Introduction
Severe trauma can cause local tissue damage,
systemic inflammatory response syndrome, as well as multiple organ
dysfunction syndrome (MODS), shock and even death (1,2).
MODS is one of the major, serious complications following severe
trauma and is the progressive dysfunction of one or more organ
systems resulting from an exaggerated and long-term inflammatory
response to severe illness and/or injury. Multiple factors
including severe shock, infection, burns, trauma and severe
pancreatitis are involved in the pathologic process of MODS
(3–7). In recent years, the mechanism
underlying MODS caused by trauma is under investigation, and there
are a number of hypotheses to explain the pathological mechanism.
However, the mechanisms underlying MODS caused by trauma have not
yet been fully elucidated. At present, due to the lack of effective
treatment, MODS is still a major cause of morbidity and mortality
in intensive care units (8,9).
Nuclear factor (NF)-κB is a protein complex that
controls transcription of DNA, cytokine production and cell
survival. NF-κB is found in almost all animal cell types and is
involved in cellular responses to stimuli such as stress, cytokines
and free radicals (10–12). Abnormal expression of NF-κB has
been reported to be linked to cancer, inflammatory and autoimmune
diseases, septic shock, viral infection and improper immune
development (13,14). Recent studies have reported that
NF-κB is activated during the progression of MODS (15,16).
TNIP2 (also termed ABIN2) interacts with several components of the
NF-κB signaling pathway and can both positively and negatively
regulate NF-κB-dependent transcription of target genes (17). The TNIP2 gene encodes a
protein that represses the activation of NF-κB, and it is generally
accepted that TNIP2 has an essential role in the NF-κB signaling
pathway (18). Moreover, studies
have indicated that TNIP2 plays an important role in acute
pancreatitis-induced myocardial injury and lupus nephritis via
regulating the NF-κB pathway (19,20).
To the best of our knowledge, no study has been
conducted concerning the role of TNIP2 in the development and
progression of MODS. Therefore, the aim of the present study was to
investigate the role and molecular mechanism of TNIP2 in MODS
following severe trauma.
Materials and methods
Clinical specimens
Peripheral blood samples (5 ml per individual) from
16 patients with MODS and peripheral blood samples from 16 healthy
individuals were collected at the Affiliated Hospital of Nantong
University (Nantong, China) from August 2014 to April 2017. The
inclusion criteria were as follows: i) No history of injury; ii)
injury severity score (ISS), ≥16; iii) age, ≥18 years; and iv) time
interval between trauma and admission, <90 h. The exclusion
criteria were as follows: i) Mortality within 24 h after trauma;
ii) main diagnosis, cardiac trauma; iii) intracranial hemorrhage;
iv) heart, liver, kidney or endocrine history. Following admission,
the age, sex, cause of trauma and medical history of the patients
were recorded. Blood samples were collected from MODS patients 48 h
after sever trauma and stored at −85°C. Informed consent was
obtained from every patient enrolled and the present study was
approved by the Ethics Committee of the Affiliated Hospital of
Nantong University.
Animal model of MODS
A total of 40 male Sprague-Dawley (SD) rats (~200 g)
were purchased from Vital River Company (Beijing, China). All rats
were fed ad libitim and maintained under standard conditions
at 22–30°C and a 12-h light/dark cycle. The experimental protocol
was approved by the Animal Care Committee of Nantong University,
and experiments were performed under the guidelines of guide for
the care and use of laboratory animals (21). The rat model of MODS was designed
and established according to a previous study (22). To investigate the role of TNIP2 in
MODS, the rats were randomly assigned into 4 groups: Control, MODS
model, MODS model + control-plasmid [C; intraperitoneal (i.p.)
injection] and MODS model + TNIP2-plasmid (T; i.p. injection). The
rats in the control and MODS model groups received saline (0.9%
NaCl) solution i.p. instead of the plasmids. All rats were
anesthetized with 30 mg/kg pentobarbital and handled at certain
time points (6, 12, 24 and 48 h). Following the specific treatment,
the subsequent experiments were conducted.
To confirm multiple organ injury/dysfunction, the
following biochemical indicators in the plasma were measured. Liver
injury was assessed by detecting the enhanced levels of alanine
aminotransferase (ALT; a specific marker for hepatic parenchymal
injury), aspartate aminotransferase (AST; a nonspecific marker for
hepatic injury) and lactate dehydrogenase (LDH) in the plasma.
Renal dysfunction was assessed by the increased levels of creatine
(Cr) and blood urea nitrogen (BUN) (indicator of reduced glomerular
filtration rate and hence renal failure) in the plasma (23).
Serum biochemical analyses
At 6, 12, 24 and 48 h after treatment, 3 ml venous
blood was collected from the rats in all groups. Serum was prepared
through centrifugation (4°C, 1,000 × g, 15 min) and stored at
−80°C. To detect the levels of ALT, AST, LDH, BUN, CK and Cr, a
Hitachi Automatic Analyzer 7170 (Hitachi, Ltd., Japan) was
utilized.
Enzyme-linked immunosorbent assay
(ELISA)
At 6, 12, 24, and 48 h after treatment, the serum of
rats were harvested by centrifugation (1,000 × g, 10 min) to
determine the secretion of TNF-α, HMGB-1, MDA and TAC using an
ELISA kit (Abcam, Cambridge, MA, USA) following the manufacturer's
protocols. Every sample was detected at least three times by
utilizing a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Western blot analysis
Western blot analysis was carried out as previously
described (24). Total proteins
were extracted from tissues/blood using radioimmunoprecipitation
asay lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate] supplemented with phenylmethylsulfonyl
fluoride at a final concentration of 1 mM. Protein concentration
was determined using the bicinchoninic acid protein assay (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal amounts of
protein (30 µg/lane) were resolved using SDS-PAGE on 10% gels, and
then transferred to a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% skimmed
milk in TBS-Tween at room temperature for 1.5 h, the samples were
probed with antibodies against TNIP2 (cat no. sc-271850; dilution
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-p65
(cat no. 3033; dilution 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), p65 (cat no. 8242; dilution 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), and β-actin (cat no.
4970; 1:1,000; Cell Signaling Technology, Inc.). After three times
of washing, the blots were then incubated with horseradish
peroxidase-conjugated secondary antibody (anti-rabbit IgG; 1:2,000;
cat no. 7074; Cell Signaling Technology, Inc.) at room temperature
for 2 h. Immunoreactive bands were visualized using the enhanced
chemiluminescence detection system (Applygen Technologies, Inc.,
Beijing, China). ImageJ 1.38X (National Institutes of Health,
Bethesda, MD, USA) was used to perform densitometry.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously described
(24). In brief, total RNA was
extracted from tissues/blood samples using RNAiso Plus (Takara Bio,
Inc., Otsu, Japan) following the manufacturer's instructions. RT
was performed to synthesize cDNAs using the ThermoScript RT-PCR
system (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was
performed to analyze the synthesized cDNA using SYBR Green PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on
a 7900 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The conditions of qPCR used for amplification
were as follows: 95°C for 5 min, 40 cycles at 95°C for 30 sec, 55°C
for 30 sec, and 72°C for 30 sec, then 72°C for 10 min. The primer
sequences used for qPCR are as follows: TNIP2, forward
5′-CTAAAGAGGCGGCAGGTCCCTC-3′ and reverse
5′-CAAGATGACCTTCCAGTGAC-3′; GAPDH, forward
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse
5′-GTAGAGGCAGGGATGATGTTCT-3′. GAPDH served as an internal control.
The relative gene expression was assessed by using the
2−ΔΔCq method (25).
The experiment was repeated at least three times.
Statistical analysis
All data analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Results are expressed as
mean ± standard deviation. Student's t-tests and one-way analysis
of variance followed by Student-Newman-Keuls tests were performed
to analyze the differences between groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Basic patient characteristics
The present study included 16 patients. The basic
characteristics of the patients are presented in Table I.
 | Table I.Basic patient characteristics and
clinical data 24 h after admission (n=16). |
Table I.
Basic patient characteristics and
clinical data 24 h after admission (n=16).
| Patient
characteristics | Values |
|---|
| Sex
(male/female) | 10/6 |
| Age range
(years) | 34-57 |
| ISS | 34.9±6.7 |
| APACHE II score | 25.3±8.1 |
| CRP (mg/l) | 104.7±13.7 |
| WBCs
(×109/l) | 13.2±9.1 |
| Neutrophils (%) | 83.9±5.5 |
TNIP2 is significantly reduced in MODS
patients and rats
To detect the expression level of TNIP2 in the blood
of MODS patients and rats, RT-qPCR and western blot assay were
used, respectively. As shown in Fig.
1A and B, compared with the healthy control, the protein and
mRNA levels of TNIP2 were significantly decreased in the blood
samples of patients with MODS. The data indicated that TNIP2 may be
involved in the development of MODS.
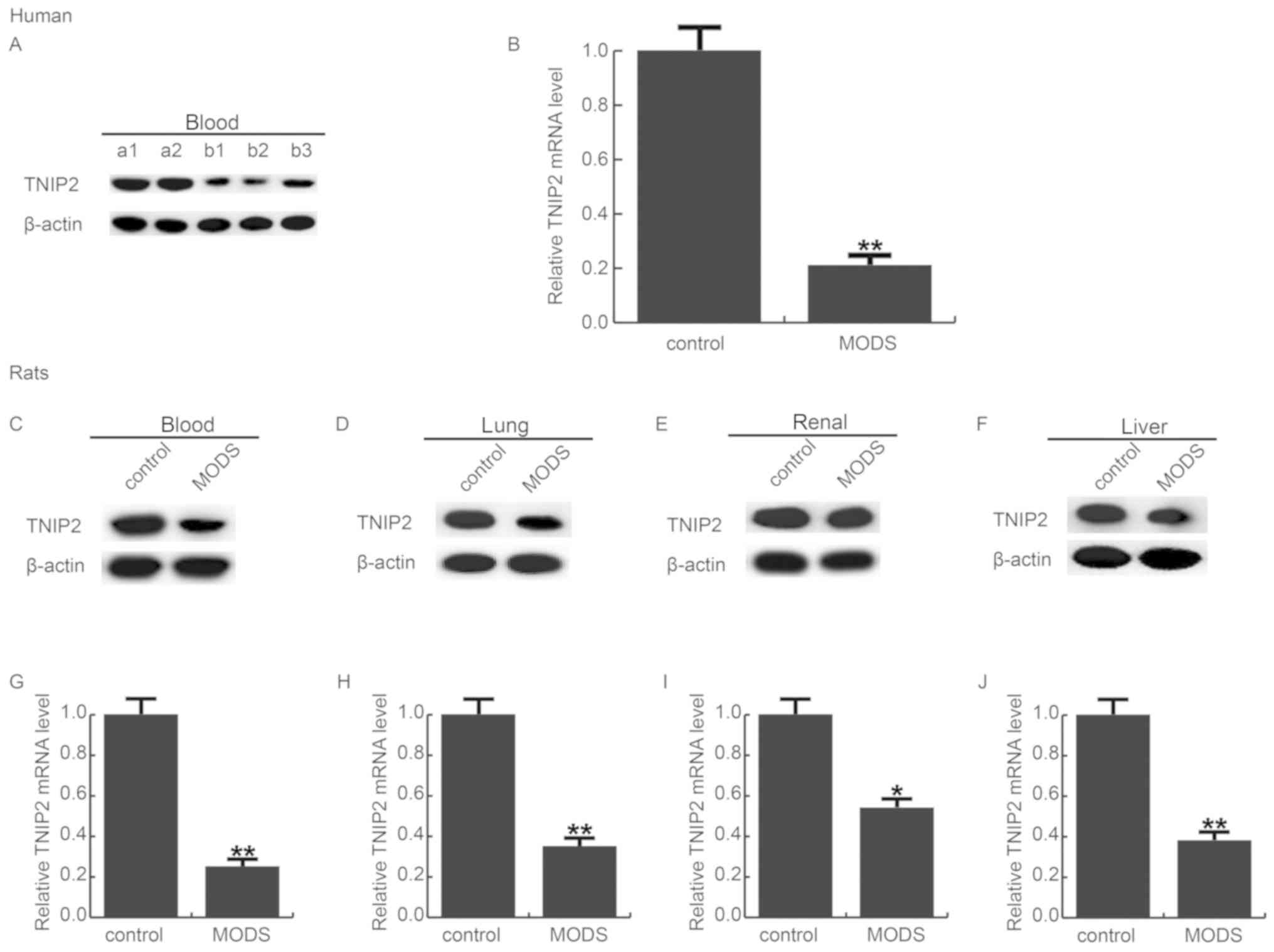 | Figure 1.TNIP2 expression in MODS. The level of
TNIP2 in MODS was detected using western blot analysis and qRT-PCR,
respectively. (A) Protein level of TNIP2 in the blood of MODS
patients; a1, a2 indicate the control patients; b1-b3 indicate the
MODS patients. (B) Relative mRNA levels of TNIP2 in the blood of
MODS patients. Protein levels of TNIP2 in the (C) blood, (D) lung,
(E) renal and (F) liver tissues of MODS rats, respectively. The
relative mRNA levels of TNIP2 in the (G) blood, (H) lung, (I) renal
and (J) liver tissues of MODS rats, respectively. Data are
expressed as mean ± standard deviation. *P<0.05, **P<0.01 vs.
the control group. MODS, multiple organ dysfunction syndrome;
TNIP2, TNFAIP3-interacting protein 2. |
Then the levels of TNIP2 were detected in the blood
and in the pulmonary, renal, and hepatic tissues of MODS rats. The
findings revealed that TNIP2 was significantly downregulated in the
blood and the pulmonary, renal and hepatic tissues of the MODS rats
(Fig. 1C-J).
Blood levels of ALT, AST, LDH, BUN, Cr
and CK
To assess changes in the cellular integrity and
functionality in the organs at 6, 12, 24 and 48 h after trauma,
blood tests were performed. As shown in Fig. 2, the levels of ALT, AST, LDH, BUN,
Cr and CK in the blood of the MODS rats were significantly higher
at different time points after injury than those in the rats of the
control group. TNIP2-plasmid administration significantly reduced
the levels of ALT, AST, LDH, BUN, Cr and CK in the blood of the
MODS rats.
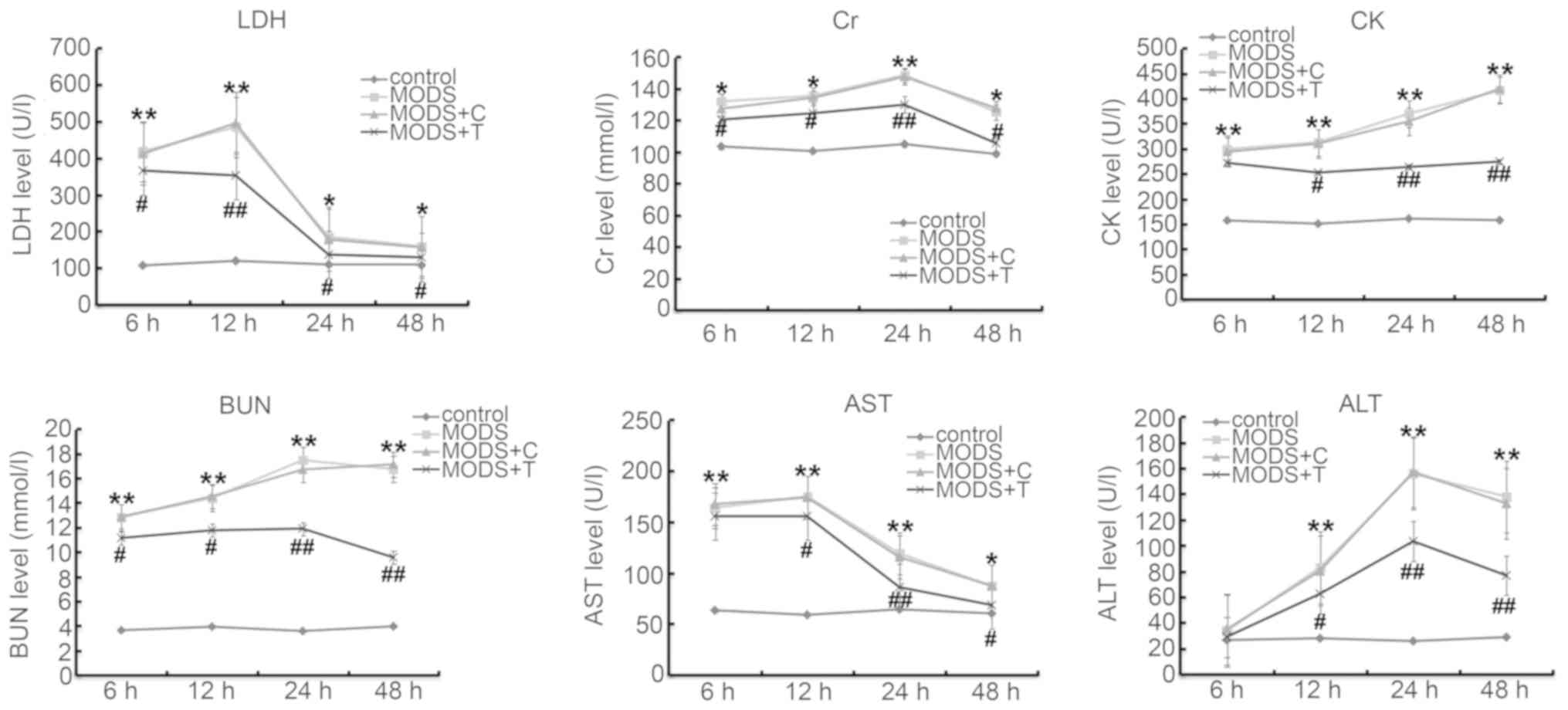 | Figure 2.Serum concentrations of major blood
biochemical parameters in the control and MODS group at different
time points. The levels of LDH, Cr, CK, BUN, AST and ALT in the
blood of the MODS rats was measured using ELISA assay. Data are
expressed as mean ± standard deviation. *P<0.05, **P<0.01 vs.
control group; #P<0.05, ##P<0.01 vs.
MODS group. Groups: Control, MODS (MODS model), MODS model +
control-plasmid (MODS + C) and MODS model + TNIP2-plasmid (MODS +
T). MODS, multiple organ dysfunction syndrome; LDH, lactate
dehydrogenase; Cr, creatine; CK, creatine kinase; BUN, blood urea
nitrogen; AST, aspartate aminotransferase; ALT, alanine
aminotransferase. |
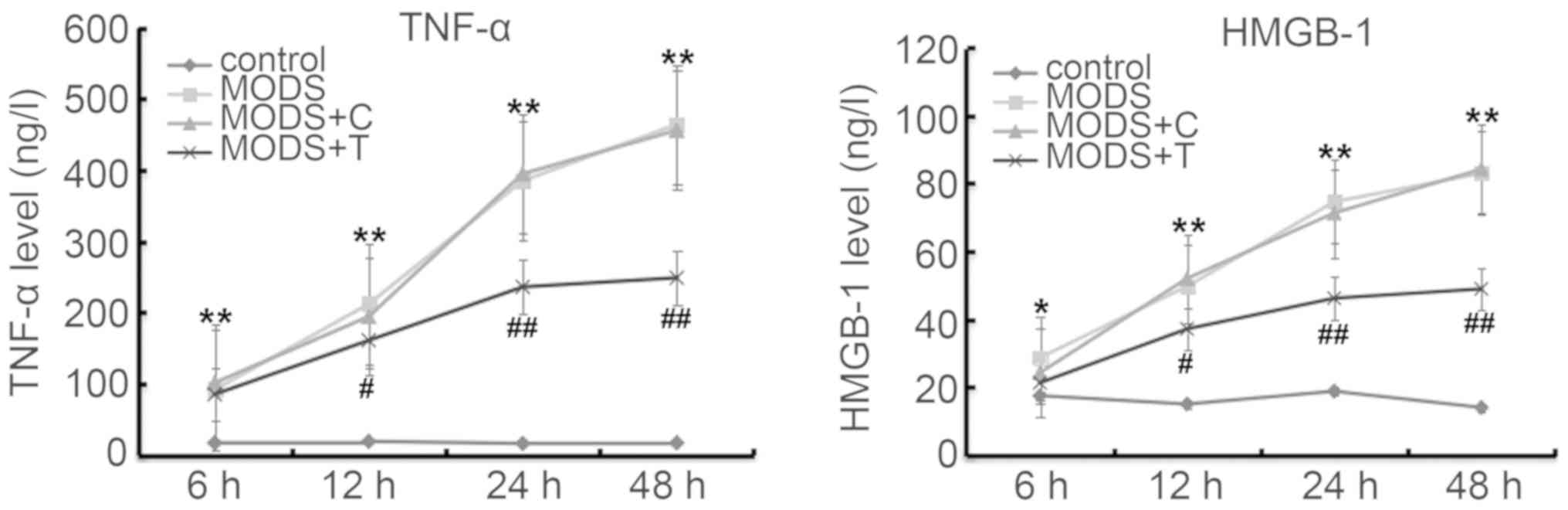
Blood levels of TNF-α and HMGB-1
As shown in Fig. 3,
the production of TNF-α and HMGB-1 in the blood of the MODS rats
was markedly higher at different time points after injury than
those in the rats of the control group. TNIP2-plasmid
administration significantly reduced the levels of TNF-α and HMGB-1
in the blood of the MODS rats.
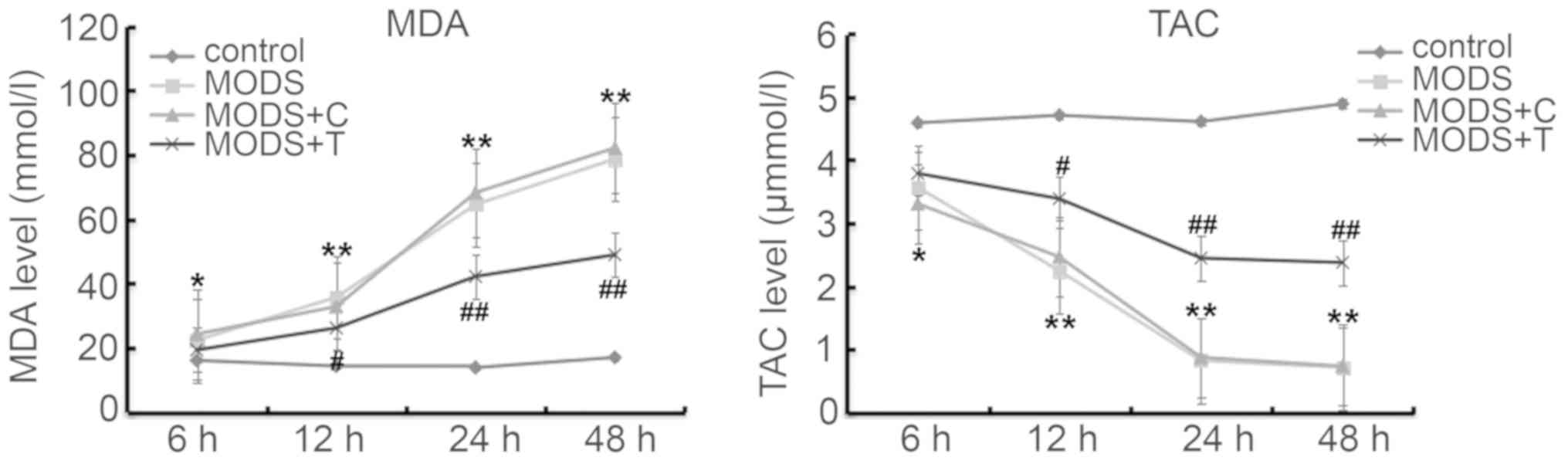
Blood levels of MDA and TAC
As shown in Fig. 4,
the production of MDA in the blood of the MODS rats was higher, and
TAC was lower, at different time points after injury than those in
the rats of the control group. However, TNIP2-plasmid
administration significantly decreased the MDA level and increased
TAC in the blood of the MODS rats.
Effect of TNIP2 on the activation of
NF-κB in MODS rats
To investigate the mechanism of the effect of TNIP2
on MODS rats, the NF-κB pathway was detected (Fig. 5). Forty-eight hours after
treatment, total protein and RNA were extracted from tissues/blood
of rats in the different groups and measured by western blot assay
and qRT-PCR. Our results showed that compared with the control
group, the mRNA and protein level of TNIP2 was significantly
decreased in the MODS model group, and compared with the MODS model
group, TNIP2-plasmid notably increased TNIP2 expression.
Phosphorylation of p65 was significantly enhanced in the MODS rats
compared to the control rats, and this enhancement was eliminated
by TNIP2-plasmid administration (Fig.
5).
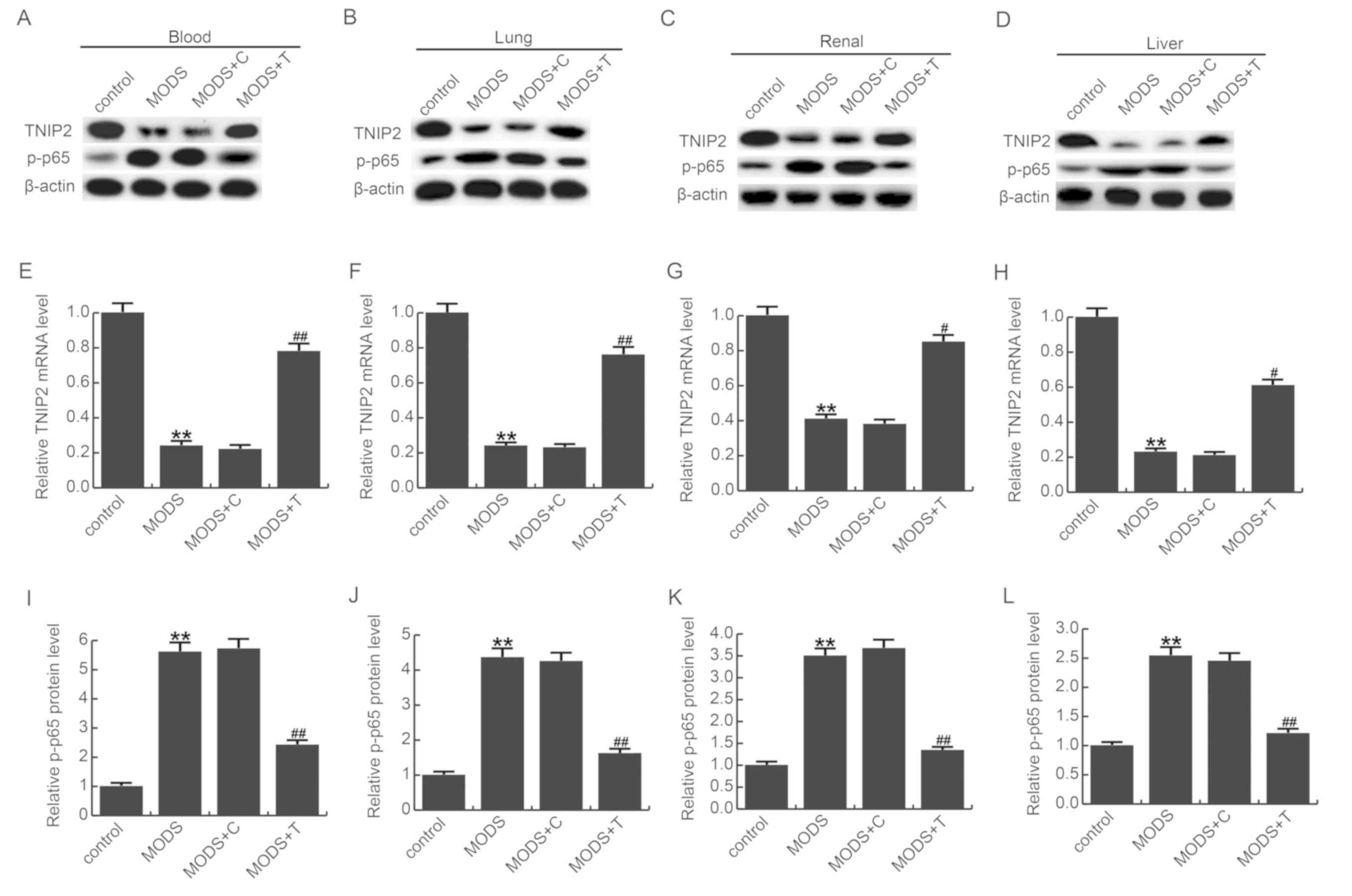 | Figure 5.Effect of TNIP2 on p-NF-κB (p-p65)
expression. Protein levels of TNIP2 and p-p65 in the (A) blood, (B)
lung, (C) renal and (D) liver tissues of MODS rats were detected
using western blot assay. mRNA levels of TNIP2 in the (E) blood,
(F) lung, (G) renal and (H) liver tissues of MODS rats was detected
using reverse transcription-quantitative polymerase chain reaction.
Relative protein levels of p-p65 were analyzed in the (I) blood,
(J) lung, (K) renal and (L) liver tissues of MODS rats. Data are
expressed as mean ± SD. **P<0.01 vs. the control group.
#P<0.05, ##P<0.01 vs. the MODS group.
Groups: Control, MODS (MODS model), MODS model + control-plasmid
(MODS + C) and MODS model + TNIP2-plasmid (MODS + T). MODS,
multiple organ dysfunction syndrome; TNIP2, TNFAIP3-interacting
protein 2; p-, phospho-; NF-κB, nuclear factor-κB. |
Discussion
In the present study, it was determined that TNIP2
is significantly decreased in MODS following severe trauma. Our
findings also showed that TNIP2 plays a protective role in MODS
development by the inhibiting inflammation response and oxidative
stress through regulation of NF-κB activation. We revealed that
TNIP2 may be a promising therapeutic target for the treatment of
MODS.
Severe post-traumatic complications, such as sepsis
and systemic inflammatory response syndrome (SIRS), are the leading
cause of mortality in hospitals with a mortality rate of 30–50%.
SIRS can eventually lead to (26).
Multiple organ failure (MOF) is common in trauma patients with the
most severe injuries, and 29% of trauma patients present with one
or more systemic organ failure (27,28).
MOF is the leading cause of morbidity and delayed mortality in
survivors immediately after injury. The mortality rate of trauma
patients with failure of three organ systems is approximately 67%,
and the mortality rate of patients with failure of four organ
systems is close to 100%. There is a distinct difference between
MODS and MOF. The function of MODS organs is not completely lost
and will not fail, while the organs of MOF patients have sequential
failure of function. Early detection of MODS and interventions can
improve organ function. At present, the therapeutic effect of MODS
remains unsatisfactory. Therefore, it is urgent to find new
effective diagnostic markers and therapeutic targets for MODS.
Severe trauma can lead to the release of various
inflammatory mediators and inflammatory cytokines in the body and
cause systemic inflammatory response syndrome and oxidative stress
(29,30). Inflammation response and oxidative
stress promote the occurrence and development of MODS (31,32).
NF-κB is an important transcription factor involved in the
regulation of proliferation, survival, apoptosis, immune, oxidative
stress, and inflammatory reaction (33–36).
In addition, studies have revealed the important role of NF-κB in
the progression of MODS (15,37).
Over-activated NF-κB may significantly enhance the inflammatory
response and oxidative stress in MODS patient and cause tissue
injury, thus leading to patient death. TNIP2 gene encodes a protein
that can repress the activation of NF-κB, thus we hypothesized that
it may be involved in the development of MODS.
We firstly investigated the expression level of
TNIP2 in MODS patients and MODS rats, and the MODS rat model was
conducted. The findings indicated that TNIP2 was significantly
decreased in the blood of MODS patients, and significant
downregulation of TNIP2 was also observed in the blood, pulmonary,
renal, and hepatic tissues of MODS rats, indicating the
downregulation of TNIP2 in MODS. Then, to study the effect of TNIP2
on MODS rats, the rats were treated with or without the
TNIP2-plasmid. It was found that the increased expression levels of
markers of cellular integrity and organ function including ALT,
AST, LDH, BUN, Cr and CK in the blood of rats induced by MODS were
inhibited by TNIP2-plasmid administration. Moreover, it was found
that blood levels of TNF-α, HMGB-1 and MDA were significantly
enhanced in MODS rats, while TAC was notably decreased, and these
changes were notably reversed by TNIP2-plasmid treatment,
indicating that TNIP2-plasmid administration prevented inflammation
response and oxidative stress in MODS rats. Finally, to investigate
the mechanism of the effect of TNIP2 on MODS rats, the NF-κB
pathway was detected. The results revealed that the phosphorylation
of p65 was significantly enhanced in MODS rats compared to the
control rats, and these enhancements were eliminated by
TNIP2-plasmid administration.
Taken together, it was found for the first time that
TNIP2 is decreased in MODS, and it plays a protective role in MODS
development by inhibiting inflammation response and oxidative
stress via repressing the activation of the NF-κB pathway. TNIP2
may be a potential diagnostic marker and therapeutic target for
MODS treatment.
Acknowledgements
Thanks to Dr Dongbo Zhu, chief physician of Nantong
University Affiliated Hospital (Nantong, China) for his help and
guidance.
Funding
No funding was recieved.
Availability of data and materials
The data sets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HG contributed to study design; HG, XS and JX
contributed to data collection, statistical analysis and data
interpretation; DZ contributed to data collection, manuscript
preparation and literature searching; All authors contributed to
the development of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from every
patient enrolled, and the present study was approved by the Ethics
Committee of the Affiliated Hospital of Nantong University. The
experimental protocol was approved by the Animal Care Committee of
Nantong University, and experiments were performed following with
the guidance of guide for the care and use of laboratory animals
(21).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uzzan B, Cohen R, Nicolas P, Cucherat M
and Perret GY: Procalcitonin as a diagnostic test for sepsis in
critically ill adults and after surgery or trauma: A systematic
review and meta-analysis. Crit Care Med. 34:1996–2003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fabiano G, Pezzolla A, Filograna MA and
Ferrarese F: Traumatic shock-physiopathologic aspects. G Chir.
29:51–57. 2008.PubMed/NCBI
|
|
3
|
Hanisch E, Brause R, Paetz J and Arlt B:
Review of a large clinical series: Predicting death for patients
with abdominal septic shock. J Intensive Care Med. 26:27–33. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mera S, Tatulescu D, Cismaru C, Bondor C,
Slavcovici A, Zanc V, Carstina D and Oltean M: Multiplex cytokine
profiling in patients with sepsis. APMIS. 119:155–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen LN and Nguyen TG: Characteristics
and outcomes of multiple organ dysfunction syndrome among
severe-burn patients. Burns. 35:937–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walsh CR: Multiple organ dysfunction
syndrome after multiple trauma. Orthop Nurs. 24:324–333. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatia M: Inflammatory response on the
pancreatic acinar cell injury. Scand J Surg. 94:97–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Yang S, Liu W, Tang B, Zhang W,
Zhang C, Liao W and Hu A: Early thrombolytic failure in a patient
with massive pulmonary embolism combined with multiple organ
dysfunction syndrome: What next? J Int Med Res. 46:3440–3445. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huster D, Härtel F, Nuding S, Schroeder J,
Zhang Y, Werdan K and Ebelt H: Prognostic relevance of tissue
oxygen saturation in patients in the early stage of multiple organ
dysfunction syndrome. Med Klin Intensivmed Notfmed. 2018.PubMed/NCBI
|
|
10
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brasier AR: The NF-kappaB regulatory
network. Cardiovas Toxicol. 6:111–130. 2006. View Article : Google Scholar
|
|
12
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasparakis M: Regulation of tissue
homeostasis by NF-κB signalling: Implications for inflammatory
diseases. Nat Rev Immunol. 9:778–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell metabolism. 13:11–22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang YT, Fu JJ, Li XL, Li YR, Li CF and
Zhou CY: Effects of hemodialysis and hemoperfusion on inflammatory
factors and nuclear transcriptionfactors in peripheral blood cell
of multiple organ dysfunction syndrome. Eur Rev Med Pharmacol Sci.
20:745–750. 2016.PubMed/NCBI
|
|
16
|
Wang YL, Shen HH, Cheng PY, Chu YJ, Hwang
HR, Lam KK and Lee YM: 17-DMAG, an HSP90 inhibitor, ameliorates
multiple organ dysfunction syndrome via induction of HSP70 in
endotoxemic rats. PLoS One. 11:e01555832016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banks CAS, Boanca G, Lee ZT, Eubanks CG,
Hattem GL, Peak A, Weems LE, Conkright JJ, Florens L and Washburn
MP: TNIP2 is a hub protein in the NF-κB network with both protein
and RNA mediated interactions. Mol Cell Proteomics. 15:3435–3449.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verstrepen L, Carpentier I, Verhelst K and
Beyaert R: ABINs: A20 binding inhibitors of NF-kappa B and
apoptosis signaling. Biochem Pharmacol. 78:105–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie H, Yang M, Zhang B, Liu M and Han S:
Protective role of TNIP2 in myocardial injury induced by acute
pancreatitis and its mechanism. Med Sci Monit. 23:5650–5656. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Gao J and Wang F:
MiR-663a/MiR-423-5p are involved in the pathogenesis of lupus
nephritis via modulating the activation of NF-κB by targeting
TNIP2. Am J Transl Res. 9:3796–3803. 2017.PubMed/NCBI
|
|
21
|
Bayne K: Revised Guide for the Care and
Use of Laboratory Animals available. American Physiological
Society. Physiologist. 39:208, 199–211. 1996.
|
|
22
|
Teng L, Yu M, Li JM, Tang H, Yu J, Mo LH,
Jin J and Liu XZ: Matrix metalloproteinase-9 as new biomarkers of
severity in multiple organ dysfunction syndrome caused by trauma
and infection. Mol Cell Biochem. 360:271–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuzzocrea S, Mazzon E, Di Paola R,
Genovese T, Serraino I, Dugo L, Cuzzocrea E, Fulia F, Caputi AP and
Salvemini D: Protective effect of M40401, selective superoxide
dismutase mimetic, on zymosan-induced nonseptic shock. Crit Care
Med. 32:157–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Wang L, Chen W, Zhao S, Yin C, Lin
Y, Jiang A and Zhang P: USP35 activated by miR let-7a inhibits cell
proliferation and NF-κB activation through stabilization of ABIN-2.
Oncotarget. 6:27891–27906. 2015.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brongel L: Guidelines for severe multiple
and multiorgan traumatic injuries. Przegl Lek. 60:56–62.
2003.PubMed/NCBI
|
|
27
|
Frohlich M, Lefering R, Probst C, Paffrath
T, Schneider MM, Maegele M, Sakka SG, Bouillon B and Wafaisade A;
Committee on Emergency Medicine, Intensive Care, Trauma Management
of the German Trauma Society Sektion NIS, : Epidemiology and risk
factors of multiple-organ failure after multiple trauma: An
analysis of 31,154 patients from the TraumaRegister DGU. J Trauma
Acute Care Surg. 76:921–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vogel JA, Liao MM, Hopkins E, Seleno N,
Byyny RL, Moore EE, Gravitz C and Haukoos JS: Prediction of
postinjury multiple-organ failure in the emergency department:
Development of the denver emergency department trauma organ failure
score. J Trauma Acute Care Surg. 76:140–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu MH, Tian J, Su YP, Wang T, Xiang Q and
Wen L: Cervical sympathetic block regulates early systemic
inflammatory response in severe trauma patients. Med Sci Monit.
19:194–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pierce A and Pittet JF: Inflammatory
response to trauma: Implications for coagulation and resuscitation.
Curr Opin Anaesthesiol. 27:246–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Chen R, Zhu Z, Zhang X and Wang S:
Effects of insulin combined with ethyl pyruvate on inflammatory
response and oxidative stress in multiple-organ dysfunction
syndrome rats with severe burns. Am J Emerg Med. 34:2154–2158.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osterbur K, Mann FA, Kuroki K and DeClue
A: Multiple organ dysfunction syndrome in humans and animals. J Vet
Intern Med. 28:1141–1151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leotoing L, Chereau F, Baron S, Hube F,
Valencia HJ, Bordereaux D, Demmers JA, Strouboulis J and Baud V:
A20-binding inhibitor of nuclear factor-κB (NF-κB)-2 (ABIN-2) is an
activator of inhibitor of NF-κB (IκB) kinase α (IKKα)-mediated
NF-κB transcriptional activity. J Biol Chem. 286:32277–32288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang Y, Zhou Y and Shen P: NF-kappaB and
its regulation on the immune system. Cell Mol Immunol. 1:343–350.
2004.PubMed/NCBI
|
|
35
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-κB transcription factors in the immune system.
Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mariappan N, Elks CM, Sriramula S,
Guggilam A, Liu Z, Borkhsenious O and Francis J: NF-kappaB-induced
oxidative stress contributes to mitochondrial and cardiac
dysfunction in type II diabetes. Cardiovasc Res. 85:473–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu S, Zhang J, Pang Q, Song S, Miao R,
Chen W, Zhou Y and Liu C: The protective role of curcumin in
zymosan-induced multiple organ dysfunction syndrome in mice. Shock.
45:209–219. 2016. View Article : Google Scholar : PubMed/NCBI
|