Introduction
Thyroid cancer is a common endocrine malignancy,
accounting for ~2.1% of all cancers worldwide (1); it includes four major types:
Papillary, medullary, follicular and anaplastic thyroid cancer
(ATC) (2). Among them, ATC is a
rare form of thyroid cancer, comprising <2% of all thyroid
cancers, but causing >50% of thyroid-related mortality (3,4).
Most patients with ATC develop distant metastasis, which often
makes traditional treatments ineffective (5); thus, there is an urgent requirement
for effective diagnostic markers and therapeutic targets.
SRY-related HMG box-2 (SOX2) is a
pluripotency-associated transcription factor, which is involved in
growth, metastasis, tumorigenicity and drug resistance in multiple
different types of cancer (6); for
example, the expression of SOX2 may be implicated in the metastasis
and invasion of pancreatic cancer (7) and gastric and prostatic
carcinogenesis (8,9). In addition, SOX2 has been observed to
promote cell proliferation and tumorigenesis through inducing cell
cycle arrest and apoptosis (10).
SOX2 was previously found to be upregulated in ATC tissues, and the
inhibition of SOX2 expression improved the sensitivity of ATC cell
lines to antitumor drugs (11,12).
These investigations indicated that SOX2 may be involved in the
growth of ATC cells; however, the mechanism remains largely
unknown.
Fibronectin 1 (FN1) is an important regulatory
factor of cell migration and metastasis, which is reported to be
involved in the development of human cutaneous squamous cell
carcinoma (13). FN1 was observed
to be upregulated in thyroid cancer, and its silencing inhibited
the proliferation, invasion and migration of thyroid cancer,
indicating that FN1 may be involved in the development of thyroid
cancer (14,15). Furthermore, FN1 stimulated cell
proliferation and inhibited apoptosis through the activation of
PI3K and NF-κB (16). A previous
study demonstrated that SOX2 targeted FN1 and mediated the
migration and invasion of ovarian cancer cells (17); thus, it was hypothesized that SOX2
may also regulate the expression of FN1 to mediate cellular
migration and invasion in ATC. The present study aimed to explore
the role of SOX2 and FN1 and determine whether SOX2 affected the
aggressive phenotype of ATC through affecting FN1 and PI3K/AKT
signaling.
Materials and methods
Cell lines and culture
Human ATC cell lines FRO and 8505c and the normal
human thyroid follicular epithelial cell line Nthy-ori 3–1 were
obtained from the China Infrastructure of Cell Line Resources,
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences. Cells were cultured in DMEM (Beyotime Institute of
Biotechnology), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin,
and maintained in a humidified atmosphere at 37°C with 5%
CO2.
Patient studies
The study was performed in accordance with the
Declaration of Helsinki and approved by The Ethics Committee of
Zhejiang Cancer Hospital (Hangzhou, China). Written informed
consent was obtained from each participant prior to the study.
Twenty pairs of surgical specimens (undifferentiated ATC tissue and
para-carcinoma tissue) were collected from patients (11 females and
9 males; age range, 41–67 years; mean age, 51.25±10.20 years) in
Zhejiang Cancer Hospital (Hangzhou, China) between July 2018 and
May 2019. Para-carcinoma tissue [negative control (NC)] was defined
as tissue located >0.5 cm away from the tumor. Patients >18
years of age with an ACT diagnosis were eligible for this study,
whereas patients with other malignancies were excluded from this
study. Tissue samples were collected, immediately frozen with
liquid nitrogen and stored in a refrigerator at −80°C until
use.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 1×106 FRO,
8505c and Nthy-ori 3-1 cells, and 50 mg ATC and para-carcinoma
tissue samples using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using the PrimeScript
RT-PCR kit (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. qPCR was subsequently performed in
triplicate using the SYBR® GreenER™ qPCR Supermix kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, and a StepOnePlus RT-PCR system (Thermo
Fisher Scientific, Inc.). The following primer pairs were used for
the qPCR: SOX2 forward, 5′-GTGGAAACTTTTGTCGGAGAC-3′ and reverse,
5′-CGAGTAGGACATGCTGTAGGT-3′; and GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. The following thermocycling conditions
were used for the qPCR: Initial denaturation at 94°C for 15 min; 38
cycles at 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec; and
a final extension at 72°C for 7 min. SOX2 mRNA expression levels
were quantified using the 2−ΔΔCq method (18) and GAPDH was used as the internal
loading control.
Immunohistochemistry (IHC)
analysis
IHC was performed using the standard
streptavidin-peroxidase method (19). Tissue samples were fixed with 10%
formalin fixative for 24 h at room temperature, embedded into
paraffin and prepared into 4-µm sections. The sections were
subsequently deparaffinized in xylene at 92°C and rehydrated in a
descending alcohol series. Following antigen retrieval, endogenous
peroxidase activity was blocked using 3% H2O2
for 10 min at 22°C. Sections were then blocked with 5% goat serum
(Thermo Fisher Scientific, Inc.) for 1 h at room temperature and
subsequently incubated overnight at 4°C with primary antibodies
against SOX2 (1:300; cat. no. sc-365823; Santa Cruz Biotechnology,
Inc.). Primary antibodies were substituted with 0.2 mol/l PBS (pH
7.4) for the negative control. Following the primary antibody
incubation, the sections were incubated with a biotinylated horse
anti-mouse IgG secondary antibody (1:100; cat. no. BA-2001; Vector
Laboratories; Maravai Life Sciences) at room temperature for 30
min, followed by incubation with a streptavidin horseradish
peroxidase complex (1:5,000; cat. no. SA-5004; Vector Laboratories;
Maravai Life Sciences) at room temperature for 20 min. Finally,
sections were counterstained with hematoxylin for 3 min at room
temperature. Chromogen detection was performed using a Tris-HCl
solution containing 0.02% 3,3′-diaminobenzidine (Dako; Agilent
Technologies, Inc.) for 5 min at room temperature. Stained tissue
was visualized using a light microscope (magnification, ×200;
Olympus Corporation). Positive staining was defined as >10% of
cells appearing as brown granules and was quantified using ImageJ
version 1.37 software (National Institutes of Health).
Cell transfection
Cell transfection and small interfering (si)RNA
interference were performed using the Lipofectamine®
2000 Transfection kit (Invitrogen; Thermo Fisher Scientific, Inc.).
A total of 4×105 FRO cells were seeded into 6-well
plates and cultured to 90–95% confluence. A pcDNA3.1 plasmid (4 µg;
Invitrogen; Thermo Fisher Scientific, Inc.) carrying a SOX2 cDNA
insert (pcDNA3.1-SOX2) was used to construct FRO cells
overexpressing SOX2, and the empty pcDNA3.1 (4 µg) plasmid was used
as the NC. The amplification of SOX2 expression used the following
primers: Forward, 5′-GGAGACCCAAGCTGGCTAGCATGTACAACATGATGGAGACGG-3′
and reverse, 5′-GTTTAAACGGGCCCTCTAGATCACATGTGTGAGAGGGGC-3′. Genetic
knockdown of SOX2 was performed using 2 µg siRNA against SOX2
(siSOX2) and 2 µg control non-targeting siRNA (siNC) was used as
the NC. The siNC sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′; and the
siSOX2 sequence was 5′-CAGGTTGATATCGTTGGTAAT-3′. Cells were
transfected for 48 h prior to subsequent experimentation and the
overexpression or inhibition of the expression of SOX2 was
confirmed using western blotting following transfection.
Western blotting
Total protein was extracted from 1×104
FRO cells/well using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a bicinchoninic
acid assay and 30 µg protein/lane was separated by 10% SDS-PAGE.
The separated proteins were subsequently transferred onto a PVDF
membrane (EMD Millipore) and blocked for 2 h at 25°C in 5% non-fat
milk. The membranes were incubated with the following primary
antibodies at 4°C overnight: Anti-SOX2 (1:400; cat. no. sc-365823;
Santa Cruz Biotechnology, Inc.), anti-FN1 (1:400; cat. no.
sc-69681; Santa Cruz Biotechnology, Inc.), anti-p65 (1:400; cat.
no. sc-8008; Santa Cruz Biotechnology, Inc.), anti-PI3K (1:500;
cat. no. sc-1637; Santa Cruz Biotechnology, Inc.),
anti-phosphorylated (p)-PI3K (1:1,000; cat. no. 4228S, Cell
Signaling Technology, Inc.), anti-AKT (1:400; cat. no. sc-8312;
Santa Cruz Biotechnology, Inc.), anti-p-AKT (1:400; cat. no.
sc-135650; Santa Cruz Biotechnology, Inc.) and anti-GADPH (1:2,000;
cat. no. sc-47724; Santa Cruz Biotechnology, Inc.). Following the
primary incubation, membranes were incubated with horseradish
peroxidase-conjugated anti-mouse or anti-rabbit secondary
antibodies (1:5,000; cat. nos. sc-2357 and sc-2005; Santa Cruz
Biotechnology, Inc.) for 2 h at 25°C. Protein bands were visualized
using enhanced chemiluminescence (Thermo Fisher Scientific, Inc.).
Protein expression was quantified using Quantity One 1-D version
4.6.2 software (Bio-Rad Laboratories, Inc.), with GAPDH as the
loading control.
Cell viability and proliferation
assays
A total of 1×103 FRO cells/well were
seeded into a 96-well plate and the cell viability was analyzed
using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology) assay, according to the manufacturer's protocol.
Cell viability was measured at 48 h post-transfection at an
absorbance of 450 nm using a µQuant™ MQX200 microplate reader
(Bio-Tek Instruments, Inc.).
A colony formation assay was performed based on the
standard two-layer soft agar culture to assess proliferation. A
total of 500 transfected FRO cells/well were cultured in DMEM in a
humidified atmosphere at 37°C with 5% CO2 for 12 days.
Subsequently, cells were fixed with 1:3 acetic acid methanol at
room temperature for 15 min and then stained with 0.1% crystal
violet at room temperature for 30 min. The colonies were visualized
and manually counted using an Olympus CKX41 light microscope
(magnification, ×100; Olympus Corporation).
Wound healing assay
The migratory ability of transfected FRO cells was
detected using a wound healing assay. A total of 2×105
FRO cells were seeded into a 6-well plate and cultured in
serum-free DMEM to ≥95% confluence. A 10-µl pipette tip was used to
generate the wound in the monolayer cells and the cells were washed
with serum-free medium. At the 0 and 24-h timepoint following wound
induction, cells were visualized using an inverted light microscope
(magnification, ×100; Olympus Corporation). The relative migratory
distance was calculated using the following formula: Relative
migratory distance (%) = [(width of cell wound at 0 h-width of cell
wound at 24 h)/width of cell wound at 0 h] ×100.
Matrigel invasion assay
Cell invasion was assessed using a Matrigel-coated
Transwell chamber (BD Biosciences), according to the manufacturer's
protocol (20). A total of
1×105 transfected FRO cells/well were plated in the
upper chambers of Matrigel-coated 8-µm Transwell plates in 100 µl
serum-free DMEM and incubated at 37°C and 5% CO2. DMEM
supplemented with 10% FBS as a chemoattractant was plated in the
lower chambers. Following incubation for 24 h at 37°C, the
non-invasive cells were removed from the upper surface of the
Matrigel using a cotton swab. The migratory cells on the underside
of the membrane were fixed with cold methanol and acetic acid (3:1
v/v) for 30 min at room temperature and subsequently stained with
Giemsa (Sigma-Aldrich; Merck KGaA) at room temperature for 20 min.
Stained cells were visualized using a light microscope
(magnification, ×400; Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.) and all data are presented as the mean ± SD of
three experimental repeats. Differences between two groups were
determined using Student's t-test; differences between multiple
groups were determined using one-way ANOVA with Fisher's Least
Significant Difference post hoc test. P<0.05 was considered to
indicate a statistically signifcant difference.
Results
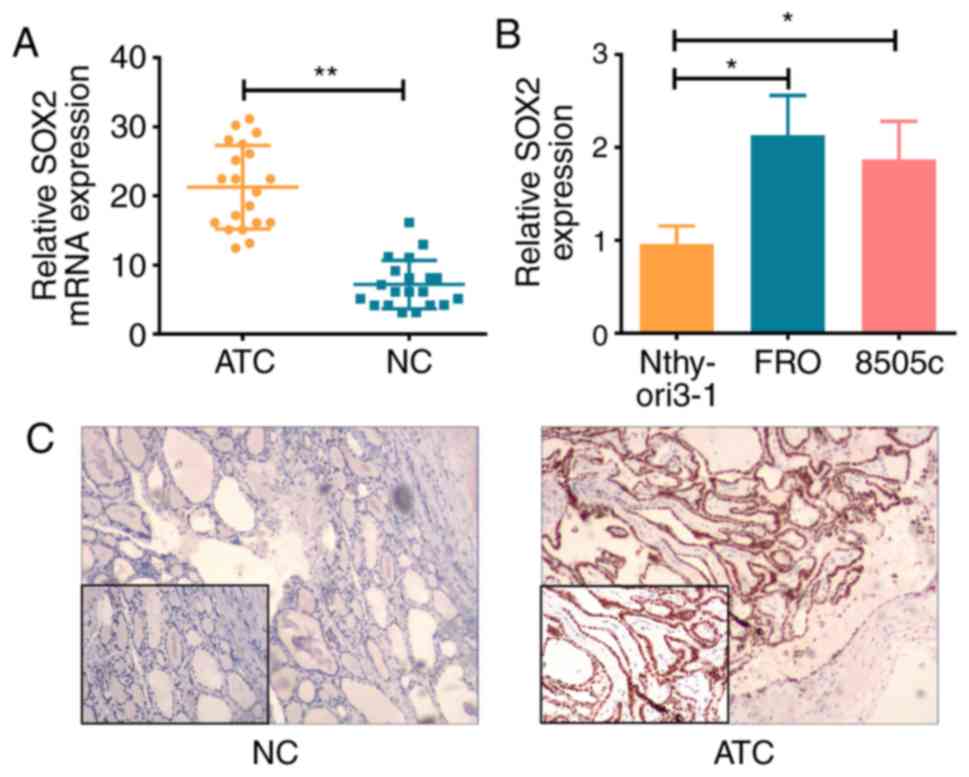
SOX2 expression levels are increased
in ATC tissue and cell lines
The mRNA expression levels of SOX2 were
significantly higher in ATC tissue and cell lines compared with the
NC tissue (P<0.01; Fig. 1A) and
Nthy-ori 3-1 cells (P<0.05; Fig.
1B), respectively. IHC analysis demonstrated that SOX2 protein
expression levels were also increased in the ATC tissue compared
with the NC tissue (Fig. 1C).
Overall, these results suggested that the expression levels of SOX2
may be increased in ATC, indicating that SOX2 may be involved in
the development of ATC.
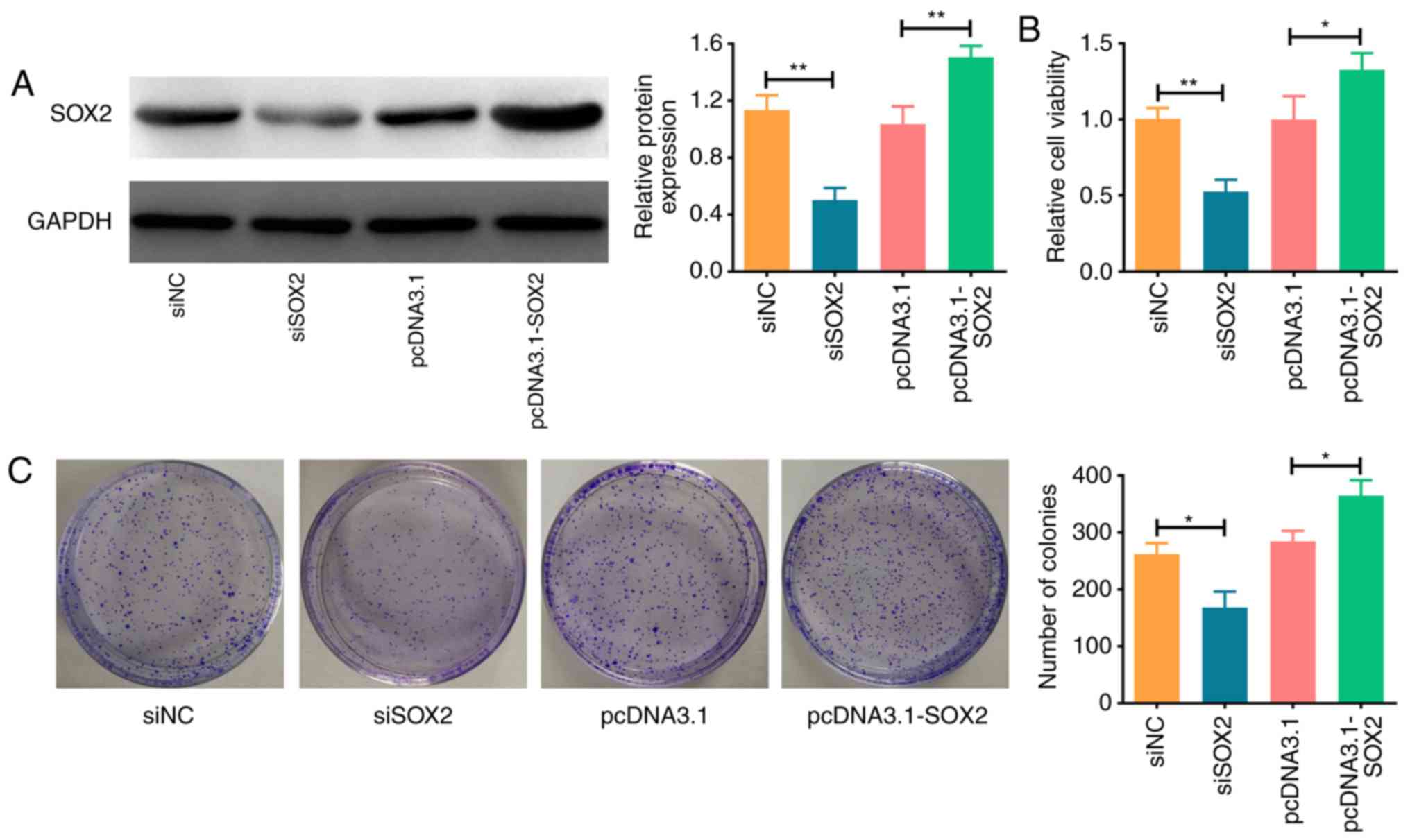
SOX2 promotes ATC cell viability
To determine the function of SOX2 in ATC cell lines,
SOX2 was knocked down with siRNA or overexpressed using cDNA
constructs in the FRO ATC cell line. Western blotting was used to
confirm SOX2 expression levels in FRO cells following the
transfections; SOX2 expression levels were significantly decreased
in cells transfected with siSOX2 compared with the siNC-transfected
cells (P<0.01), whereas levels were significantly increased in
FRO cells transfected with pcDNA3.1-SOX2 compared with pcDNA3.1
(P<0.01; Fig. 2A). A CCK-8
assay was performed to evaluate the influence of SOX2 on cell
viability. In FRO cells transfected with siSOX2, the viability was
significantly decreased compared with cells transfected with siNC
(P<0.01; Fig. 2B). Conversely,
the cell viability was significantly increased in cells transfected
with pcDNA3.1-SOX2 compared with pcDNA3.1-transfected cells
(P<0.05; Fig. 2B). The effect
of SOX2 on cell proliferation was evaluated using a colony
formation assay. Cells transfected with siSOX2 exhibited a
significantly lower proliferative ability compared with
siNC-transfected cells (P<0.05; Fig. 2C), whereas cells transfected with
pcDNA3.1-SOX2 demonstrated significantly enhanced proliferative
abilities compared with pcDNA3.1-transfected cells (P<0.05;
Fig. 2C). Overall, these results
indicated that SOX2 may promote ATC cell proliferation and
viability.
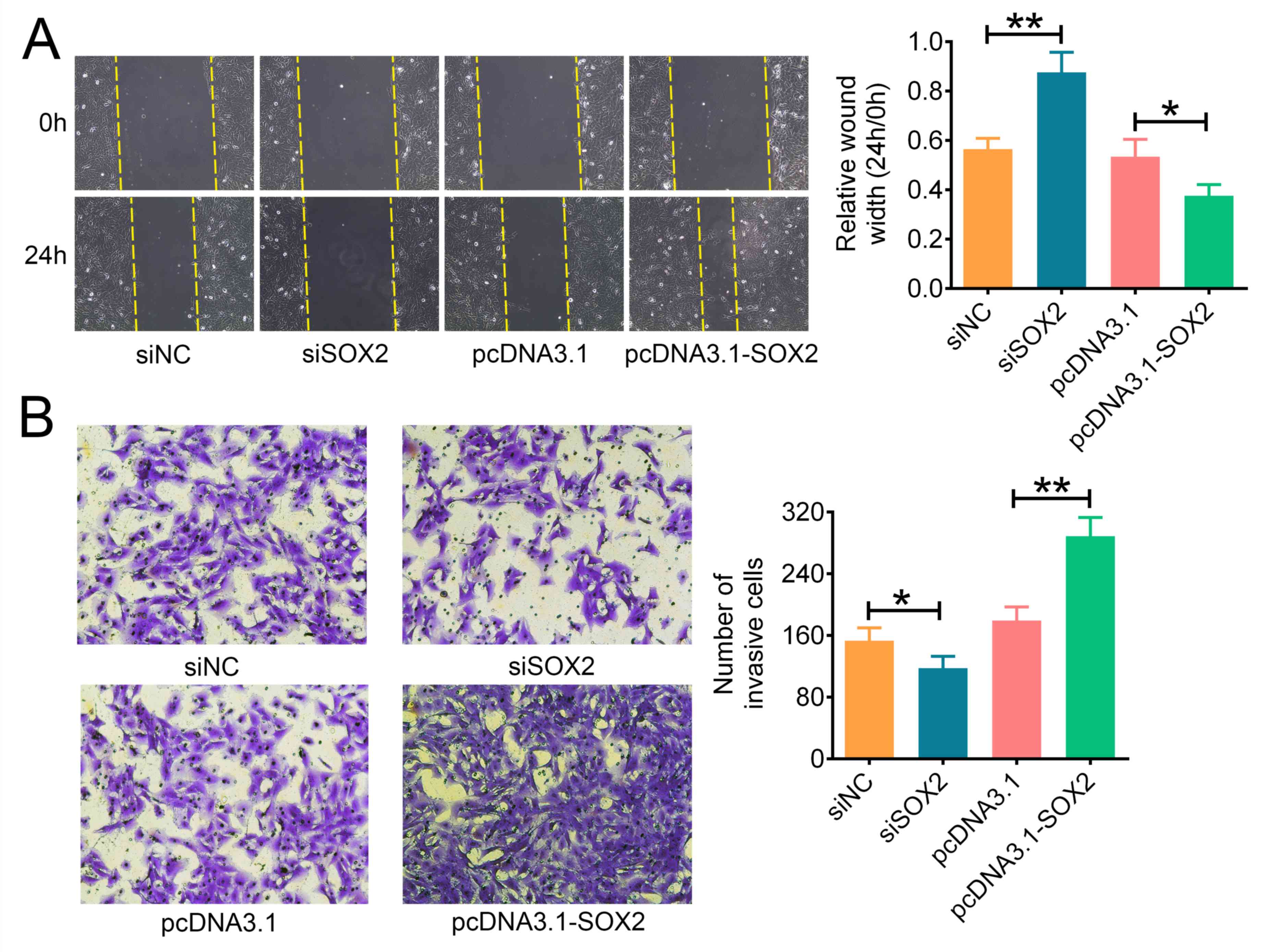
SOX2 promotes ATC cell migration and
invasion
The effects of SOX2 on the migratory and invasive
abilities of ATC cell lines in vitro were analyzed using
wound healing and Matrigel Transwell assays, respectively.
siSOX2-transfected cells were observed to have significantly
decreased migratory ability compared with siNC-transfected cells,
whereas the overexpression of SOX2 with pcDNA3.1-SOX2 significantly
enhanced the migratory ability of FRO cells compared with the
pcDNA3.1 transfected cells (Fig.
3A). In addition, the genetic knockdown of SOX2 with siSOX2
significantly inhibited cell invasion compared with
siNC-transfected cells, whereas the overexpression of SOX2 with
pcDNA3.1-SOX2 significantly increased the invasive ability of FRO
cells compared with pcDNA3.1-transfected cells (Fig. 3B). Overall, these results indicated
that SOX2 may promote the migration and invasion of ATC cells.
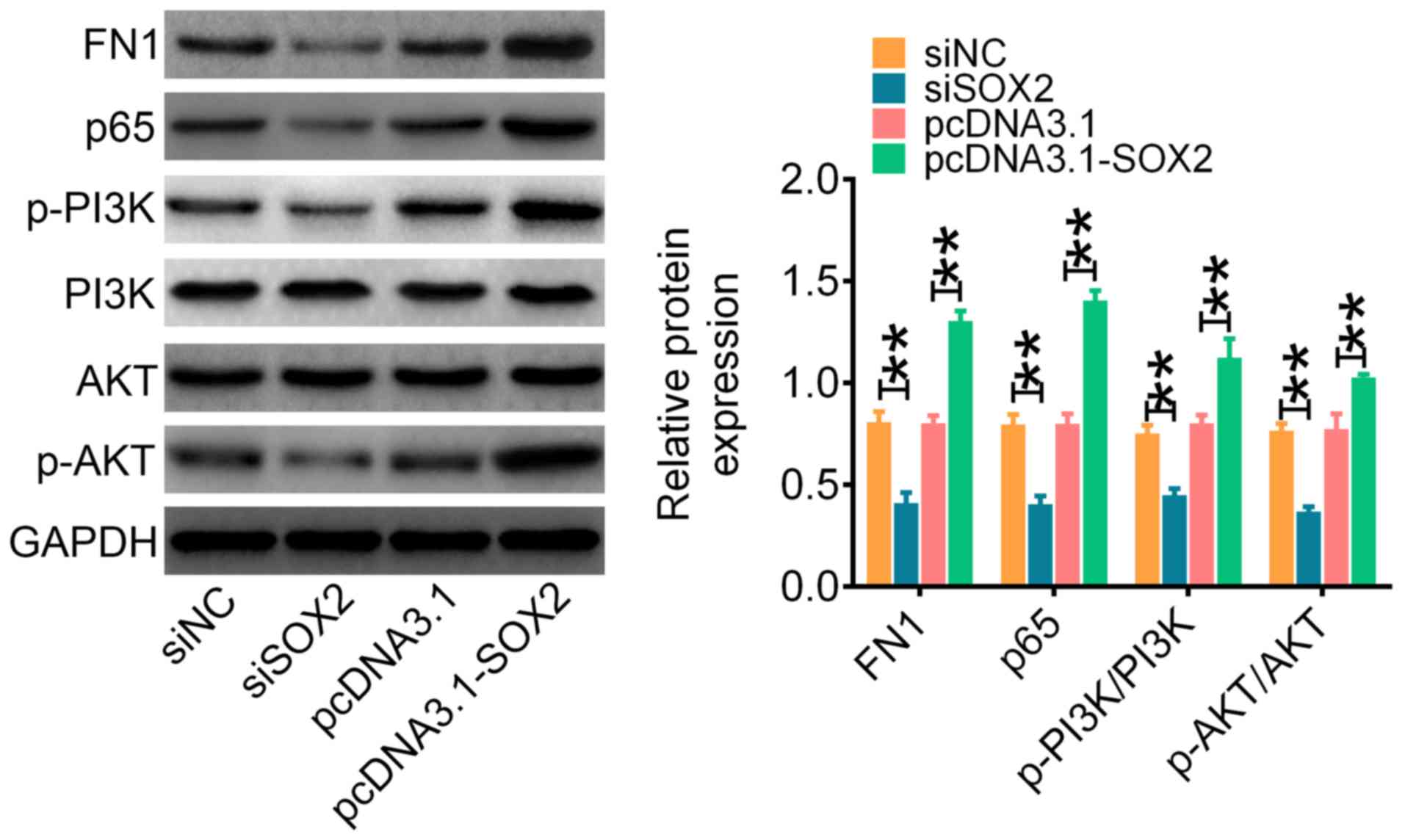
SOX2 targets FN1 to promote aggressive
phenotypes in vitro
To clarify the possible mechanism of the
SOX2-mediated increase in the aggressive phenotypes observed in FRO
cells, western blotting was used to assess the effect of SOX2
expression on tumor proliferation-related signaling proteins.
siSOX2-transfected cells significantly decreased FN1 and p65
expression levels compared with the siNC group, whereas the
overexpression of SOX2 in pcDNA3.1-SOX2 cells significantly
increased FN1 and p65 expression levels compared with the
pcDNA3.1-transfected cells (Fig.
4). Similarly, the genetic knockdown of SOX2 in FRO cells
significantly decreased the phosphorylation levels of PI3K and AKT
compared with siNC-transfected cells, whereas
pcDNA3.1-SOX2-transfected cells demonstrated significantly
increased phosphorylation levels of PI3K and AKT compared with
pcDNA3.1-transfected cells (Fig.
4). Overall, these results indicated that SOX2 may promote
aggressive phenotypes in ATC cell lines by upregulating FN1
expression levels through the activation of the PI3K/AKT signaling
pathway.
Discussion
Previous studies have reported that SOX2 expression
levels were increased in numerous types of cancer, including
glioblastoma, ovarian cancer, small cell lung cancer and squamous
cell carcinoma, which affected cancer cell physiology owing to the
involvement of SOX2 in numerous protein-protein interactions and
complex cell signaling pathways, such as the Hippo, Hedgehog and
Ras Homolog C/Rho-Associated Coiled-Protein Kinase signaling
pathways (21–24). Thus, the pathogenic function of
SOX2 in multiple types of cancer is of interest. It is reported
that FN1 is a direct target of SOX2 in ovarian cancer cells
(17), however, the role of SOX2
and its relationship with FN1 have not been investigated in ATC.
Given that FN1 expression levels were increased in thyroid cancer
(15), it was hypothesized that
SOX2 may also regulate the expression of FN1 to mediate the
migration and invasion of cells in ATC. Thus, to the best of our
knowledge, the present study was the first to focus on the role of
SOX2 in ATC cells, including its potential regulatory mechanism.
The results demonstrated that SOX2 was overexpressed in ATC tissues
and cell lines, which subsequently increased the viability, and
proliferative, migratory and invasive ability of ATC cells in
vitro. Moreover, SOX2 was found to increase the expression
levels of FN1 and other proteins involved in the PI3K/AKT signaling
pathway, including p65, PI3K and AKT. These proteins serve
important roles in tumorigenesis and regulate critical cellular
functions including survival, proliferation, apoptosis and
metabolism (25); for example, the
PI3K/AKT signaling pathway has been implicated in modulating a more
aggressive phenotype through regulating cholesterol ester formation
in prostate cancer cells (26);
and PI3K/AKT signaling also been observed to reduce apoptosis,
stimulate cell growth and increase proliferation (27). Notably, a previous study also
demonstrated that FN1 induced cell proliferation and inhibited
apoptosis through the PI3K/AKT signaling pathway (16). Thus, the present study presented a
possible model of SOX2-mediated gene regulation in ATC cell lines,
in which the overexpression of SOX2 may promote FN1 expression
through the PI3K/AKT signaling pathway to induce the aggressive
phenotype of ATC.
SOX2 is a pluripotency-associated transcription
factor that induces the proliferation, migration, metastasis and
invasion of cancer stem cells through various signaling pathways
depending on the cancer type (28). Previously, the overexpression of
SOX2 has been observed in various types of tumor, but it has not
been reported in ATC. In the present study, SOX2 was overexpressed
in ATC tissue, FRO and 8505c cell lines, which was in agreement
with previous studies into other types of cancer (17,29).
To determine the effect of SOX2 on the aggressive cell phenotype,
SOX2 expression was overexpressed using cDNA constructs or knocked
down using siRNA in FRO cell lines. The results demonstrated that
cell activity, migration and invasion were significantly enhanced
by SOX2 overexpression and inhibited by SOX2 knockdown. Therefore,
the present study concluded that SOX2 overexpression in ATC may
regulate the proliferation, migration and invasion of ATC
cells.
FN1 promoted tumor invasion and metastasis through
activating specific matrix metalloproteinases (MMPs) in breast
cancer (30). SOX2/FN1 signaling
pathway is an important pathway mediating the migration and
invasion of ovarian cancer cells; SOX2 directly targeted FN1 to
induce the expression of MMP-2 and MMP-9 (17). In the present study, FN1 expression
levels were increased in SOX2-overexpressed cells, and the protein
expression levels of tumor proliferation-related signaling
pathways, including p65, p-PI3K/PI3K, AKT/p-AKT, were also
increased by SOX2. SOX2 induced the activation of AKT and PI3K by
phosphorylation, indicating that SOX2 may mediate FN1 upregulation
through the activation of the PI3K/AKT signaling pathway. This is
similar to a previous study by Liao et al (31), which reported that FN1 stimulated
glioma growth, invasion and survival through the activation of the
PI3K/AKT signaling pathway. Besides, p65 as a subunit of NF-κB was
also upregulated by SOX2; it is widely accepted that NF-κB
contributes to the metastasis and invasion of cancer stem cells
(32), and it is hypothesized that
SOX2 may also mediate FN1 upregulation through activating NF-κB
signaling. For example, FN1 was observed to induce an aggressive
phenotype following the activation of the NF-κB pathway in
nasopharyngeal carcinoma (33).
These results illustrated that the overexpression of SOX2 promoted
FN1 expression mainly through the PI3K/AKT/NF-κB signaling pathway,
which may induce the aggressive phenotype of ATC.
In conclusion, to the best of our knowledge, this is
the first study to report on the function of SOX2 in promoting
aggressive phenotypes in ATC and its possible regulatory
mechanisms; the present study identified a possible model of
SOX2-mediated gene regulation in ATC cell lines. These findings
suggested that the observed overexpression of SOX2 in ATC tissues
and cell lines may promote the aggressive phenotypes of ATC cells
by inducing FN1 expression through the PI3K/AKT signaling pathway.
These finding provided crucial molecular insights into ATC
pathogenesis and indicated that SOX2 may be useful as a therapeutic
target for ATC. Investigations on the detailed molecular mechanism
of SOX2 in ATC remains to be further researched in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding obtained
from The Major Science and Technology Projects of Zhejiang Medical
and Health Program (grant nos. WKJ-ZJ-1712 and 2015ZDA007) and The
General Research Projects of Zhejiang Medical and Health Program
(grant nos. 2018KY310, 2017KY029 and 2015KYA036).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PW and JS designed all the experiments and revised
the paper. JZ, KW and LG performed the experiments. JG and WW
analyzed and interpreted the data. All liabilities related to the
content of this article will be borne by the authors.
Ethics approval and consent to
participate
The study was performed in accordance with the
Helsinki Declaration and it was approved by The Ethics Committee of
Zhejiang Cancer Hospital (Hangzhou, China). Written informed
consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qu Y, Zhuo L, Li N, Hu Y, Chen W, Zhou Y,
Wang J, Tao Q, Hu J, Nie X and Zhan S: Prevalence of post-stroke
cognitive impairment in china: A community-based, cross-sectional
study. PLoS One. 10:e01228642015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American Thyroid Association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuffrida D and Gharib H: Anaplastic
thyroid carcinoma: Current diagnosis and treatment. Ann Oncol.
11:1083–1089. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao SN, Zafereo M, Dadu R, Busaidy NL,
Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, et
al: Patterns of treatment failure in anaplastic thyroid carcinoma.
Thyroid. 27:672–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wuebben EL and Rizzino A: The dark side of
SOX2: Cancer-a comprehensive overview. Oncotarget. 8:44917–44943.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanada Y, Yoshida K, Ohara M, Oeda M,
Konishi K and Tsutani Y: Histopathologic evaluation of stepwise
progression of pancreatic carcinoma with immunohistochemical
analysis of gastric epithelial transcription factor SOX2:
Comparison of expression patterns between invasive components and
cancerous or nonneoplastic intraductal components. Pancreas.
32:164–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XL, Eishi Y, Bai YQ, Sakai H, Akiyama
Y, Tani M, Takizawa T, Koike M and Yuasa Y: Expression of the
SRY-related HMG box protein SOX2 in human gastric carcinoma. Int J
Oncol. 24:257–263. 2004.PubMed/NCBI
|
|
9
|
Sattler HP, Lensch R, Rohde V, Zimmer E,
Meese E, Bonkhoff H, Retz M, Zwergel T, Bex A, Stoeckle M and
Wullich B: Novel amplification unit at chromosome 3q25-q27 in human
prostate cancer. Prostate. 45:207–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Shi L, Zhang L, Li R, Liang J, Yu
W, Sun L, Yang X, Wang Y, Zhang Y and Shang Y: The molecular
mechanism governing the oncogenic potential of SOX2 in breast
cancer. J Biol Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alonso MM, Diez-Valle R, Manterola L,
Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, Lopez de
Munain A, Sampron N, et al: Genetic and epigenetic modifications of
Sox2 contribute to the invasive phenotype of malignant gliomas.
PLoS One. 6:e267402011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carina V, Zito G, Pizzolanti G, Richiusa
P, Criscimanna A, Rodolico V, Tomasello L, Pitrone M, Arancio W and
Giordano C: Multiple pluripotent stem cell markers in human
anaplastic thyroid cancer: The putative upstream role of SOX2.
Thyroid. 23:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dooley TP, Reddy SP, Wilborn TW and Davis
RL: Biomarkers of human cutaneous squamous cell carcinoma from
tissues and cell lines identified by DNA microarrays and qRT-PCR.
Biochem Biophys Res Commun. 306:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
da Silveira Mitteldorf CA, de
Sousa-Canavez JM, Leite KR, Massumoto C and Camara-Lopes LH: FN1,
GALE, MET, and QPCT overexpression in papillary thyroid carcinoma:
Molecular analysis using frozen tissue and routine fine-needle
aspiration biopsy samples. Diagn Cytopathol. 39:556–561. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sponziello M, Rosignolo F, Celano M,
Maggisano V, Pecce V, De Rose RF, Lombardo GE, Durante C, Filetti
S, Damante G, et al: Fibronectin-1 expression is increased in
aggressive thyroid cancer and favors the migration and invasion of
cancer cells. Mol Cell Endocrinol. 431:123–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han SW and Roman J: Fibronectin induces
cell proliferation and inhibits apoptosis in human bronchial
epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and
NF-kappaB. Oncogene. 25:4341–4349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lou X, Han X, Jin C, Tian W, Yu W, Ding D,
Cheng L, Huang B, Jiang H and Lin B: SOX2 targets fibronectin 1 to
promote cell migration and invasion in ovarian cancer: New
molecular leads for therapeutic intervention. OMICS. 17:510–518.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He WP, Zhou J, Cai MY, Xiao XS, Liao YJ,
Kung HF, Guan XY, Xie D and Yang GF: CHD1L Protein is overexpressed
in human ovarian carcinomas and is a novel predictive biomarker for
patients survival. BMC Cancer. 12:4372012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Repesh LA: A new in vitro assay for
quantitating tumor cell invasion. Invasion Metastasis. 9:192–208.
1989.PubMed/NCBI
|
|
21
|
Wen Y, Hou Y, Huang Z, Cai J and Wang Z:
SOX2 is required to maintain cancer stem cells in ovarian cancer.
Cancer Sci. 108:719–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Basu-Roy U, Bayin NS, Rattanakorn K, Han
E, Placantonakis DG, Mansukhani A and Basilico C: Sox2 antagonizes
the Hippo pathway to maintain stemness in cancer cells. Nat Commun.
6:64112015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kar S, Sengupta D, Deb M, Pradhan N and
Patra SK: SOX2 function and Hedgehog signaling pathway are
co-conspirators in promoting androgen independent prostate cancer.
Biochim Biophys Acta Mol Basis Dis. 1863:253–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng J, Xu L, Pan Y, Yu S, Wang H,
Kennedy D and Zhang Y: Sox2 modulates motility and enhances
progression of colorectal cancer via the Rho-ROCK signaling
pathway. Oncotarget. 8:98635–98645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang F, Lee JT, Navolanic PM, Steelman
LS, Shelton JG, Blalock WL, Franklin RA and McCubrey JA:
Involvement of PI3K/Akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: A target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weina K and Utikal J: SOX2 and cancer:
Current research and its implications in the clinic. Clin Transl
Med. 3:192014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gut A, Moch H and Choschzick M: SOX2 gene
amplification and overexpression is linked to HPV-positive vulvar
carcinomas. Int J Gynecol Pathol. 37:68–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian P, Zuo Z, Wu Z, Meng X, Li G, Wu Z,
Zhang W, Tan S, Pandey V, Yao Y, et al: Pivotal role of reduced
let-7g expression in breast cancer invasion and metastasis. Cancer
Res. 71:6463–6474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao YX, Zhang ZP, Zhao J and Liu JP:
Effects of fibronectin 1 on cell proliferation, senescence and
apoptosis of human glioma cells through the PI3K/AKT signaling
pathway. Cell Physiol Biochem. 48:1382–1396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rinkenbaugh AL and Baldwin AS: The NF-κB
pathway and cancer stem cells. Cells. 5:E162016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Deng L, Huang J, Cai R, Zhu X, Liu
F, Wang Q, Zhang J and Zheng Y: High expression of Fibronectin 1
suppresses apoptosis through the NF-κB pathway and is associated
with migration in nasopharyngeal carcinoma. Am J Transl Res.
9:4502–4511. 2017.PubMed/NCBI
|