Introduction
Human parvovirus B19 (B19V) is a member of the
Parvoviridae family and has been demonstrated to cause
various human diseases, including erythema infectiosum,
arthropathies, hemolytic disorders, hydrops fetalis and fetal death
(1–4). Although the role of B19V in inducing
autoimmunity is still unknown, numerous studies have reported an
association between B19V infection and autoimmune disorders, such
as systemic lupus erythematous (SLE) and rheumatoid arthritis (RA)
(5,6). The VP1 unique region (VP1u) of B19V
exhibits activity of secreted phospholipidase A2 and has been
demonstrated to have an essential role in viral infectivity, and
the induction of inflammation and autoimmunity (5,7–10).
Indeed, evidence indicates a link between B19V and autoimmune
disorders, particularly an association between the B19V–VP1u and
anti-phospholipid syndrome (APS) (8,10–12).
APS is an autoimmune state characterized by
thrombosis, which affects the venous or arterial vascular systems,
and obstetrical morbidity (13).
APS is associated with numerous autoimmune diseases, including SLE,
systemic sclerosis, Sjögren's syndrome, dermatomyositis and RA
(13,14). APS is considered to be an
autoantibody-mediated disease diagnosed by the presence of
anti-phospholipid antibodies (aPLs), such as anti-cardiolipin and
anti-β-2-glycoprotein I (β2GPI) antibodies (13). A number of studies have reported an
association between B19V infection and the induction of APL
(12,15). The induction of APS-like syndromes
and aPLs has been observed in naïve mice that received antibodies
against B19V–VP1u (8). Notably,
similar activity between APL and B19V–VP1u antibodies on the
activation of adhesion molecules has been reported (16). Our previous study demonstrated that
B19V–VP1u antibodies induce the expression of adhesion molecules,
including intracellular cell adhesion molecule 1 (ICAM-1), vascular
cell adhesion molecule 1 (VCAM-1) and E-selectin, in human vascular
endothelial cells by activating the phosphorylated (p)-p38
mitogen-activated protein kinase (MAPK) signaling pathway (17). Since similar results were also
reported in previous studies (18,19),
these findings indicated that both aPL and B19V–VP1u antibodies
induce the expression of adhesion molecules through the p38 MAPK
pathway.
Endothelial dysfunction is an important pathological
process that is associated with APS. Evidence indicates that aPLs,
including β2GPI antibodies, significantly reduce the length of the
tubules that are formed in an in vitro endometrial
endothelial cell angiogenesis assay and reduce vessel formation in
an aPL-inoculated animal (20). In
the presence of monoclonal aPLs or immunoglobulin Gs (IgGs) that
have been isolated from patients with APS, impairments in vascular
remodeling and angiogenesis have also been reported (21). In contrast, β2GPI antibodies that
have been isolated from patients with APS induce angiogenesis by
activating the mTOR pathway in cultured vascular endothelial cells
(22). Although antibodies against
B19V–VP1u have been demonstrated to induce APS-like symptoms in
naïve mice, the effect of B19V–VP1u antibodies on angiogenesis
remains unclear. The present study investigated the effect of
antibodies against B19V–VP1u on mTOR signaling to identify the role
of B19V–VP1u in angiogenesis in human umbilical vein endothelial
cells (HUVEC) and the expression of vascular endothelial growth
factor (VEGF) protein in A549 cells, another well-known model for
angiogenesis study (23,24).
Materials and methods
Antibody preparations
Human parvovirus B19V-NS1 and B19V–VP1u recombinant
proteins, and antibodies against B19V-NS1 and B19V–VP1u were
prepared as described in our previous studies (17,25).
The yields of the purified recombinant B19-NS1 and B19-VP1u
proteins were 3.8 and 2.3 µg/ml, respectively, with purities of
~96.2 and ~98.3%, respectively. The antibodies against B19V-NS1 and
B19V–VP1u were purified by protein G agarose (Roche Diagnostics)
chromatography. The purified antibodies were filtered through a
0.22-µm microporous membrane (EMD Millipore). Normal human serum
IgG (NH IgG; 200 µg/ml) and human aPL IgG (200 µg/ml; 250 units)
were obtained from a commercial APhL ELISA IgG and IgM horseradish
peroxidase (HRP) kit (cat. nos. LAPL-K-HRP-00GM and
LAPL-K-HRP-01GM; Louisville APL Diagnostics, Inc.). A limulus
amebocyte lysate endochrome assay (Charles River Laboratories,
Inc.) was used to detect the endotoxin levels of the antibody
preparations. The endotoxin levels were identified to be below the
detection limit (0.25 endotoxin U/ml) for all IgG preparations at
the concentrations used in the present study.
Cell culture and treatment
HUVECs (BCRC no. H-UV001; cat. no. 01193) and a
non-small cell lung cancer cell line (A549) were purchased from
Bioresource Collection and Research Center, Food Industry Research
and Development Institute. The culture dishes were pre-coated with
1% gelatin and HUVECs were then cultured in 90% M199 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 25 U/ml
heparin, 30 µg/ml endothelial cell growth supplement, 1.5 g/l
sodium bicarbonate, 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin/streptomycin. A549 cells were cultured in
DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin at 37°C in a 5% CO2 incubator.
The activation of p-AKT-mTOR-S6 ribosomal protein (S6RP) angiogenic
signaling was performed as described previously (22). HUVECs were cultured in M199 medium
containing 2% fetal calf serum (FCS; Gibco; Thermo Fisher
Scientific, Inc.) for 12 h at 37°C and subsequently treated with 5
µg/ml β2GP1 (cat. no. OPPA01429; Aviva Systems Biology, Corp.) for
1 h at 37°C. After washing with 1X PBS, the cells were cultured in
M199 medium containing 2% FCS and treated with 10% FCS or 100 µg/ml
NH IgG, aPL IgG, B19V–VP1u IgG or B19V-NS1 IgG for a further 5 min
at 37°C. The reactions were then stopped by washing with ice-cold
PBS and solubilizing in RIPA buffer (cat. no. 89901; Thermo Fisher
Scientific, Inc.). The treatments with 10% FCS or NH IgG were
considered as the positive control and negative control,
respectively.
Viability assay
For cell survival determination, the Trypan blue
exclusion method was conducted. Briefly, 5×105
HUVECs/well were cultured in a 6-well plate overnight at 37°C in an
incubator. After 24 h, HUVECs were cultured in M199 medium
containing 2% FCS for 12 h at 37°C and subsequently treated with 5
µg/ml β2GP1 for 1 h at room temperature. After washing with 1X PBS,
the cells were then cultured in M199 medium containing 2% FCS and
treated with 10% FCS or 100 µg/ml NH IgG, aPL IgG, B19V–VP1u IgG or
B19V-NS1 IgG for a further 10 min at 37°C. Following the
experimental treatments, the morphology of cells was observed and
photographed under a phase-contrast microscope (Zeiss Axiovert 200;
magnification, ×200). Next, the culture medium was discarded, and
the cells were harvested with 0.25% trypsin-EDTA solution. The
number of viable cells was counted using Trypan blue solution for
1–2 min at room temperature under a Zeiss Axiovert 200 light
microscope (magnification, ×200). The viability of the control
(untreated cells) was set as 100%.
Protein preparation and
immunoblotting
The cells were collected by centrifugation at 800 ×
g for 5 min at 4°C and suspended in 600 µl PRO-PREP™ buffer (iNtRON
Biotechnology, Inc.) for lysis. The supernatant of protein extracts
was then collected by centrifugation at 16,600 × g for 5 min at
4°C. Protein concentration was determined by a modified Bradford's
assay using a spectrophotometer (Hitachi U3000) at 595 nm with BSA
(Sigma-Aldrich; Merck KGaA) as the standard. For immunoblotting,
extracted proteins (20 µg/lane) were separated by 8–10% SDS-PAGE
and electrophoretically transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). After blocking with 5% non-fat dry
milk for 1 h at 4°C, antibodies against p-mTOR (Ser2481; 1:500;
cat. no. 09343; EMD Millipore), p-AKT (Ser473; 1:500; cat. no.
sc-7985-R), p-AKT (Thr308; 1:1,000; cat. no. sc-135650), p-S6RP
(1:500; cat. no. sc-293144), vascular cell adhesion molecule 1
(VCAM-1; 1:250; cat. no. sc-13160), ICAM-1 (1:500; cat. no.
sc-8439), integrin β1 (1:1,000; cat. no. sc-8978; all Santa Cruz
Biotechnology, Inc.), human inducible factor-1α (HIF-1α; 1:500;
cat. no. NB100-105), VEGF (1:500; cat. no. NB100-664; both Novus
Biologicals, LLC) or β-actin (1:5,000; cat. no. MAB1501; EMD
Millipore) were diluted in PBS with 2.5% BSA and incubated with the
membranes overnight at 4°C with gentle agitation. The membranes
were subsequently incubated with diluted HRP-conjugated secondary
antibodies (1:5,000; cat. nos. sc-2004 or sc-2005; Santa Cruz
Biotechnology, Inc.) for a further 1 h at 4°C. An Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore) and a
chemiluminescence imaging analyzer (GE ImageQuant TL 8.1; GE
Healthcare Life Sciences) were used to detect the antigen-antibody
complexes. Finally, a densitometric apparatus (Appraise
densitometer; Beckman Coulter, Inc.) was used to quantify the
density of the blot.
Statistical analysis
The statistical significance among experimental
groups was evaluated using GraphPad Prism 5 software (GraphPad
Software, Inc.) by one-way ANOVA followed by Tukey's multiple
comparisons test. All data are presented at the mean ± SEM. Three
independent experiments were performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of B19V–VP1u antibodies on
HUVEC survival
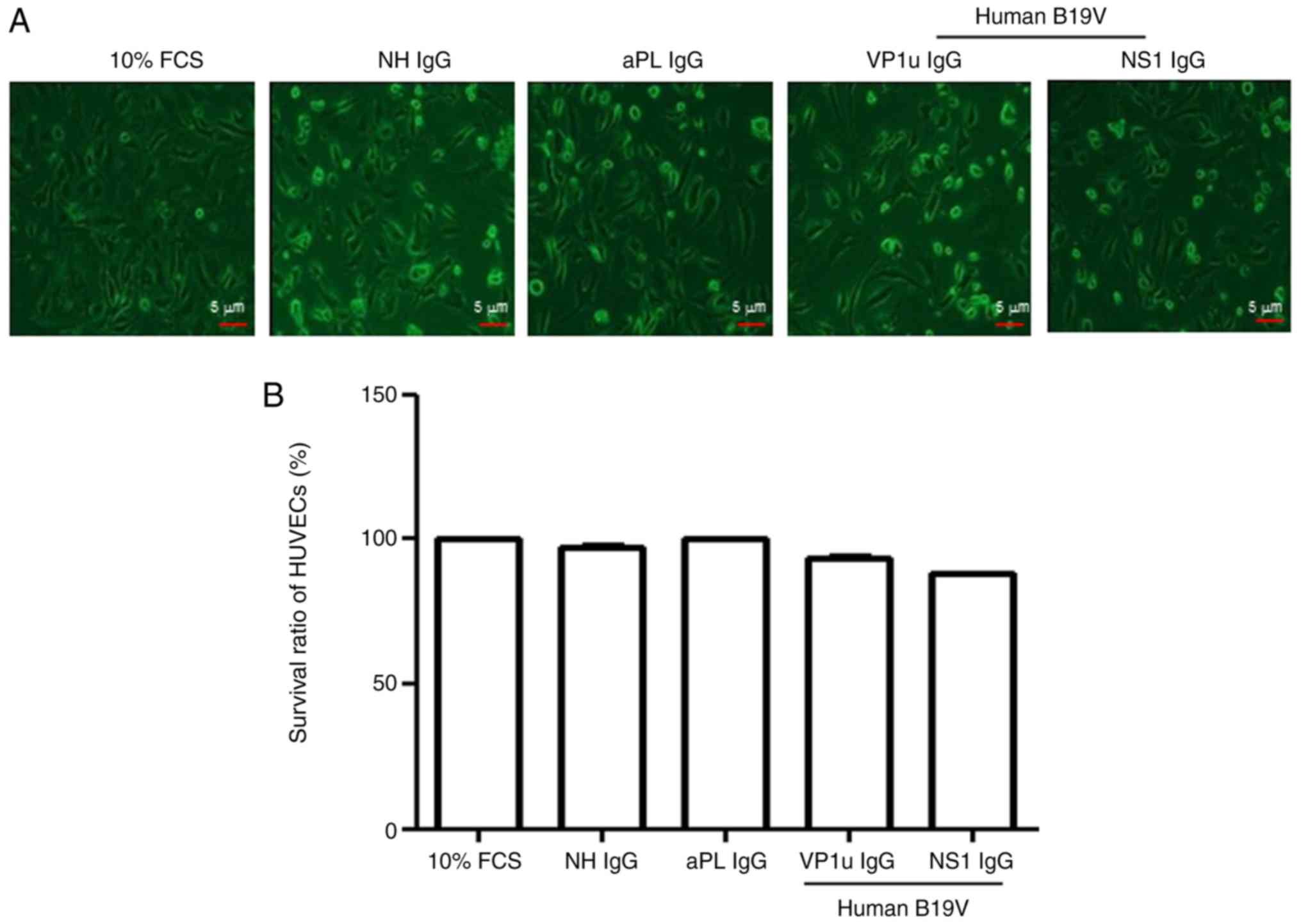
To detect the toxic effects of the antibodies used
in this study on HUVECs, the Trypan blue exclusion method was
performed. The survival of HUVECs was unchanged by the presence of
10% FCS, NH IgG, aPL IgG, B19V–VP1u IgG and B19V-NS1 IgG (Fig. 1). Although the survival of HUVECs
in the presence of B19V-NS1 IgG was slightly lower compared with
those in the presence of 10% FCS or the other antibodies, no
statistical significance was detected among these treatments
(Fig. 1).
Effects of B19V–VP1u antibodies on
adhesion molecules
To detect the effects of B19V–VP1u antibodies on
adhesion molecules, the protein expression levels of VCAM-1, ICAM-1
and integrin β1 were measured by immunoblotting. No significant
difference in VCAM-1 level was observed among the HUVECs treated
with 10% FCS, NH IgG, aPL IgG or B19V-NS1 IgG, whereas
significantly greater VCAM-1 expression was detected in the HUVECs
that were treated with B19V–VP1u IgG compared with those treated
with NH IgG (Fig. 2A and B).
Significantly higher ICAM-1 expression was detected in the HUVECs
treated with 10% FCS, aPL IgG, B19V–VP1u IgG or B19V-NS1 IgG
compared with those treated with NH IgG (Fig. 2C). Significantly higher integrin β1
expression was detected in the HUVECs treated with aPL IgG or
B19V–VP1u IgG compared with those treated with NH IgG (Fig. 2D). No difference in integrin β1
expression was observed among the HUVECs treated with 10% FCS, NH
IgG or B19V-NS1 IgG (Fig. 2D).
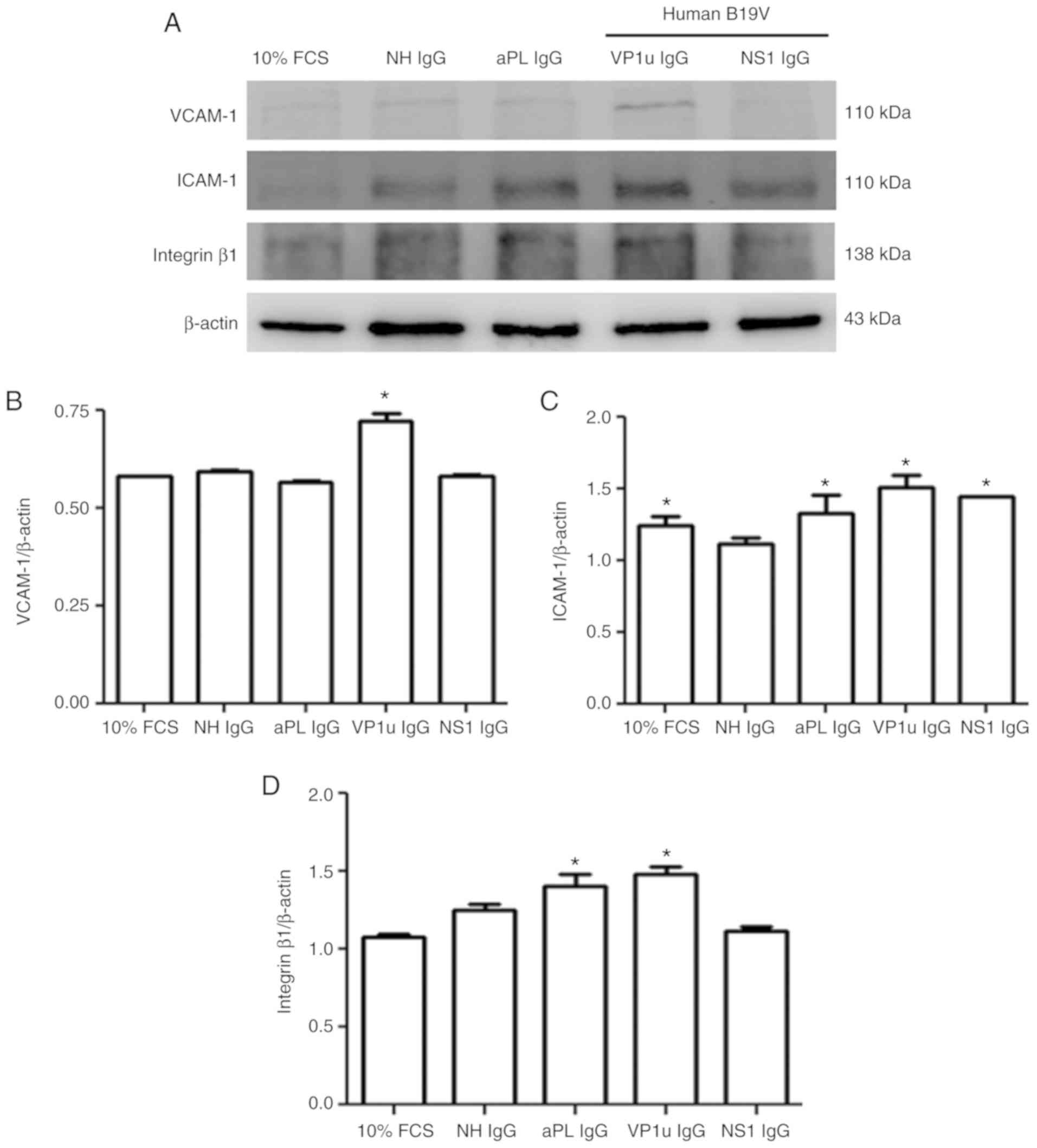 | Figure 2.Expression of VCAM-1, ICAM-1 and
integrin β1. (A) Protein expression levels of VCAM-1, ICAM-1 and
integrin β1 in human umbilical vein endothelial cells treated with
different antibodies were detected by immunoblotting. (B-D)
Relative levels of (B) VCAM-1, (C) ICAM-1 and (D) integrin β1 are
based on β-actin expression. Similar results were observed in
triplicate experiments. *P<0.05 vs. cells treated with NH IgG.
VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intracellular
adhesion molecule 1; NH, normal human; IgG, immunoglobulin G; FCS,
fetal calf serum; aPL, anti-phospholipid antibodies; B19V,
parvovirus B19; VP1u, VP1 unique region. |
Effects of B19-VP1u antibodies on
AKT-mTOR-S6RP signaling
To detect the effects of B19V antibodies on
angiogenic signaling, the expression levels of proteins associated
with the AKT-mTOR-S6RP pathway were detected by immunoblotting.
Significantly higher protein expression levels of p-mTOR, p-AKT
(Ser473), p-AKT (Thr308) and p-S6RP were detected in the HUVECs
treated with 10% FCS compared with those treated with NH IgG
(Fig. 3A). Significantly higher
protein expression levels of p-mTOR, p-AKT (Thr308) and p-S6RP were
detected in HUVECs treated with aPL IgG compared with those treated
with NH IgG (Fig. 3B-E). p-mTOR,
p-AKT (Ser473 and Thr308) and p-S6RP expression levels in the
HUVECs treated with B19V–VP1u IgG or B19V-NS1 IgG did not differ
significantly compared with those cells treated with NH IgG
(Fig. 3B-E). The protein
expression levels of HIF-1α and VEGF were also measured.
Significantly higher protein expression levels of HIF-1α and VEGF
were detected in the HUVECs treated with 10% FCS or aPL IgG
compared with those treated with NH IgG (Fig. 3F and G). Significantly higher
expression of HIF-1α was detected in the HUVECs treated with
B19V–VP1u IgG or B19V-NS1 IgG compared with those treated with NH
IgG (Fig. 3F). Notably, no
significant difference in VEGF expression was observed among the
HUVECs that were treated with NH IgG, B19V–VP1u IgG or B19V-NS1 IgG
(Fig. 3G).
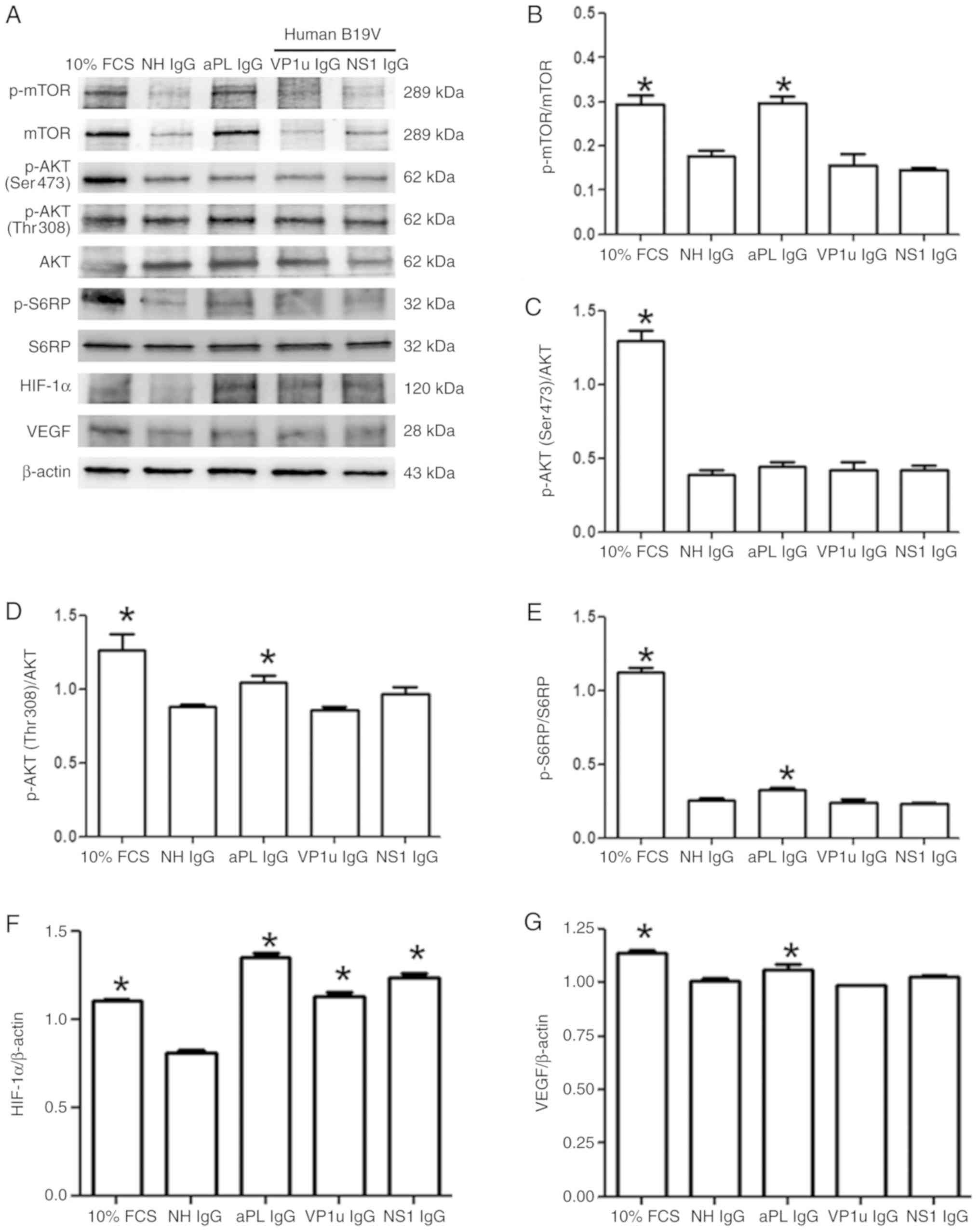 | Figure 3.Expression and phosphorylation of
mTOR, AKT, S6RP, HIF-1α and VEGF proteins. (A) Protein expression
levels in human umbilical vein endothelial cells treated with
different antibodies were detected by immunoblotting: p-mTOR; mTOR;
p-AKT (Ser473); p-AKT (Thr308); AKT; p-S6RP; S6RP; HIF-1α; and
VEGF. (B-G) Relative levels of (B) p-mTOR (C) p-AKT (Ser473) (D)
p-AKT (Thr308) (E) p-S6RP (F) HIF-1α and (G) VEGF are based on its
total protein expression or β-actin expression. Similar results
were observed in triplicate experiments. *P<0.05 vs. cells
treated with NH IgG. p-, phosphorylated; S6RP, S6 ribosomal
protein; NH, normal human; HIF-1α, human inducible factor-1α; VEGF,
vascular endothelial growth factor; IgG, immunoglobulin G; FCS,
fetal calf serum; aPL, anti-phospholipid antibodies; B19V;
parvovirus B19, VP1u, VP1 unique region. |
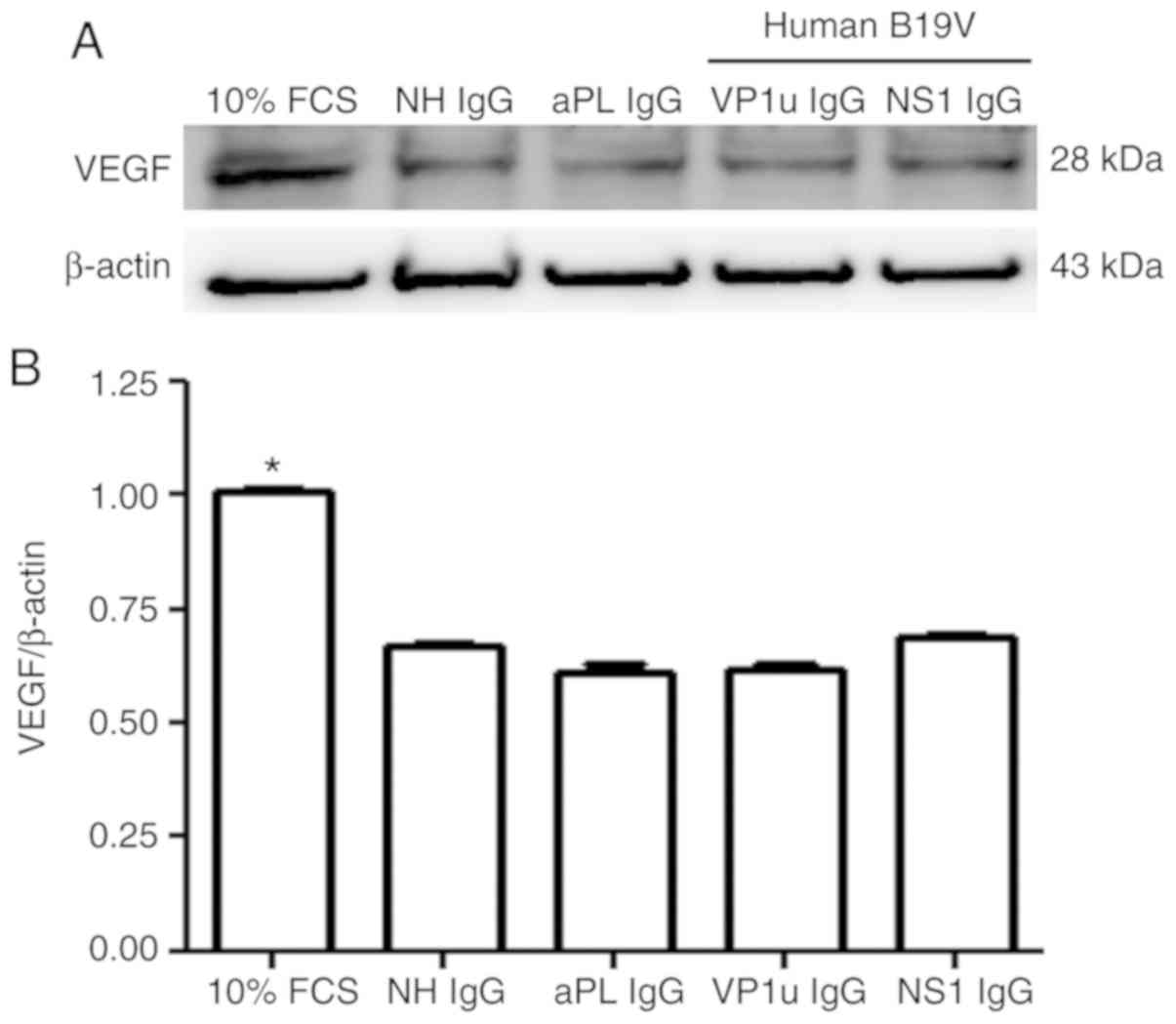
Effects of B19V–VP1u antibodies on
VEGF expression in A549 cells
Since the A549 cell line is a well-known and popular
model for studying angiogenesis in cancer (23,24),
the present study further investigated the effects of B19-VP1u
antibodies on angiogenesis by detecting VEGF expression in A549
cells via immunoblotting. Significantly higher protein expression
of VEGF was detected in A549 cells that were treated with 10% FCS
compared with those treated with NH IgG, whereas no significant
difference in VEGF protein expression was observed in the A549
cells treated with NH IgG, aPL IgG, B19V–VP1u IgG or B19V-NS1 IgG
(Fig. 4).
Discussion
The engagement of β2GPI molecules at vascular
endothelial cell membranes and antibodies against β2GPI can induce
pro-coagulant and pro-inflammatory phenotypes, such as elevated
expression levels of ICAM-1, VCAM-1, E-selectin and various
pro-inflammatory cytokines (18,26,27).
Similar results have indicated that antibodies against B19-VP1u
upregulate adhesion molecules, including ICAM-1, VCAM-1 and
E-selectin in HUVECs by activating p-p38 MAPK signaling (17). Notably, some studies have indicated
that antibodies against β2GPI phosphorylate AKT (Ser473) at the
plasma membrane and activate PI3K-dependent AKT-mTOR signaling and
lead to the development of vascular lesions and/or angiogenesis
(22,28,29).
Although antibodies against B19V–VP1u have been reported to exhibit
activity similar to that of aPL by inducing pro-inflammatory
cytokines and adhesion molecules in APS (8,17),
little is known regarding the role of B19V–VP1u antibody in
vascular intimal hyperplasia. In the present study, the induction
of adhesion molecules, such as VCAM-1, ICAM-1 and integrin β1, but
not AKT-mTOR signaling, was observed in HUVECs that had been
treated with B19V–VP1u antibody. These findings are the first to
reveal the role of antibodies against B19V–VP1u in activating
adhesion molecules but not angiogenic signaling. However, the
precise mechanism by which B19V–VP1u antibodies induce adhesion
molecules requires further investigation.
The role of aPLs in angiogenesis is controversial. A
previous study reported that aPLs in serum from patients with APS
reduce in vitro angiogenesis to a level below that induced
by serum from normal individuals (30). An in vivo experiment in
which angiogenesis was impaired in aPL-inoculated mice revealed a
similar result (20). In contrast,
the induction of mTOR signaling, a key pathway of angiogenesis
(31), has been observed in an
in vitro study (22). These
findings have attracted attention and motivated discussion of the
role of aPLs in angiogenesis. Notably, the aPLs that were obtained
from patients with APS in a previous study were a mixture of
anti-β2GPI, cardiolipin and anti-lupus anticoagulant antibodies
(22). Since the role of each
antibody in aPLs in angiogenesis is unclear, further investigations
are required to identify the actual role of each type of antibody
in aPLs in angiogenic signaling.
As well as being associated with autoimmune
disorders, B19V has been associated with a variety of cancer types
(32). A systematic review and
meta-analysis of the literature revealed an association between
B19V infection, and both testicular cancer and leukemia (33,34).
Nested PCR has revealed that B19V DNA and VP1/VP2 protein are more
prevalent in tissues of colon adenocarcinomas compared with in
adjacent noncancerous tissues, polyps and normal controls (35). A previous study reported
significantly higher B19V capsid protein levels in thyroid tissues
from patients with papillary thyroid carcinoma (PTC) than in the
non-neoplastic adjacent tissues and in thyroid tissues from healthy
controls, suggesting a possible role of B19V in the pathogenesis of
PTC (36). These findings imply
the possible involvement of B19V in cancer development. Since
antibodies against B19V–VP1u exhibit similar activity to that of
β2GPI antibodies, the present study investigated the effects of
antibodies against B19V–VP1u on angiogenic signaling, which is an
important indicator of tumorigenesis. Notably, antibodies against
B19V–VP1u had no effect on the induction of mTOR angiogenic
signaling and VEGF expression, which suggests that B19V–VP1u
antibodies may have no effect on angiogenesis in cancer.
In summary, the present study, to the best of our
knowledge, is the first to report that B19V–VP1u antibodies induce
the expression of adhesion molecules, but not AKT-mTOR signaling
and VEGF expression. Therefore, this implies a differential role of
B19V–VP1u antibodies compared with aPLs on angiogenesis in APS.
Acknowledgements
Not applicable.
Funding
This study was supported by the Chung Shan Medical
University and Chi-Mei Medical Center Cooperative Project (grant
nos. CSMU-CMMC-107-01 and CMCSMU10701). Consumptive materials were
partially supported by the Chunghua Christian Hospital (grant no.
104-CCH-IRP-018) and Ministry of Science and Technology (grant no.
MOST-106-2314-B040-023), Taiwan. The funders had no role in study
design, data collection and analysis, decision to publish, or
preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CCS, CCC, TCH and BST conceived and designed the
experiments and analyzed the data; CHH and TCH performed the
experiments and analyzed the data; TCH and BST drafted and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson MJ, Jones SE, Fisher-Hoch SP,
Lewis E, Hall SM, Bartlett CL, Cohen BJ, Mortimer PP and Pereira
MS: Human parvovirus, the cause of erythema infectiosum (fifth
disease)? Lancet. 1:13781983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reid DM, Reid TM, Brown T, Rennie JA and
Eastmond CJ: Human parvovirus-associated arthritis: A clinical and
laboratory description. Lancet. 1:422–425. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serjeant GR, Topley JM, Mason K, Serjeant
BE, Pattison JR, Jones SE and Mohamed R: Outbreak of aplastic
crises in sickle cell anaemia associated with parvovirus-like
agent. Lancet. 2:595–597. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown T, Anand A, Ritchie LD, Clewley JP
and Reid TM: Intrauterine parvovirus infection associated with
hydrops fetalis. Lancet. 2:1033–1034. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyer O: Parvovirus B19 and autoimmune
diseases. Joint Bone Spine. 70:6–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerr JR: The role of parvovirus B19 in the
pathogenesis of autoimmunity and autoimmune disease. J Clin Pathol.
69:279–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dorsch S, Liebisch G, Kaufmann B, von
Landenberg P, Hoffmann JH, Drobnik W and Modrow S: The VP1 unique
region of parvovirus B19V and its constituent phospholipase A2-like
activity. J Virol. 76:2014–2018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzang BS, Lee YJ, Yang TP, Tsay GJ, Shi
JY, Tsai CC and Hsu TC: Induction of antiphospholipid antibodies
and antiphospholipid syndrome-like autoimmunity in naive mice with
antibody against human parvovirus B19 VP1 unique region protein.
Clin Chim Acta. 382:31–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tzang BS, Tsay GJ, Lee YJ, Li C, Sun YS
and Hsu TC: The association of VP1 unique region protein in acute
parvovirus B19 infection and anti-phospholipid antibody production.
Clin Chim Acta. 378:59–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CY, Chiu CC, Cheng J, Lin CY, Shi YF,
Tsai CC, Tzang BS and Hsu TC: Antigenicity analysis of human
parvovirus B19-VP1u protein in the induction of anti-phospholipid
syndrome. Virulence. 9:208–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Von Landenberg P, Lehmann HW, Knöll A,
Dorsch S and Modrow S: Antiphospholipid antibodies in pediatric and
adult patients with rheumatic disease are associated with
parvovirus B19 infection. Arthritis Rheum. 48:1939–1947. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lunardi C, Tinazzi E, Bason C, Dolcino M,
Corrocher R and Puccetti A: Human parvovirus B19 infection and
autoimmunity. Autoimmun Rev. 8:116–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyakis S, Lockshin MD, Atsumi T, Branch
DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni
PL, et al: International consensus statement on an update of the
classifi cation criteria for definite antiphospholipid syndrome
(APS). J Thromb Haemost. 4:295–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Linnemann B: Antiphospholipid syndrome -
an update. Vasa. 47:451–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DY, Tzang BS, Chen YM, Lan JL, Tsai
CC and Hsu TC: The association of anti-parvovirus B19-VP1 unique
region antibodies with antiphospholipid antibodies in patients with
antiphospholipid syndrome. Clin Chim Acta. 411:1084–1089. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loizou S, Cazabon JK, Walport MJ, Tait D
and So AK: Similarities of specificity and cofactor dependence in
serum antiphospholipid antibodies from patients with human
parvovirus B19 infection and from those with systemic lupus
erythematosus. Arthritis Rheum. 40:103–108. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tzang BS, Tsai CC, Chiu CC, Shi JY and Hsu
TC: Up-regulation of adhesion molecule expression and induction of
TNF-alpha on vascular endothelial cells by antibody against human
parvovirus B19 VP1 unique region protein. Clin Chim Acta.
395:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
George J, Blank M, Levy Y, Meroni P,
Damianovich M, Tincani A and Shoenfeld Y: Differential effects of
anti-beta2-glycoprotein I antibodies on endothelial cells and on
the manifestations of experimental antiphospholipid syndrome.
Circulation. 97:900–906. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vega-Ostertag M, Casper K, Swerlick R,
Ferrara D, Harris EN and Pierangeli SS: Involvement of p38 MAPK in
the up-regulation of tissue factor on endothelial cells by
antiphospholipid antibodies. Arthritis Rheum. 52:1545–1554. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Simone N, Di Nicuolo F, D'Ippolito S,
Castellani R, Tersigni C, Caruso A, Meroni P and Marana R:
Antiphospholipid antibodies affect human endometrial angiogenesis.
Biol Reprod. 83:212–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Velásquez M, Rojas M, Abrahams VM,
Escudero C and Cadavid ÁP: Mechanisms of endothelial dysfunction in
antiphospholipid syndrome: Association with clinical
manifestations. Front Physiol. 9:18402018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canaud G, Bienaimé F, Tabarin F, Bataillon
G, Seilhean D, Noël LH, Dragon-Durey MA, Snanoudj R, Friedlander G,
Halbwachs-Mecarelli L, et al: Inhibition of the mTORC pathway in
the antiphospholipid syndrome. N Engl J Med. 371:303–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakamoto Y, Terashita N, Muraguchi T,
Fukusato T and Kubota S: Effects of epigallocatechin-3-gallate
(EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosci
Biotechnol Biochem. 77:1799–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amann A, Zwierzina M, Koeck S, Gamerith G,
Pechriggl E, Huber JM, Lorenz E, Kelm JM, Hilbe W, Zwierzina H and
Kern J: Development of a 3D angiogenesis model to study
tumour-endothelial cell interactions and the effects of
anti-angiogenic drugs. Sci Rep. 7:29632017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai CC, Chiu CC, Hsu JD, Hsu HS, Tzang BS
and Hsu TC: Human parvovirus B19 NS1 protein aggravates liver
injury in NZB/W F1 mice. PLoS One. 8:e5972420132013.
|
|
26
|
Raschi E, Testoni C, Borghi MO, Fineschi S
and Meroni PL: Endothelium activation in the anti-phospholipid
syndrome. Biomed Pharmacother. 57:282–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meroni PL, Raschi E, Testoni C and Borghi
MO: Endothelial cell activation by antiphospholipid antibodies.
Clin Immunol. 112:169–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi T, Giannakopoulos B, Yan X, Yu P,
Berndt MC, Andrews RK, Rivera J, Iverson GM, Cockerill KA, Linnik
MD and Krilis SA: Anti-beta2-glycoprotein I antibodies in complex
with beta2-glycoprotein I can activate platelets in a dysregulated
manner via glycoprotein Ib-IX–V. Arthritis Rheum. 54:2558–2567.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Laat B, Derksen RH, van Lummel M,
Pennings MT and de Groot PG: Pathogenic anti-beta2-glycoprotein I
antibodies recognize domain I of beta2-glycoprotein I only after a
conformational change. Blood. 107:1916–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
D'Ippolito S, Marana R, Di Nicuolo F,
Castellani R, Veglia M, Stinson J, Scambia G and Di Simone N:
Effect of low molecular weight heparins (LMWHs) on antiphospholipid
antibodies (aPL)-mediated inhibition of endometrial angiogenesis.
PLoS One. 7:e296602012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitsiades CS, Mitsiades N and Koutsilieris
M: The Akt pathway: Molecular targets for anti-cancer drug
development. Curr Cancer Drug Targets. 4:235–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jitschin R, Peters O, Plentz A, Turowski
P, Segerer H and Modrow S: Impact of parvovirus B19 infection on
paediatric patients with haematological and/or oncological
disorders. Clin Microbiol Infect. 17:1336–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yousif L, Hammer GP, Blettner M and Zeeb
H: A systematic literature review and meta-analysis. J Med Virol.
85:2165–2175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ibrahem WN, Hasony HJ and Hassan JG: Human
parvovirus B19 in childhood acute lymphoblastic leukaemia in
Basrah. J Pak Med Assoc. 64:9–12. 2014.PubMed/NCBI
|
|
35
|
Li Y, Wang J, Zhu G, Zhang X, Zhai H,
Zhang W, Wang W and Huang G: Detection of parvovirus B19 nucleic
acids and expression of viral VP1/VP2 antigen in human colon
carcinoma. Am J Gastroenterol. 102:1489–1498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JH, Zhang WP, Liu HX, Wang D, Li YF,
Wang WQ, Wang L, He FR, Wang Z, Yan QG, et al: Detection of human
parvovirus B19 in papillary thyroid carcinoma. Br J Cancer.
98:611–618. 2008. View Article : Google Scholar : PubMed/NCBI
|