed glaucoma as the second leading cause of
irreversible blindness (2).
According to World Health Organization statistics, ~6,680 million
patients suffer from primary glaucoma and 600 million patients are
affected by secondary glaucoma, resulting in 670 million patients
exhibiting blindness (3). It is
estimated by Quigley and Broman (4) that the number of patients with global
primary glaucoma will grow to 7,960 million by the year 2020, while
blindness caused by glaucoma will increase to 1,120 million.
It is widely known that elevated intraocular
pressure (IOP) is the most critical risk factor that leads to optic
nerve damage in glaucoma (5).
Therefore, controlling IOP has been suggested as one of the main
strategies to prevent the development of glaucoma from ocular
hypertension, eliminate the progression of early glaucoma disease
and block the occurrence of progressive visual function damage in
the clinic (6–8). However, current clinically employed
treatments for reducing IOP are not ideal. The treatment of
glaucoma in clinical settings can be divided into drug therapy and
surgical procedures (9). There are
a wide range of drugs available that reduce IOP, but constant
medication is required due to their limited effects. Consequently,
a number of patients must resort to surgery after drug treatment
(10). Anti-glaucoma surgery can
be classified into two types: Procedures that facilitate the
outflow of the aqueous humor, including aqueous humor shunts, and
trabecular, goniotomy and iridotomy surgeries; and procedures that
reduce aqueous humor secretion by destroying cells of the ciliary
body, such as cyclocryotherapy and cyclophotocoagulation (11). However, the former group of
procedures is flawed, due to scarring problems and their limited
long-term effects, while the latter group lacks precise control and
can only be utilized for patients with absolute glaucoma who have
already lost their vision (12–15).
Therefore, there is an urgent need to develop more safe and
effective therapeutic approaches for glaucoma.
Radiofrequency ablation (RFA) is a new, minimally
invasive technique (16). During
treatment, microelectrodes are directly punctured into the focal
site, and high-power radiofrequency energy is delivered inside
through the catheter, making the local temperature high and
resulting in irreversible coagulating necrosis (17). RFA has a number of advantages: It
offers precise control, it is non-invasive and it demonstrates high
effectiveness. Therefore, it has been applied in various
departments, including cancer, cardiology, otorhinolaryngology,
digestion, orthopedics, obstetrics, stomatology, surgery, physical
therapy and dermatology (18).
Currently, conductive keratoplasty has also been utilized in the
clinic for the treatment of presbyopia, in which the radiofrequency
energy is employed to change the shape of the eye's surface
(19,20). The successful application of
conductive keratoplasty indicates that radiofrequency treatment may
be safe and effective for ocular diseases.
In the present study, the minimally invasive
technique of radiofrequency catheter ablation was used, guided by
ultrasound biomicroscopy (UBM), to treat glaucoma. The ciliary body
was specifically targeted and ablated, following which the
production of the aqueous humor was reduced, which significantly
lowered IOP. RFA technology not only controls IOP precisely,
reliably, safely and durably, but it is also simple and repeatable,
with a relatively low cost (21).
Thus, it was hypothesized that minimally invasive RFA may be a
highly desirable treatment procedure for glaucoma, from a social
and economic standpoint.
Materials and methods
Experimental animals
A total of 30 New Zealand rabbits (age, 4–6 months;
weight, 2.0–2.5 kg; sex, 16 female and 14 male) were purchased from
the Animal Research Center of the Chinese PLA General Hospital. Eye
disease was excluded before research. All rabbits were allowed free
access to food and water, and lived in an environment with 12-h
light/dark cycles, a temperature of 25°C and ~50% humidity. Animals
were randomly divided into 3 groups: The model group (n=10), in
which the drug was injected into the anterior chamber to induce
glaucoma, but no treatment was applied; the RFA group (n=10), where
rabbits were treated with RFA after the induction of glaucoma; and
the sham group (n=10), in which saline instead of the drug was
injected into the anterior chamber. All protocols were conducted in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals (22) and approved by the Institutional
Animal Care and Use Committee of the Chinese PLA General
Hospital.
Establishment of the glaucoma model in
rabbits
Glaucoma was induced by injection of carbomer and
dexamethasone solution. Rabbits were anesthetized after intravenous
administration of sumianxin (0.1 ml/kg; consists of
dihydroetorphine hydrochloride, dimethylaniline thiazole,
ethylenediam-inetetraacetic acid and haloperidol) (23) and topical application of 0.5%
dicaine (24). Firstly, the
corneal limbus of the paranasal side was punctured with a 1-ml
syringe needle to form a pinhole. Then, 1 ml of aqueous humor was
extracted from the corneal limbus on the temporal side, followed by
an injection of 0.2 ml of carbomer solution containing 0.3%
carbomer and 0.025% dexamethasone (Sigma-Aldrich; Merck KGaA).
After 24 h, IOP was checked and a value of IOP >22 mmHg was used
to determine the successful establishment of the glaucoma model
(success ratio was 85% in the present study).
RFA treatment for glaucoma
RFA surgery was performed based on an XL-1-type RF
exposure system developed by the Chinese PLA General Hospital (with
independent intellectual property rights), which can produce
~300–500-µm wide, 1–1.5-mm deep tissue ablation. After local
anesthesia (0.5% dicaine, one drop) (24) combined with general anesthesia
(sumianxin mixture, 0.1 ml/kg) (23), 5 model eyes were randomly selected,
and the sclera was punctured at 1.5 mm from the corneal margin with
a depth of 1 mm. This was exposed to a probe that generated a
500-KHz radiofrequency field at specific absorption rate of 0.5–0.8
W/kg. Output power was set at 0.6–1.0 W, duration was 1–2 sec and
the number of ablation sites was 8.
IOP measurement
Under local anesthesia, IOP was measured before and
on days 1, 3 and 7 post-RFA with a Schiotz tonometer (Rudolf
Riester GmbH). Scalp acupuncture connected to the Schiotz tonometer
was inserted into the anterior chamber through the margin between
the sclera and the cornea. After 5 min of stabilization, the value
was read three times and the average value was calculated. The
value obtained before ablation surgery was considered as the basal
level of IOP.
Investigation of changes to the
eyeball and anterior segments with UBM and slit lamp
Investigation with the slit lamp was carried out
before surgery and on days 1, 3 and 7 post-surgery. Before
investigation, rabbits were anesthetized with 3.5% sodium
phenobarbital (35 mg/kg) (25).
After the corneal reflex and corneal touch reflection disappeared,
the transparency of the cornea, shades of the anterior chamber, the
oscillation or opacity of the aqueous humor, and damages to the
iris and lens were checked. Subsequently, our self-designed
lens-water bath transformation system (patent no. ZL201420207350.0)
was utilized to investigate the rabbit eyes with UBM for the
purpose of assessing the influence of ablation on the ciliary body.
During the UBM test, 0.2 ml 0.1% (w/v) hyaluronic acid sodium drops
were administered to the junctival sac with the external optic cup
utilized.
Histopathological assay after RFA
On 1, 3, 7 and 14 days after RFA, 2 rabbits on each
day were anesthetized with an intravenous injection of 3.5% sodium
phenobarbital (35 mg/kg) and sacrificed by ear vein air embolism
(10–20 ml air). Following this, the eyeball was removed and 0.1 ml
4% formaldehyde was injected into the vitreous body through the
site of the optic nerve. The eye was then soaked in 4% formaldehyde
at 25°C for 24 h. The cornea, lens and posterior segment were
removed from the fixed eyes. Then, the ciliary body and the sclera
were collected, embedded in paraffin and cut into 5-µm sections.
They were stained with hematoxylin for 5 min and eosin for 3 min,
respectively, to observe the pathological changes in the retinal
ganglion cells using light microscopy (magnification, ×400).
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for data
analysis. Results of IOP were expressed as mean ± SD. Comparisons
of IOP among three groups were performed using one-way ANOVA,
followed by LSD post hoc test for multiple comparisons. P<0.05
was considered to be statistically significant.
Results
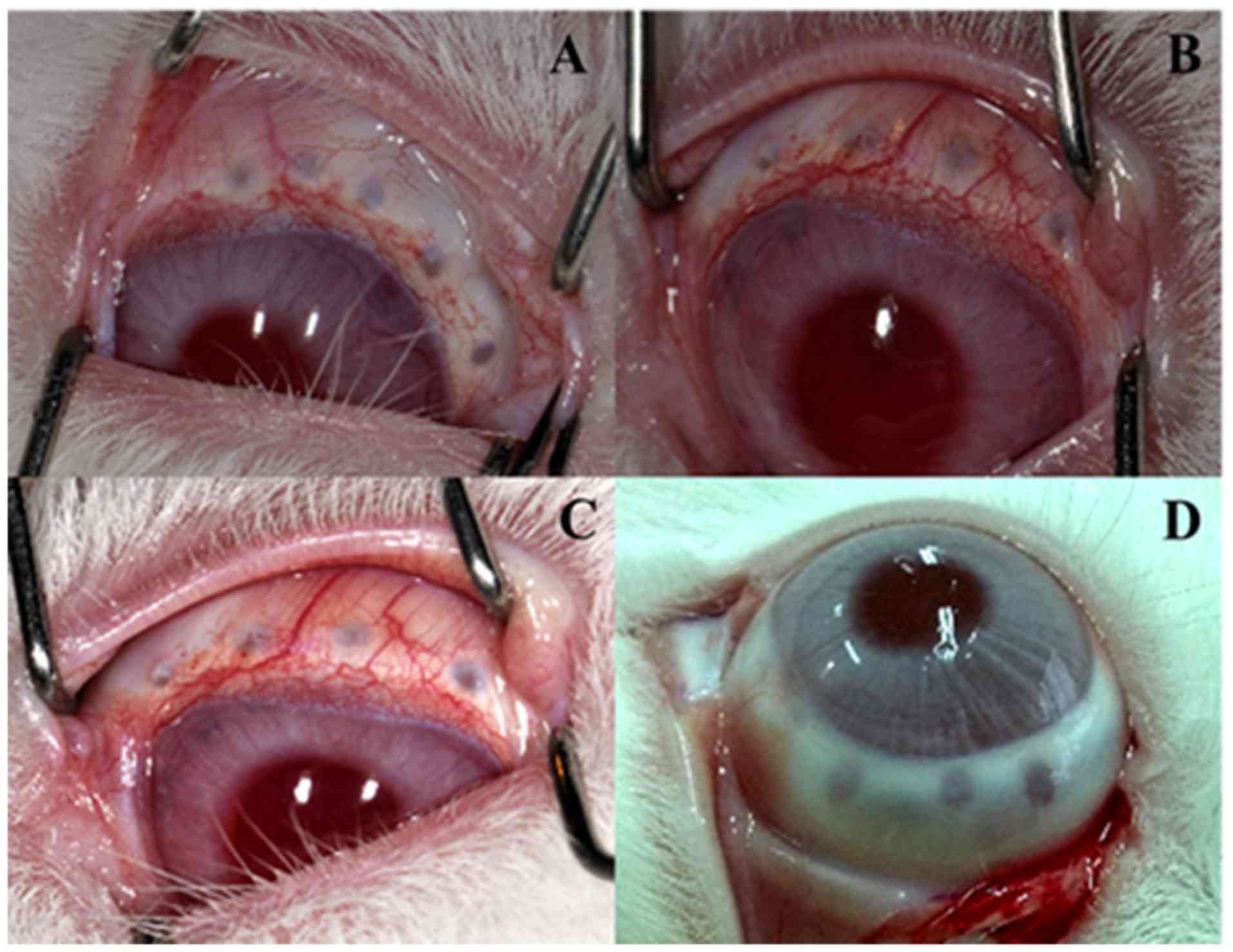
General observation after RFA
After radiofrequency catheter ablation of the
ciliary body, the eyes were examined with UBM and a slit lamp. A
melting spot was observed (Fig.
1), but no puncture point penetrating the ciliary body into the
eye was shown in the UBM check. Slit-lamp examination results
showed no significant anterior chamber reaction.
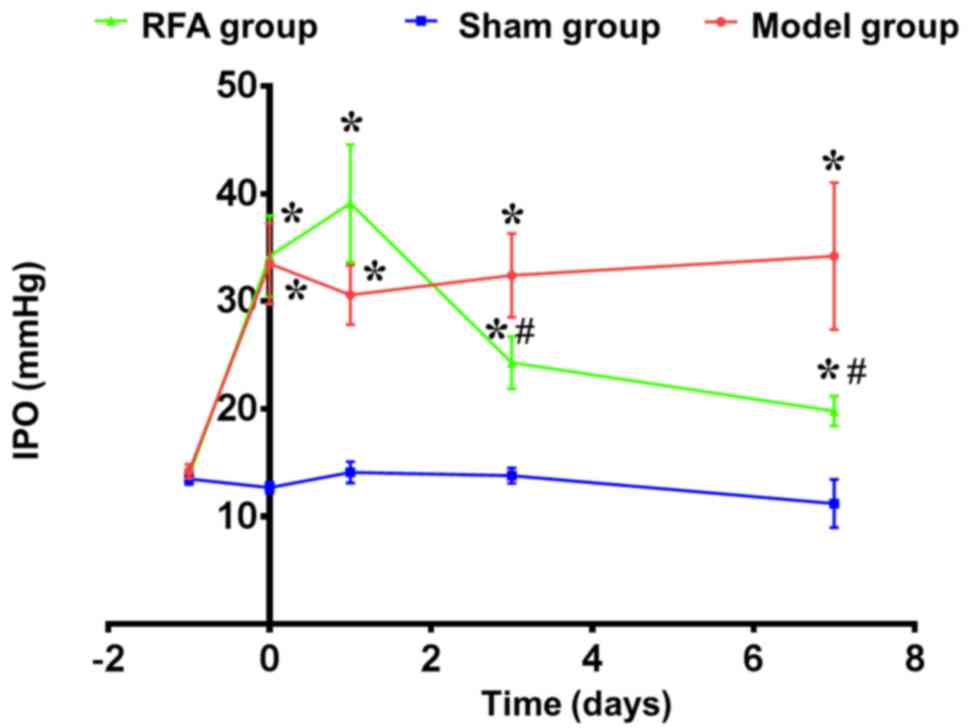
Changes in IOP after RFA
In comparison with the sham group, rabbits of the
glaucoma model showed ~2.5× higher IOP (~33 mmHg). At 1 day after
RFA of the ciliary body, IOP was still higher than in the sham
group (~15%), but 3 days post-RFA, IOP was decreased by ~40%, and
significantly lower than the model group (P=0.047). At day 7, IOP
of the RFA group continued to decrease (~25%), although it was not
completely restored to the level of the sham group. However, it was
significantly lower compared with the model group
(P=3.64×10−4; Fig.
2).
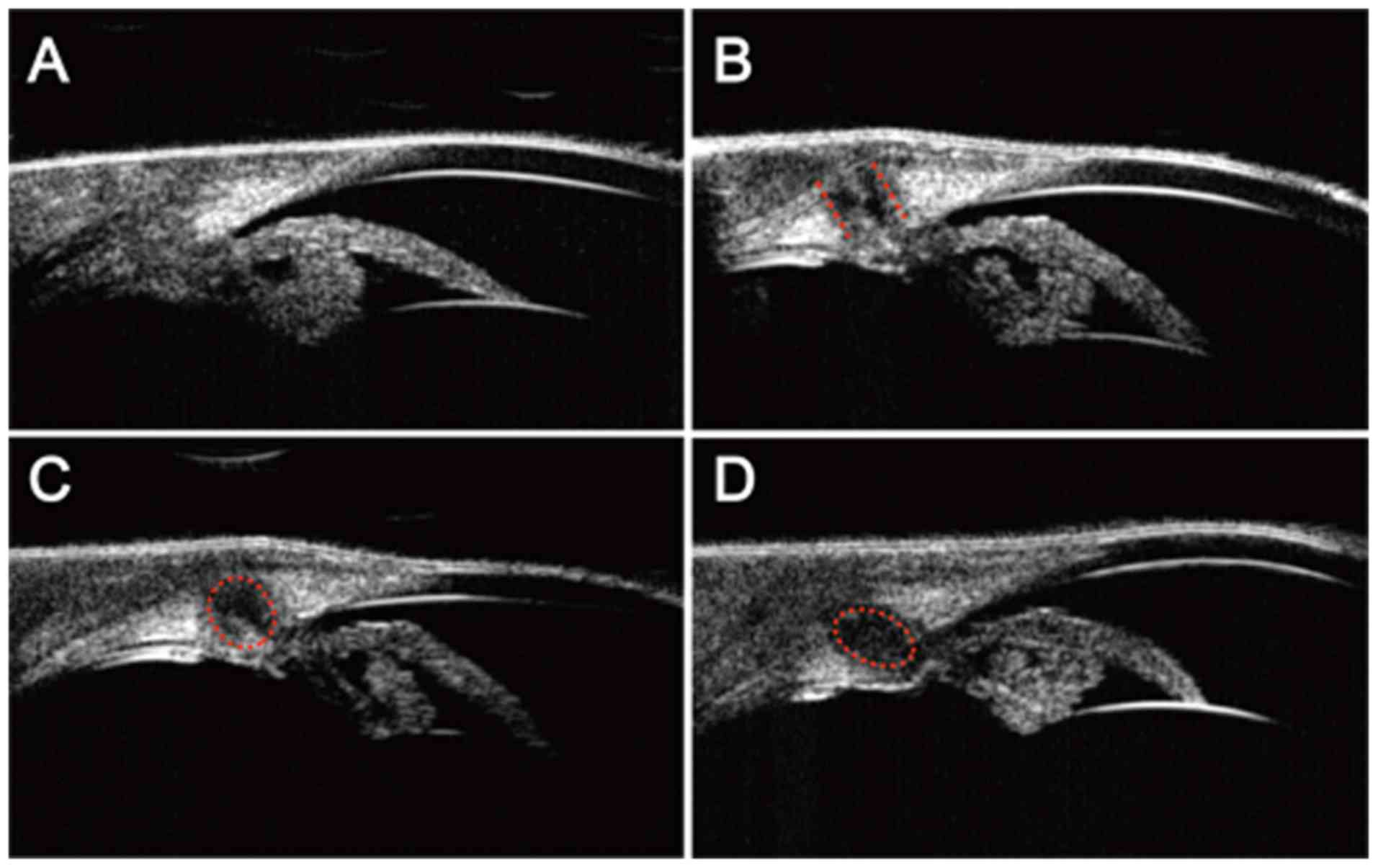
UBM investigation of anterior segment
after RFA
Stable UBM images were obtained by utilizing the
objective-bath conversion device coupled with UBM that we
developed. Based on this method, the changes of the anterior
segments at different time points after ablation were investigated.
Fig. 3A shows the anterior
segments of the sham group, in which the corneal, anterior chamber
angle, iris and the ciliary body could be seen clearly. Fig. 3B-D are days 1, 3 and 7
post-ablation, respectively. At 1 day post-RFA, a straight RFA
pathway was displayed in the ciliary body, damages within the
ciliary body can be seen, but no penetration of the ciliary body
was observed (Fig. 3B). At day 3,
ablation-caused damage had decreased, peripheral damage was
repaired and the ablation pathway showed a rounded rectangular
shape (Fig. 3C). At day 7
post-RFA, the area of damages continued to shrink, displaying an
oval shape (Fig. 3D).
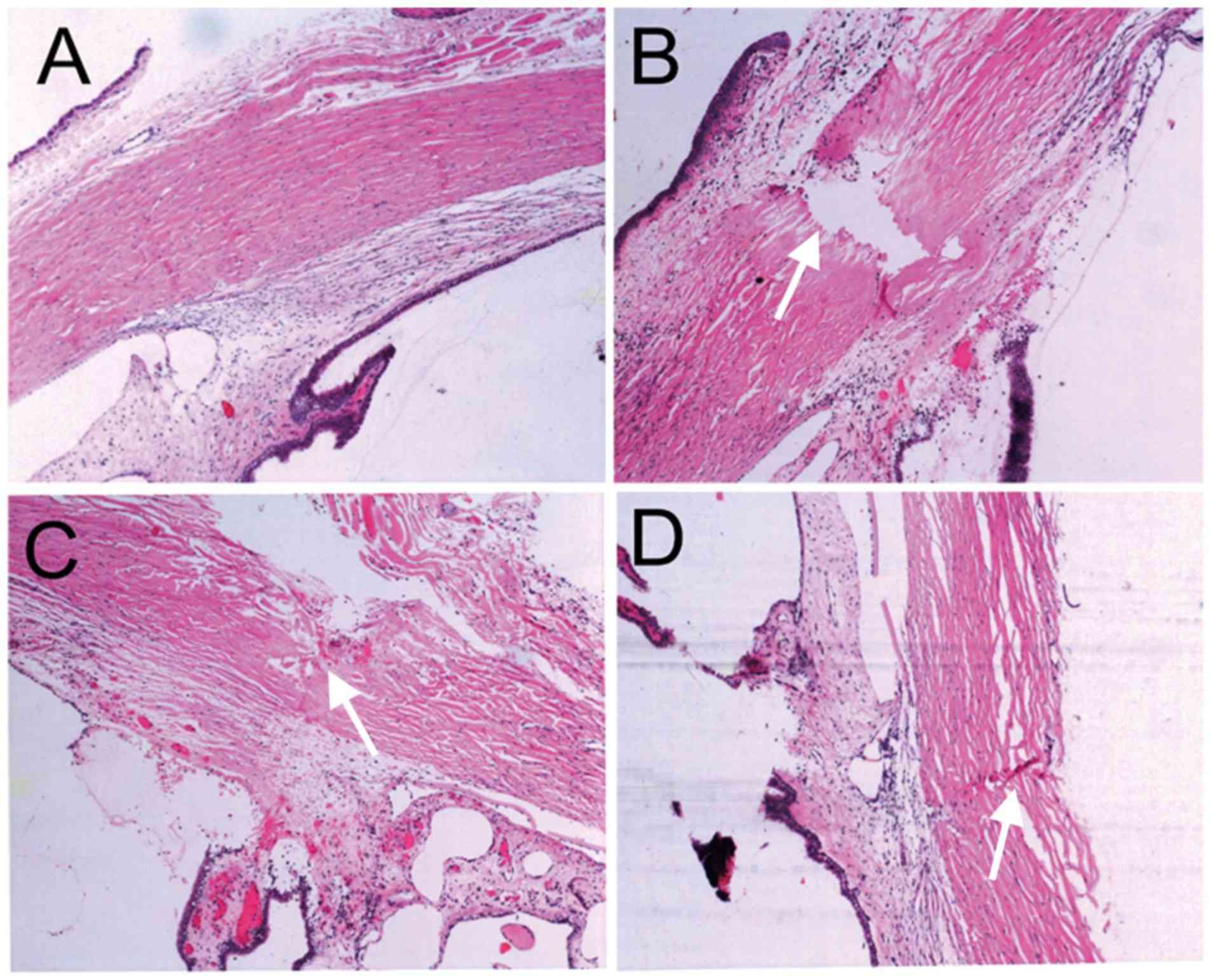
H&E staining and pathological
changes after RFA
At 1, 3, 7 and 14 days post-RFA of the ciliary body,
pathological changes were assessed in H&E stained slices with a
light microscope. The sham group displayed a normal chamber angle,
the muscle layers of the ciliary body were arranged densely and
regularly, and the epithelium was intact (Fig. 4A). At day 1 post-RFA, the sclera
fiber was fractured at the site of the ablation pathway and the
endothelium of the ciliary body had become disordered, but the
ciliary body was not penetrated (Fig.
4B). At 7 days post-RFA, the layer boundaries of the ciliary
body had become clear, muscle layer fibers on the ablation pathway
were arranged loosely and the scar had healed (Fig. 4C). At 14 days post-RFA, the scleral
ablation pathway had almost healed, with only a few cells
infiltrated, the ciliary body had healed and the epithelium was
integrated (Fig. 4D).
Discussion
RFA was first introduced by Harvey Cushing and WT
Bovie for the clinical treatment of neurological diseases, followed
by Huang in 1987 (26) who applied
it in the treatment of arrhythmia disease (27). In the 21st century, RFA
technologies began to be widely utilized in minimally invasive
surgery for the treatment of various diseases (18). Based on the characteristics of
glaucoma and the structure of eyes, the aim of the present study
was to find out whether RFA technology could also be applicable in
the treatment of glaucoma, which has not been reported previously.
In the current study, a New Zealand white rabbit model of glaucoma
was used to examine the potential of RFA of the ciliary body for
the treatment of glaucoma. As expected, the results of the present
study showed that region-specific ablation of the ciliary body
could reduce the production of aqueous humor, which is the cause of
increased ocular pressure, and gradually restore IOP to a normal
level over a long period of time.
Radiofrequency (RF) techniques have been used to
heat biological tissues for a number of years. Laser ablation (LA)
is also a thermal ablation technique (28). However, their heating mechanisms
are different. In RFA, resistive heating is generated by the
agitation of ions, due to the alternating directions of the
electric field, while in LA, hyperthermia is produced by increased
light absorption and heat conduction (29). To achieve a reduction in IOP, the
output power required is 1.5–2 W for 1.5–2 sec, and the temperature
of the tissue is set between 70°C and 110°C in LA (30), which seem to be higher than those
used for RFA in the present study (output power, 0.6 W; duration,
0.6 sec; temperature, 55–75°C). Thus, LA surgery could induce an
inflammatory reaction and lead to several related complications,
such as cystoid macular edema, hyphema and persistent hypotony
(31). In addition, RFA is
completed by moving the electrode under the guide of UBM and
thereby precisely acting on the target lesion regions (32), and thermometers can be incorporated
into the tips of the electrodes that allow for the continuous
monitoring of tissue temperatures (33). These both prevent underlying damage
to the adjacent structures. Furthermore, the fiber used in the LA
may be difficult to place into the target sites, so the treatment
efficiency and safety may be lower than RFA. Therefore, RFA may be
more suitable for the treatment of glaucoma.
The technique of minimally invasive RFA demonstrated
here has a high potential for application in the clinical treatment
of glaucoma (34,35). First of all, the rabbit's eye has
the power to proliferate and self-repair (36), while its size is comparable to the
human eye. The body size of the rabbit is small, which facilitates
the use of the slit lamp microscope. These advantages make the
rabbit model of glaucoma more suited to study common clinical
issues than other animal models, and consequently it is widely used
for the preclinical study of glaucoma (37). This is the reason that the ocular
hypertension model in rabbits was chosen for the present study.
Secondly, invasive RFA technology can not only control IOP
precisely, reliably, safely and durably, but it is also easy to
operate, it is a repeatable therapy, and it is relatively less
expensive, resulting in a high socio-economic value.
Since standard wands were used, some scleral damage
occurred after ablation. This damage could be seen in the images
obtained using ultrasound biomicroscopy, UBM anterior segment
imaging and pathological imaging. To this end, a new type of RF
wand was designed for ablation of the ciliary body (patent
pending). This new wand is loaded with a specially designed
insulating sheath that can more effectively protect the tissue
around the sclera puncture track, making ablation more restricted
to the needle tip of the wand or handle and thus, the ciliary
processes (Fig. 5).
However, there are some limitations to the present
study. Firstly, this was only an animal model study and further
clinical studies with short or long-term follow up are also needed
to confirm the effectiveness and safety of RFA. Risks may include
cataract development, cognitive effects and possible carcinogenic
effects due to the frequency used, which is within the range of 30
kHz to 300 GHz. Secondly, a direct comparison between RFA and LA
for glaucoma is lacking.
The results of the present study indicated that XL-1
meter-based RFA of the ciliary body for controlling ocular pressure
in patients with glaucoma is mostly safe and effective, showing
only a few anterior chamber reactions and almost no influence on
intraocular structures. In further studies, the new handle for the
ciliary body ablation, the dose-effect relationship of the ciliary
body ablation in the treatment of glaucoma and the application of
RFA of the ciliary body for treatment of absolute glaucoma in
clinical settings will be investigated.
Acknowledgements
The authors would like to thank Dr Xinglin Wang
(Chinese PLA General Hospital) for assistance using the RF
instruments and Dr Zhigang Song for helping to analyze the
pathological results (Chinese PLA General Hospital).
Funding
The present study was supported by The National Key
Research and Development Project (grant no. 2016YFC1305504).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived and designed the experiments. ZY
analyzed the data. BH and FW performed the experiments and wrote
the article for publication. ZY, XJ, YF and ZL helped to perform
the experiments. All authors read and approved the final version of
the manuscript to be published.
Ethics approval and consent to
participate
All protocols were conducted in accordance with the
National Institutes of Health Guidelines for the Care and Use of
Laboratory Animals and approved by the Institutional Animal Care
and Use Committee of Chinese PLA General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haffner DS, Smedley GT, Tu H and Burns TW:
Devices and methods for glaucoma treatment. US Patent 9,597,230.
Filed March 20, 2017; issued February 8, 2018.
|
|
2
|
Gupta N and Yücel YH: Glaucoma as a
neurodegenerative disease. Curr Opin Ophthalmol. 18:110–114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cook C and Foster P: Epidemiology of
glaucoma: What's new? Can J Ophthalmol. 47:223–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quigley HA and Broman AT: The number of
people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol.
90:262–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang JJ, Frankfort BJ, Gross RL and Wu SM:
Elevated intraocular pressure decreases response sensitivity of
inner retinal neurons in experimental glaucoma mice. Proc Natl Acad
Sci USA. 112:2593–2598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy Chowdhury U and Fautsch MP:
Intracranial pressure and its relationship to glaucoma: Current
understanding and future directions. Med Hypothesis Discov Innov
Ophthalmol. 4:712015.PubMed/NCBI
|
|
7
|
Stevens A, Iliev ME, de Jong L, Grobeiu I
and Hommer A: A combined analysis of four observational studies
evaluating the intraocular pressure-lowering ability and
tolerability of bimatoprost 0.01% in patients with primary
open-angle glaucoma or ocular hypertension. Clin Ophthalmol.
10:635–641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heijl A, Leske MC, Bengtsson B, Hyman L,
Bengtsson B and Hussein M; Early Manifest Glaucoma Trial Group, :
Reduction of intraocular pressure and glaucoma progression: Results
from the Early Manifest Glaucoma Trial. Arch Ophthalmol.
120:1268–1279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dietlein TS, Hermann MM and Jordan JF: The
medical and surgical treatment of glaucoma. Dtsch Arzteblatt Int.
106:597–605. 2009.
|
|
10
|
Calissendorff BM: Costs of medical and
surgical treatment of glaucoma. Acta Ophthalmol Scand. 79:286–288.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenfeld C, Price MO, Lai X, Witzmann FA
and Price FW Jr: Distinctive and pervasive alterations in aqueous
humor protein composition following different types of glaucoma
surgery. Mol Vis. 21:911–918. 2015.PubMed/NCBI
|
|
12
|
Stryker JE, Beck AD, Primo SA, Echt KV,
Bundy L, Pretorius GC and Glanz K: An exploratory study of factors
influencing glaucoma treatment adherence. J Glaucoma. 19:66–72.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baudouin C, Denoyer A and Rosténe W:
Glaucoma today: Detection and therapeutic progress. Biol
Aujourdhui. 207:87–95. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed S, Khan Z, Si F, Mao A, Pan I, Yazdi
F, Tsertsvadze A, Hutnik C, Moher D, Tingey D, et al: Summary of
glaucoma diagnostic testing accuracy: An evidence-based
meta-analysis. J Clin Med Res. 8:641–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gedde SJ and Vinod K: Resident surgical
training in glaucoma. Curr Opin Ophthalmol. 27:151–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wahl DR, Stenmark MH, Tao Y, Pollom EL,
Caoili EM, Lawrence TS, Schipper MJ and Feng M: Outcomes after
stereotactic body radiotherapy or radiofrequency ablation for
hepatocellular carcinoma. J Clin Oncol. 34:452–459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haemmerich D: Biophysics of radiofrequency
ablation. Crit Rev Biomed Eng. 38:53–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldberg SN: Radiofrequency tumor
ablation: Principles and techniques. Multi-treatment modalities of
liver tumours. Springer, Boston, MA. 87–118. 2002.
|
|
19
|
Ye PP, Xu W, Xu HS, Li ZC, Shi JT, He FY
and Yao K: Conductive keratoplasty: An approach for the correction
of residual hyperopia in post-lasik pseudophakia. Int J Ophthalmol.
5:630–633. 2012.PubMed/NCBI
|
|
20
|
Sy ME, Kovoor TA, Tannan A, Choi D, Deng
SX, Danesh J and Hamilton DR: Combined astigmatic keratotomy and
conductive keratoplasty to correct high corneal astigmatism. J
Cataract Refract Surg. 41:1050–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng CH, Sanders GD, Hlatky MA,
Heidenreich P, McDonald KM, Lee BK, Larson MS and Owens DK:
Cost-effectiveness of radiofrequency ablation for supraventricular
tachycardia. Ann Intern Med. 133:864–876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Institute of Laboratory Animal Resources
(US). Committee on Care, the 2. Laboratory Animal Husbandry in Use
of Laboratory Animals, . Guide for the care and use of laboratory
animals. US Department of Health and Human Services, Public Health
Service, National Institutes of Health. 1986.
|
|
23
|
Yan D, Zhang J, Liang W, Sun J, Liu BY,
Tian W and Cheng XG: Magnetic resonance imaging and
histopathological analysis of experimental muscle injuries in a
rabbit. Biomed Environ Sci. 26:841–848. 2013.PubMed/NCBI
|
|
24
|
Zhang ZY, Chu RY, Zhou XT, Dai JH, Sun XH,
Hoffman MR and Zhang XR: Morphologic and histopathologic changes in
the rabbit cornea produced by femtosecond laser-assisted multilayer
intrastromal ablation. Invest Ophthalmol Visual Sci. 50:2147–2153.
2009. View Article : Google Scholar
|
|
25
|
Balazs T, Hooper W, Farber TM, Van Loon
EJ, Earl FL and Weinberger MA: Studies on the effects of
anticonvulsant drugs on the activity of vitamin D in rats and dogs.
Toxicol Appl Pharmacol. 29:47–52. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang SK, Bharati S, Graham AR, Lev M,
Marcus FI and Odell RC: Closed chest catheter desiccation of the
atrioventricular junction using radiofrequency energy-a new method
of catheter ablation. J Am Coll Cardiol. 9:349–358. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao Y, Ni Y, Yu J, Zhang H and Marchal G:
Evaluation of radiofrequency ablation as an alternative for the
treatment of brain tumor in rabbits. J Neurooncol. 56:119–126.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen A, Wong SH, Patel S and Tsai JC:
Endoscopic cyclophotocoagulation for the treatment of glaucoma.
Surv Ophthalmol. 62:357–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Costanzo GG, Tortora R, D'Adamo G, De
Luca M, Lampasi F, Addario L, Galeota Lanza A, Picciotto FP,
Tartaglione MT, Cordone G, et al: Radiofrequency ablation versus
laser ablation for the treatment of small hepatocellular carcinoma
in cirrhosis: A randomized trial. J Gastroenterol Hepatol.
30:559–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fenelon G, Pereira KP and de Paola AA:
Epicardial radiofrequency ablation of ventricular myocardium:
Factors affecting lesion formation and damage to adjacent
structures. J Interve Card Electrophysiol. 15:57–63. 2006.
View Article : Google Scholar
|
|
31
|
Kaplowitz K, Kuei A, Klenofsky B, Abazari
A and Honkanen R: The use of endoscopic cyclophotocoagulation for
moderate to advanced glaucoma. Acta Ophthalmol. 93:395–401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vogl TJ, Farshid P, Naguib NN, Darvishi A,
Bazrafshan B, Mbalisike E, Burkhard T and Zangos S: Thermal
ablation of liver metastases from colorectal cancer:
Radiofrequency, microwave and laser ablation therapies. Radiol Med.
119:451–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH,
Baek SH, Døssing H and Hegedüs L: Comparative efficacy of
radiofrequency and laser ablation for the treatment of benign
thyroid nodules: Systematic review including traditional pooling
and bayesian network meta-analysis. J Clin Endocrinol Metab.
100:1903–1911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bong JJ, Kumar R and Spalding D: A novel
technique of partial splenectomy using radiofrequency ablation. J
Gastrointest Surg. 15:371–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nierkens S, den Brok MH, Ruers TJ and Adem
GJ: Radiofrequency ablation in cancer therapy: Tuning in to in situ
tumor vaccines. Tumor Ablation. Springer; Dordrecht: pp. 39–59.
2013, View Article : Google Scholar
|
|
36
|
Abrams GW, Topping TM and Machemer R:
Vitrectomy for injury: The effect on intraocular proliferation
following perforation of the posterior segment of the rabbit eye.
Arch Ophthalmol. 97:743–748. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Esson DW, Neelakantan A, Iyer SA, Blalock
TD, Balasubramanian L, Grotendorst GR, Schultz GS and Sherwood MB:
Expression of connective tissue growth factor after glaucoma
filtration surgery in a rabbit model. Invest Ophthalmol Visual Sci.
45:485–491. 2004. View Article : Google Scholar
|