Introduction
Temporal lobe epilepsy (TLE) is the most common form
of adult epilepsy (1). Mossy fiber
sprouting (MFS) has been observed in experimental models of TLE and
in the epileptic human hippocampus (2–5);
however, the mechanisms underlying this structural alteration are
not fully understood. Previously, it was demonstrated that axon
guidance molecules served important roles in epilepsy (6).
The repulsive guidance molecule (RGM) was originally
reported as a membrane-bound protein involved in guiding axons in
the developing chick retina (7).
As a homolog of RGM, RGMa is mainly expressed in the central
nervous system (CNS), particularly in the hippocampus (8). Previous studies have revealed that
RGMa served a critical role in neural circuit formation,
potentially via focal adhesion kinase (FAK) dephosphorylation at
Tyr397 (9–11). Dephosphorylation of FAK (Tyr397)
inhibits the interaction between p120GAP-FAK and promotes the
interaction between p120GAP and GTP-Ras, downregulating the
activation of Ras; p120GAP has been reported a Ras-specific
GTPase-activating protein (11).
In our previous study, we hypothesized that RGMa may be involved in
TLE via the RGMa-FAK-Ras signaling pathway (12); however, the exact role of RGMa and
FAK in epileptogenesis and MFS remains unclear. The present study
aimed to investigate whether RGMa and FAK dephosphorylation
(Tyr397) inhibits epileptogenesis and MFS in vivo.
Materials and methods
Intracerebroventricular injection
The present study was approved by the Ethics
Committee of Xiangya Hospital of Central South University
(Changsha, China). Rats were anesthetized with 10% chloral hydrate
(300 mg/kg) intraperitoneally and positioned in a stereotaxic frame
(RWD Life Science Co., Ltd., Shenzhen, China). A stainless-steel
guide cannula (RWD Life Science Co., Ltd.) was inserted into the
lateral ventricle at the following coordinates relative to the
Bregma: Antero-posterior-0.9 mm, medio-lateral-1.4 mm and
dorso-ventral-3.3 mm. The guide cannula was fixed into the skull
with adhesive and dental acrylic cement. A stainless steel cannula
served as an injection cannula that was connected via a
polyethylene tube to a microsyringe, which was inserted via the
guide cannula and extended 2 mm beyond the tip of the guide cannula
to reach the lateral ventricle. Following surgery, the rats were
allowed to recover for 1 week. Recombinant RGMa protein (R&D
Systems, Inc., Minneapolis, MN, USA) dissolved in PBS (0.04 µg/µl),
and FAK inhibitor 14 (R&D Systems, Inc.) dissolved in PBS (0.04
mg/µl) (13) were administrated
intracerebroventricularly at a volume of 10 µl every 3 days;
control rats were injected with PBS.
Animals and the pentylenetetrazol
(PTZ) model
Male Sprague-Dawley rats (n=200; age, 40–45
days-old; weight, 120–180 g) were purchased from the Center for
Experimental Animals of Central South University (Changsha, China).
Rats were housed under controlled conditions (18-25°C; 50–60%
humidity; 12 h light/dark cycle) with food pellets and water
available ad libitum. A total of 50 rats were randomly
divided into the control (n=15) and PTZ groups (n=35). The PTZ
kindling model was established as previously described (12). Briefly, the rats in the PTZ group
received an intraperitoneal dose of 30 mg/kg PTZ (10 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) once daily until the
rats were kindled or sacrificed. If persistent generalized
tonic-clonic seizure lasted ≥20 sec, an intraperitoneal injection
of 300 mg/kg chloral hydrate was administered. Following seizure
termination, if the rat breathed irregularly, had no autonomous
respiration or death appeared imminent, it was sacrificed
immediately. Rats in the control group were injected with an equal
dose of saline (3 ml/kg). Rats were scored according to the Racine
scale: Score 0, behavior arrest; score 1, facial twitches; score 2,
chewing and/or head nodding or ‘wet dog’ shakes; score 3, forelimb
clonus; score 4, rearing, falling on forelimbs; score 5, imbalance
and falling on side or back (14).
A score ≥3 for 5 consecutive days indicated that the rat was
kindled. At day 3, and 1, 2, 4 and 6 weeks following the first
injection, the rats were sacrificed for co-immunoprecipitation
(Co-IP) analysis, Timm staining and western blot analysis. Rats in
the day 3 and 1 week group were administrated PTZ/saline daily
until they were sacrificed on day 3 and at the end of week 1,
respectively. For the 2 weeks, 4 weeks and 6 weeks group, rats were
only administrated PTZ/saline daily until they were kindled and
then sacrificed at each time-point. If the rat was not kindled,
PTZ/saline was administered daily until they were sacrificed at
each time-point. A total of 150 rats were divided into experimental
and intervention groups. The experimental group (PTZ + PBS)
received PTZ intraperitoneally and PBS intracerebroventricularly
(n=39, with the exception of 10 rats that succumbed to mortality
due to persistent generalized tonic-clonic seizure, and 1 rat that
was not kindled). The intervention group were divided into two
subgroups, receiving PTZ intraperitoneally and recombinant RGMa
protein (PTZ + RGMa; n=36, with the exception of 9 rats that
succumbed to mortality due to persistent generalized tonic-clonic
seizure, and 5 rats that were not kindled) or FAK inhibitor 14
intracerebroventricularly (PTZ + FAK inhibitor 14; n=39, with the
exception of 7 rats that succumbed to mortality due to persistent
generalized tonic-clonic seizure, and 4 rats that were not
kindled), respectively. The rates of mortality seen in the present
study were similar to previous studies (15–17).
In our previous study, it was observed that the PTZ group rats were
kindled in accordance with the kindling criterion at 23.6±2 days
following PTZ injection, and the expression levels of RGMa, FAK
(Tyr397) and Ras were the lowest or peaked at 4 weeks (12). The present study was approved and
conducted in accordance with the guidelines of the Animal Ethics
Committee of Central South University (Changsha, China). All
efforts were made to minimize the number of animals employed and
their suffering in the present study.
Timm staining
The method of Timm staining was the conducted as
previously described (12). In
brief, rats were injected with 10% chloral hydrate (300 mg/kg) and
perfused intracardially with 300 ml saline, followed by 200 ml 0.4%
sodium sulfide in 0.1 M phosphate buffer and 200 ml 4%
paraformaldehyde (PFA) at 4°C. The brains were removed, fixed in 4%
PFA at 4°C overnight and in a 30% solution of sucrose in fixative.
Coronal sections (30 µm) were mounted on slides, air-dried and
developed for 90 min in the dark at 26°C in 60 ml gum arabic (50%),
10 ml citrate buffer (2 M), 30 ml hydroquinone (0.5 M) and 0.5 ml
silver nitrate (17%). The inner molecular layer of the dentate
gyrus, as well as the pyramidal and infrapyramidal CA3 region were
analyzed using three randomly chosen fields under an optical
microscope (magnification, ×200).
Western blot analysis and Co-IP
assay
At the various time-points, rats were deeply
anesthetized with chloral hydrate and sacrificed as aforementioned.
Proteins of the hippocampus were extracted using
radioimmunoprecipitation assay lysate buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was
determined using a bicinchoninic acid protein assay. For western
blotting, equal amounts of protein (40 µg) were subjected to 10%
SDS-PAGE. The separated proteins were electro-transferred onto 0.45
or 0.22 µm polyvinylidene difluoride (PVDF) membranes (Pall Life
Sciences, Port Washington, NY, USA). The membranes were blocked
with 5% dried skim milk in TBS at room temperature for 2 h and were
then incubated with the following primary antibodies overnight at
4°C: Anti-RGMa polyclonal antibodies (1:800; cat. no. ab26287;
Abcam, Cambridge, UK), anti-FAK Tyr397 polyclonal antibodies
(1:500; cat. no. ab81298; Abcam), anti-FAK polyclonal antibodies
(1:1,000; cat. no. sc-558; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-Ras polyclonal antibodies (1:500; cat. no.
sc-166691; Santa Cruz Biotechnology, Inc.) and anti-GAPDH
monoclonal antibodies (1:1,000; sc-66163, Santa Cruz Biotechnology,
Inc.). Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit/anti-mouse secondary
antibodies (1:2,000; cat. no. A0208/A0216; Beyotime Institute of
Biotechnology) for 1 h at room temperature. The immunoreactive
bands were visualized via enhanced chemiluminescence (Pierce;
Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol, and quantified using Image Lab version 4.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). For Co-IP,
antibodies were applied to lysates (100–500 µg total protein) plus
1–2 µg rabbit anti-rat FAK polyclonal antibody (cat. no. sc-558;
Santa Cruz Biotechnology, Inc.) for 4.5 h at 4°C and collected by
binding to protein G plus- or protein A-agarose beads and washed in
lysis buffer (Beyotime Institute of Biotechnology) without SDS and
sodium deoxycholate. The precipitated proteins were separated by
10% SDS-PAGE and transferred to PVDF membranes followed by
immunoblotting using anti-p120GAP antibodies (cat. no. sc-63;
1:500; Santa Cruz Biotechnology, Inc.) as aforementioned.
Experiments were independently repeated three times.
Statistical analysis
The results were presented as the mean ± standard
deviation. Analysis was performed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA). Statistical significance was assessed
using one-way analysis of variance followed by the Least
Significant Difference post-hoc test for multiple group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
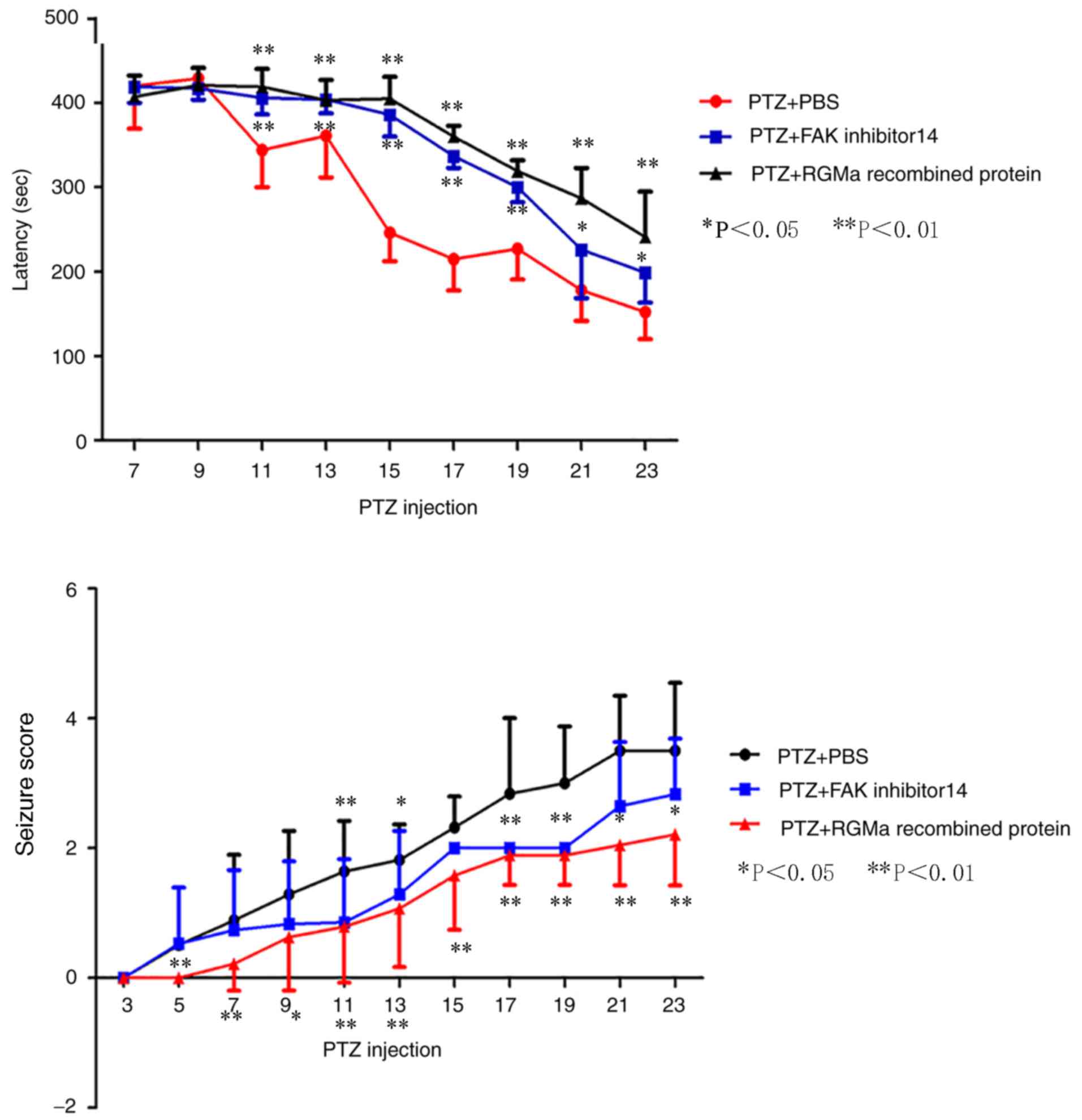
Behavioral outcomes
To determine the exact role of RGMa and FAK (Tyr397)
in the PTZ model, the seizure latency and severity was assessed.
With the exception of 10 rats that succumbed to mortality due to
persistent generalized tonic-clonic seizure, and 1 rat that was not
kindled, rats of the PTZ + PBS group developed seizure activities
of varying degrees following continuous PTZ injections for 19–25
days (an average of 22.06±2.32 days) and the success rate was 92.3%
(kindled/surviving rats). The PTZ-induced seizure activity was
observed to occur 2–8 min following treatment (an average of
420±50.73 sec at 7 days, 429±11.57 sec at 9 days, 344±43.86 sec at
11 days, 361±49.47 sec at 13 days, 246±33.76 sec at 15 days,
215±37.15 sec at 17 days, 227±36.29 sec at 19 days, 178±36.33 sec
at 21 days and 152±31.99 sec at 23 days following treatment)
(Fig. 1). No epileptic-associated
behavior was noted in the control rats.
Similar to the PTZ + PBS group, the seizure severity
of the PTZ + RGMa and PTZ + FAK inhibitor 14 groups gradually
increased following continuous PTZ administration; however, in the
PTZ + RGMa group (with the exception of 9 rats that had succumbed
to mortality due to persistent generalized tonic-clonic seizure,
and 5 rats that were not kindled), compared with in the PTZ + PBS
group, the average kindling duration significantly increased to
29.0±3.13 days (P<0.01; data not shown) and the success rate of
PTZ kindling model significantly decreased to 52.6% (P<0.05).
The seizure latency was significantly increased from 11 days
post-PTZ administrationexhibited no significant difference compared
with that of the PTZ + PBS group. The results indicated that
intracerebroventricular injection of recombinant RGMa protein and
FAK inhibitor 14 exhibited an increase in seizure latency and
decreased seizure severity scores (Fig. 1).
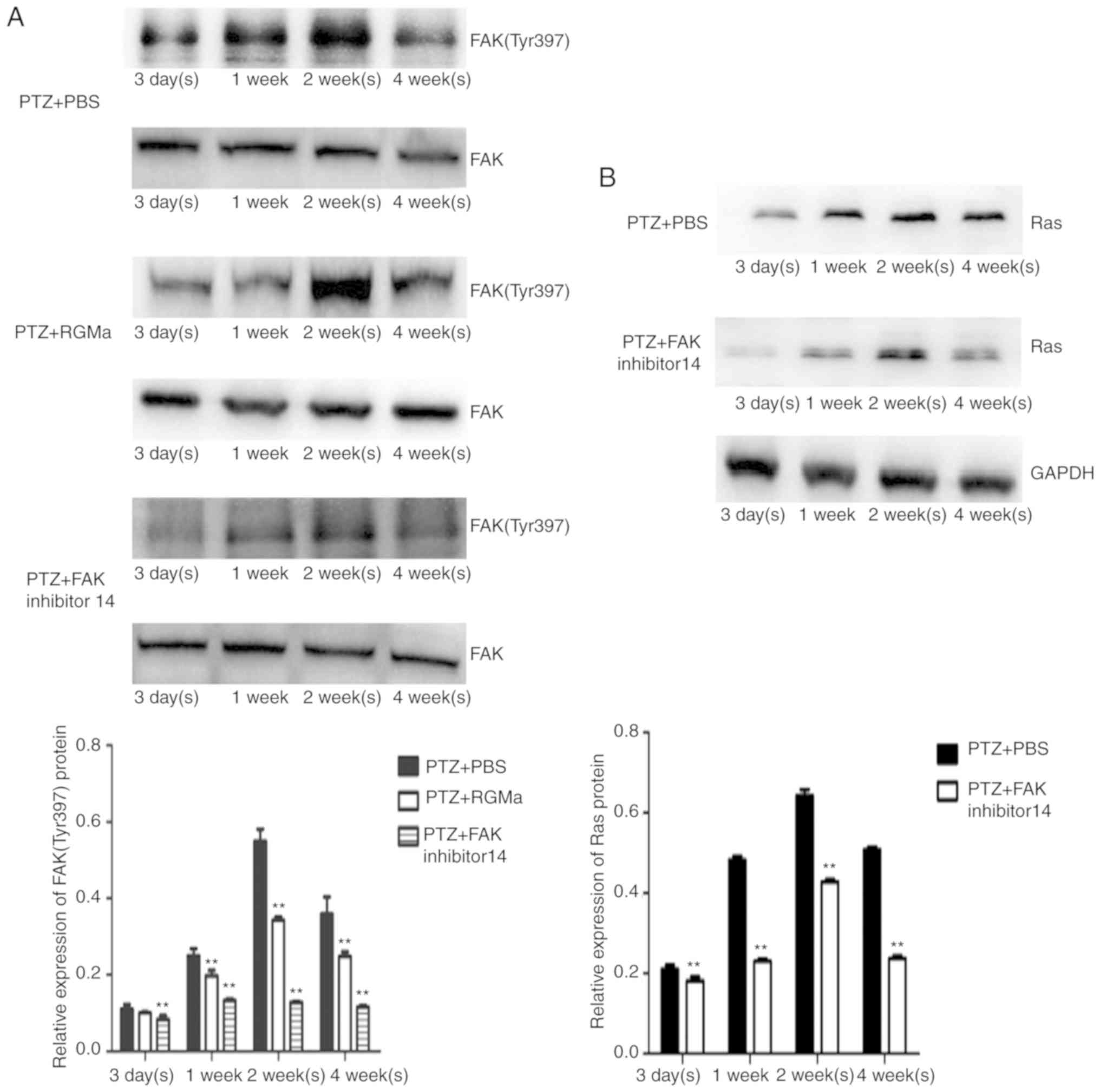
Intracerebroventricular injection of
recombinant RGMa protein reduces the phosphorylation of FAK
(Tyr397), the expression of Ras and suppresses MFS
As reported in our previous study (11), in the PTZ + PBS group, the
expression of FAK (Tyr397) increased from 1 week and peaked at 2
weeks, then declined at 4 weeks following PTZ administration;
however, high phosphorylation levels were maintained (Fig. 2A; Table I). Conversely, in the PTZ + RGMa
and PTZ + FAK inhibitor 14 groups, the phosphorylation levels of
FAK (Tyr397) were significantly decreased at all investigated
time-points (P<0.01) except at 3 days compared with the PTZ +
PBS group (Fig. 2A; Table I).
 | Table I.Relative expression of FAK (Tyr397) in
PTZ + PBS group, PTZ + RGMa group and PTZ + FAK inhibitor 14
group. |
Table I.
Relative expression of FAK (Tyr397) in
PTZ + PBS group, PTZ + RGMa group and PTZ + FAK inhibitor 14
group.
| Group | 3 days | 1 week | 2 weeks | 4 weeks |
|---|
| PTZ + PBS | 0.111±0.012 | 0.250±0.018 | 0.550±0.031 | 0.359±0.045 |
| PTZ + RGMa | 0.100±0.002 |
0.198±0.014a |
0.343±0.009a |
0.248±0.012a |
| PTZ + FAK inhibitor
14 |
0.084±0.009a |
0.133±0.004a |
0.128±0.003a |
0.116±0.004a |
The expression of Ras also increased at weeks 1 and
2, but decreased at 4 weeks (Fig.
2B); the PTZ + FAK inhibitor 14 group exhibited a similar
phosphorylation profile to that of the PTZ + PBS group; however,
the expression levels of Ras were significantly reduced (Fig. 2B).
Similar to the PTZ + PBS group (Fig. 3A), the PTZ + RGMa group (Fig. 3B) at day 3 and 1 week following PTZ
administration, exhibited weak black Timm staining in the stratum
pyramidale of the CA3 region. During the progression of PTZ-induced
kindling, MFS in the PTZ + RGMa group rats was notably suppressed
than that in PTZ + PBS group. Timm score analysis demonstrated that
the PTZ + RGMa group possessed a low density band of Timm staining
at 2, 4 and 5 weeks (Table II).
The results indicated that recombinant RGMa protein may reduce the
phosphorylation of FAK (Tyr397) and suppress MFS. Whether inhibited
FAK (Tyr397) phosphorylation may suppress MFS requires further
investigation. In contrast to the PTZ + RGMa group, black Timm
staining gradually increased at 2 weeks (Fig. 3C) and significantly increased at 4
weeks in PTZ + FAK inhibitor 14 group compared with in the PTZ +
PBS group (Table II), there was
no significant difference compared with in PTZ + PBS group except
at 2 weeks.
 | Table II.Timm score analysis in PTZ + PBS
group, PTZ + RGMa group and PTZ + FAK inhibitor 14 group. |
Table II.
Timm score analysis in PTZ + PBS
group, PTZ + RGMa group and PTZ + FAK inhibitor 14 group.
| Group | 3 days | 1 week | 2 weeks | 4 weeks | 5 weeks |
|---|
| PTZ + PBS | 1.00±0.00 | 2.20±0.45 | 3.20±0.84 | 4.25±0.50 | 4.5±0.58 |
| PTZ + RGMa | 0.75±0.50 | 1.66±0.58 |
1.75±0.50a |
2.50±0.58a | 3.0±0.71a |
| PTZ + FAK inhibitor
14 | 1.00±0.00 | 1.50±0.58 | 2.25±0.50 |
2.75±0.50a | 4.00±0.82 |
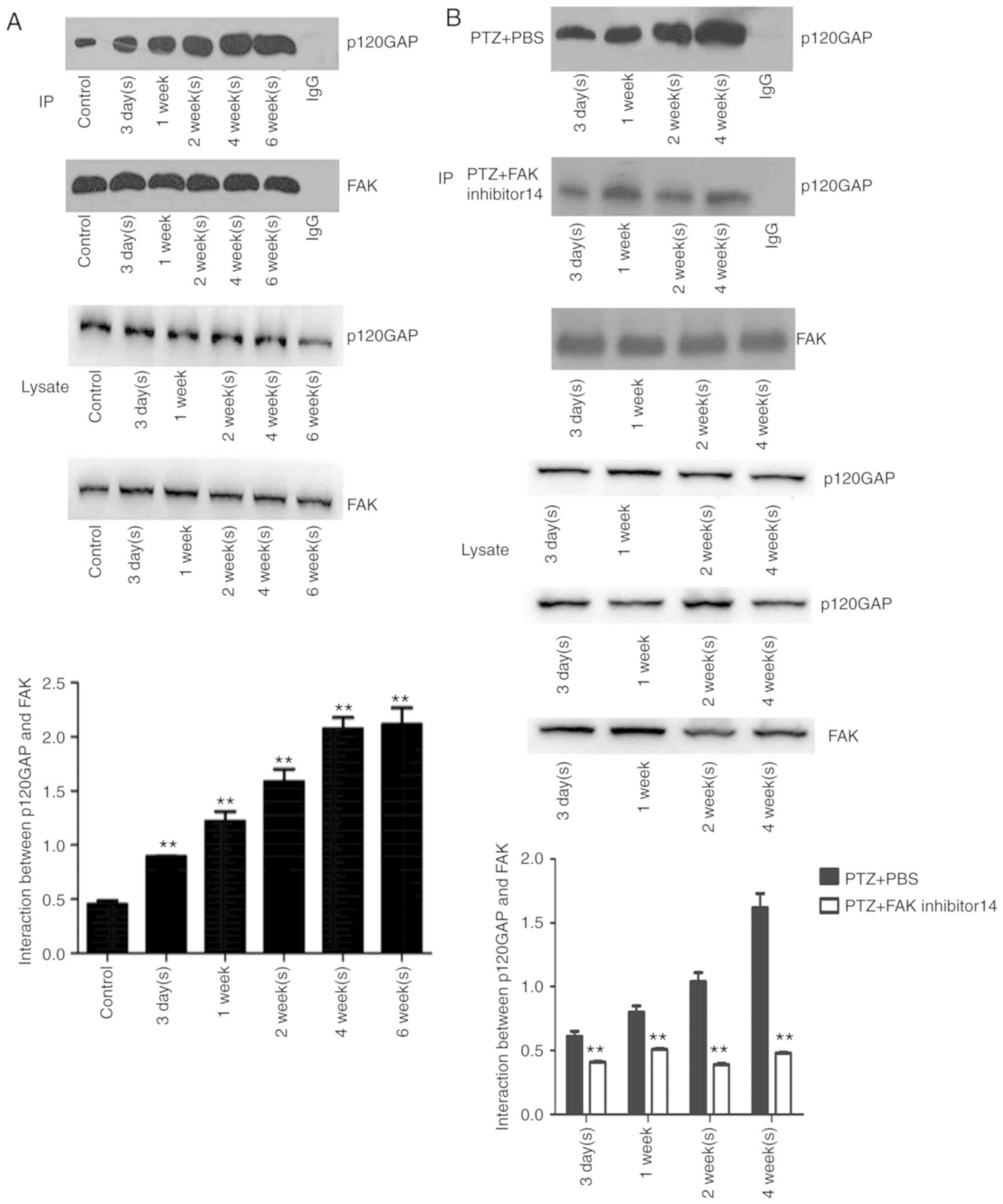
Intracerebroventricular injection FAK
inhibitor 14 inhibits the interaction between FAK and p120GAP, but
does not suppress MFS
From the Co-IP assay, the interaction between FAK
and p120GAP was detected in the PTZ and control groups, which was
significantly upregulated (P<0.01) from day 3 in the PTZ group
than that of control group, and peaked at weeks 4 and 6 week
(Fig. 4A). Similar to the PTZ
group, interactions between FAK and p120GAP increased at 1 week,
and peaked at 4 weeks in the PTZ + PBS group (Fig. 4B); however the interactions between
p120GAP were significantly reduced in the PTZ + FAK inhibitor 14
group compared with in the PTZ + PBS group.
Discussion
RGMa is a membrane-associated glycoprotein that
regulates axonal guidance and inhibits axon outgrowth. (7). In a case report, a child with
deletion of the RGMa gene exhibited epilepsy and mental deficiency
(18). In a previous study, we
reported that the expression of RGMa was significantly
downregulated in the PTZ kindling model, suggesting that decreased
expression of RGMa may be associated with the development of
epilepsy (12). In the present
study, it was demonstrated that intracerebroventricular injection
of RGMa suppressed MFS, increased seizure latency and decreased
seizure severity score, which indicated that RGMa reduced MFS and
inhibited epileptogenesis in a PTZ-induced rat model. The results
of the present study were consistent with a previous report that
recombinant RGMa protein could inhibit hyperexcitability-induced
MFS in cultured slices (19); the
underlying mechanism remains unknown.
A previous study revealed that RGMa may exert its
biological effects by dephosphorylating FAK at Tyr397.
Dephosphorylation of FAK (Tyr397) decreased the interaction between
p120GAP-FAK and increased frequency of interactions between p120GAP
and GTP-Ras (p120GAP is a Ras-specific GTPase-activating protein),
which reduced the activation of Ras (11). Our previous study reported that FAK
(Tyr397) and Ras were significantly upregulated during the
progression of PTZ-associated kindling (12). In the present study, it was
demonstrated that the interactions between FAK and p120GAP were
significantly increased during PTZ-associated kindling progression.
Intracerebroventricularly injected recombinant RGMa protein reduced
the phosphorylation levels of FAK at Tyr397; the interactions
between FAK and p120GAP, and the expression of Ras were
significantly decreased in response to intracerebroventricularly
injected FAK inhibitor 14. Collectively, these results indicated
that by decreasing the phosphorylation levels of FAK (Tyr397),
p120GAP may mediate RGMa-induced Ras inactivation, thereby inducing
MFS inhibition. However, the present study reported that seizures,
but not MFS, may be suppressed by intracerebroventricular injection
of FAK inhibitor 14. The possible reasons were as follows: i)
Tyr397 phosphorylation may serve an important role in FAK-dependent
signaling; however, FAK is also phosphorylated at numerous serine
residues, including Ser-722, 732, 910 and 843. The proximity of
these phosphorylated serine residues to sites at which FAK
interacts with other proteins suggests a possible function in the
regulation of the assembly of FAK signaling complexes (20). Semaphorin induced Ser-732 and
Tyr397 phosphorylation of FAK serves a previously unreported role
in regulating the dendritic development of newborn neurons, and the
phosphorylation process of these two residues were independent of
each other (21). The association
between these phosphorylated serine residues and MFS, and the
interaction between tyrosine and serine phosphorylation residues
requires further investigation; and ii) in addition to MFS,
hippocampal neuronal apoptosis is another common pathological
phenomenon (22). When neuronal
loss or apoptosis is present in the CA3 region, the mossy fibers of
granule cells lose normal connections with CA3 neurons, or
otherwise develop collaterals in an abnormal location, such as
inner molecular layer subfields and/or stratum oriens of the CA3
(23). A previous study revealed
that tyrosine phosphorylation of FAK may confer anti-neuronal
apoptosis; the inhibition of Tyr397 phosphorylation may induce
hippocampal neuronal apoptosis, which may partly offset the
function of inhibiting axonal growth by Ras.
In conclusion, the present study demonstrated that
intracerebroventricular injection of recombinant RGMa protein
attenuated PTZ-induced seizures and ameliorated MFS. Additionally,
RGMa may exert these effects, partly via the FAK-p120GAP-Ras
signaling pathway. Thus, the present study proposed that RGMa may
be considered as a potential therapeutic agent in the treatment of
epilepsy. Furthermore, intracerebroventricular-injected protein and
inhibitor may not only affect the hippocampus; however, as TLE is
characterized by several histological aberrations in the
hippocampus, the effects of RGMa on MFS in a TLE model were
investigated only in this region in the present study. The
particular effects of RGMa in other brain tissues on TLE require
further investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by National Nature Science
Foundation of China (grant no. 81571276).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS and FT conceived and designed the study. MS, HX
and YX performed the experiments and data analysis. MS wrote the
manuscript. FT reviewed and edited the manuscript. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Xiangya Hospital of Central South University
(Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dewar SR and Pieters HC: Perceptions of
epilepsy surgery: A systematic review and an explanatory model of
decision-making. Epilepsy Behav. 44:171–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrade-Valença LP, Valença MM, Velasco
TR, Carlotti CG Jr, Assirati JA, Galvis-Alonso OY, Neder L, Cendes
F and Leite JP: Mesial temporal lobe epilepsy: Clinical and
neuropathologic findings of familial and sporadic forms. Epilepsia.
6:1046–1054. 2008. View Article : Google Scholar
|
|
3
|
Lamont SR, Stanwell BJ, Hill R, Reid IC
and Stewart CA: Ketamine pre-treatment dissociates the effects of
electro-convulsive stimulation on mossy fiber sprouting and
cellular proliferation in the dentate gyrus. Brain Res. 1053:27–32.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo LW, Lee CY, Chen JH, Wedeen VJ, Chen
CC, Liou HH and Tseng WY: Mossy fiber sprouting in
pilocarpine-induced status epilepticus rat hippocampus: A
correlative study of diffusion spectrum imaging and histology.
Neuroimage. 41:789–800. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buckmaster PS, Zhang GF and Yamawaki R:
Axon sprouting in a model of temporal lobe epilepsy creates a
predominantly excitatory feedback circuit. J Neurosci.
22:6650–6658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yaron A and Zheng B: Navigating their way
to the clinic: Emerging roles for axon guidance molecules in
neurological disorders and injury. Dev Neurobiol. 67:1216–1231.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monnier PP, Sierra A, Macchi P,
Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B,
Bonhoeffer F and Mueller BK: RGM is a repulsive guidance molecule
for retinal axons. Nature. 419:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brinks H, Conrad S, Vogt J, Oldekamp J,
Sierra A, Deitinghoff L, Bechmann I, Alvarez-Bolado G, Heimrich B,
Monnier PP, et al: The repulsive guidance molecule RGMa is involved
in the formation of afferent connections in the dentate gyrus. J
Neurosci. 24:3862–3869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hata K, Fujitani M, Yasuda Y, Doya H,
Saito T, Yamagishi S, Mueller BK and Yamashita T: RGMa inhibition
promotes axonal growth and recovery after spinal cord injury. J
Cell Biol. 173:47–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang T, Wu X, Yin C, Klebe D, Zhang JH and
Qin X: CRMP-2 is involved in axon growth inhibition induced by RGMa
in vitro and in vivo. Mol Neurobiol. 47:903–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Endo M and Yamashita T: Inactivation of
Ras by p120GAP via focal adhesion kinase dephosphorylation mediates
RGMa-induced growth cone collapse. J Neurosci. 29:6649–6662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song MY, Tian FF, Wang YZ, Huang X, Guo JL
and Ding DX: Potential roles of the RGMa-FAK-Ras pathway in
hippocampal mossy fiber sprouting in the pentylenetetrazole
kindling model. Mol Med Rep. 11:1738–1744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Topkoru BC, Altay O, Duris K, Krafft PR,
Yan J and Zhang JH: Nasal administration of recombi nant
osteopontin attenuates early brain injury after subarachnoid
hemorrhage. Stroke. 44:3189–3194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Racine RJ, Gartner JG and Burnham WM:
Epileptiform activity and neural plasticity in limbic structures.
Brain Res. 47:262–268. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoeller AA, de Carvalho CR, Franco PLC,
Formolo DA, Imthon AK, Dos Santos HR, Eidt I, Souza GR, Constantino
LC, Ferreira CL, et al: Behavioral and neurochemical consequences
of pentylenetetrazol-induced kindling in young and middle-aged
rats. Pharmaceuticals (Basel). 10:e752017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lian XY, Zhang ZZ and Stringer JL:
Anticonvulsant activity of ginseng on seizures induced by chemical
convulsants. Epilepsia. 46:15–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ullah I, Badshah H, Naseer MI, Lee HY and
Kim MO: Thymoquinone and vitamin C attenuates
pentylenetetrazole-induced seizures via activation of GABAB1
receptor in adult rats cortex and hippocampus. Neuromol Med.
17:35–45. 2015. View Article : Google Scholar
|
|
18
|
Capelli LP, Krepischi AC, Gurgel-Giannetti
J, Mendes MF, Rodrigues T, Varela MC, Koiffmann CP and Rosenberg C:
Deletion of the RMGA and CHD2 genes in a child with epilepsy and
mental deficiency. Eur J Med Genet. 55:132–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata K, Nakahara S, Shimizu E,
Yamashita T, Matsuki N and Koyama R: Repulsive guidance molecule a
regulates hippocampal mossy fiber branching in vitro. Neuroreport.
24:609–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang X, Sinnett-Smith J and Rozengurt E:
Differential FAK phosphorylation at Ser-910, Ser-843 and Tyr-397
induced by angiotensin II, LPA and EGF in intestinal epithelial
cells. Cell Signal. 19:1000–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ng T, Ryu JR, Sohn JH, Tan T, Song H, Ming
GL and Goh EL: Class 3 semaphorin mediates dendrite growth in adult
newborn neurons through Cdk5/FAK pathway. PloS One. 8:e655722013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavazos JE and Cross DJ: The role of
synaptic reorganization in mesial temporal lobe epilepsy. Epilepsy
Behav. 8:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Guo J, Wang Q and Chen Y:
MicroRNA-132 silencing decreases the spontaneous recurrent
seizures. Int J Clin Exp Med. 7:1639–1649. 2014.PubMed/NCBI
|