Introduction
Low back pain (LBP) is one of the most common
complaints worldwide (1).
Approximately 70 to 85% of the Western population will develop LBP
at least once during their lifetime (2). It has been reported that 40 to 50% of
cases of LBP are attributed to discogenic origin (3,4).
The mechanisms of discogenic pain are complicated,
consisting of a complex biochemical cascade in which
proinflammatory cytokines such as interleukin (IL)-1β and tissue
necrosis factor (TNF)-α play an important role (5). Cytokines induce discogenic pain by
promoting the expression of pain mediators and inducing
inflammatory cascades (6). The
upregulation of TNF-α in intervertebral discs (IVDs) may not only
contribute to catabolic process, but also be related with
discogenic pain (7,8), especially following neural and
vascular ingrowth (9,10). Tissue inhibitor of
metalloproteinase-3 (TIMP3) was reported to be a suppressor of
TNF-α which induces inflammation in various tissues and organs
(11,12). However, further studies are needed
to clarify the association between TIMP3 and discogenic pain.
Healthy IVDs are aneural and avascular, while neural
and vascular ingrowth has been frequently found in degenerated IVDs
(9,10), and the neovascularization of IVDs
is believed to be associated with discogenic pain (13). Nucleus pulposus (NP) cells were
found to induce endothelial cell (EC) invasion by expressing
vascular endothelial growth factor (VEGF), especially under
inflammatory condition (13). ECs
further express nerve growth factor (NGF) that accompany ingrowing
nerves (13) and the release of
pain mediators (10). VEGF
expression in NP cells could be induced by degeneration or
inflammation (13,14). TIMP3 is an angiogenesis inhibitor,
and the anti-angiogenesis ability of TIMP3 has been demonstrated in
many types of tumor tissues (15–17).
However, whether TIMP3 is associated with the neovascularization of
IVDs remains unknown.
Substance P (SP) is a sensory marker related to
pain. SP-positive nerve fibers were demonstrated to be related to
discogenic pain (18). SP
expressed in small nociceptive dorsal root ganglion (DRG) neurons
is believed to be the sensory transmitter of nociceptive
information (19). Numerous
studies have demonstrated an association between the expression of
SP in ingrowth nerves and the extent of disc degeneration (13). SP expression is important for pain
transmission from the IVD and occurrence of discogenic pain.
Considering the potential anti-inflammatory and anti-angiogenesis
abilities of TIMP3, the relationship between TIMP3 expression and
SP release should be further investigated.
Previous studies indicated that TIMP3 expression
decreases with age and IVD degeneration (20–23).
In addition, previous research has confirmed that this unbalanced
expression of TIMP3 may lead to intervertebral disc degeneration
(IDD) (24). Despite the finding
that the loss of TIMP3 expression leads to IDD, whether TIMP3
expression is related with discogenic pain still lacks direct
evidence. In the present study, we explored the relationship
between TIMP3 expression and discogenic pain using in vitro
and in vivo models.
Materials and methods
Reagents
The antibodies and reagents used in the present
study are as follows: Rat Vascular Endothelial Growth Factor-164
(rVEGF164; cat. no. 5874, Cell Signaling Technology,
Inc., Danvers, MA, USA), fetal bovine serum (FBS; Gibco, Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and lipopolysaccharides
(LPS; L5543, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany).
Primary antibodies against TIMP3 (ab155749, Abcam, Cambridge, UK),
collagen-2 (ab34712, Abcam), GAPDH (ab181603, Abcam), Substance P
(ab14184, Abcam), aggrecan (ab36861, Abcam) and CD34 (50589-R013,
Sino Biological, Beijing, China) were used in the study. Secondary
antibodies for western blotting (ab205718, Abcam) and
immunohistochemical analysis (ab205719, Abcam) were also used in
the study.
Cell culture
According to previously reported methods, primary
nucleus pulposus (NP) cells and rat aorta endothelial cells (RAECs)
were isolated from Sprague-Dawley (SD) rats (24,25).
A total of 34 SD rats were used for the present study. The SD rats
(6 weeks of age) were euthanized using an abdominal injection of
pentobarbital sodium (150 mg/kg). Briefly, NP cells were isolated
from lumbar spines and cultured in complete media (high-glucose
DMEM with 10% FBS and 1% antibiotic). RAECs were isolated from
aortas of SD rats and cultured with DMEM/F12 media (with 10% FBS
and 1% antibiotic). The primary cell procurement and animal
experiments were approved by the Animal Experimental Ethics
Committee of the Beijing Anzhen Hospital (approval no.
20170614).
Adenovirus vector transfection
Adenovirus vectors loading the coding sequences of
rat TIMP3 (NM_012886) or a scramble control were purchased from
Sino Biological (Beijing, China). Vectors were amplified on 293
cells (American Type Culture Collection, Manassas, VA. USA),
purified, titered and then the particle concentration was measured
by optical absorbance. NP cells were transfected with adenovirus
vector (TIMP3) or a scrambled control at 50 multiplicity of
infection (MOI) according to standard procedure. The transfection
efficacy was verified by western blotting 3 days after
transfection.
Endothelial cell migration and tube
formation assays
Different NP cells were cultured for 48 h and the
medium was isolated as conditioned medium (26). For tube formation assays, RAECs
were seeding at a density of 1×104/well in 96-well
plates precoated with Matrigel (356234, BD Biosciences, Franklin
Lakes, NJ, USA), and then incubated with different reagents (100
ng/ml VEGF, NP-TIMP3 or NP conditioned medium) for 6 h according to
the different groupings. For cell migration assays,
1×105 RAECs were seeding on a Matrigel-coated
polycarbonate membrane insert (8.0-µm pores) in a Transwell
apparatus (Costar, Corning, NY, USA). Different NP cells (NP and
NP-TIMP3) were also cultured with or without 100 ng/ml VEGF in the
lower chamber for 24 h. The cells on the bottom surface of the
insert were fixed with 4% paraformaldehyde and stained with 0.1%
crystal violet. Then the stained cells were observed and counted
using a microscope. The formation of tube-like structures and
migrated cells were observed under a light microscope (×40
magnification, Olympus). Complete medium without cells was used as
the blank control.
Gene expression assay
The total RNA of the various NP cells was isolated
using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Reverse transcription
was carried out using the 1st Strand cDNA Synthesis Kit (Takara
Biotechnology Co., Ltd., Dalian, China). DNA amplification was
carried out using the SYBR Premix Ex Taq kit (Takara) followed by
real-time PCR. The primers were designed and synthesized by
GenePharma (Shanghai, China). Gene expression was measured using
the 2−ΔΔCq method (27). The primer sequences are summarized
in Table I, and GAPDH was selected
as a reference gene.
 | Table I.Sequences of the primers used in
PCR. |
Table I.
Sequences of the primers used in
PCR.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| TACE | Forward |
CCGAACGAGTTTACGGGGAT |
|
| Reverse |
TGTGCGTCGCCTAGAACTAC |
| TIMP3 | Forward |
ACAGACGCCAGAGTCTCCTA |
|
| Reverse |
ACCTCAAGTCTGTCCGGGTA |
| Substance
P | Forward |
TTCATCTCCATCTGTGTCCGC |
|
| Reverse |
GTCTGAGGAGGTCACCACATT |
| TNF-α | Forward |
TCGTAGCAAACCACCAAGCA |
|
| Reverse |
TCGTAGCAAACCACCAAGCA |
| GAPDH | Forward |
AACCTTCTTGCAGCTCCTCCG |
|
| Reverse |
CCATACCCACCATCACACCCT |
Western blot analysis
Aggrecan, Col-2 and TIMP3 protein expression levels
were assessed by western blot analysis in NP cells treated with
lipopolysaccharide (1 µg/ml) for 2 or 5 days. Briefly, for cell
samples, cells were lysed with RIPA buffer and total proteins were
isolated. For tissue samples, a tissue homogenate was made and
total proteins were isolated. A total of 20 µg of each protein
sample was separated by 10% SDS-PAGE, then transferred onto PVDF
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% fat-free milk at room temperature for 1 h,
incubated with primary antibodies (1:1,000) at 4°C overnight,
followed by secondary antibodies (1:5,000) at room temperature for
1 h. Immunoreactive bands were detected using the Odyssey infrared
imaging system (LI-COR). Densitometrical analysis of the bands was
completed using Image-Pro Plus 6.0 software supplied by Media
Cybernetics, Inc. GAPDH was used as the internal reference.
ELISA assessments
Various NP cells were exposed to LPS (1 µg/ml) for 5
days. The TNF-α level in the culture medium was measured using
commercially available enzyme-linked immunosorbent assay (ELISA)
kits according to the manufacturer's instructions (R&D Systems,
Inc., Minneapolis, MN, USA).
Animal experiments
Twenty-four male SD rats (6–8 weeks weighing 200–250
g), were purchased from the Experimental Animal Center of Beijing
Anzhen Hospital. Rats were housed under controlled conditions,
including a 12-h light/dark cycle, 21±2°C and 60–70% humidity, and
rats were also allowed free access to standard dry rat diet and tap
water. The surgical procedure was performed as previously described
(28). Briefly, rats were
anesthetized by an abdominal injection of pentobarbital sodium (40
mg/kg), and percutaneously punctured with a 21G needle in the
coccygeal vertebra (puncture and TIMP3+puncture group). For the
TIMP3+puncture group, rats were injected with adenovirus vector
(1×109 pfu/level) immediately after puncture. At day 28
after puncture, rats were euthanized by intraperitoneal injection
of an overdose of pentobarbital sodium (150 mg/kg) and nucleus
pulposus tissues were isolated for western blot assay and
histologic analysis.
Histologic analysis
Discs from rats were fixed and serial sagittal
sections of discs (5-µm thick) were obtained to prepare slides.
TIMP3, CD34 and substance P expression levels were determined by
immunohistochemical (IHC) staining. All staining procedures were
performed following standard histochemical protocols. We analyzed 3
sections from each disc samples, and for each section, we
calculated 3 fields and took the average. The positive staining was
calculated and analyzed using Image-Pro Plus 6.0 software supplied
by Media Cybernetics, Inc.
Statistical analysis
All the experiments were repeated at least 3 times.
The data are expressed as the mean ± SD. Statistical analysis was
performed with a one-way analysis of variance (ANOVA), followed by
Duncan's post hoc test using SPSS 22.0 software (IBM, Inc.). A
P-value <0.05 was considered to indicate a statistically
significant result.
Results
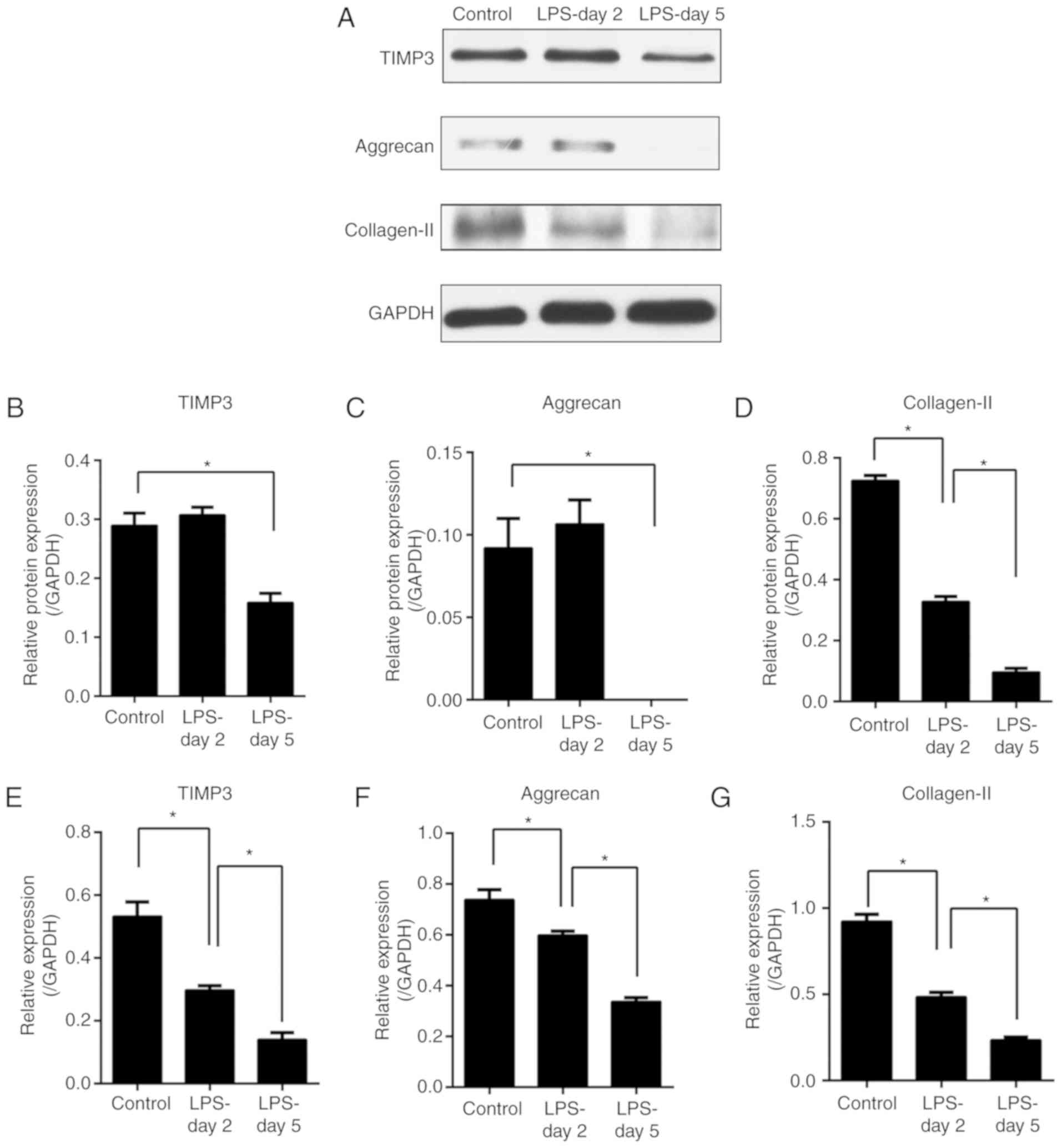
LPS induces TIMP3, aggrecan and
collagen-II downregulation in the NP cells
To study the regulation of TIMP3 expression and the
matrix degradation in the presence of inflammation, NP cells were
treated with or without LPS for 2 or 5 days. The gene and protein
expression of TIMP3, aggrecan and collagen-2 were assessed by PCR
(Fig. 1E-G) and western blot
analysis (Fig. 1A-D). Our results
revealed that gene expression of TIMP3, aggrecan and collagen-2
were downregulated from day 2 after exposure to LPS (Fig. 1E-G), while significant protein
expression reduction was observed only at day 5 except for
collagen-II (Fig. 1A-D). These
results suggest that the downregulation of TIMP3 plays a role in
the matrix degradation of NP cells.
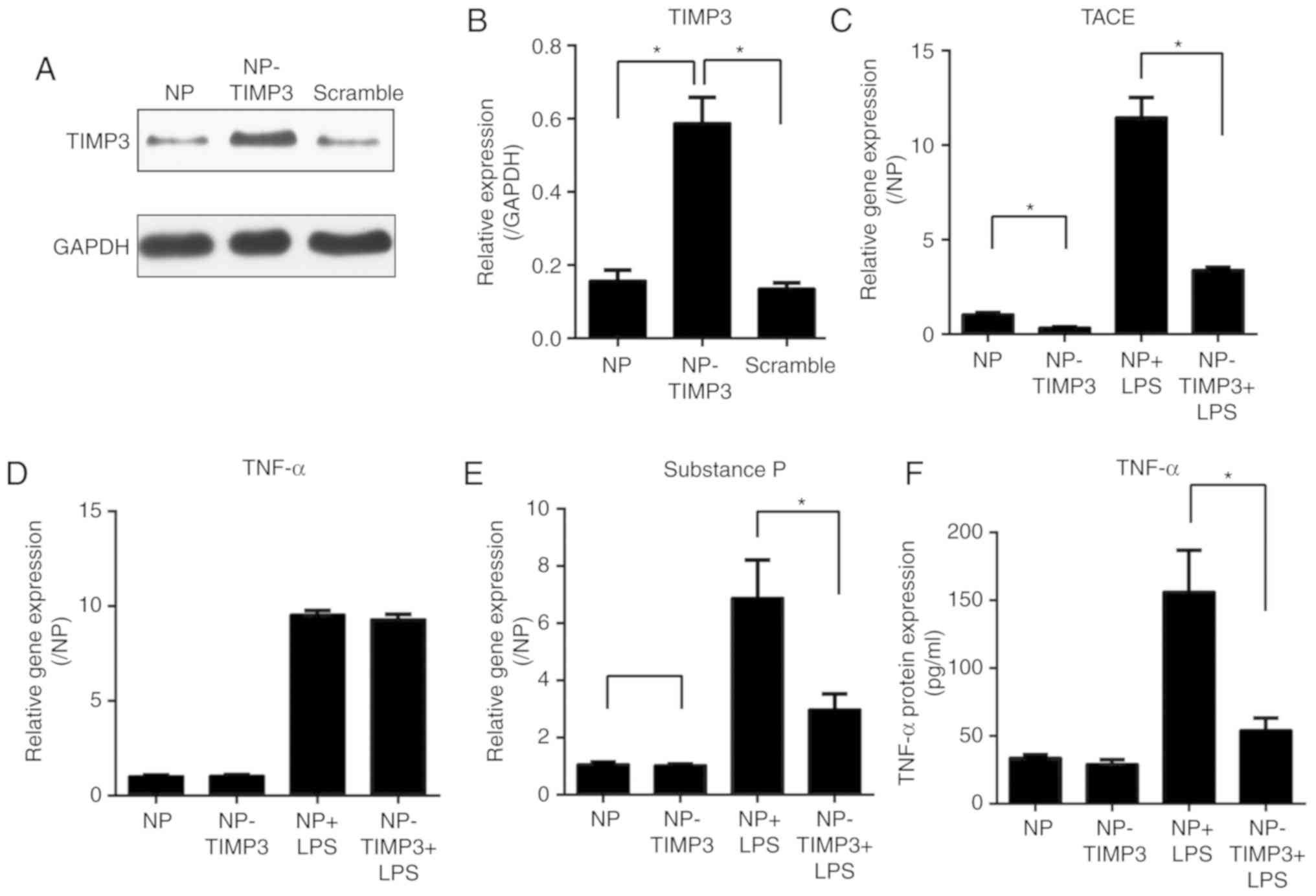
TIMP3 regulates TNF-α and pain
mediator expression in NP cells
Considering that the expression of TIMP3 was found
to be downregulated after exposure to LPS, we aimed to increase
TIMP3 expression in an inflammatory condition and explore the role
of TIMP3 in inflammation. TIMP3 was overexpressed by transfection
of an adenovirus vector in NP cells. TIMP3 overexpression was
confirmed by western blot analysis (Fig. 2A and B). After exposure to LPS, the
gene expression of TNF-α converting enzyme (TACE), TNF-α and
substance P in different NP cells was measured by qPCR. The results
showed that TIMP3 overexpression downregulated TACE and substance P
expression which were induced by LPS (Fig. 2C and E). Although the gene
expression of TNF-α was not affected upon overexpression of TIMP3
(Fig. 2D), the ELISA measurement
indicated that the protein level of TNF-α in medium was decreased
in the TIMP3+LPS group (Fig. 2F).
Taken together, our results suggest that instead of directly
inhibiting TNF-α expression, TIMP3 was more likely to suppress the
activation of TNF-α induced by TACE.
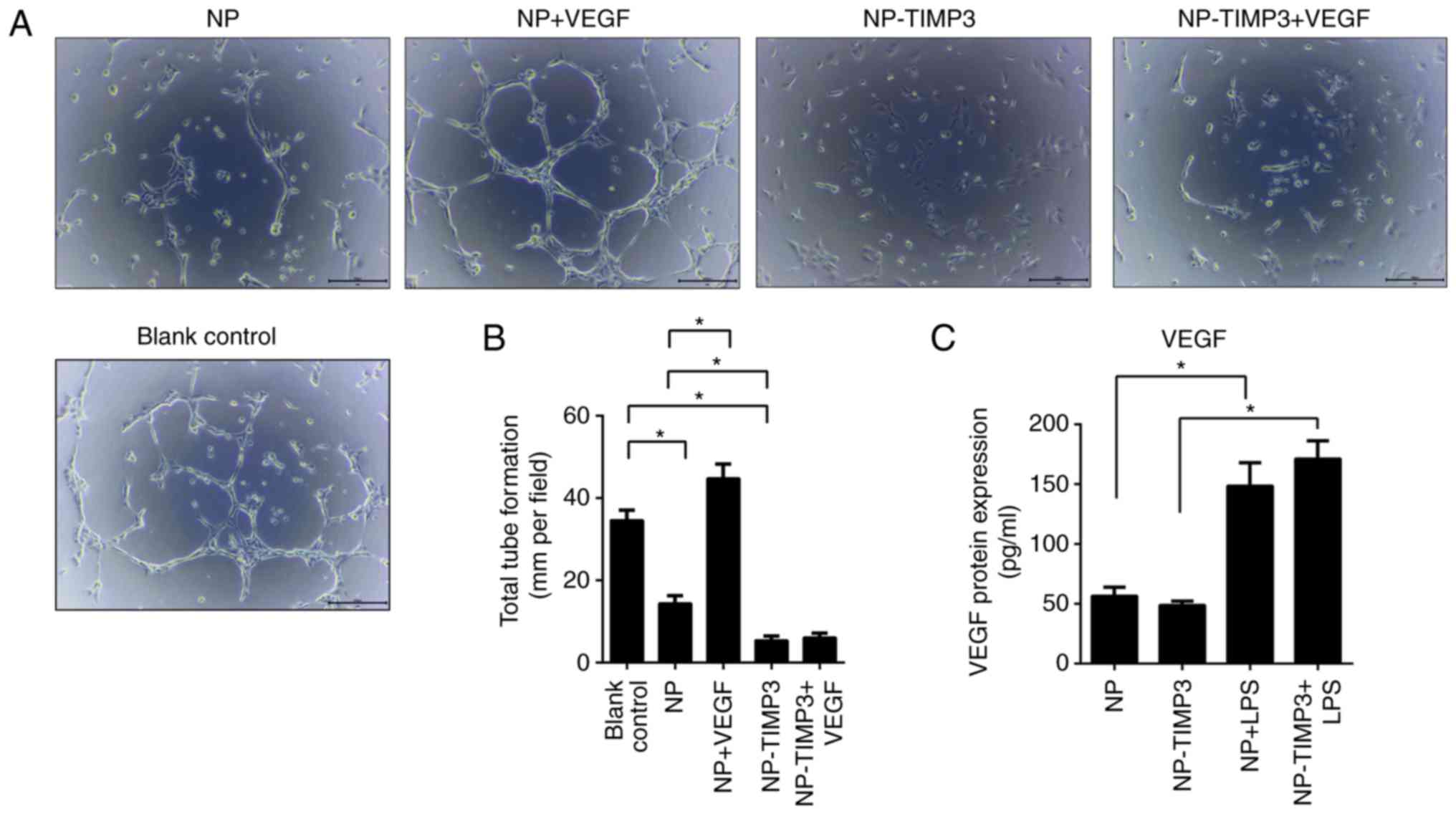
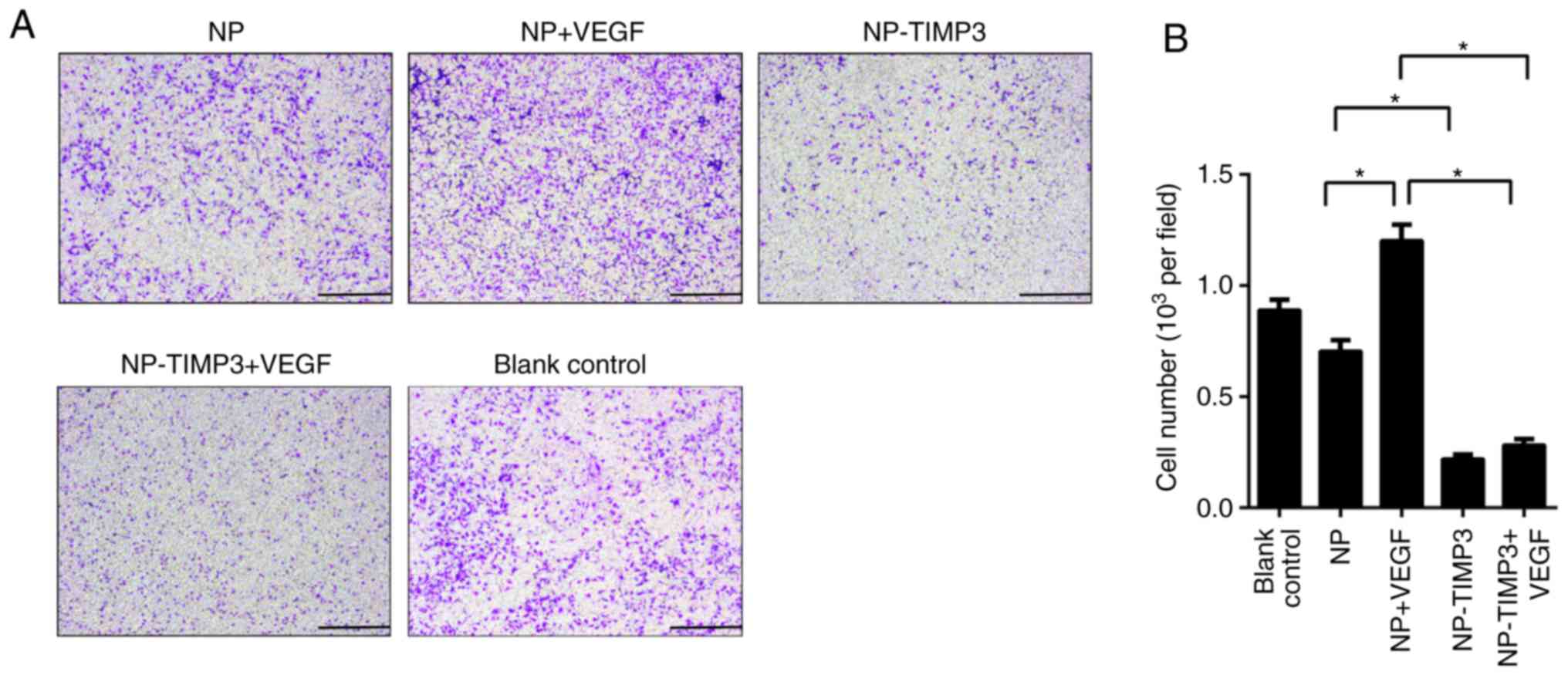
TIMP3 inhibits tube formation and
migration of RAECs
According to ELISA measurement, NP cells secreted
VEGF after stimulation with LPS (Fig.
3C). To further investigate the association between
angiogenesis and TIMP3, RAECs were treated with different
conditioned medium and VEGF or not. Tube formation was observed
under a light microscope. As shown in Fig. 3, NP cell medium significantly
suppressed tube formation of RAECs, while overexpression of TIMP3
further significantly inhibited tube formation. Treatment with VEGF
promoted tube formation of RAECs, however, this promoting effect
was blocking by overexpression of TIMP3 (Fig. 3). The results of the cell migration
assay were similar to that of the tube formation assay (Fig. 4). TIMP3 significantly inhibited the
migration ability of the RAECs and eliminated the promoting effect
of VEGF. According to the above results, TIMP3 inhibits
angiogenesis without regulating VEGF expression, indicated that
TIMP3 may inhibit angiogenesis by blocking the bonding of VEGF and
VEGFR-2.
Overexpression of TIMP3 reduces pain
mediator expression in IVD
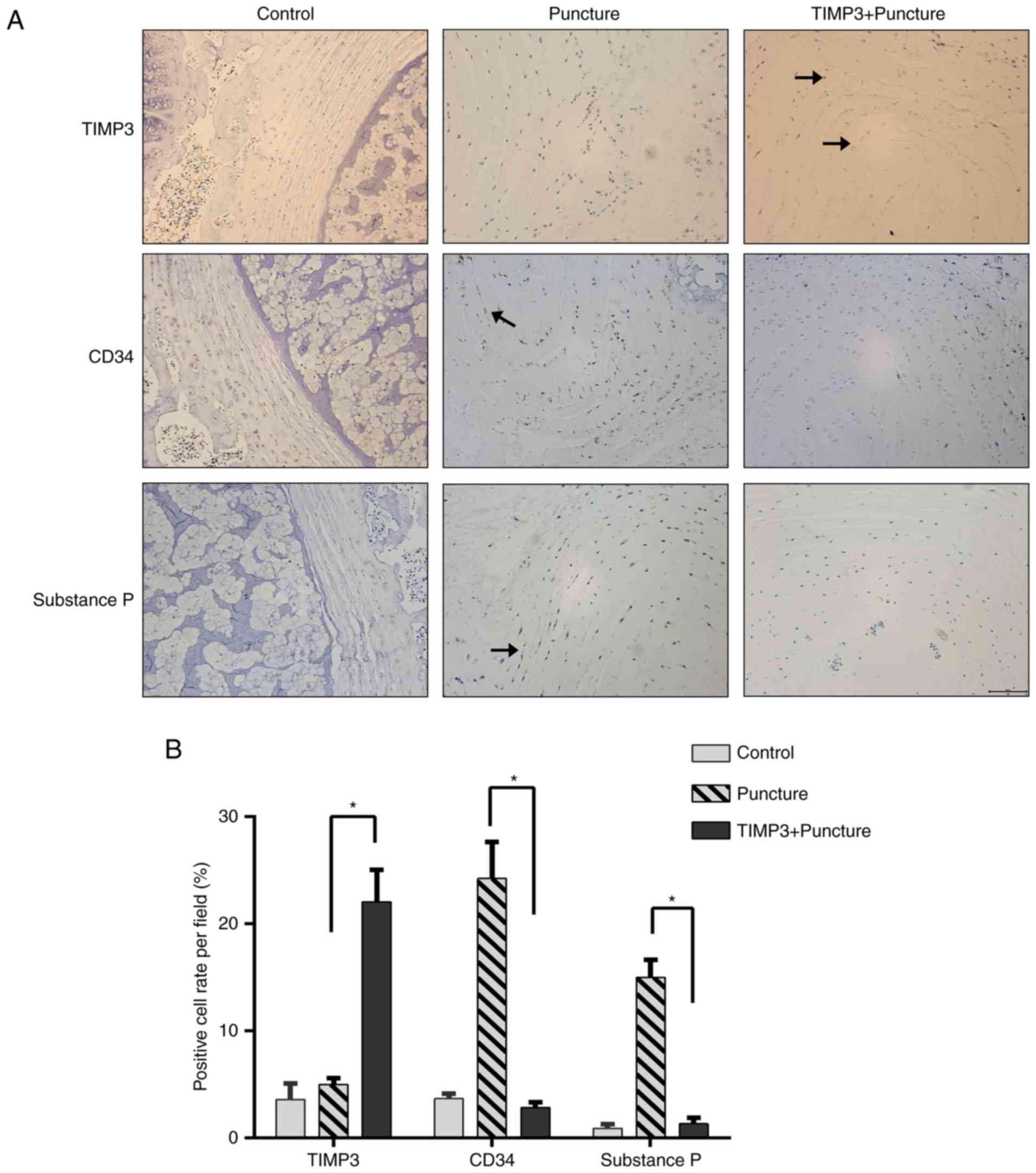
Substance P is a key pain mediator in IVD, and CD34
is a marker of angiopoiesis. Considering the inhibitory effects of
TIMP3 on substance P expression and angiopoiesis, these effects
were confirmed in an in vitro model. The inhibitory effect
of TIMP3 on discogenic pain was further investigated in an in
vivo model by assaying substance P and CD34 expression. IDD rat
model was established by puncture of IVD. After injection of an
adenovirus vector loading TIMP3, TIMP3 expression was significantly
upregulated at day 28 (Fig. 5).
The puncture group exhibited more positive CD34 and substance P
staining, which indicated the neovascularization of IVDs after
puncture. The positive staining rate of CD34 and substance P was
significantly reduced in the TIMP3+puncture group compared with
that in the control group (Fig.
5). These results indicate that TIMP3 may suppress the
angiogenesis and pain mediator expression in degenerative discs,
thus inhibiting discogenic pain in IVD. On the other hand, the loss
of TIMP3 expression may play a role in the development of
discogenic pain.
Discussion
As a key matrix-degrading enzyme inhibitor, the
imbalance of expression between TIMP3 and matrix-degrading enzymes
has been reported to be responsible for the aggrecan breakdown in
intervertebral discs (IVDs) (20–22,24).
Lipopolysaccharides (LPS) markedly induce inflammation in nucleus
pulposus (NP) cells (29). And LPS
also induce the gene and protein overexpression of various
matrix-degrading enzymes, thus leading to matrix degradation
(20,30). In the present study, exposure to
LPS induced the downregulation of collagen-II and aggrecan at both
the gene and protein levels, and the TIMP3 expression was also
inhibited by LPS, which was consistent with a previous study
(24). In addition to direct
inhibition of enzyme activity, TIMP-3 also provides a feedback gene
downregulation of matrix-degrading enzymes (24).
The relationship between TNF-α and discogenic pain
has been studied for years. There are two forms of TNF-α in humans,
membrane-bound (mTNF-α) and a secreted form (sTNF-α). The
transformation from mTNF-α to sTNF-α is processed by TACE.
Increased levels of sTNF-α were reported to present in degenerate
and herniated IVDs (31,32). TNF-α may trigger discogenic pain in
several ways, including sensitization of neurons, induction of
inflammation and upregulation of various receptors (33,34).
These modifications promote the release of pain mediators,
resulting in chronic, persistent pain (33,35).
Although there is still controversy concerning the clinical effect,
the use of anti-TNF-α has been proven effective in discogenic pain
(35–38). TIMP3 was reported to be a
suppressor of TNF-α-induced inflammation in various tissues and
organs (11,12). Our study also showed that instead
of directly inhibited TNF-α expression, TIMP3 suppressed TNF-α
secretion by downregulating TACE expression. As a previous study
reported, intradiscal administration of TNF-α inhibitor could
alleviate discogenic pain for up to 8 weeks (39). Based on our results, overexpression
of TIMP3 in NP cells may be able to relieve discogenic pain by the
inhibition of TNF-α.
SP has been reported to be a sensory marker which is
released by nerve fiber. However, in an inflammatory pain model, SP
release was also increased (40).
The interplay between inflammatory cytokines and neurotrophins,
which is produced by disc cells may explain the phenomenon.
Neurotrophins such as NGF expression was found to be increased in
painful and degenerate discs and are sensitive to TNF-α (13,35).
Neurotrophins would further promote nerve ingrowth and the release
of SP (13,14). Collectively, in light of the
present results and the research mentioned above, we deduced that
TIMP3 may suppress SP released by the inhibition of TNF-α
expression. Innervation is limited in the normal disc, while
abundance of nociceptive nerve endings was found in patients with
chronic back pain (9,13). Neural ingrowth in IVDs was found to
be accompanied by angiogenesis, and nerve growth factor (NGF) was
expressed by vascular tissue, thus promoting neural ingrowth
(13). Moreover, NGF was found to
be expressed only in blood vessels in painful degenerative IVDs
(13), which further confirms the
close relationship between angiogenesis and discogenic pain. VEGF
is an important growth factor of angiogenesis. Overexpression of
VEGF was found in NP cells under inflammatory or degenerated
situations, which was considered to be one of the key reasons
causing angiogenesis (14).
Co-culture model also showed that Fas ligand (FasL) and immune
privilege were involved in the process of IVD angiogenesis,
indicating a more complicated mechanism of neovascularization of
IVDs (41,42). TIMP3 was reported as an effective
angiogenesis inhibitor in various tumor tissues, which suppressed
vascular ingrowth by interfering with the binding of VEGF and
VEGFR-2 (15). TIMP3 also inhibits
the inflammation which was proven as a trigger of VEGF release
(11,12,14).
Moreover, aggrecan from healthy IVDs inhibited neural and vascular
ingrowth (43). Downregulation of
TIMP3 may cause matrix degradation, thus breaking the defense
effect provided by intact aggrecan leading to discogenic pain. Our
study further confirmed the anti-angiogenesis effect of TIMP3 and
interruption of the positive feedback of neural ingrowth in IVD.
However, the tube formation results seemed a bit confusing. The NP
group exhibited suppressed tube formation compared with the blank
control. We speculated that this occurred due to the influence of
notochord cells. The NP cells were collected from young rat IVDs
which consist of a certain amount of notochord cells, and notochord
cells were reported to be able to inhibit the vascular ingrowth in
IVDs (44). We did not separate
these notochord cells from NP cells in our study. The mixed state
of NP and notochord cells was more in accord with the natural state
of an organism.
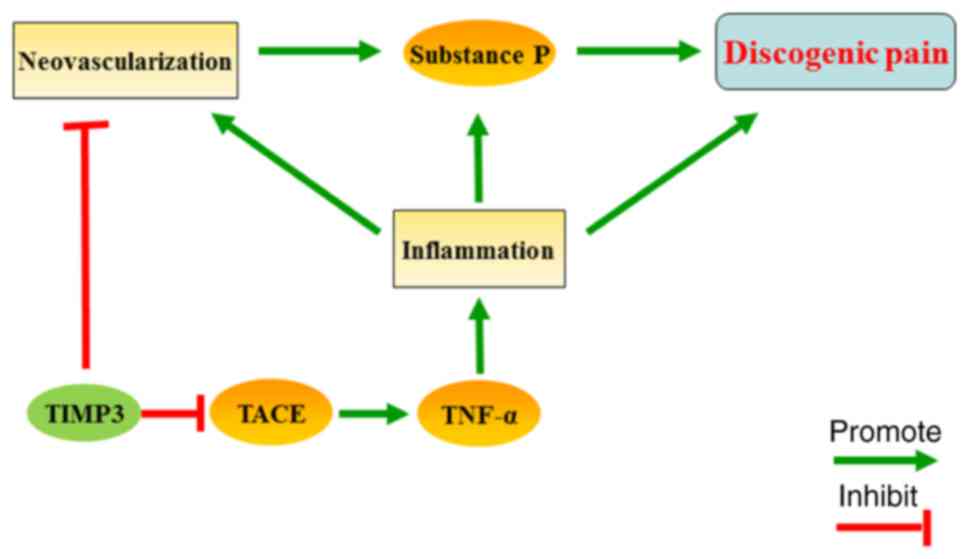
Combined with the results above, we believe that
TIMP3 may reduce the neovascularization of IVDs and suppress the
inflammation-related SP release, and ultimately inhibit discogenic
pain (Fig. 6). However, there are
still some limitations of our study. First, the detailed mechanism
of the anti-angiogenesis effect of TIMP3 was not thoroughly
studied. The interaction between VEGF and VEGFR-2 was not
demonstrated experimentally. Moreover, all of the conclusions in
the present study were based on in vivo and in vitro
experiments. More data from clinical samples are needed in the
future.
In conclusion, our in vitro and in
vivo studies indicate that overexpression of TIMP3 inhibits
discogenic pain by suppressing angiogenesis and the expression of
pain mediator in nucleus pulposus. TIMP-3 may play an important
role in the pathogenesis of discogenic pain and therefore be a
potential therapeutic target.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MWH, JLP, WPG and GRZ conceived and designed the
study. MWH, JLP and HYS performed the experiments. MWH, JLP and HYS
wrote the paper. YL and MWH analyzed the data. GRZ, YL and WPG
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The primary cell procurement and animal experiments
were approved by the Animal Experimental Ethics Committee of the
Beijing Anzhen Hospital (approval no. 20170614).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersson GB: Epidemiological features of
chronic low-back pain. Lancet. 354:581–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DePalma MJ, Ketchum JM and Saullo T: What
is the source of chronic low back pain and does age play a role?
Pain Med. 12:224–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzer AC, Aprill CN, Derby R, Fortin
J, Kine G and Bogduk N: The prevalence and clinical features of
internal disc disruption in patients with chronic low back pain.
Spine (Phila Pa 1976). 20:1878–1883. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wuertz K and Haglund L: Inflammatory
mediators in intervertebral disk degeneration and discogenic pain.
Global Spine J. 3:175–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bachmeier BE, Nerlich AG, Weiler C,
Paesold G, Jochum M and Boos N: Analysis of tissue distribution of
TNF-alpha, TNF-alpha-receptors, and the activating
TNF-alpha-converting enzyme suggests activation of the TNF-alpha
system in the aging intervertebral disc. Ann N Y Acad Sci.
1096:44–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igarashi T, Kikuchi S, Shubayev V and
Myers RR: 2000 Volvo Award winner in basic science studies:
Exogenous tumor necrosis factor-alpha mimics nucleus
pulposus-induced neuropathology. Molecular, histologic, and
behavioral comparisons in rats. Spine (Phila Pa 1976).
25:2975–2980. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boos N, Weissbach S, Rohrbach H, Weiler C,
Spratt KF and Nerlich AG: Classification of age-related changes in
lumbar intervertebral discs: 2002 Volvo Award in basic science.
Spine (Phila Pa 1976). 27:2631–2644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freemont AJ, Peacock TE, Goupille P,
Hoyland JA, O'Brien J and Jayson MI: Nerve ingrowth into diseased
intervertebral disc in chronic back pain. Lancet. 350:178–181.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smookler DS, Mohammed FF, Kassiri Z,
Duncan GS, Mak TW and Khokha R: Tissue inhibitor of
metalloproteinase 3 regulates TNF-dependent systemic inflammation.
J Immunol. 176:721–725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohammed FF, Smookler DS, Taylor SE,
Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B,
Yeh WC and Khokha R: Abnormal TNF activity in Timp3-/- mice leads
to chronic hepatic inflammation and failure of liver regeneration.
Nat Genet. 36:969–977. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Freemont AJ, Watkins A, Le Maitre C, Baird
P, Jeziorska M, Knight MT, Ross ER, O'Brien JP and Hoyland JA:
Nerve growth factor expression and innervation of the painful
intervertebral disc. J Pathol. 197:286–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Binch AL, Cole AA, Breakwell LM, Michael
AL, Chiverton N, Cross AK and Le Maitre CL: Expression and
regulation of neurotrophic and angiogenic factors during human
intervertebral disc degeneration. Arthritis Res Ther. 16:4162014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janssen A, Hoellenriegel J, Fogarasi M,
Schrewe H, Seeliger M, Tamm E, Ohlmann A, May CA, Weber BH and
Stöhr H: Abnormal vessel formation in the choroid of mice lacking
tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci.
49:2812–2822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das AM, Koljenović S, Oude Ophuis CM, van
der Klok T, Galjart B, Nigg AL, van Cappellen WA, Noordhoek Hegt V,
Dinjens WN, Atmodimedjo PN, et al: Association of TIMP3 expression
with vessel density, macrophage infiltration and prognosis in human
malignant melanoma. Eur J Cancer. 53:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Välimäki J and Uusitalo H: Matrix
metalloproteinases (MMP-1, MMP-2, MMP-3 and MMP-9, and TIMP-1,
TIMP-2 and TIMP-3) and markers for vascularization in functioning
and non-functioning bleb capsules of glaucoma drainage implants.
Acta Ophthalmol. 93:450–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamauchi K, Inoue G, Koshi T, Yamashita M,
Ito T, Suzuki M, Eguchi Y, Orita S, Takaso M, Nakagawa K, et al:
Nerve growth factor of cultured medium extracted from human
degenerative nucleus pulposus promotes sensory nerve growth and
induces substance P in vitro. Spine (Phila Pa 1976). 34:2263–2269.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuraishi Y, Hirota N, Sato Y, Hino Y,
Satoh M and Takagi H: Evidence that substance P and somatostatin
transmit separate information related to pain in the spinal dorsal
horn. Brain Res. 325:294–298. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Localization of degradative enzymes and their inhibitors in the
degenerate human intervertebral disc. J Pathol. 204:47–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang M, Wang HQ, Zhang Q, Yan XD, Hao M
and Luo ZJ: Alterations of ADAMTSs and TIMP-3 in human nucleus
pulposus cells subjected to compressive load: Implications in the
pathogenesis of human intervertebral disc degeneration. J Orthop
Res. 30:267–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuji T, Chiba K, Imabayashi H, Fujita Y,
Hosogane N, Okada Y and Toyama Y: Age-related changes in expression
of tissue inhibitor of metalloproteinases-3 associated with
transition from the notochordal nucleus pulposus to the
fibrocartilaginous nucleus pulposus in rabbit intervertebral disc.
Spine (Phila Pa 1976). 32:849–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Li K, Han X, Mao C, Zhang K, Zhao T
and Zhao J: The imbalance between TIMP3 and matrix-degrading
enzymes plays an important role in intervertebral disc
degeneration. Biochem Biophys Res Commun. 469:507–514. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng F, Zhao Y, Wang B, Li B, Sheng Y, Liu
M, Li H and Xiu R: Endothelial cells promote calcification in
aortic smooth muscle cells from spontaneously hypertensive rats.
Cell Physiol Biochem. 49:2371–2381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang JH, Zheng ZY, Liu JY, Xie C, Zhang ZJ
and Zhuang SM: Regulatory role of the MicroRNA-29b-IL-6 signaling
in the formation of vascular mimicry. Mol Ther Nucleic Acids.
8:90–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Cheng X, Wang J, Feng X and Zhang
L: Time-course investigation of intervertebral disc degeneration
induced by different sizes of needle punctures in rat tail disc.
Med Sci Monit. 24:6456–6465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JS, Ellman MB, Yan D, An HS, Kc R, Li
X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, et al: Lactoferricin
mediates anti-inflammatory and anti-catabolic effects via
inhibition of IL-1 and LPS activity in the intervertebral disc. J
Cell Physiol. 228:1884–1896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ellman MB, Kim JS, An HS, Chen D, KC R, An
J, Dittakavi T, van Wijnen AJ, Cs-Szabo G, Li X, et al: Toll-like
receptor adaptor signaling molecule MyD88 on intervertebral disk
homeostasis: In vitro, ex vivo studies. Gene. 505:283–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Le Maitre CL, Hoyland JA and Freemont AJ:
Catabolic cytokine expression in degenerate and herniated human
intervertebral discs: IL-1beta and TNFalpha expression profile.
Arthritis Res Ther. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schaible HG, Schmelz M and Tegeder I:
Pathophysiology and treatment of pain in joint disease. Adv Drug
Deliv Rev. 58:323–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calich AL, Domiciano DS and Fuller R:
Osteoarthritis: Can anti-cytokine therapy play a role in treatment?
Clin Rheumatol. 29:451–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schäfers M, Svensson CI, Sommer C and
Sorkin LS: Tumor necrosis factor-alpha induces mechanical allodynia
after spinal nerve ligation by activation of p38 MAPK in primary
sensory neurons. J Neurosci. 23:2517–2521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olmarker K, Nutu M and Størkson R: Changes
in spontaneous behavior in rats exposed to experimental disc
herniation are blocked by selective TNF-alpha inhibition. Spine
(Phila Pa 1976). 28:1635–1641. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schäfers M and Sommer C: Anticytokine
therapy in neuropathic pain management. Expert Rev Neurother.
7:1613–1627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olmarker K and Larsson K: Tumor necrosis
factor alpha and nucleus-pulposus-induced nerve root injury. Spine
(Phila Pa 1976). 23:2538–2544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sainoh T, Orita S, Miyagi M, Inoue G,
Kamoda H, Ishikawa T, Yamauchi K, Suzuki M, Sakuma Y, Kubota G, et
al: Single intradiscal administration of the tumor necrosis
factor-alpha inhibitor, etanercept, for patients with discogenic
low back pain. Pain Med. 17:40–45. 2016.PubMed/NCBI
|
|
40
|
Noguchi K and Ruda MA: Gene regulation in
an ascending nociceptive pathway: Inflammation-induced increase in
preprotachykinin mRNA in rat lamina I spinal projection neurons. J
Neurosci. 12:2563–2572. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Z, Wan ZY, Guo YS, Wang HQ and Luo ZJ:
FasL on human nucleus pulposus cells prevents angiogenesis in the
disc by inducing Fas-mediated apoptosis of vascular endothelial
cells. Int J Clin Exp Pathol. 6:2376–2385. 2013.PubMed/NCBI
|
|
42
|
Liu ZH, Sun Z, Wang HQ, Ge J, Jiang TS,
Chen YF, Ma Y, Wang C, Hu S, Samartzis D and Luo ZJ: FasL
expression on human nucleus pulposus cells contributes to the
immune privilege of intervertebral disc by interacting with
immunocytes. Int J Med Sci. 10:1053–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johnson WE, Caterson B, Eisenstein SM,
Hynds DL, Snow DM and Roberts S: Human intervertebral disc aggrecan
inhibits nerve growth in vitro. Arthritis Rheum. 46:2658–2664.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cornejo MC, Cho SK, Giannarelli C,
Iatridis JC and Purmessur D: Soluble factors from the
notochordal-rich intervertebral disc inhibit endothelial cell
invasion and vessel formation in the presence and absence of
pro-inflammatory cytokines. Osteoarthritis Cartilage. 23:487–496.
2015. View Article : Google Scholar : PubMed/NCBI
|