Introduction
Liver cancer causes more than 70,000 deaths every
year worldwide (1,2). The incidence of liver cancer is quite
different among different regions. In China, the high infection
rate of hepatitis B and C leads to the high prevalence of liver
cancer (3). Hepatocellular
carcinoma (HCC) accounts for the majority of liver cancer and death
among patients with cirrhosis is usually caused by HCC, which is
mainly caused by chronic hepatitis C virus (HCV), and hepatitis B
virus (HBV) infection (4).
Survival of patients with early stages of HCC is generally
satisfactory (5). However, once
metastasis occurs, treatment outcomes will be extremely poor
(6).
Rho associated coiled-coil containing protein kinase
2 (ROCK2) plays pivotal roles in regulating smooth muscle
contraction, cytokinesis and the formation of focal adhesions as
well as actin stress fibers (7).
Previous studies have shown that ROCK kinases including ROCK2 also
have critical functions in the development of human cancers
including HCC (8,9). ROCK2 inhibition provides new insights
to the treatment of certain malignancies (10,11).
HAND2-AS1 suppresses several types of human cancers (12–15),
but its functions in HCC are unknown. The present study performed
deep sequencing-based transcriptome analysis and observed the
inverse correlation between HAND2-AS1 and ROCK2 (data not shown).
The present study revealed that HAND2-AS1 might mediate the
downregulation of ROCK2 in HCC to inhibit cancer cell
behaviors.
Materials and methods
Patients and serum specimens
A total of 44 HCC patients (44–68 years) and 38
hepatitis B (HB, 44–68 years) patients who were treated in
Zhongshan Hospital, Shanghai were enrolled from January 2017 to May
2018. HBV infections were determined by sensitive PCR. Inclusion
criteria are: i) Patients diagnosed in the Zhongshan hospital; ii)
patients received treatment for the first time; iii) HCC patients
at AJCC stage IA-IIIA. Exclusion criteria include: i) Patients
complicated with other severe diseases or liver diseases; ii)
patients who were treated within 3 months before this study; iii)
patients have difficulties in understanding the experimental
protocol. During the same time period, 32 healthy volunteers were
also included to be control group of the present study. Blood (5
ml) was extracted from the elbow vein of each participant before
breakfast. Blood samples were used to prepare serum using
conventional methods. Patient characteristics are presented in
Table I. Clinical factors were
compared among groups using a chi-squared test. No significant
differences in age (P=0.63), gender (P=0.52), smoking habit
(P=0.36) and drinking habit (P=0.34) were found among groups. The
present study received approval from The Ethics Committee of
Zhongshan Hospital, Shanghai before patient admission. Informed
consent was signed by all participants.
 | Table I.General information of 3 groups of
participants. |
Table I.
General information of 3 groups of
participants.
|
| HCC patients | HB patients | Controls |
|---|
| Cases | 44 | 38 | 32 |
| Sex |
|
|
|
| Male | 24 | 20 | 17 |
|
Female | 20 | 18 | 15 |
| Habits |
|
|
|
|
Smoking | 22 (50.0%) | 17 (44.7%) | 15 (46.9%) |
|
Drinking | 24 (54.5%) | 18 (47.4%) | 19 (59.4%) |
ELISA
Serum levels of ROCK2 were measured through an ELISA
using a kit (MBS705283, MyBioSource). The detection sensitivity of
the kit was 156 pg/ml and the detection range was 625–40,000
pg/ml.
Reverse transcription-quantitative
(RT-q)PCR
RNAzol® RT (Sigma-Aldrich; Merck KGaA)
was mixed with serum and cells to extract RNAs. Following reverse
transcription (25°C for 5 min, 55°C for 30 min and 75°C for 10
min), PCR reaction systems were prepared using SuperScript III
Platinum One-Step RT-qPCR kit (Thermo Fisher Scientific, Inc.).
Thermocycling conditions: 95°C for 57 sec and then 40 cycles of
95°C for 16 sec and 56.5°C for 33 sec. Sequences of primers:
5′-GGGTGTTTACGTAGACCAGAACC-3′ (forward) and
5′-CTTCCAAAAGCCTTCTGCCTTAG-3′ (reverse) for HAND2-AS1;
5′-GACCTCTATGCCAACACAG-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGG-3′
(reverse) for β-actin. All Cq values were processed through
2−ΔΔCq method (16).
Cell lines, cell culture and cell
transfection
SNU-398 human HCC cell line American Type Culture
Collection (ATCC) and THLE-3 human normal liver epithelial cell
line (ATCC) were included. HAND2-AS1 and ROCK2 expression pIRSE2
vectors as well as empty vectors were bought from GeneCopoeia, Inc.
HAND2-AS1 and ROCK2 vectors (10 nM) or empty vectors (10 nM;
negative control) were transfected into cells using lipofectamine
2000 (11668-019; Invitrogen; Thermo Fisher Scientific, Inc.). The
control group included untransfected cells. Before the following
experiments, overexpression of HAND2-AS1 and ROCK2 (overexpression
rate: 200–240%) was confirmed by RT-qPCR. The following experiments
were performed at 24 h post-transfection.
Cell proliferation analysis
Cell proliferation analysis was performed by Cell
Counting Kit-8 (CCK-8) assay at 24 h post-transfection. Briefly,
cells were harvested to prepare cell suspensions
(5×104/ml). Cells were cultivated in a 96-well plate
(0.1 ml per well) and 10 µl CCK-8 solution was added 4 h before the
end of cell culture. Following the addition of 10 µl DMSO, optical
density values at 450 nm were measured.
Cell migration and invasion
analysis
Cell migration and invasion were analyzed at 24 h
post-transfection following the same procedure except that Matrigel
(356234; EMD Millipore) was used to coat the upper chamber before
invasion assay. Transwell inserts (8 µm Dojindo Molecular
Technologies, Inc.) were used. Cell suspensions
(5×104/ml) were prepared using serum free RPMI 1640
medium (ATCC). A total of 0.1 ml cell suspension was transferred to
the upper chamber and culture medium containing 20% fetal bovine
serum (Sigma-Aldrich, Merck KGaA) was used to fill the lower
chamber. Membranes were collected 24 h later, being followed by
staining with 0.5% crystal violet (Sigma-Aldrich, Merck KGaA) at
room temperature for 15 min. Under a light microscope, migrating
cells and invading cells were observed.
Western-blotting
Following total protein extraction using RIPA
solution, BCA assay (both from Sigma-Aldrich, Merck KGaA) was
performed to measure protein concentrations. Electrophoresis was
carried out using 12% SDS-PAGE gel with 30 µg denatured protein per
well. After protein transfer to PVDF membranes, blocking in 5%
non-fat milk was performed for 2 h at room temperature. Then
membranes were incubated with primary antibodies of rabbit
anti-human ROCK2 (1:1,300; cat. no. ab66320; Abcam) and GAPDH
(1:1,300; cat. no. ab8245; Abcam) overnight at 4°C. Followed by
incubation with IgG-HRP goat anti-rabbit secondary antibody (1:800;
cat. no. MBS435036; MyBioSource) at room temperature for 2 h. ECL
Detection Reagent (Sigma-Aldrich; Merck KGaA) was used to develop
signals. Image J v1.6 software (National Institute of Health) was
used for signal normalization.
Statistical analysis
Experiments were performed in sets of 3 repeats and
mean ± standard error of the mean values were calculated.
Differences among groups were explored using analysis of variance
(one-way) by Tukey test. Correlation analysis was performed by
Pearson correlation coefficient. Diagnostic analyses were performed
using receiver operating characteristic (ROC) curve. P<0.05 was
considered to indicate a statistically significant difference.
Results
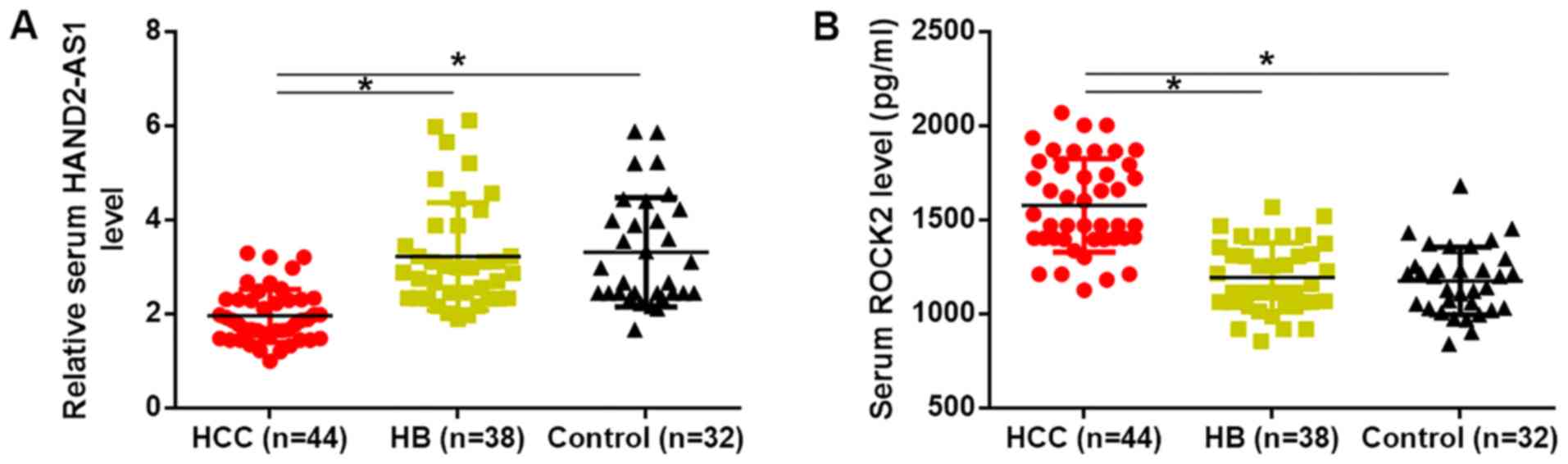
Expression of HAND2-AS1 and ROCK2 are
altered only in HCC patients
The present study first detected the expression of
HAND2-AS1 and ROCK2 in the serum of all 3 groups of participants.
Results showed that serum levels of HAND2-AS1 were significantly
downregulated (P<0.05; Fig.
1A), while serum levels of ROCK2 were significantly increased
(P<0.05; Fig. 1B) in HCC
patients compared with in HB patients and healthy controls.
However, no significant differences in serum levels of HAND2-AS1
and ROCK2 were found between HB patients and healthy controls. It
is worth noting that, HAND2-AS1 expression levels decreased, while
ROCK2 expression levels increased with the increase of clinical
stages, however the changes were not statistically significant
(data not shown).
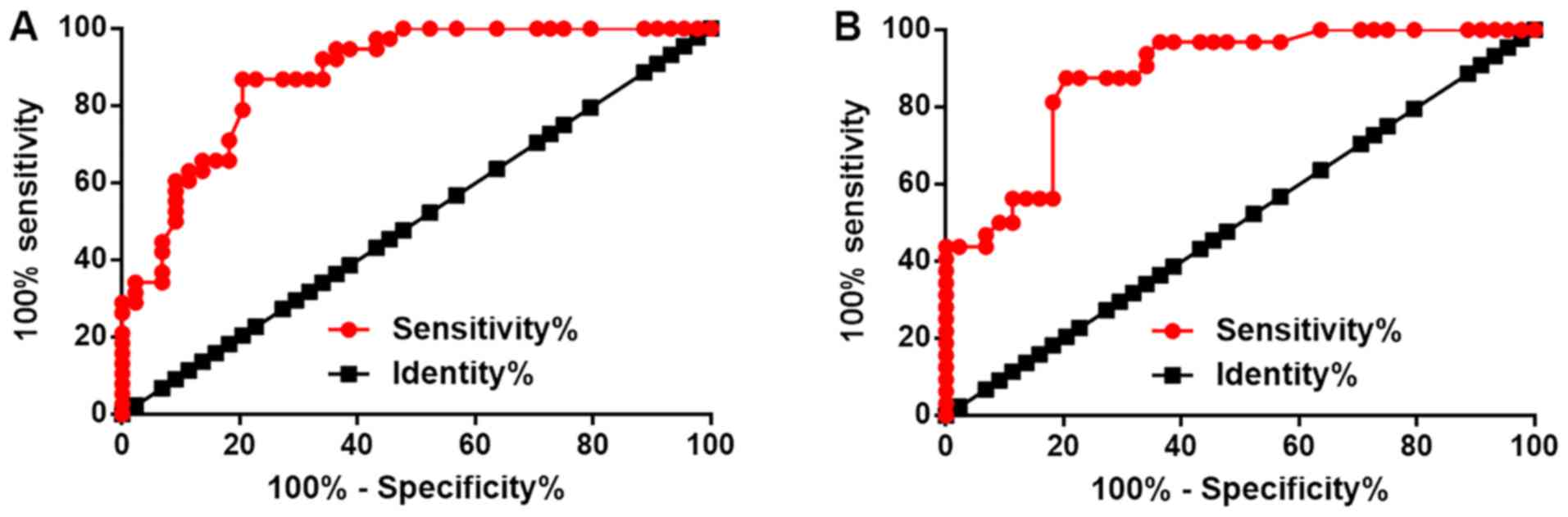
Decreased HAND2-AS1 levels
distinguishes HCC patients from HB patients and healthy
controls
ROC curve analysis was performed with HCC patients
as true positive subjects and HB patients or healthy controls as
true negative subjects (Fig. 2).
With HB patients as references, the area under the curve (AUC) was
0.8792 (standard error: 0.03662; 95% confidence interval:
0.8074–0.9510). With healthy controls as references, the AUC was
0.8800 (standard error: 0.03795; 95% confidence interval:
0.8056–0.9544).
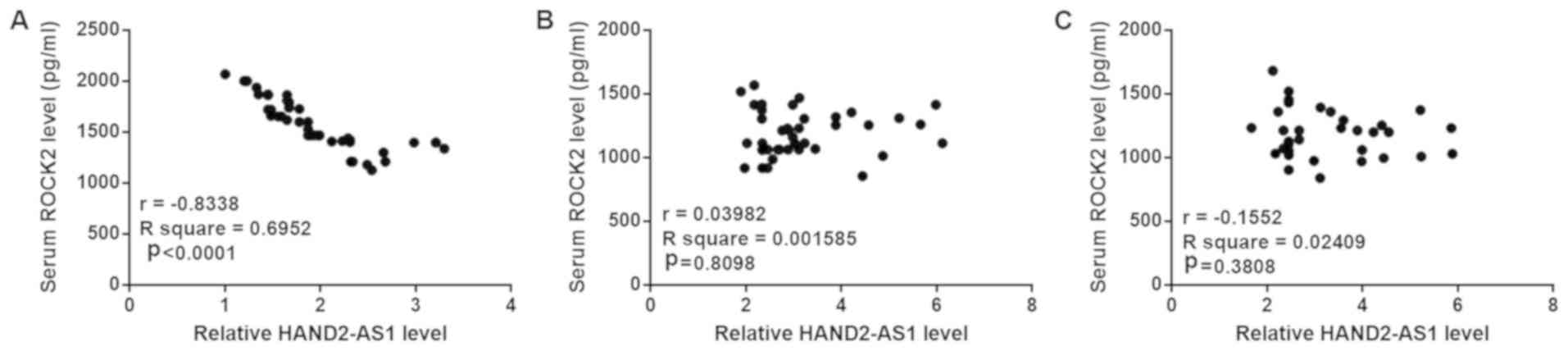
HAND2-AS1 and ROCK2 are negatively
correlated in HCC patients
Pearson correlation analysis was performed to
investigate the correlations between serum levels of HAND2-AS1 and
ROCK2. Significant negative correlation between serum levels of
HAND2-AS1 and ROCK2 was observed in HCC patients (P<0.0001;
Fig. 3A). However, the correlation
between serum levels of HAND2-AS1 and ROCK2 was not strong in HB
patients (Fig. 3B) and healthy
controls (Fig. 3C).
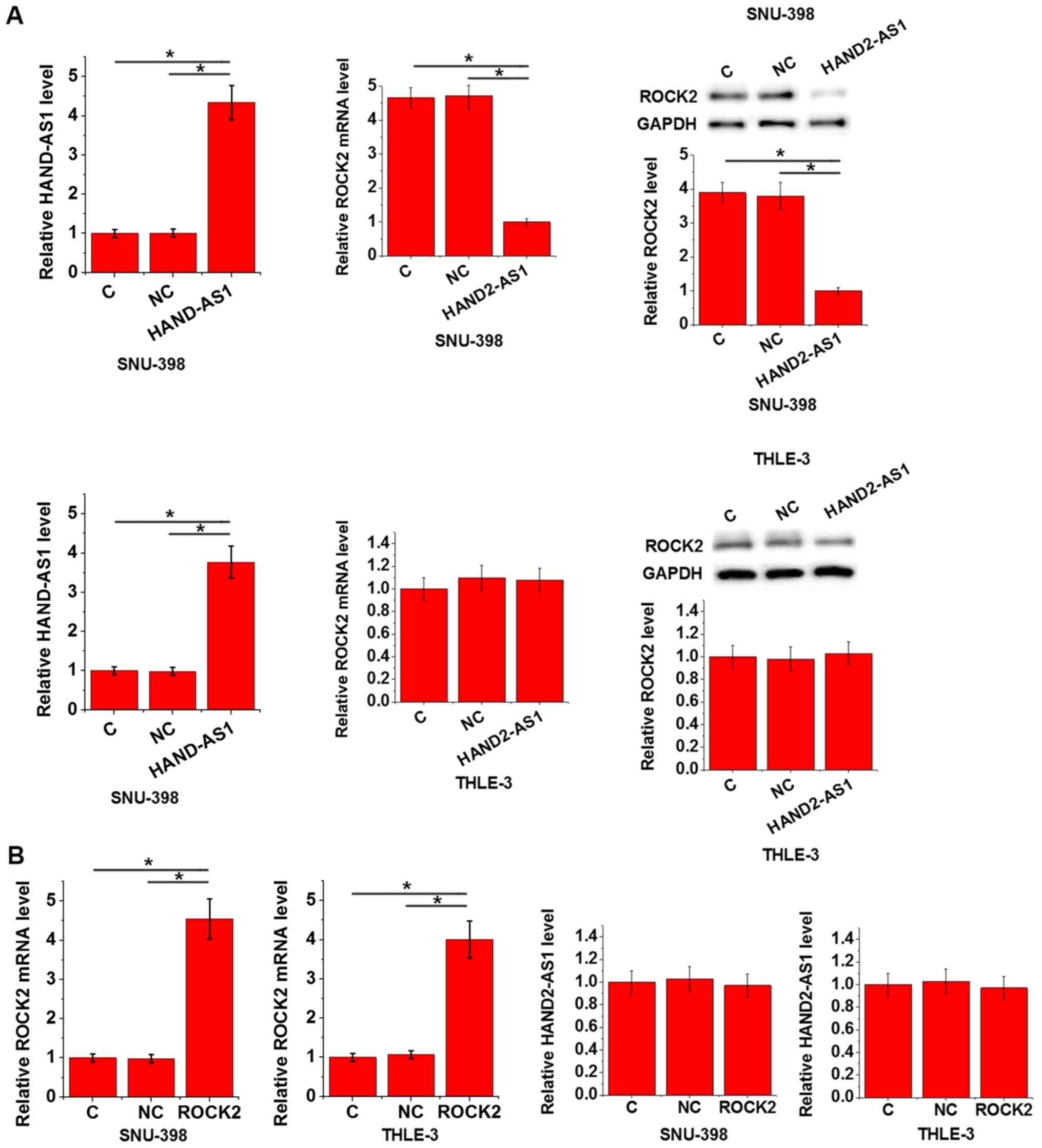
HAND2-AS1 overexpression leads to
inhibited ROCK2 expression in HCC cells but not in normal liver
epithelial cells
In this experiment, the control (C) group was the
untransfected cells and the negative control (NC) group was the
cells transfected with empty vectors. Compared with C and NC,
HAND2-AS1 overexpression led to significantly inhibited ROCK2
expression in cells of SNU-398 human HCC cell line (P<0.05) but
not in cells of THLE-3 human normal liver epithelial cell line
(Fig. 4A). In contrast, ROCK2
overexpression did not significantly affect HAND2-AS1 expression in
cells of those two cell lines (Fig.
4B).
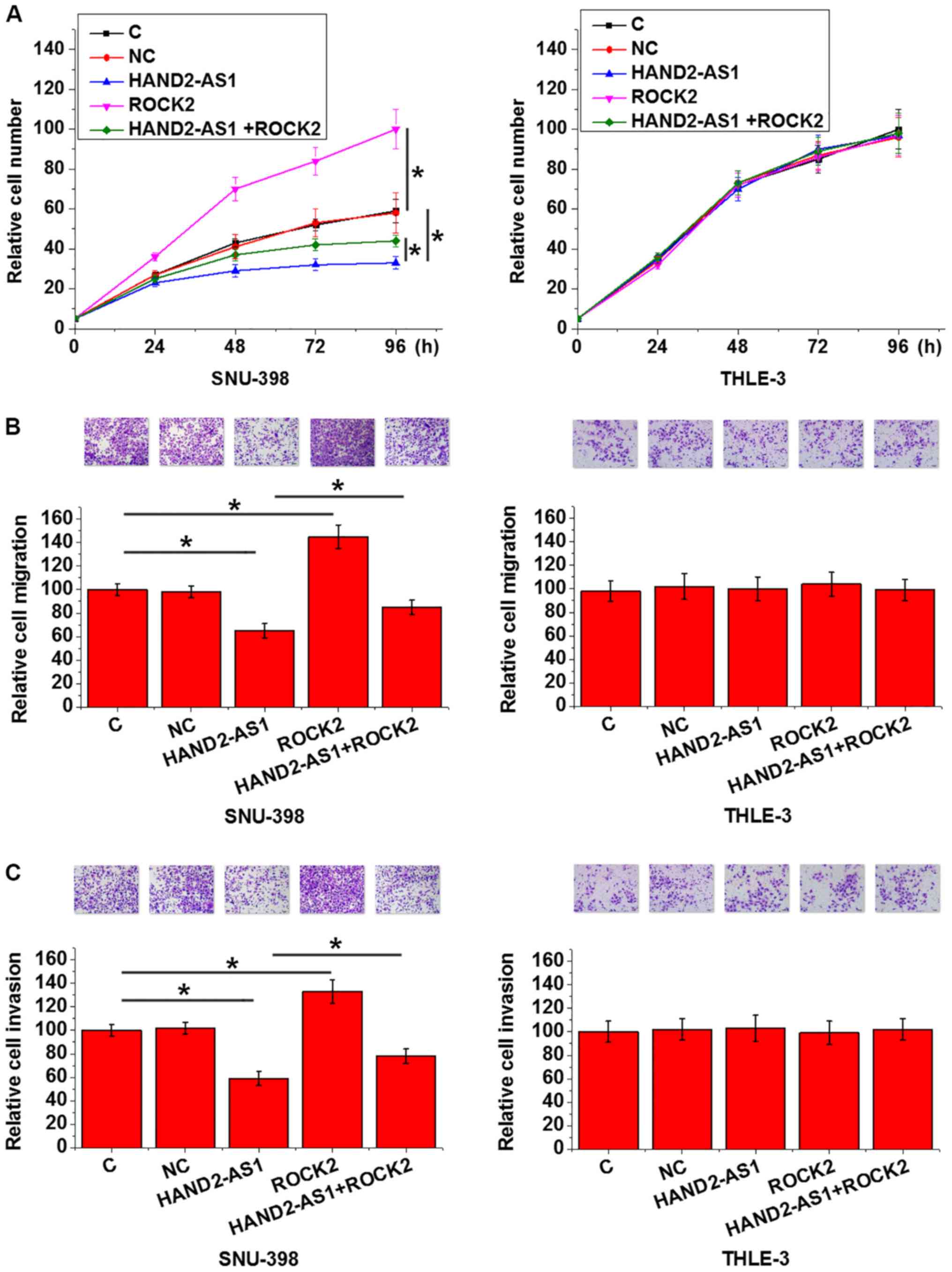
HAND2-AS1 and ROCK2 play opposite
roles in regulating HCC cell behaviors
In this experiment, the control group was the
untransfected cells and NC group was the cells transfected with
empty vectors. Compared with C (untransfected cells) and NC (empty
vector transfection), HAND2-AS1 overexpression significantly
inhibited, while ROCK2 overexpression significantly promoted the
proliferation (P<0.05; Fig.
5A), migration (P<0.05; Fig.
5B) and invasion (P<0.05; Fig.
5C) of SNU-398 human HCC cell line, but not cells of THLE-3
human normal liver epithelial cell line. In addition, compared with
SNU-398 cells with HAND2-AS1 overexpression only, SNU-398 cells
with both HAND2-AS1 and ROCK2 overexpression showed significantly
promoted proliferation (P<0.05; Fig. 5A), migration (P<0.05; Fig. 5B) and invasion (P<0.05; Fig. 5C).
Discussion
HAND2-AS1 as a tumor suppressor has been reported in
several human malignancies (12–15),
its role in liver cancer is unclear. The present study to the best
of our knowledge first reported the involvement of HAND2-AS1 in
HCC. The actions of HAND2-AS1 in HCC were also proved to be
achieved probably through the downregulation of ROCK2.
Tumor metastasis globally affects gene expression
(17). To simplify the story, the
present study only included HCC patients at stages IA-IIIA (early
stages before lymph node metastasis). Treatment outcomes of
patients with metastatic HCC are extremely poor (6). Therefore, early diagnosis and
treatment are still critical for the survival of patients with HCC.
Only HCC patients were enrolled at early stages to investigate the
application potentials of HAND2-AS1 in the early detection of HCC.
HAND2-AS1 is a tumor suppressor gene with downregulated expression
pattern in colorectal cancer, endometrioid endometrial carcinoma
and osteosarcoma (12–15). The present study revealed that
serum levels of HAND2-AS1 were also reduced in HCC patients
compared with HB patients and healthy controls. In effect,
downregulation of HAND2-AS1 effectively distinguished HCC patients
from HB patients and healthy controls. Therefore, HAND2-AS1 may
have application potential in the early diagnosis of HCC. HBV or
HCV infection is the major cause of HCC (4). No significant differences in serum
levels of HAND2-AS1 and healthy controls were observed in the
present study. Therefore, the downregulated HAND2-AS1 in HCC
patients is unlikely to be caused by HBV infection. This study
didn't include hepatitis C patients since most HCC patients (32/44)
included in this study were infected by HBV and only 2 cases were
infected by HCV.
As an oncogenic kinase protein, ROCK2 usually showed
an upregulated expression pattern in the development of human
cancers (18). Overexpression of
ROCK2 promotes HCC through multiple pathways (19). Being consistent with a previous
study (18), the present study
found that the serum levels of ROCK2 were increased in HCC patients
compared with those in HB patients and healthy controls, further
confirming the oncogenic role of ROCK2 in HCC.
It has been well established that ROCK2 achieves its
biological functions through the interactions with different
functional molecules including lncRNAs (20), while crosstalk between ROCK2 and
lncRNAs in HCC still hasn't been reported. The present study proved
that HAND2-AS1 is likely to be an upstream inhibitor of ROCK2 in
the regulation of HCC cell proliferation, migration and invasion.
The present study also speculated that the interaction between
HAND2-AS1 and ROCK2 is indirect due to following observations: i)
No significant correlation between serum levels of HAND2-AS1 and
ROCK2 was observed in healthy controls; ii) HAND2-AS1
overexpression showed no significant effects on ROCK2 expression in
normal liver cells.
It is also worth noting that HAND2-AS1 and ROCK2
showed no significant effects on the proliferation, migration and
invasion of normal liver cells. This is possibly a result of
certain signaling pathways activated in HCC may be mediating the
actions of HAND2-AS1 and ROCK2 in HCC. Normal liver cells lack the
activation of those pathways. Therefore HAND2-AS1 overexpression
may serve as a promising target for the treatment of HCC by
downregulating ROCK2. The present study was limited by the small
sample size. Larger sample size studies may be needed in the future
to further confirm the conclusions. Only one HCC cell line was used
in this study. The authors' future studies will include more HCC
cell lines to further confirm the present study's conclusions.
In conclusion, HAND2-AS1 levels in serum were
decreased in HCC. Overexpression of HAND2-AS1 may inhibit the
behaviors of HCC cells by downregulating ROCK2.
Acknowledgements
Not applicable.
Funding
The present study was funded by financial support
from The Shanghai Science and Technology Committee (grant no.
13ZR1436500).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, YH and XL designed experiments. LJ, YH and GS
performed experiments. JN, ZX, HL and YC collected and analyzed
data. XL drafted the manuscript. All authors approved this
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The present study received approval from The Ethics
Committee of Zhongshan Hospital, Shanghai before patient admission.
Informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
Informed consent was obtained from all individual
participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maucort-Boulch D, de Martel C, Franceschi
S and Plummer M: Fraction and incidence of liver cancer
attributable to hepatitis B and C viruses worldwide. Int J Cancer.
142:2471–2477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singal AG, Pillai A and Tiro J: Early
detection, curative treatment, and survival rates for
hepatocellular carcinoma surveillance in patients with cirrhosis: A
meta-analysis. PLoS Med. 11:e10016242014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang D, Du X, Yuan R, Chen L, Liu T, Wen
C, Huang M, Li M, Hao L and Shao J: Rock2 promotes the invasion and
metastasis of hepatocellular carcinoma by modifying MMP2
ubiquitination and degradation. Biochem Biophys Res Commun.
453:49–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng F, Jiang J, Yu Y, Tian R, Guo X, Li
X, Shen M, Xu M, Zhu F, Shi C, et al: Direct targeting of
SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma
tumourigenesis and metastasis. Br J Cancer. 109:3092–3104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Zhou X and Wei M: MicroRNA-144
suppresses osteosarcoma growth and metastasis by targeting ROCK1
and ROCK2. Oncotarget. 6:10297–10308. 2015.PubMed/NCBI
|
|
12
|
Yang X, Zhao YU, Sun K, Li Y, Zhou J, Wang
J, Sun H, Wang CC, Kwong J, Wang H, et al: LncRNA HAND2-AS1
inactivates neuromedin U (NMU) and inhibits tumor invasion and
metastasis in endometrioid endometrial carcinoma. Cancer Rec.
3448:2017.f.
|
|
13
|
Kang Y, Zhu X, Xu Y, Tang Q, Huang Z, Zhao
Z, Lu J, Song G, Xu H, Deng C and Wang J: Energy stress-induced
lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and
inhibits osteosarcoma progression. Am J Cancer Res. 8:526–537.
2018.PubMed/NCBI
|
|
14
|
Zhou J, Lin J, Zhang H, Zhu F and Xie R:
LncRNA HAND2-AS1 sponging miR-1275 suppresses colorectal cancer
progression by upregulating KLF14. Biochem Biophys Res Commun.
503:1848–1853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Wang CC, Lee WYW, Trovik J, Chung
TKH and Kwong J: Long non-coding RNA HAND2-AS1 inhibits invasion
and metastasis in endometrioid endometrial carcinoma through
inactivating neuromedin U. Cancer Lett. 413:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM,
Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu
J, et al: Gene-expression profiles to predict distant metastasis of
lymph-node-negative primary breast cancer. Lancet. 365:671–679.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei L, Surma M, Shi S, Lambert-Cheatham N
and Shi J: Novel insights into the roles of Rho kinase in cancer.
Arch Immunol Ther Exp (Warsz). 64:259–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zhou W, Yuan R, Chen L, Liu T, Huang
D, Hao L, Xie Y and Shao J: ROCK2 promotes HCC proliferation by
CEBPD inhibition through phospho-GSK3β/β-catenin signaling. FEBS
Lett. 589:1018–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Li X, Li X, Li X and Chen Z:
LncRNA H19 promotes epithelial-mesenchymal transition (EMT) by
targeting miR-484 in human lung cancer cells. J Cell Biochem.
119:4447–4457. 2018. View Article : Google Scholar : PubMed/NCBI
|