Introduction
Intestinal ischemia/reperfusion (I/R), a common
clinical event, is always related to medical conditions, such as
serious trauma, hemorrhagic shock, vascular occlusion, or organ
transplantation (1). I/R injury
has been divided into an initial phase, which is due to the
synthesis of oxygen-free radicals, followed by a later phase
involving activation of the innate immune system (2). Ultimately, remote organ injury leads
to systemic inflammatory response syndrome and multiple organ
dysfunction syndrome. Therefore, interference with the activation
or activity of the innate immune system may be beneficial for
alleviation and prevention of I/R-induced intestinal damage.
6-Formylindolo(3,2-b)carbazole (FICZ) is a ligand of
the aryl hydrocarbon receptor (AhR), which is part of the basic
helix-loop-helix/Per-Arnt-Sim superfamily (3). Generally, the AhR is bound to several
co-chaperones in an inactive form in the cytosol. Once bound by a
ligand, the AhR translocates to the nucleus to initiate the
transcription of a variety of genes (4). The best characterized target genes of
the AhR include the cytochrome P450 family members, CYP1A1 and
CYP1A2 (5). AhR signaling plays an
important role in the control of several components of the innate
immune system, including dendritic cells (DCs), macrophages, and
innate lymphoid cells (ILCs) (6).
Indeed, the AhR in macrophages limits lipopolysaccharide
(LPS)-induced inflammation (7) and
the AhR in DCs mediates the anti-inflammatory activities of lipoxin
(8). Moreover, the AhR serves to
induce expression of Bcl-2, c-Kit, IL-7R and Notch2, which together
promote the survival of ILCs. Therefore, the AhR provides a
molecular pathway by which the environment can affect the innate
immune response and the development of innate immune-mediated
injury.
Intestinal intraepithelial lymphocytes (IELs), which
are involved in regulating mucosal immune responses, are essential
intestinal innate immune cells. It has been shown that IELs not
only maintain the intestinal epithelial barrier function and
initiate the early response to infection in the intestine (9), but regulate activation of immune
cells (10). Gamma-delta (γδ) IELs
constitute 30–50% of IELs in the small intestine (11). A role for γδIELs has been reported
in maintaining the protective barrier in DSS-treated mice and
γδIELs are indispensable for restricting the mucosal penetration of
commensal bacteria (12). It has
also been shown that γδIELs contribute to mucosal homeostasis
(13) by secreting keratinocyte
growth factor (KGF) (14) and
antimicrobial peptides (15) and
by suppressing CD4+ T cell expansion through the
production of TGF-β and IL-10 (13). Our group has demonstrated that
I/R-induced acute intestinal mucosal barrier damage significantly
reduced the proportion of small intestine γδIELs and that I/R can
trigger inflammatory responses and increase the apoptosis of IELs
(16). Moreover, we also
demonstrated that FICZ-mediated AhR activation inhibits
inflammation in the gastrointestinal tract in mice with DSS-induced
colitis (17). The AhR is highly
expressed in γδIELs and has been reported to function in γδIELs.
Mice without the AhR exhibit a >95% loss of γδIELs in the small
intestine (18). We also
demonstrated that administration of FICZ increases the proportion
of γδIELs and downregulates inflammatory responses in colitis. In
the present study, we investigated the effect of FICZ on changes in
γδIELs and on the subsequent inflammatory response to I/R injury of
the small intestine. Indeed, AhR activation may be a crucial factor
for preventing I/R-induced intestinal mucosal injury.
Materials and methods
Animal experiments
Male C57BL/6 mice weighing 20±2 g were purchased
from the Laboratory Animal Center of the Army Medical University
(Chongqing, China). All animals were 6–8 weeks of age and specific
pathogen-free. At the beginning of the study, the animals were
housed under temperature-, humidity-, and light-controlled
conditions. The laboratory animals were cared for according to the
Guidelines for the Care and Use of Laboratory Animals, as set forth
and approved by the University Committee at the Army Medical
University.
Operative procedures
After a 12-h fast, the mice were anesthetized with
an intraperitoneal injection of sodium pentobarbital (50 ml/kg).
The abdomens were opened via midline incisions. The mice were
randomly assigned to the sham group and two I/R groups, as follows:
i) sham group, no intestinal ischemia treatment; ii) I/R group, the
superior mesenteric vessels (SMVs) were occluded for 30 min and an
intraperitoneal injection of saline (0.3 ml) was administered 6 h
before reperfusion; and iii) I/R+FICZ group, an intraperitoneal
injection of FICZ [1 µg/mouse (0.3 ml)] was given before
reperfusion. Then, the mice in the three groups were sacrificed by
cervical dislocation. The mice were sprayed with 70% ethanol, an
abdominal midline incision was performed and then the mesenteric
lymph nodes were isolated and collected from the entire small
intestine. The same individual performed these manipulations. The
experiment was repeated at least three times.
Histologic examination
The same intestinal segment (2 cm) from each animal
was removed and immediately placed in 4% paraformaldehyde. Paraffin
blocks were prepared from the tissue pieces. The blocks were cut
into sections (5-µm thick), stained with hematoxylin and eosin
(H&E) and observed by light microscopy. Histologic evaluations
were graded from 0–5 by a single observer, as follows: grade 0,
normal morphology; grade 1, sub-epithelial edema and partial
separation of apical cells; grade 2, moderate lifting of
enterocytes from the tips of the villi; grade 3, lifting of
enterocytes from both the tips and sides of the villi, including
the superficial crypts; grade 4, partial mucosal necrosis of the
lamina propria; and grade 5, total mucosal necrosis.
Detection of intestinal
permeability
After the small intestine was harvested, a 3-cm
section of the Peyer's patch-free jejunum along the mesenteric
border was opened and gently washed three times with cool RPMI-1640
medium to cleanse the luminal contents. The full-thickness small
intestine was fixed in a modified Ussing chamber (Physiologic
Instruments, San Diego, CA, USA). Each half cell (mucosal and
serosal) was filled with 5 ml of pre-heated 37°C Krebs-Ringer
solution buffer containing 110.0 mM NaCl, 5.5 mM KCl, 3.0 mM
CaCl2, 1.2 mM MgCl2, 1.4 mM
KH2PO4 and 29.0 mM NaHCO3,
adjusted to pH 7.4. The cells were continuously oxygenated with
O2/CO2 (95%/5%) and stirred by gas flow in
the chambers. One pair of Ag/AgCl electrodes (Physiologic
Instruments) with 3 mM KCl in 3% agar bridges was used to calculate
the transepithelial potential difference and another pair of Pt
electrodes was used for current passage. The transmembrane
resistance (TER) was determined using Ohm's law. The baseline short
circuit current (ISC) was recorded for up to 120 min after a 20-min
equilibration period.
IEL isolation
The entire small intestine was removed and placed in
tissue culture media (RPMI-1640). The small intestine was cut
longitudinally into 5-mm pieces, washed 3 times with the same
tissue culture media, and incubated in isolation buffer (54 ml of
PBS, 22.8 mg of EDTA, 9.6 mg of DTT, and 6 ml of 10% fetal calf
serum) with continuous gentle shaking at 37ºC for 30
min. After centrifugation, the pellets were purified in 40%
isotonic Percoll (GE Healthcare Biosciences) and reconstituted in
RPMI-1640 tissue culture media. The viability of the IELs exceeded
96% based on trypan blue exclusion staining.
Quantitative PCR (qPCR)
Total RNA was isolated from the sorted IELs using
Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
Briefly, RNA was reverse-transcribed into complementary DNA (cDNA)
using a SuperScript First-Strand Synthesis System RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc.), and this cDNA was
used as a template for the amplification of β-actin, TNF-α, IL-1β,
IL-6, Bcl2, Bcl2l1, KGF, CYP1A1 and CD127. The primer sequences are
presented in Table I (gene symbol:
forward, reverse). Gene expression was determined by qPCR, which
was performed using a SYBR PrimeScript RT kit, as described by the
manufacturer (Takara Bio Inc., Kusatsu, Japan). The PCR mixture (20
µl final volume per reaction) was prepared per the manufacturer's
protocol. Amplifications were performed under the following
conditions on a 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.): 94ºC for 5 min; 35
cycles of 94ºC for 30 sec, 59ºC for 30 sec,
and 72ºC for 30 sec; and 72ºC for 10 min. The
expression of each target gene was calculated by the
2−ΔΔCq method (19).
 | Table I.Primer sequences used for qPCR assays
of inflammatory mediators. |
Table I.
Primer sequences used for qPCR assays
of inflammatory mediators.
| Gene name | 5′-3′ primer
sequence |
|---|
| β-actin | F:
CTTCTTTGCAGCTCCTTCGTT |
|
| R:
AGGAGTCCTTCTGACCCATTC |
| TNF-α | F:
TCTTCTCATTCCTGCTTGTGG |
|
| R:
CACTTGGTGGTTTGCTACGAC |
| IL-1β | F:
CCAGCTTCAAATCTCACAGCAG |
|
| R:
CTTCTTTGGGTATTGCTTGGGATC |
| IL-6 | F:
TCCAGTTGCCTTCTTGGGAC |
|
| R:
GTACTCCAGAAGACCAGAGG |
| Bcl2 | F:
AGTACCTGAACCGGCATCTG |
|
| R:
AGGTATGCACCCAGAGTGATG |
| Bcl2l1 | F:
ATGACCACCTAGAGCCTTTGGA |
|
| R:
GAAGAGTGAGCCCAGCAGAAC |
| KGF | F:
CGCAAATGGATACTGACACG |
|
| R:
GGGCTGGAACAGTTCACACT |
| CYP1A1 | F:
CCAAGAGCTGCTCAGCATAG |
|
| R:
GGCATCCAGGGAAGAGTTAG |
| CD127 | F:
GCGGACGATCTCCTTCTG |
|
| R:
AGCCCCACATATTTGAAATTCCA |
Flow cytometric analysis
The IEL subtype was confirmed by fluorescent
antibody staining, which was detected using flow cytometry. The
following anti-mouse monoclonal antibodies (eBioscience, Inc., San
Diego, CA, USA) were used: CD45-PE (dilution 1:100; cat. no.
12-0451-82); CD45-PerCP-Cy5.5 (dilution 1:100; cat. no.
45-0451-82); CD4-PE (dilution 1:100; cat. no. 12-0041-82);
CD69-FITC (dilution 1:100; cat. no. 11-0691-82); CD8α-APC (dilution
1:100; cat. no. 17-0081-82); CD8β-PE (dilution 1:100; cat. no.
12-0083-82); TCRβ-APC (dilution 1:100; cat. no. 17-5961-82);
CD127-PE-CY7 (dilution 1:100; cat. no. 25-1271-82); and
TCRγδ-FITC (dilution 1:100; cat. no. 11-5711-82). Isotype-matched,
irrelevant antibodies (eBioscience, Inc.) were used as negative
controls: Rat IgG2b kappa Isotype Control (eB149/10H5), PE (cat.
no. 12-4031-82); Rat IgG2b kappa Isotype Control (eB149/10H5),
PerCP-Cyanine5.5 (cat. no. 45-4031-80); Armenian Hamster IgG
Isotype Control (eBio299Arm), FITC (cat. no. 11-4888-81); Rat IgG2a
kappa Isotype Control (eBR2a), APC (cat. no. 17-4321-81). The
apoptotic ratios of γδIELs among the different groups were detected
by flow cytometry per the manufacturer's protocol. γδIELs were
stained with PE-Annexin V and cellular DNA was stained
with7-aminoactinomycin D (7-AAD), as described in the protocol. The
acquisition and analysis were performed using MoFlo XDP (Beckman
Coulter, Inc., Brea, CA, USA).
Total γδIEL isolation
To study associated gene expression in γδIELs, total
IELs were stained with the following anti-mouse monoclonal
antibodies (eBioscience, Inc.): FITC anti-mouse TCRγδ (dilution
1:100; cat. no. 11-5711-82); and PE anti-mouse CD8β (dilution
1:100; cat. no. 12-0083-82). γδIELs were purified using a cell
sorter (Beckman Coulter). The TCRδ+/CD8β−
subsets were isolated from γδIELs and the purity was ≥98%.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). The results were analyzed by one-way analysis of
variance (ANOVA) followed by Tukey's post-hoc test using SPSS
statistical software package. A P-value <0.05 was considered to
indicate statistical significance.
Results
I/R induces changes in intestinal
morphology and FICZ ameliorates inflammation following intestinal
I/R injury
Based on our previous study, the destruction of
intestinal morphology was more severe 6 h after intestinal I/R.
Therefore, this time-point was chosen for subsequent experiments.
H&E was used to stain the small intestines from mice in the
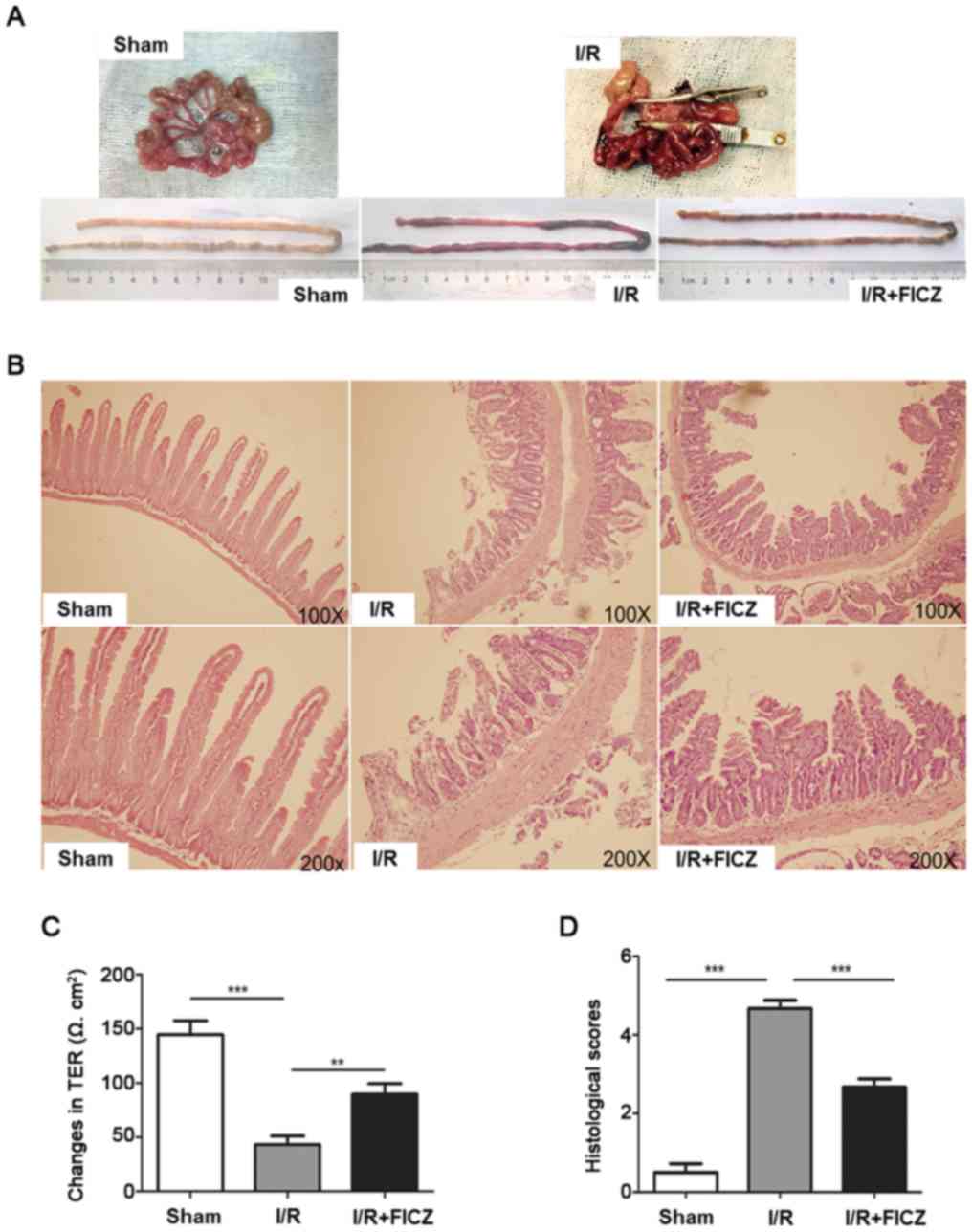
sham, I/R and I/R+FICZ groups. Representative images from each
group are presented in Fig. 1A.
The intestines of the sham group showed an intact epithelium, a
normal villous length, well-defined glands, and minimal leukocyte
infiltration in the mucosa. In contrast, severe mucosal damage,
including villous blunting, epithelial denudation, gland
distortion, leukocyte inflammation, and lamina propria
disintegration, were observed in the I/R group (Fig. 1B). FICZ attenuated these changes.
The histologic scores of intestinal mucosal injury are presented as
the mean ± SD (Fig. 1D). Moreover,
changes in intestinal permeability were detected using Ussing
chambers. Compared with the I/R+FICZ group, the TER of the I/R
group was significantly lower (P<0.01; Fig. 1C). These results showed that FICZ
attenuates the IR-induced destruction of intestinal morphology and
intestinal barrier function.
FICZ downregulates pro-inflammatory
mediator mRNA expression in small intestinal IELs
During reperfusion injury, pro-inflammatory
molecules, such as IL-1β, IL-6 and TNF-α, directly induce tissue
damage to affect intestinal barrier function and are potent
activators of other neutrophils. Based on the attenuation of
decreased intestinal barrier function, the effect of FICZ was
confirmed on IL-1β, IL-6 and TNF-α gene expression. The expression
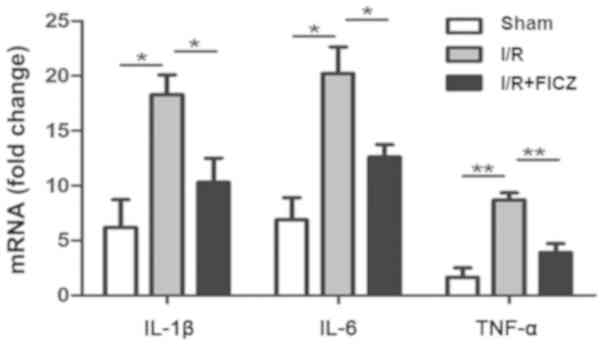
of IL-1β, IL-6 and TNF-α mRNA in intestinal IELs is shown.
Real-time PCR showed that IEL-derived IL-1β (P<0.05; Fig. 2), IL-6 (P<0.05; Fig. 2), and TNF-α (P<0.01; Fig. 2) mRNA expression was significantly
increased after I/R compared with the sham group. Importantly, the
expression of IL-1β (P<0.05; Fig.
2), IL-6 (P<0.05; Fig. 2)
and TNF-α (P<0.01; Fig. 2) was
significantly decreased in the I/R+FICZ group when compared with
the I/R group.
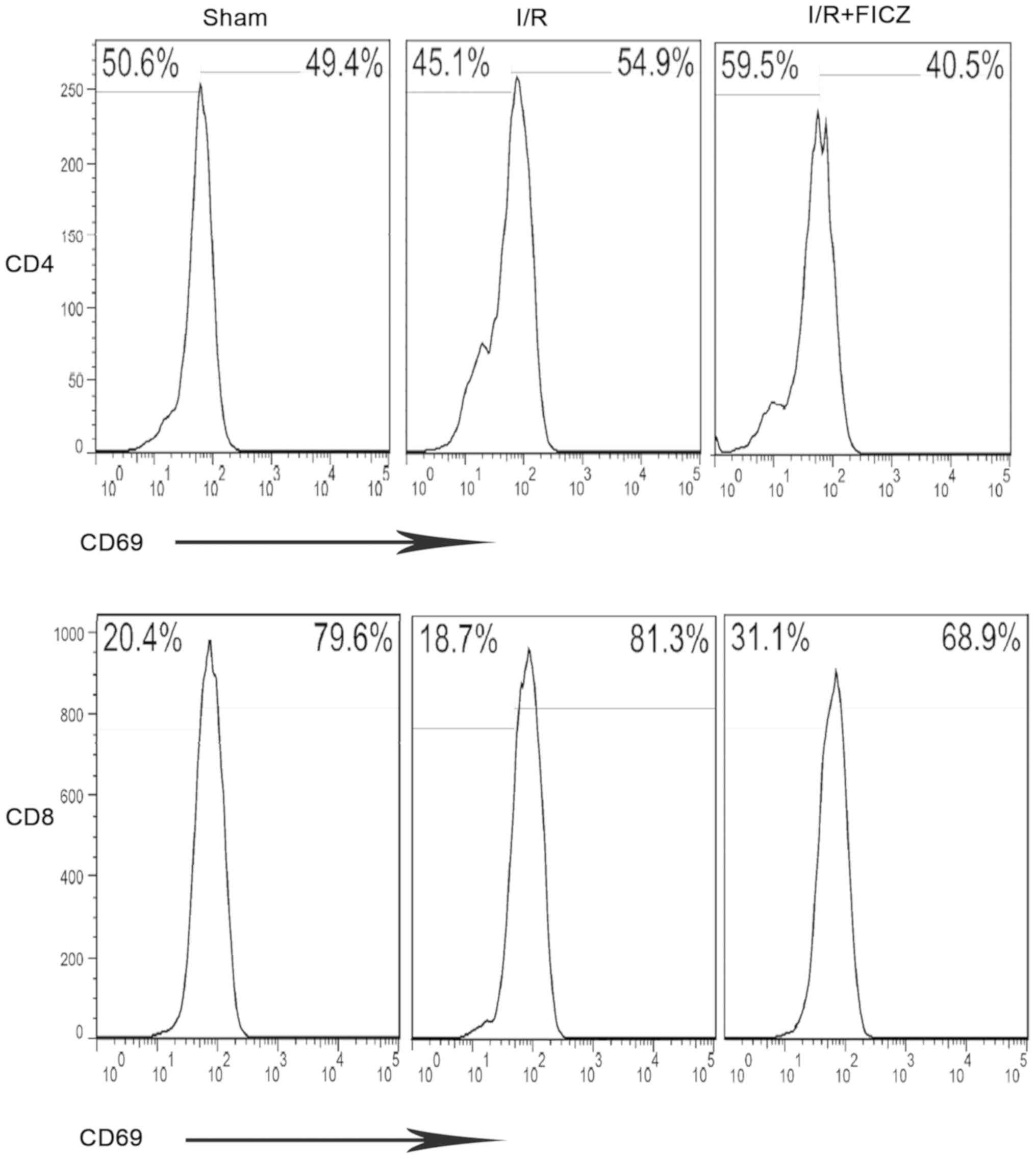
FICZ reduces the percentage of activated
CD4+ and CD8+ IEL subpopulations. CD69, an
early T cell activation marker, is expressed in most IELs,
indicating the intensity of inflammatory responses. In the present
study, CD69 was used to assess CD4+ and CD8+
IEL subsets. Compared with the sham group,
CD4+CD69+ and CD8+CD69+
IEL subpopulations were significantly increased in the I/R group
(55.30±1.92% vs. 49.40±2.22%, P<0.05; 85.60±3.22% vs.
77.40±2.00%, P<0.05; Fig. 3).
Notably, the I/R+FICZ group had a decreased population of IELs
compared with the I/R group (43.60±2.90% vs. 55.30±1.92%,
P<0.01; 72.10±2.86% vs. 85.60±3.22%, P<0.01; Fig. 3).
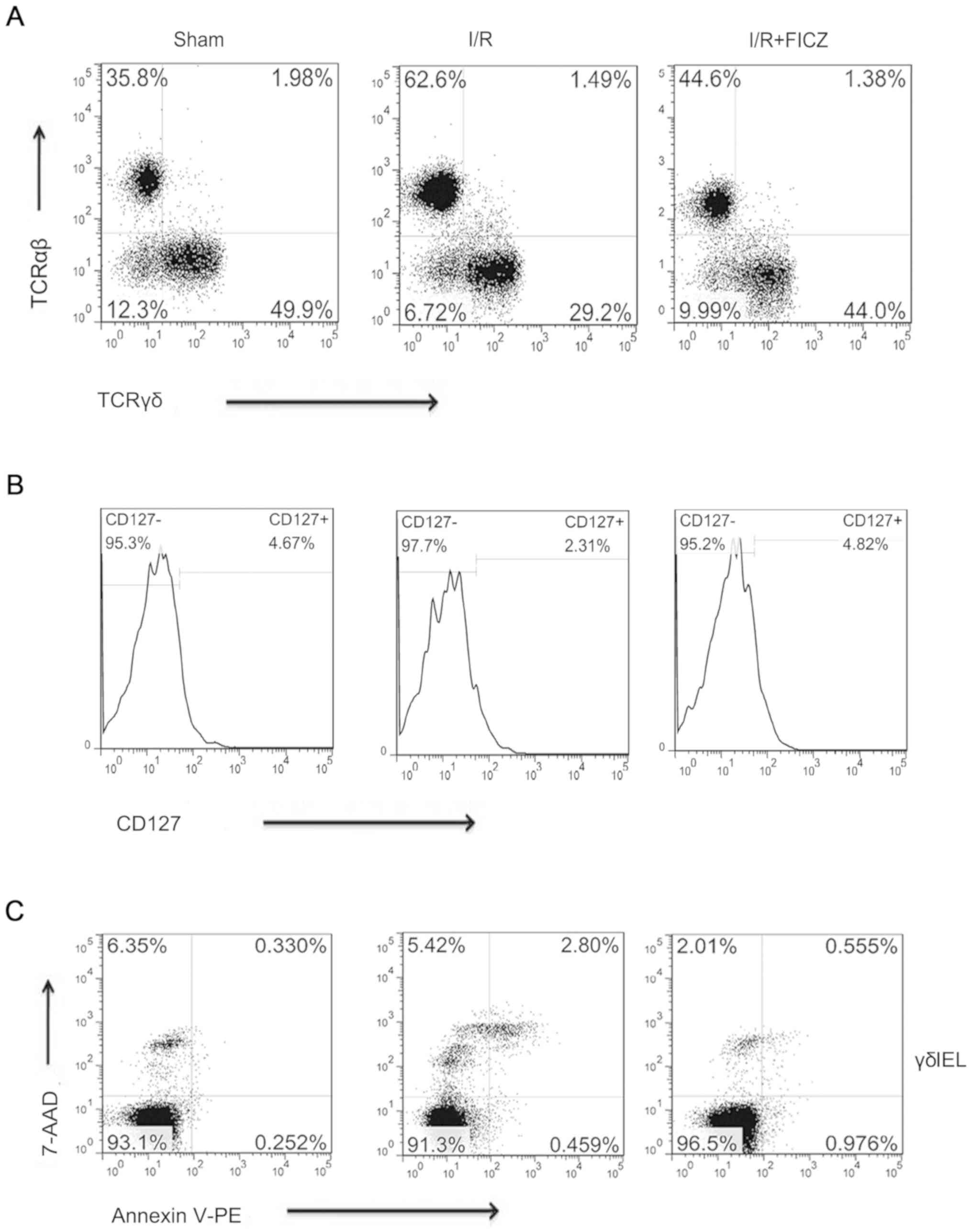
FICZ enhances the percentage of
TCRγδ+/CD45+and
CD127+/TCRγδ+ T cells and decreases apoptosis
in γδIELs
Reductions in intestinal inflammation suggested that
FICZ may alter IEL phenotypes. Thus, a series of phenotypic
analyses were performed by flow cytometry. The T-cell receptor
(TCR) subpopulations were examined. Compared with the sham group,
intestinal I/R treatment resulted in lower percentages of small
intestinal IEL TCRγδ+/CD45+ T cells
(29.20±2.43% vs. 51.30±1.87%, P<0.001; Fig. 4A) and
CD127+/TCRγδ+ T cells (2.31±0.26% vs.
4.65±0.32%, P<0.05; Fig. 4B).
The percentage of TCRγδ+/CD45+ T cells
(40.80±.72% vs. 29.20±2.43%, P<0.01; Fig. 4A) and
CD127+/TCRγδ+ T cells (4.82±0.50% vs.
2.31±0.26%, P<0.01; Fig. 4B)
among small intestinal IELs was significantly higher in the
I/R+FICZ group than the I/R group. Considering the decreased
percentage of TCRγδ cells, the survival of γδIELs was subsequently
investigated by assaying I/R apoptosis using flow cytometry. There
were significant changes in the apoptosis of these cells. Compared
with the sham group, the apoptosis rate (early and late) of γδIELs
was significantly increased after I/R (3.26±0.51% vs. 0.78±0.23%,
P<0.01; Fig. 4C). FICZ
administration significantly attenuated apoptosis induced by
intestinal I/R (1.53±0.33% vs. 3.26±0.51%, P<0.05; Fig. 4C).
CYP1A1, KGF, Bcl2, Bcl2l1 and CD127
expression in small intestinal γδIELs
The expression of CD127, which binds to IL-7, is
regulated throughout T cell development, activation, and survival,
and CD127 signals are thus required for γδ T cell development and
survival. Since the proportion of
TCRγδ+/CD45+ T cells was decreased after I/R,
it was determined whether or not the expression of CD127 mRNA in
small intestinal γδIELs was altered. CD127 gene expression was
significantly lower in the I/R group than that noted in the sham
group (P<0.05; Fig. 5B). Bcl2
and Bcl2l1 expression is maintained by IL-7R signaling. Thus, we
detected the expression of Bcl2 and Bcl2l1 mRNA in small intestinal
γδIELs. The expression of Bcl2 and Bcl2l1 was significantly
decreased after I/R (P<0.05; Fig.
5B). KGF, which plays a critical role in intestinal epithelial
growth and maintenance, is only expressed by γδIELs. Intestinal I/R
resulted in a significant decrease in the expression of KGF mRNA
(P<0.05; Fig. 5A). As a
standard indicator of AhR activation, the expression of CYP1A1 mRNA
in γδIELs was significantly decreased after I/R when compared with
the sham group (P<0.01; Fig.
5A). Interestingly, the I/R+FICZ group had significantly higher
CYP1A1expression when compared with the I/R group (P<0.01;
Fig. 5A). Bcl2 and Bcl2l1 gene
expression in the I/R+FICZ group increased significantly compared
with the I/R group (P<0.05; Fig.
5B). Moreover, compared with the I/R group, CD127 expression
was significantly increased after FICZ treatment (P<0.01;
Fig. 5B). As expected, KGF gene
expression was increased after FICZ administration compared with
I/R alone (P<0.01; Fig.
5A).
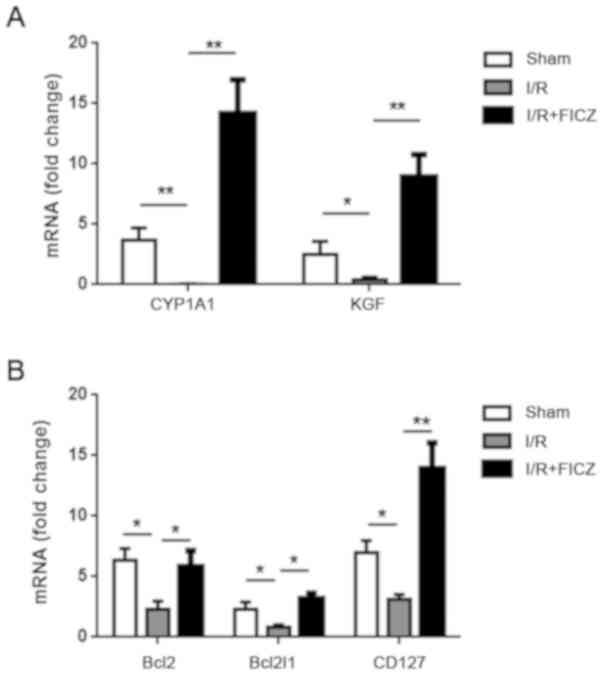 | Figure 5.FICZ upregulates the expression of
anti-apoptosis genes (Bcl2 and Bcl2l1) and CYP1A1, KGF and CD127 in
γδIELs. (A) The expression of CYP1A1 and KGF in γδELs was analyzed
by qPCR. (B) The expression of Bcl2, Bcl2l1, and CD127 mRNA in
γδIELs was analyzed by qPCR. Each group had 2–3 mice and every
experiment was repeated three times. *P<0.05; **P<0.01. FICZ,
6-formylindolo(3,2-b)carbazole; IELs, intraepithelial lymphocytes;
Bcl2, B-cell lymphoma 2; Bcl2l1, BCL2-like 1; KGF, keratinocyte
growth factor; CYP1A1, cytochrome P450 1A1; CD127, cluster of
differentiation 127. |
Discussion
Intestinal ischemia/reperfusion (I/R) injury that
occurs in a variety of clinical conditions may lead to local or
systemic inflammatory responses (10,20).
As a source of pro-inflammatory stimuli and an important barrier
against intestinal bacteria, the small intestine is vulnerable
during local I/R (21). Intestinal
I/R is considered an abdominal emergency and is associated with
high morbidity and mortality rates. It has been reported that
intestinal I/R injuries feature acute inflammation, oxygen
radicals, and cytolysis in the intestine (22). Furthermore, the present study
showed that the expression of inflammatory-associated mediator
genes was increased in the small intestine, thus indicating the
induction of an inflammatory response in the small intestine in
response to I/R.
Intraepithelial lymphocytes (IELs) play an important
role in maintaining the intestinal barrier function. Previous
studies from our group have shown that the changes in IEL subset
markers and functions are significant under some pathologic
circumstances in the intestine (16). Moreover, there is a crucial
positive correlation between CD3+CD8+IELs and
disease activity in patients with ulcerative colitis (23). In a previous study, it was shown
that TPN significantly affects the CD4+CD8−
and CD4+CD8+ IEL subpopulations and induces
loss of CD8αβ+ IELs (16). Furthermore, intestinal I/R
significantly reduced the CD8αα+ subpopulation and
increased the CD8aβ+ subpopulation (16). CD69 expression is an index of T
cell function (24), and CD69
expression might reflect the activated cytotoxic nature of IELs
(25).
CD4+CD69+ and CD8+CD69+
co-staining is used to predict immune reactivity (26). This study showed that
CD4+ and CD8+ had much higher percentages of
CD69, which is consistent with the previous findings by our group
in a DSS-induced mouse model (17). Treatment with FICZ decreased the
percentage of the CD69 subpopulation, which could indicate
attenuation of an inflammatory response. KGF is secreted by
activated small intestinal γδIELs and this mitogenic growth factor
plays an important role in restoring or maintaining the integrity
of epithelial tissues after I/R injury (27). In this study, it was demonstrated
that expression of KGF mRNA in γδIELs was downregulated in mice
with intestinal I/R, suggesting that γδIELs are affected by
I/R-induced small intestine injury, and as expected, FICZ
significantly increased expression of KGF mRNA expression in the
small intestine of mice after I/R.
The IL-7 receptor is composed of an α-chain (CD127)
and a β-chain (CD132). The expression of CD127, which binds to
IL-7, is strictly regulated throughout T cell development,
activation and survival. Therefore, its signals are required for γδ
T cell development and survival (28). IL-7 secreted by intestinal
epithelial cells (IECs) directly binds to IL-7R, and this
interaction maintains the survival of γδIELs. Many previous studies
in animals have shown that the IL-7/IL-7R-dependent signaling
pathway plays an important role in attenuating the inflammatory
response in the intestinal mucosa and that γδIELs are absent when
IL-7 and IL-7R genes are inactivated (29). Moreover, a previous study from our
group showed that IL-7 expression in the small intestine is
increased after I/R (30). In the
present study, it was found that the proportion of γδIELs was
decreased, and lower CD127 expression was detected on γδIELs after
small intestine I/R. The decrease in CD127 may be the key event
that results in a decreased number of γδIELs. Importantly,
intraperitoneal treatment with FICZ prior to reperfusion increased
CD127 expression on γδIELs.
IELs consist of TCRαβ and TCRγδ T cells, and several
studies have suggested that these subpopulations of IELs play a
crucial role in modulating inflammatory diseases in the intestine
(31,32); however, consistent data from our
group have shown that the population of αβIELs is always positively
correlated with the intensity of the inflammatory response, but
γδIELs have yielded contrary results (16,17).
Consistent with a previous study (33), small intestinal I/R increased the
apoptosis of γδIELs. Whether or not the stable population of γδIELs
deterred the progressive inflammatory response remains unknown.
Notably, our results showed that FICZ attenuated apoptosis and the
inflammatory response, which explained why it is so important to
sustain survival of γδIELs. These changes might partly explain why
FICZ can ameliorate IR-induced acute inflammation of the intestine.
Bcl-xL, also known as Bcl2l1, is a known inhibitor of apoptosis,
the expression of which sustains the survival of CD4+ T
cells (34). In the present study,
increased expression of CD127 and Bcl-xL was detected after
administration of FICZ, indicating that FICZ-activated AhR
attenuated the decreased expression of CD127 and Bcl-xL in γδIELs
after I/R.
In the present study, severe epithelial damage was
observed 6 h after intestinal I/R. The histologic features in the
I/R group were consistent with previous studies on intestinal I/R
in animals (35,36). Destruction of the epithelium is
often accompanied by increased permeability in the damaged mucosa
(16,37). Consistent with previous results on
morphology and physiology, this study showed that intestinal
mucosal damage was less severe in the I/R+FICZ group. Therefore,
AhR activation results in a greater restoration and higher
anti-infection-associated gene expression, thus attenuating the
severity of small intestinal epithelial injury. Based on these
findings, FICZ, in ameliorating IR-induced small intestinal
epithelial injury, could be involved in a complex mechanism;
however, modulating the changes in γδIEL gene expression may be one
of several crucial contributory mechanisms. A previous study by our
group showed that intraperitoneal FICZ injection increased the
percentage of the γδIEL subset in mice with DSS-induced colitis
(17). It has been widely
established that functional activation of the AhR causes nuclear
translocation, which results in the transcriptional activation of
phase I and II detoxification enzymes, such as CYP1A1. Therefore,
we determined the functional activation of the AhR by analyzing the
expression of CYP1A1 mRNA in γδIELs.
In summary, it was demonstrated that intestinal I/R
injury results in a decrease in CD127 on the membrane of small
intestinal γδIELs, which is accompanied by apoptosis. FICZ
administration enhanced CD127 expression and the γδIEL subset and
downregulated inflammatory mediator genes expressed by IELs.
Together these results indicate that FICZ administration after
intestinal I/R attenuates apoptosis of γδIELs and maintains the
small intestinal γδIEL population, as well as reducing the
expression of intestinal inflammatory cytokines, which probably
ameliorates the severity of IR-induced injury to the small
intestinal barrier.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (NSFC 81330013 to HY, NSFC
81501661 to MY, NSFC 81770524 and 81470803 to WX and NSFC 81370054
to YC) and the Innovative Research Team of Ministry of Education of
China (IRT_17R16 to HY).
Availability of data and materials
All datasets used in the current study are available
from the corresponding author upon reasonable request.
Authors' contributions
HY and WX conceived and designed the study. ZZ, AP,
MY and LS performed the experiments. ZZ and AP collected the data
and YC performed the data analysis. ZZ, AP and YC wrote the
manuscript. All authors have read and approved the final manuscript
and agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed with the mice were
according to the Guidelines for the Care and Use of Laboratory
Animals, as set forth and approved by the University Committee at
the Army Medical University, Chongqing, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gennaro M, Ascer E, Matano R, Jacobowitz
IJ, Cunningham JN Jr and Uceda P: Acute mesenteric ischemia after
cardiopulmonary bypass. Am J Surg. 166:231–236. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan YF, Pritts TA and Montrose MH:
Ischemic post-conditioning to counteract intestinal
ischemia/reperfusion injury. World J Gastrointest Pathophysiol.
1:137–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen LP and Bradfield CA: The search for
endogenous activators of the aryl hydrocarbon receptor. Chem Res
Toxicol. 21:102–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kewley RJ, Whitelaw ML and Chapman-Smith
A: The mammalian basic helix-loop-helix/PAS family of
transcriptional regulators. Int J Biochem Cell Boil. 36:189–204.
2004. View Article : Google Scholar
|
|
5
|
Schrenk D: Impact of dioxin-type induction
of drug-metabolizing enzymes on the metabolism of endo- and
xenobiotics. Biochem Pharmacol. 55:1155–1162. 1998.PubMed/NCBI
|
|
6
|
Esser C: Biology and function of the aryl
hydrocarbon receptor: Report of an international and
interdisciplinary conference. Arch Toxicol. 86:1323–1329. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lahoti TS, Boyer JA, Kusnadi A, Muku GE,
Murray IA and Perdew GH: Aryl hydrocarbon receptor activation
synergistically induces lipopolysaccharide-mediated expression of
proinflammatory chemokine (c-c motif) ligand 20. Toxicol Sci.
148:229–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gras D, Chanez P, Vachier I, Petit A and
Bourdin A: Bronchial epithelium as a target for innovative
treatments in asthma. Pharmacol Ther. 140:290–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zimmerman BJ and Granger DN: Mechanisms of
reperfusion injury. Am J Med Sci. 307:284–292. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye Y, Yue M, Jin X, Chen S and Li Y:
Isolation of murine small intestinal intraepithelial gammadeltat
cells. Immunol Invest. 39:661–673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuhl AA, Pawlowski NN, Grollich K,
Loddenkemper C, Zeitz M and Hoffmann JC: Aggravation of intestinal
inflammation by depletion/deficiency of gammadelta T cells in
different types of IBD animal models. J Leukoc Boil. 81:168–175.
2007. View Article : Google Scholar
|
|
13
|
Inagaki-Ohara K, Chinen T, Matsuzaki G,
Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y and
Yoshimura A: Mucosal T cells bearing TCRgammadelta play a
protective role in intestinal inflammation. J Immunol.
173:1390–1398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Antony PA, Wildhaber BE and
Teitelbaum DH: Intestinal intraepithelial lymphocyte gamma delta-T
cell-derived keratinocyte growth factor modulates epithelial growth
in the mouse. J Immunol. 172:4151–4158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ismail AS, Behrendt CL and Hooper LV:
Reciprocal interactions between commensal bacteria and gamma delta
intraepithelial lymphocytes during mucosal injury. J Immunol.
182:3047–3054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu Y, Yu M, Yang Y, Sheng H, Wang W, Sun
L, Chen G, Liu Y, Xiao W and Yang H: Disturbance of intraepithelial
lymphocytes in a murine model of acute intestinal
ischemia/reperfusion. J Mol Histol. 45:217–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y,
Xiao W and Yang H: Aryl hydrocarbon receptor activation
down-regulates IL-7 and reduces inflammation in a mouse model of
DSS-induced colitis. Dig Dis Sci. 60:1958–1966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Innocentin S, Withers DR, Roberts
NA, Gallagher AR, Grigorieva EF, Wilhelm C and Veldhoen M:
Exogenous stimuli maintain intraepithelial lymphocytes via aryl
hydrocarbon receptor activation. Cell. 147:629–640. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vajdovich P: Free radicals and
antioxidants in inflammatory processes and ischemia-reperfusion
injury. Vet Clin North Am Small Anim Pract. 38:31–123. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edrees WK, Lau LL, Young IS, Smye MG,
Gardiner KR, Lee B, Hannon RJ and Soong CV: The effect of lower
limb ischaemia-reperfusion on intestinal permeability and the
systemic inflammatory response. Eur J Vasc Endovasc Surg.
25:330–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kannan KB, Colorado I, Reino D, Palange D,
Lu Q, Qin X, Abungu B, Watkins A, Caputo FJ, Xu DZ, et al:
Hypoxia-inducible factor plays a gut-injurious role in intestinal
ischemia reperfusion injury. Am J Physiol Gastrointest Liver
Physiol. 300:G853–G861. 2001. View Article : Google Scholar
|
|
23
|
Vidali F, Di Sabatino A, Broglia F,
Cazzola P, Biancheri P, Viera FT, Vanoli A, Alvisi C, Perego M and
Corazza GR: Increased CD8+ intraepithelial lymphocyte infiltration
and reduced surface area to volume ratio in the duodenum of
patients with ulcerative colitis. Scand J Gastroenterol.
45:684–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindsey WB, Lowdell MW, Marti GE, Abbasi
F, Zenger V, King KM and Lamb LS Jr: CD69 expression as an index of
T-cell function: Assay standardization, validation and use in
monitoring immune recovery. Cytotherapy. 9:123–132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Montufar-Solis D, Garza T and Klein JR:
T-cell activation in the intestinal mucosa. Immunol Rev.
215:189–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ekong UD, Luo X, Yu M, Wang D, Miller SD
and O'Gorman MR: Lymphocyte activation markers may predict the
presence of donor specific alloreactivity in pediatric living
related liver transplant recipients. Hum Immunol. 72:392–397. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boismenu R and Havran WL: Modulation of
epithelial cell growth by intraepithelial gamma delta T cells.
Science. 266:1253–1255. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schluns KS and Lefrancois L: Cytokine
control of memory T-cell development and survival. Nat Rev Immunol.
3:269–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maki K, Sunaga S, Komagata Y, Kodaira Y,
Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI and Ikuta K:
Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc
Natl Acad Sci USA. 93:7172–7177. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai YJ, Wang WS, Liang HY, Sun LH,
Teitelbaum DH and Yang H: Keratinocyte growth factor up-regulates
Interleukin-7 expression following intestinal ischemia/reperfusion
in vitro and in vivo. Int J Clin Exp Pathol. 5:569–580.
2012.PubMed/NCBI
|
|
31
|
Weitkamp JH, Rosen MJ, Zhao Z, Koyama T,
Geem D, Denning TL, Rock MT, Moore DJ, Halpern MD, Matta P and
Denning PW: Small intestinal intraepithelial TCRgammadelta+ T
lymphocytes are present in the premature intestine but selectively
reduced in surgical necrotizing enterocolitis. PloS One.
9:e990422014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haas E, Rutgen BC, Gerner W, Richter B,
Tichy A, Galler A, Bilek A, Thalhammer JG, Saalmüller A and
Luckschander-Zeller N: Phenotypic characterization of canine
intestinal intraepithelial lymphocytes in dogs with inflammatory
bowel disease. J Vet Intern Med. 28:1708–1715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee WY, Hu YM, Ko TL, Yeh SL and Yeh CL:
Glutamine modulates sepsis-induced changes to intestinal
intraepithelial gammadeltaT lymphocyte expression in mice. Shock.
38:288–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khoshnan A, Tindell C, Laux I, Bae D,
Bennett B and Nel AE: The NF-kappa B cascade is important in Bcl-xL
expression and for the anti-apoptotic effects of the CD28 receptor
in primary human CD4+ lymphocytes. J Immunol. 165:1743–1754. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai Y, Wang W, Liang H, Sun L, Teitelbaum
DH and Yang H: Keratinocyte growth factor improves epithelial
structure and function in a mouse model of intestinal
ischemia/reperfusion. PloS One. 7:e447722012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maretta M, Toth S, Bujdos M, Toth S Jr,
Jonecova Z and Vesela J: Alterations of epithelial layer after
ischemic preconditioning of small intestine in rats. J Mol Histol.
43:171–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cerqueira NF, Hussni CA and Yoshida WB:
Pathophysiology of mesenteric ischemia/reperfusion: A review. Acta
Cir Bras. 20:336–343. 2005. View Article : Google Scholar : PubMed/NCBI
|