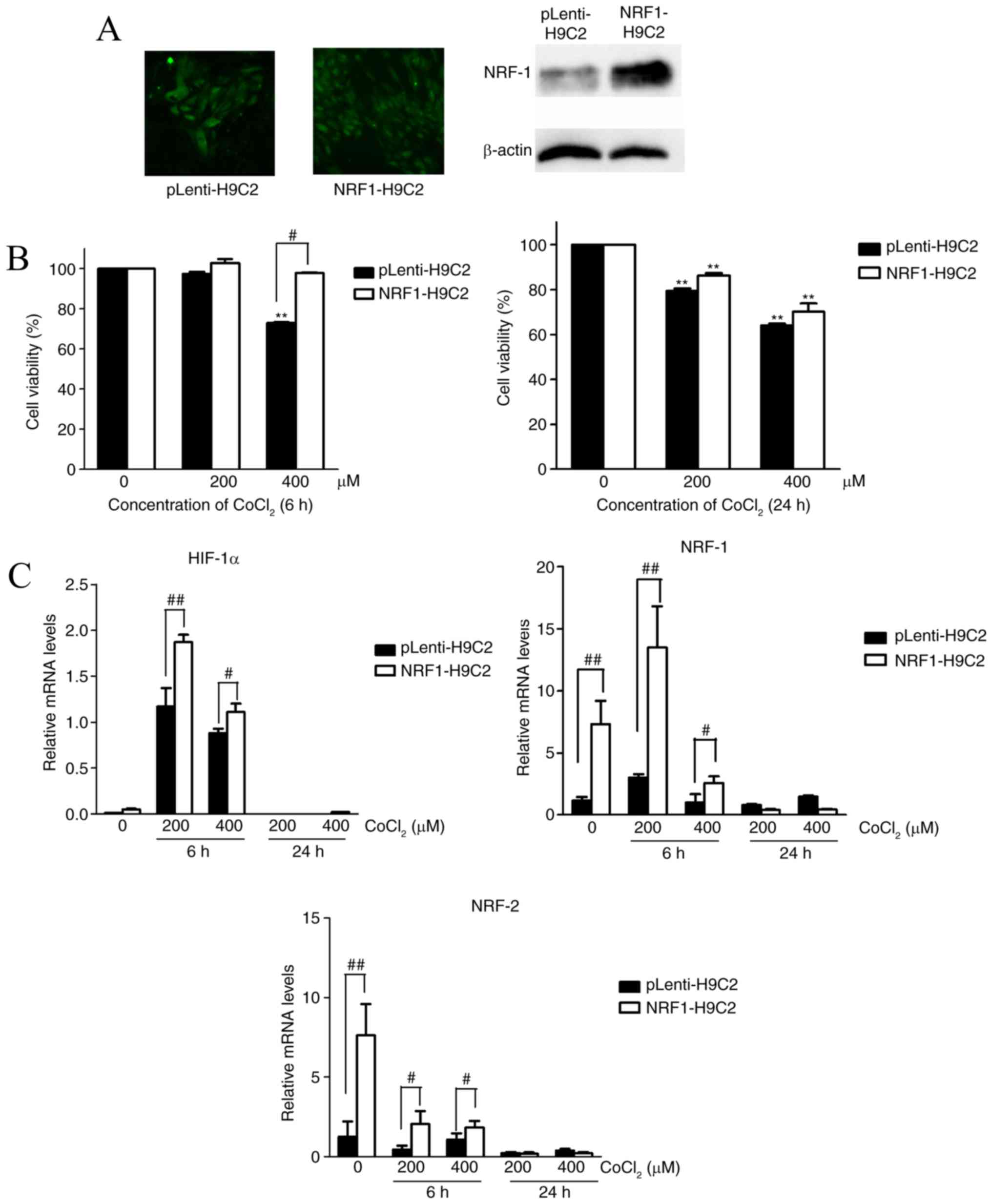
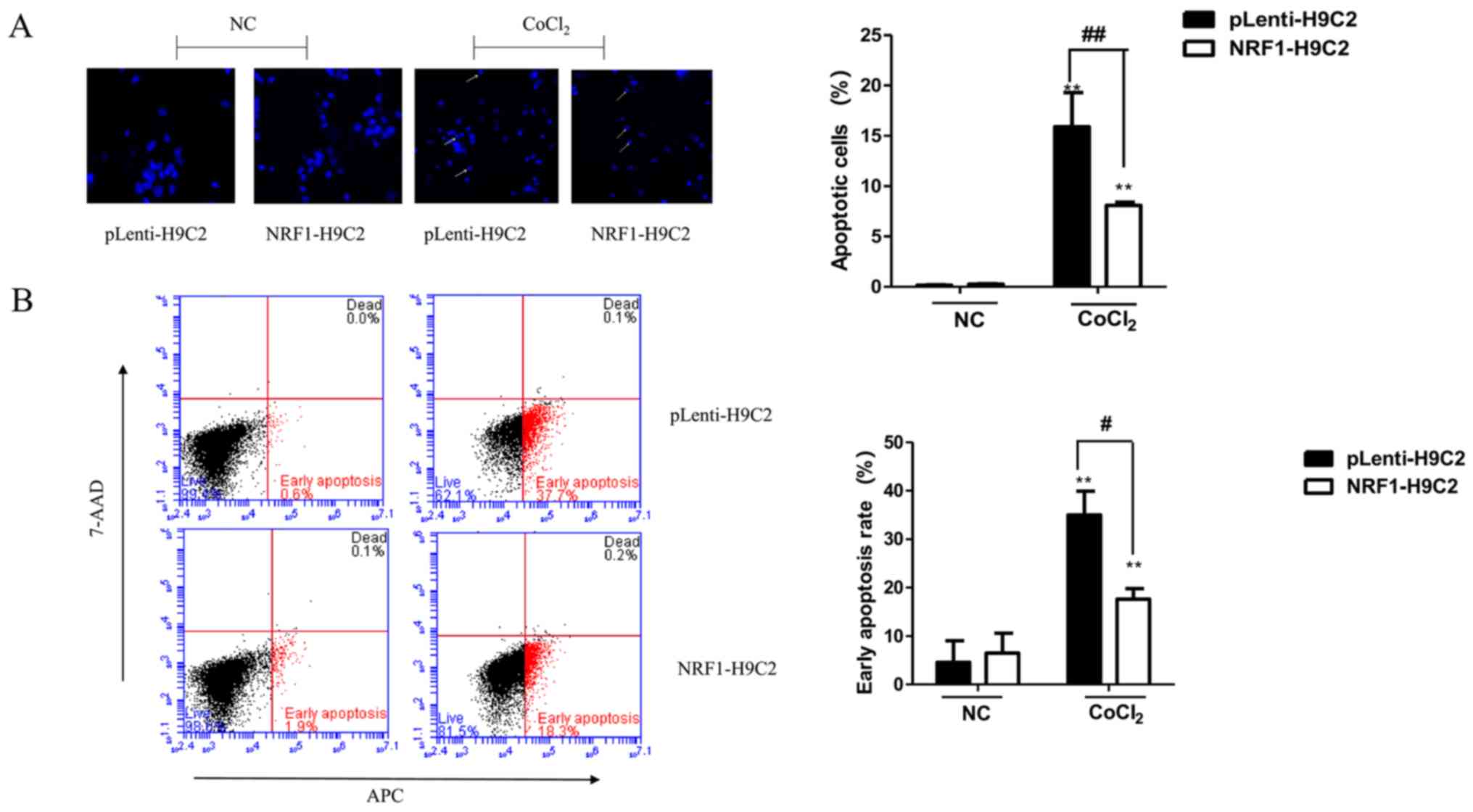
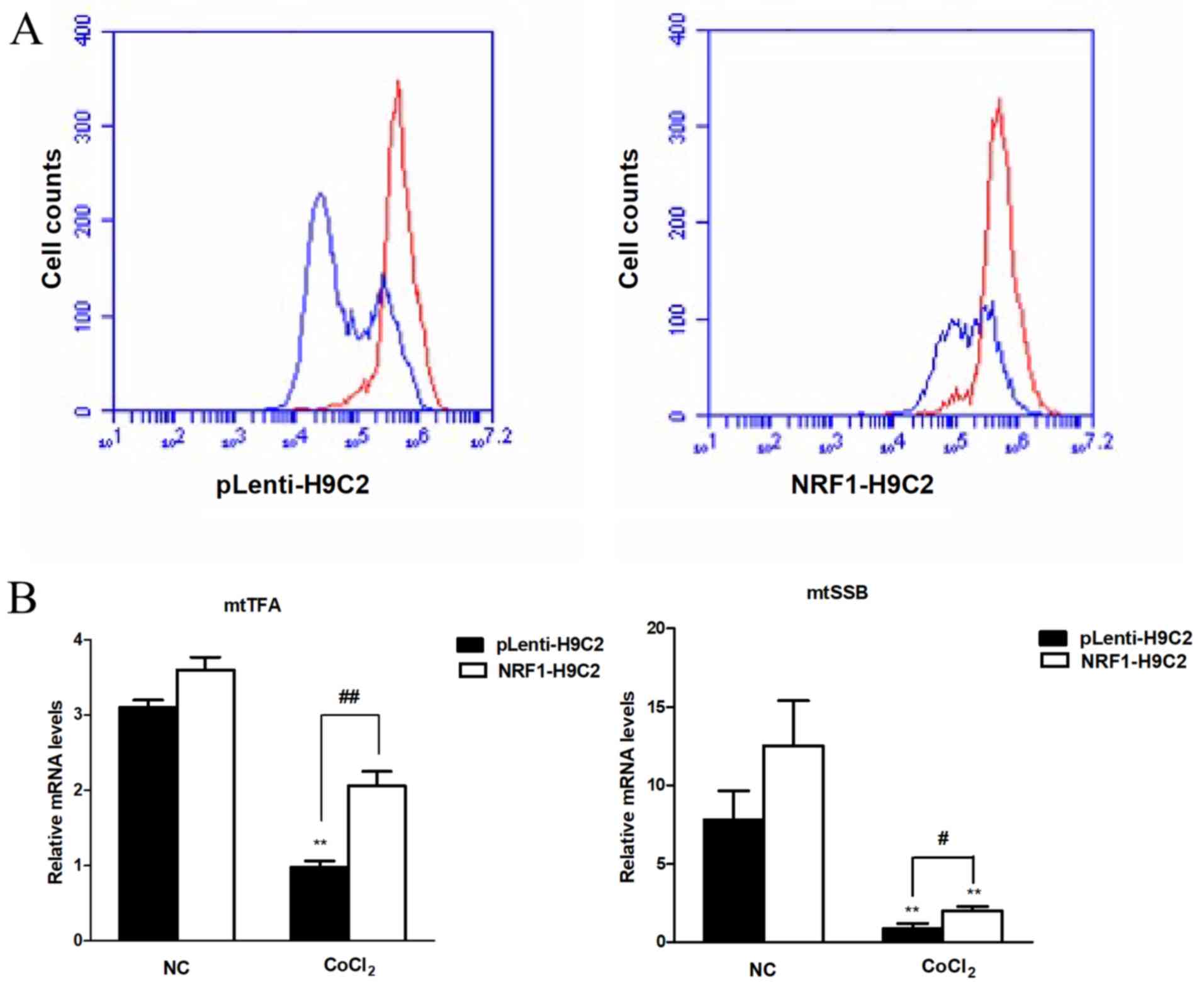
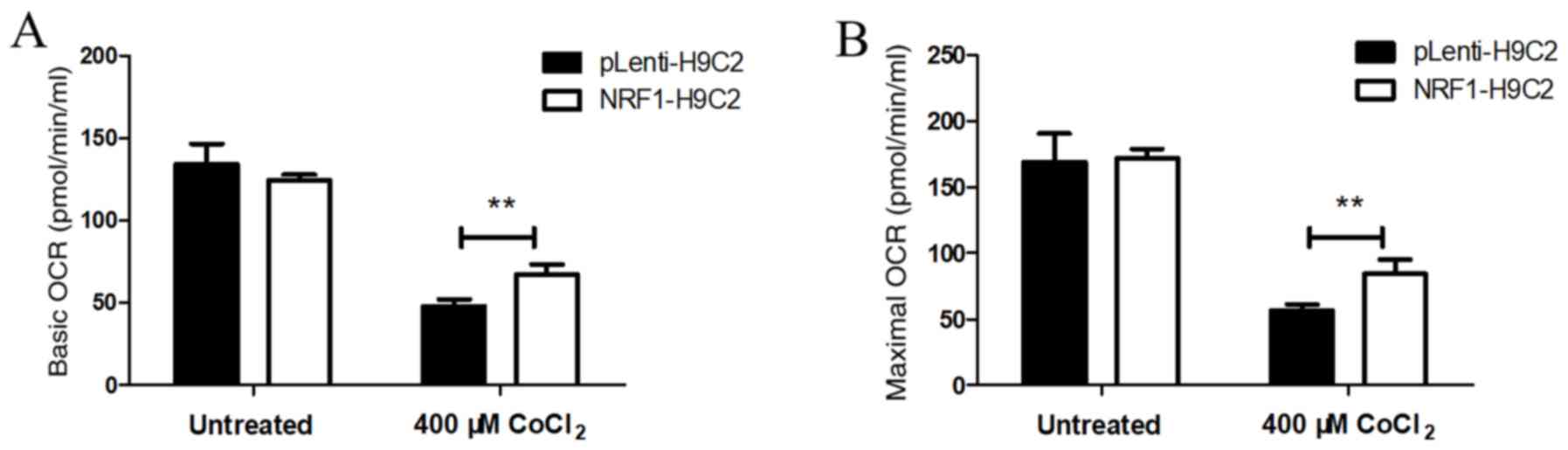
|
1
|
Ratcliffe P, Koivunen P, Myllyharju J,
Ragoussis J, Bovée JV, Batinic-Haberle I, Vinatier C, Trichet V,
Robriquet F, Oliver L and Gardie B: Update on hypoxia-inducible
factors and hydroxylases in oxygen regulatory pathways: From
physiology to therapeutics. Hypoxia (Auckl). 5:11–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romero JI, Holubiec MI, Tornatore TL,
Rivière S, Hanschmann EM, Kölliker-Frers RA, Tau J, Blanco E,
Galeano P, Rodríguez de Fonseca F, et al: Neuronal damage induced
by perinatal asphyxia is attenuated by postinjury glutaredoxin-2
administration. Oxid Med Cell Longev 2017. 41624652017.
|
|
3
|
Keel M and Trentz O: Pathophysiology of
polytrauma. Injury. 36:691–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grifka RG: Cyanotic congenital heart
disease with increased pulmonary blood flow. Pediatr Clin North Am.
46:405–425. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostadal B, Ostadalova I and Dhalla NS:
Development of cardiac sensitivity to oxygen deficiency:
Comparative and ontogenetic aspects. Physiol Rev. 79:635–659. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bär H, Kreuzer J, Cojoc A and Jahn L:
Upregulation of embryonic transcription factors in right
ventricular hypertrophy. Basic Res Cardiol. 98:285–294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray CJ and Lopez AD: Alternative
projections of mortality and disability by cause 1990–2020: Global
burden of disease study. Lancet. 349:1498–1504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yach D, Hawkes C, Gould CL and Hofman KJ:
The global burden of chronic diseases: Overcoming impediments to
prevention and control. JAMA. 291:2616–2622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhmedov AT, Rybin V and Marín-García J:
Mitochondrial oxidative metabolism and uncoupling proteins in the
failing heart. Heart Fail Rev. 20:227–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ingwall JS: ATP and the Heart: An
Overview. Springer; US: 2002, View Article : Google Scholar
|
|
11
|
Ong SB and Hausenloy DJ: Mitochondrial
morphology and cardiovascular disease. Cardiovasc Res. 88:16–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ventura-Clapier R, Garnier A, Veksler V
and Joubert F: Bioenergetics of the failing heart. Biochim Biophys
Acta 1813. 1360–1372. 2011.
|
|
13
|
Hausenloy DJ and Ruiz-Meana M: Not just
the powerhouse of the cell: Emerging roles for mitochondria in the
heart. Cardiovasc Res. 88:5–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soubannier V and Mcbride HM: Positioning
mitochondrial plasticity within cellular signaling cascades.
Biochim Biophys Acta 1793. 154–170. 2009.
|
|
15
|
Dominic EA, Ramezani A, Anker SD, Verma M,
Mehta N and Rao M: Mitochondrial cytopathies and cardiovascular
disease. Heart. 100:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saraste M: Oxidative phosphorylation at
the fin de siècle. Science. 283:1488–1493. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bianca B and Montagna E: The advances and
new technologies for the study of mitochondrial diseases. Einstein
(Sao Paulo). 14:291–293. 2016.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Björkholm P, Harish A, Hagström E, Ernst
AM and Andersson SG: Mitochondrial genomes are retained by
selective constraints on protein targeting. Proc Natl Acad Sci USA.
112:10154–10161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Hattab AW and Fernando S: Mitochondrial
Cardiomyopathies. Front Cardiovasc Med. 3:252016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans MJ and Scarpulla RC: NRF-1: A
trans-activator of nuclear-encoded respiratory genes in animal
cells. Genes Dev. 4:1023–1034. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huo L and Scarpulla RC: Mitochondrial DNA
instability and peri-implantation lethality associated with
targeted disruption of nuclear respiratory factor 1 in mice. Mol
Cell Biol. 21:644–654. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scarpulla RC: Nuclear respiratory factors
and the pathways of nuclear-mitochondrial interaction. Trends
Cardiovasc Med. 6:39–45. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scarpulla RC: Nuclear control of
respiratory gene expression in mammalian cells. J Cell Biochem.
97:673–683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Virbasius CA, Virbasius JV and Scarpulla
RC: NRF-1, an activator involved in nuclear-mitochondrial
interactions, utilizes a new DNA-binding domain conserved in a
family of developmental regulators. Genes Dev. 7:2431–2445. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clayton DA: Transcription and replication
of animal mitochondrial DNAs. Int Rev Cytol. 141:217–232. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi YS, Kim S, Kyu LH, Lee KU and Pak YK:
In vitro methylation of nuclear respiratory factor-1 binding site
suppresses the promoter activity of mitochondrial transcription
factor A. Biochem Biophy Res Commun. 314:118–122. 2004. View Article : Google Scholar
|
|
27
|
Piantadosi CA and Suliman HB:
Mitochondrial transcription factor A induction by redox activation
of nuclear respiratory factor 1. J Biol Chem. 281:324–333. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Bao Y, Liu Y and Li J:
Downregulation of nuclear respiratory factor-1 contributes to
mitochondrial events induced by benzo(a)pyrene. Environ Toxicol.
29:780–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suliman HB, Sweeney TE, Withers CM and
Piantadosi CA: Co-regulation of nuclear respiratory factor-1 by
NFkappaB and CREB links LPS-induced inflammation to mitochondrial
biogenesis. J Cell Sci. 123:2565–2575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piantadosi CA and Suliman HB:
Transcriptional regulation of SDHa flavoprotein by nuclear
respiratory factor-1 prevents pseudo-hypoxia in aerobic cardiac
cells. J Biol Chem. 283:10967–10977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang GL and Semenza GL: Characterization
of hypoxia-inducible factor 1 and regulation of DNA binding
activity by hypoxia. J Biol Chem. 268:21513–21518. 1993.PubMed/NCBI
|
|
32
|
Wang GL and Semenza GL: Desferrioxamine
induces erythropoietin gene expression and hypoxia-inducible factor
1 DNA-binding activity: Implications for models of hypoxia signal
transduction. Blood. 82:3610–3615. 1993.PubMed/NCBI
|
|
33
|
Hervouet E, Pecina P, Demont J, Vojtísková
A, Simonnet H, Houstek J and Godinot C: Inhibition of cytochrome c
oxidase subunit 4 precursor processing by the hypoxia mimic cobalt
chloride. Biochem Biophys Res Commun. 344:1086–1093. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang YB, Wang X, Meister EA, Gong KR, Yan
SC, Lu GW, Ji XM and Shao G: The effects of CoCl2 on
HIF-1α protein under experimental conditions of autoprogressive
hypoxia using mouse models. Int J Mol Sci. 15:10999–11012. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-alpha to the von
Hippel-Lindau ubiquitylation complex by O2-regulated
prolyl hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaelin WG Jr: The von Hippel-Lindau tumor
suppressor gene and kidney cancer. Clin Cancer Res. 10:6290S–6295S.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taylor MS: Characterization and
comparative analysis of the EGLN gene family. Gene. 275:125–132.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bae S, Jeong HJ, Cha HJ, Kim K, Choi YM,
An IS, Koh HJ, Lim DJ, Lee SJ and An S: The hypoxia-mimetic agent
cobalt chloride induces cell cycle arrest and alters gene
expression in U266 multiple myeloma cells. Int J Mol Med.
30:1180–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shrivastava K, Ram MS, Bansal A, Singh SS
and Ilavazhagan G: Cobalt supplementation promotes hypoxic
tolerance and facilitates acclimatization to hypobaric hypoxia in
rat brain. High Alt Med Biol. 9:63–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shrivastava K, Shukla D, Bansal A, Sairam
M, Banerjee PK and Ilavazhagan G: Neuroprotective effect of cobalt
chloride on hypobaric hypoxia-induced oxidative stress. Neurochem
Int. 52:368–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Araya J, Maruyama M, Inoue A, Fujita T,
Kawahara J, Sassa K, Hayashi R, Kawagishi Y, Yamashita N, Sugiyama
E and Kobayashi M: Inhibition of proteasome activity is involved in
cobalt-induced apoptosis of human alveolar macrophages. Am J
Physiol Lung Cell Mol Physiol. 283:L849–L858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mecklenburgh KI, Walmsley SR, Cowburn AS,
Wiesener M, Reed BJ, Upton PD, Deighton J, Greening AP and Chilvers
ER: Involvement of a ferroprotein sensor in hypoxia-mediated
inhibition of neutrophil apoptosis. Blood. 100:3008–3016. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tello D, Balsa E, Acosta-Iborra B,
Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I,
López-Bernardo E, Perales-Clemente E, et al: Induction of the
mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen
consumption by inhibiting Complex I activity. Cell Metab.
14:768–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tischlerova V, Kello M, Budovska M and
Mojzis J: Indole phytoalexin derivatives induce
mitochondrial-mediated apoptosis in human colorectal carcinoma
cells. World J Gastroenterol. 23:4341–4353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Venkatarame G, owda Saralamma V, Lee HJ,
Hong GE, Park HS, Yumnam S, Raha S, Lee WS, Kim EH, Sung NJ, Lee
SJ, et al: Korean Scutellaria baicalensis Georgi flavonoid
extract induces mitochondrially mediated apoptosis in human gastric
cancer AGS cells. Oncol Lett. 14:607–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Gao S, Jiang W, Luo C, Xu M,
Bohlin L, Rosendahl M and Huang W: Antioxidative dietary compounds
modulate gene expression associated with apoptosis, DNA repair,
inhibition of cell proliferation and migration. Int J Mol Sci.
15:16226–16245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Yu RY and He QY: Proteomic
analysis of anticancer TCMs targeted at mitochondria. Evid Based
Complement Alternat Med 2015. 5392602015.
|
|
49
|
Kogot-Levin A, Saada A, Leibowitz G,
Soiferman D, Douiev L, Raz I and Weksler-Zangen S: Upregulation of
mitochondrial content in cytochrome c oxidase deficient
fibroblasts. PLoS One. 11:e01654172016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
van der Windt GJ, Everts B, Chang CH,
Curtis JD, Freitas TC, Amiel E, Pearce EJ and Pearce EL:
Mitochondrial respiratory capacity is a critical regulator Of CD8+
T cell memory development. Immunity. 36:68–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paradis AN, Gay MS and Zhang L:
Binucleation of cardiomyocytes: The transition from a proliferative
to a terminally differentiated state. Drug Discovery Today.
19:602–609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li F, Wang X, Capasso JM and Gerdes AM:
Rapid transition of cardiac myocytes from hyperplasia to
hypertrophy during postnatal development. J Mol Cell Cardiol.
28:1737–1746. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ahuja P, Sdek P and Maclellan WR: Cardiac
myocyte cell cycle control in development, disease, and
regeneration. Physiol Rev. 87:521–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burrell JH, Boyn AM, Kumarasamy V, Hsieh
A, Head SI and Lumbers ER: Growth and maturation of cardiac
myocytes in fetal sheep in the second half of gestation. Anat Rec A
Discov Mol Cell Evol Biol. 274:952–961. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ostádal B, Ostádalová I, Kolár F,
Charvátová Z and Netuka I: Ontogenetic development of cardiac
tolerance to oxygen deprivation-possible mechanisms. Physiol Res.
58 Suppl 2:S1–S12. 2009.
|
|
56
|
Dai M, Cui P, Yu M, Han J, Li H and Xiu R:
Melatonin modulates the expression of VEGF and HIF-1 alpha induced
by CoCl2 in cultured cancer cells. J Pineal Res.
44:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang Y, Satoh K, Watanabe S, Kusama K and
Sakagami H: Inhibition of chlorogenic acid-induced cytotoxicity by
CoCl2. Anticancer Res. 21:3349–3353. 2001.PubMed/NCBI
|
|
58
|
Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG,
Choi NK, Oh WM, Oh HK, Kim SH, Lee JH, et al: Inhibition by
epigallocatechin gallate of CoCl2-induced apoptosis in
rat PC12 cells. Life Sci. 80:1355–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen R, Xu J, She Y, Jiang T, Zhou S, Shi
H and Li C: Necrostatin-1 protects C2C12 myotubes from
CoCl2-induced hypoxia. Int J Mol Med. 41:2565–2572.
2018.PubMed/NCBI
|
|
60
|
Chen R, Jiang T, She Y, Xu J, Li C, Zhou
S, Shen H, Shi H and Liu S: Effects of cobalt chloride, a
hypoxia-mimetic agent, on autophagy and atrophy in skeletal C2C12
myotubes. Biomed Res Int 2017. 70975802017.
|
|
61
|
Rovetta F, Stacchiotti A, Faggi F,
Catalani S, Apostoli P, Fanzani A and Aleo MF: Cobalt triggers
necrotic cell death and atrophy in skeletal C2C12 myotubes. Toxicol
Appl Pharmacol. 271:196–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bartz RR, Suliman HB and Piantadosi CA:
Redox mechanisms of cardiomyocyte mitochondrial protection. Front
Physiol. 6:2912015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen L, Kwong M, Lu R, Ginzinger D, Lee C,
Leung L and Chan JY: Nrf1 is critical for redox balance and
survival of liver cells during development. Mol Cell Biol.
23:4673–4686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Doerks T, Copley RR, Schultz J, Ponting CP
and Bork P: Systematic identification of novel protein domain
families associated with nuclear functions. Genome Res. 12:47–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Saxena S, Shukla D, Saxena S, Khan YA,
Singh M, Bansal A, Sairam M and Jain SK: Hypoxia preconditioning by
cobalt chloride enhances endurance performance and protects
skeletal muscles from exercise-induced oxidative damage in rats.
Acta Physiol (Oxf). 200:249–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Priya A, Johar K and Wong-Riley M: Nuclear
respiratory factor 2 regulates the expression of the same NMDA
receptor subunit genes as NRF-1: Both factors act by a concurrent
and parallel mechanism to couple energy metabolism and synaptic
transmission. Biochim Biophys Acta 1833. 48–58. 2013.
|
|
67
|
Priya A, Johar K, Nair B and Wong-Riley
MT: Nuclear respiratory factor 2 regulates the transcription of
AMPA receptor subunit GluA2 (Gria2). Biochim Biophys Acta 1843.
3018–3028. 2014.
|
|
68
|
Vercauteren K, Gleyzer N and Scarpulla RC:
PGC-1-related coactivator complexes with HCF-1 and NRF-2beta in
mediating NRF-2(GABP)-dependent respiratory gene expression. J Biol
Chem. 283:12102–12111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector? FASEB J. 19:1061–1066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liby KT and Sporn MB: Synthetic oleanane
triterpenoids: Multifunctional drugs with a broad range of
applications for prevention and treatment of chronic disease.
Pharmacol Rev. 64:972–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Arlt A, Sebens S, Krebs S, Geismann C,
Grossmann M, Kruse ML, Schreiber S and Schäfer H: Inhibition of the
Nrf2 transcription factor by the alkaloid trigonelline renders
pancreatic cancer cells more susceptible to apoptosis through
decreased proteasomal gene expression and proteasome activity.
Oncogene. 32:4825–4835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Arlt A, Bauer I, Schafmayer C, Tepel J,
Müerköster SS, Brosch M, Röder C, Kalthoff H, Hampe J, Moyer MP, et
al: Increased proteasome subunit protein expression and proteasome
activity in colon cancer relate to an enhanced activation of
nuclear factor E2-related factor 2 (Nrf2). Oncogene. 28:3983–3996.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Calvert JW, Elston M, Nicholson CK,
Gundewar S, Jha S, Elrod JW, Ramachandran A and Lefer DJ: Genetic
and pharmacologic hydrogen sulfide therapy attenuates
ischemia-induced heart failure in mice. Circulation. 122:11–19.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Malhotra D, Thimmulappa R, Vij N,
Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A,
Wise R, et al: Heightened endoplasmic reticulum stress in the lungs
of patients with chronic obstructive pulmonary disease: The role of
Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med.
180:1196–1207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Permenter MG, Dennis WE, Sutto TE, Jackson
DA, Lewis JA and Stallings JD: Exposure to cobalt causes
transcriptomic and proteomic changes in two rat liver derived cell
lines. PLoS One. 8:e837512013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Piantadosi CA, Carraway MS, Babiker A and
Suliman HB: Heme oxygenase-1 regulates cardiac mitochondrial
biogenesis via Nrf2-mediated transcriptional control of nuclear
respiratory factor-1. Circ Res. 103:1232–1240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu Z, Ding Y, Ye N, Wild C, Chen H and
Zhou J: Direct activation of Bax protein for cancer therapy. Med
Res Rev. 36:313–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pagliari LJ, Kuwana T, Bonzon C, Newmeyer
DD, Tu S, Beere HM and Green DR: The multidomain proapoptotic
molecules Bax and Bak are directly activated by heat. Proc Natl
Acad Sci USA. 102:17975–17980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yin C, Knudson CM, Korsmeyer SJ and Van
DT: Bax suppresses tumorigenesis and stimulates apoptosis in vivo.
Nature. 385:637–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c
and dATP-dependent formation of Apaf-1/caspase-9 complex initiates
an apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rampino N, Yamamoto H, Ionov Y, Li Y,
Sawai H, Reed JC and Perucho M: Somatic frameshift mutations in the
BAX gene in colon cancers of the microsatellite mutator phenotype.
Science. 275:967–969. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Karbowski M, Norris KL, Cleland MM, Jeong
SY and Youle RJ: Role of Bax and Bak in mitochondrial
morphogenesis. Nature. 443:658–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Walensky LD and Gavathiotis E: BAX
unleashed: The biochemical transformation of an inactive cytosolic
monomer into a toxic mitochondrial pore. Trends Biochem Sci.
36:642–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kale J, Liu Q, Leber B and Andrews DW:
Shedding light on apoptosis at subcellular membranes. Cell.
151:1179–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wood WG, Igbavboa U, Muller WE and Eckert
GP: Statins, Bcl-2 and apoptosis: Cell death or cell protection?
Mol Neurobiol. 48:308–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gogvadze V and Orrenius S: Mitochondrial
regulation of apoptotic cell death. Chem Biol Interact. 163:4–14.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Park JB, Chang H and Kim KW: Expression of
Fas ligand and apoptosis of disc cells in herniated lumbar disc
tissue. Spine (Phila Pa 1976). 26:618–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Park JB, Kim KW, Han CW and Chang H:
Expression of Fas receptor on disc cells in herniated lumbar disc
tissue. Spine (Phila Pa 1976). 26:142–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Parsons MJ and Green DR: Mitochondria in
cell death. Essays Biochem. 47:99–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Evans MJ and Scarpulla RC: Interaction of
nuclear factors with multiple sites in the somatic cytochrome
c promoter. Characterization of upstream NRF-1, ATF, and
intron Sp1 recognition sequences. J Biol Chem. 264:14361–14368.
1989.PubMed/NCBI
|
|
95
|
Evans MJ and Scarpulla RC: Both upstream
and intron sequence elements are required for elevated expression
of the rat somatic cytochrome c gene in COS-1 cells. Mol
Cell Biol. 8:35–41. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Scarpulla RC and Wu R: Nonallelic members
of the cytochrome c multigene family of the rat may arise
through different messenger RNAs. Cell. 32:473–482. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chau CM, Evans MJ and Scarpulla RC:
Nuclear respiratory factor 1 activation sites in genes encoding the
gamma-subunit of ATP synthase, eukaryotic initiation factor 2
alpha, and tyrosine aminotransferase. Specific interaction of
purified NRF-1 with multiple target genes. J Biol Chem.
267:6999–7006. 1992.PubMed/NCBI
|
|
98
|
Kelly DP and Scarpulla RC: Transcriptional
regulatory circuits controlling mitochondrial biogenesis and
function. Genes Dev. 18:357–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Scarpulla RC: Nuclear activators and
coactivators in mammalian mitochondrial biogenesis. Biochim Biophys
Acta 1576. 1–14. 2002.
|
|
100
|
Scarpulla RC: Transcriptional activators
and coactivators in the nuclear control of mitochondrial function
in mammalian cells. Gene. 286:81–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Scarpulla RC: Nuclear control of
respiratory chain expression by nuclear respiratory factors and
PGC-1-related coactivator. Ann NY Acad Sci 1147. 321–334. 2008.
View Article : Google Scholar
|
|
102
|
Perry SW, Norman JP, Barbieri J, Brown EB
and Gelbard HA: Mitochondrial membrane potential probes and the
proton gradient: A practical usage guide. Biotechniques. 50:98–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang BB, Wang DG, Guo FF and Xuan C:
Mitochondrial membrane potential and reactive oxygen species in
cancer stem cells. Fam Cancer. 14:19–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Henry-Mowatt J, Dive C, Martinou JC and
James D: Role of mitochondrial membrane permeabilization in
apoptosis and cancer. Oncogene. 23:2850–2860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Campbell CT, Kolesar JE and Kaufman BA:
Mitochondrial transcription factor A regulates mitochondrial
transcription initiation, DNA packaging, and genome copy number.
Biochim Biophys Acta 1819. 921–929. 2012.
|
|
108
|
Fisher RP and Clayton DA: Purification and
characterization of human mitochondrial transcription factor 1. Mol
Cell Biol. 8:3496–3509. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Parisi MA and Clayton DA: Similarity of
human mitochondrial transcription factor 1 to high mobility group
proteins. Science. 252:965–969. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Maier D, Farr CL, Poeck B, Alahari A,
Vogel M, Fischer S, Kaguni LS and Schneuwly S: Mitochondrial
single-stranded DNA-binding protein is required for mitochondrial
DNA replication and development in Drosophila melanogaster.
Mol Biol Cell. 12:821–830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Takamatsu C, Umeda S, Ohsato T, Ohno T,
Abe Y, Fukuoh A, Shinagawa H, Hamasaki N and Kang D: Regulation of
mitochondrial D-loops by transcription factor A and single-stranded
DNA-binding protein. EMBO Rep. 3:451–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Van Dyck E, Foury F, Stillman B and Brill
SJ: A single-stranded DNA binding protein required for
mitochondrial DNA replication in S. cerevisiae is homologous to E.
coli SSB. EMBO J. 11:3421–3430. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mali VR, Pan G, Deshpande M, Thandavarayan
RA, Xu J, Yang XP and Palaniyandi SS: Cardiac mitochondrial
respiratory dysfunction and tissue damage in chronic hyperglycemia
correlate with reduced aldehyde dehydrogenase-2 activity. PLoS One.
11:e01631582016. View Article : Google Scholar : PubMed/NCBI
|