Introduction
The regenerative mechanisms of mandibular and
maxillary bones have attracted increasing attention in recent
decades. Most patients who undergo surgical resections require
mandibular/maxillary bone reconstruction; however, some cases have
reported spontaneous bone regeneration or accelerated bone repair
(1,2). Identifying the regulatory mechanisms
behind this phenomenon may support the development of novel
therapeutic strategies and provide improved treatment options for
patients.
Bone regeneration involves highly integrated
interactions between numerous types of cells and signaling pathways
(3). In 1994, it was clinically
observed that new blood vessels surrounding the site of injury
could lead to the improved healing of fractures (4); it was considered that blood vessels
served as scaffolds during bone regeneration, around which bone
formation could take place, owing to the ability of blood vessels
to provide oxygen, nutrients and other required mineral materials
(3). In addition, they could
function as routes for bone precursor cells to reach the site of
injury (5). It has also been
demonstrated that blood vessels could exert angiocrine functions,
which permit blood vessels to produce a series of paracrine signals
to coordinate multiple biological processes associated with
osteogenesis, including proliferation, differentiation, stem cell
behavior and tissue regeneration (6). It was therefore hypothesized that
angiogenesis may serve a vital role in bone regeneration (7). Osteoblasts and preosteoblasts have
important roles during bone regeneration-associated angiogenesis.
They communicate with endothelial cells, most likely through
secreting cytokines or other factors, and remodel the
microenvironment; for example, osteoblasts were reported to
regulate angiogenesis through secreting C-X-C motif chemokine
ligand 9 (8); micro (mi)RNA
(miR)-9 was observed to regulate angiogenesis through targeting the
AMP-activated protein kinase pathway in osteoblasts (9); CCN family member 1 increased VEGF
expression levels in osteoblasts, subsequently increasing
angiogenesis (10). Nonetheless,
the mechanisms regulating the interaction between osteoblasts and
angiogenesis remain largely unknown.
Transforming growth factor β1 (TGF-β1) is a
multifunctional cytokine associated with tissue remodeling
processes, including bone regeneration and angiogenesis (10). A previous in vitro
angiogenesis study revealed that TGF-β1 induced the phosphorylation
of SMAD2 and enhanced VEGF signaling, which is required for
angiogenesis (11). Multiple
upstream or downstream factors can affect angiogenesis through
regulating the TGF-β1 pathway; for example, leucine-rich
α-2-glycoprotein 1 promoted angiogenesis through modulating TGF-β1
signaling (12); thrombospondin-4
expression in endothelial cells was observed to promote
TGF-β1-mediated effects on angiogenesis (13). TGF-β1 is also associated with
osteogenesis. It promoted osteo-induction through the PI3K/AKT/mTOR
signaling pathway and synergistically functioned with bone
morphogenetic protein 2 to promote the initiation and progression
of osteogenesis (14,15). Thus, because osteogenesis and
angiogenesis are both vital processes required for bone
regeneration, the TGF-β1/SMAD pathway may contribute to mandibular
and maxillary bone repair and regeneration through promoting both
osteogenesis and angiogenesis.
Specificity protein 1 (SP1) is a transcription
factor involved in numerous cellular processes, such as cell
differentiation and proliferation; it can directly interact with
DNA and enhance gene transcription (16). SP1 was also observed to interact
with SMAD and enhance TGF-β1 signaling to promote cartilage repair
in chondrocyte proliferation (17). Furthermore, the downregulation of
SP1 by miRNAs, such as miR-29c and miR-223 inhibited TGF-β1
signaling in lung cancer and gastric carcinoma (18,19).
SP1 also serves important roles in osteogenesis and angiogenesis;
SP1 regulates human osteoblast differentiation and mineralization
(20), and it is involved in the
regulation of bone metabolism through the frizzled-1 precursor and
peroxisome proliferator-activated receptor signaling pathways
(21). In osteosarcoma cells, the
downregulation of SP1 inhibited osteoblast differentiation
(22), and in terms of
angiogenesis, it was reported that SP1 functioned through the VEGF
and epidermal growth factor receptor/p38 signaling pathways to
promote angiogenesis in ovarian and pancreatic cancers (23,24).
Thus, it was hypothesized that SP1 could also promote bone
regeneration through promoting angiogenesis and osteogenesis in
mandibular and maxillary bones.
The present study aimed to reveal the regulatory
mechanisms of mandibular and maxillary bone regeneration. The
MC3T3-E1 cell line is a mouse embryonic osteoblast precursor cell
line that is widely used to study osteoblast differentiation
(25,26). Although the cell line does not
consist of preosteoblasts of mandibular or maxillary bones, it was
used in the present study due to its differentiating potential. It
was revealed that the overexpression of SP1 increased TGF-β1
expression levels, activated the TGF-β1/SMAD2 signaling pathway and
promoted VEGF secretion, which facilitated the angiogenesis of
preosteoblasts. These findings provided an improved understanding
of mandibular and maxillary bone regeneration, and may support
future studies aimed at developing novel therapeutic strategies for
patients that undergo mandibular and maxillary bone resection.
Materials and methods
Cell culture and reagents
All cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2. MC3T3-E1 preosteoblast
cells were purchased from American Type Culture Collection and
cultured in α-minimum essential medium supplemented with
ribonucleotides and deoxyribonucleosides (12571063; Gibco; Thermo
Fisher Scientific, Inc.), 2 mM L-glutamine (Gibco; Thermo Fisher
Scientific, Inc.) and 1 mM sodium pyruvate (Gibco; Thermo Fisher
Scientific, Inc.) and 10% FBS, but without ascorbic acid. HUVECs
were purchased from the Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences, and were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
(Gibco; Thermo Fisher Scientific, Inc.) and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). For TGF-β1 treatment,
cells were treated with 5 ng/ml TGF-β1 (Gibco PHG9214; Thermo
Fisher Scientific, Inc.) for 3 h directly or following pretreatment
with 5 µM TGF-β1 inhibitor SB431542 (Selleck Chemicals) for 30 min.
Control groups received vehicle treatment. Bevacizumab, an
anti-VEGF humanized antibody, was provided by Roche Diagnostics and
10 µg/ml was used to treat cells.
Cell transfection
Overexpression vector pcDNA3.1-SP1 (p-SP1) and its
negative control (NC; pcDNA3.1-NC), and small interfering RNA
(siRNA) of SP1 (si-SP1) and its negative control, si-NC, were
transfected into cells using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. For each transfection, 0.4 µg of plasmid
and 100 nM of siRNA were used. Cells were cultured continuously for
4 h at 37°C and the medium containing Lipofectamine®
2000 was then replaced by fresh medium. Subsequent experiments were
carried out 24 h post-transfection unless otherwise indicated. The
following siRNAs were synthesized by Sangon Biotech Co., Ltd.:
si-SP1, sense
5′-AACCCACTAACACTCGGTCTACTTCACGAGCAGCTCTGGGCTGCAGAGCC-3′, antisense
5′-TGAAGTAGACCGAGTGTTAGTGGGTTCGTCGCCCAGGGACAGGAAACAC-3′; si-NC,
sense 5′-ACGCGUAACGCGGGAAUUUdTdT-3′, antisense
5′-AAAUUCCCGCGUUACGCGUdTdT-3′.
Co-culture of MC3T3-E1 and HUVECs
For the indirect co-culture, HUVECs and MC3T3-E1
cells were indirectly co-cultured in the same well, but separated
using a 0.4 µm filter insert (12 mm in diameter; Merck KGaA). In
this co-culture system, each well had two chambers, consisting of
an outer chamber (24-multiwell plate) and an inner Millicell-CM
chamber. MC3T3-E1 cells (1×104) were seeded in the outer
chamber in medium (0.5 ml). Upon MC3T3-E1 cells reaching 50%
confluence, MC3T3-E1 was transfected or treated as indicated in the
figures, and in the inner chamber, a total of 2×104
HUVECs/well were seeded in 0.5 ml medium and the inserts containing
HUVECs were placed into the wells of MC3T3-E1 cells. In control
cultures, the cell inserts without MC3T3-E1 were placed in the
control wells.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells
(5×106) using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), being treated with 1 ml TRIzol
according to the manufacturer's protocol. A total of 1 µg RNA was
reverse transcribed into cDNA using the PrimeScript™ RT reagent kit
(cat. no. RR037A; Takara Biotechnology Co. Ltd.) according to the
manufacturer's protocol. qPCR was subsequently performed using a
96-well plate ABI Prism 7500 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and SYBR®
Premix Ex Taq II master mix (cat. no. RR820A; Takara Bio,
Inc.), according to the manufacturer's protocol. The following
primer pairs (Sangon Biotech Co., Ltd.) were used for the qPCR:
SP1: Forward 5′-TGGGTACTTCAGGGATCCAG-3′, reverse
5′-TGAGGCTCTTCCCTCACTGT-3′; TGF-β1: forward
5′-AGCCCGAAGCGGACTACTAT-3′, reverse 5′-TCCACATGTTGCTCCACACT-3′. The
following thermocycling conditions were used: 94°C for 60 sec and
40 cycles of 94°C for 5 sec, 60°C for 34 sec, and 72°C for 30 sec.
mRNA expression levels were quantified using the 2−∆∆Cq
method (27), and the expression
levels of were normalized to β-actin (forward
5′-CTCCATCCTGGCCTCGCTGT-3′, reverse
5′-GCTGTCACCTTCACCGTTCC-3′).
Western blotting
Total protein was extracted from cells
(107) using 1 ml RIPA buffer (Sigma-Aldrich; Merck KGaA)
and quantified using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocol. A
total of 30 µg protein was separated by 10% SDS-PAGE. The separated
proteins were transferred onto a nitrocellulose membrane and
subsequently blocked for 1 h at room temperature with TBS-Tween 20
(TBST; 20 mM Tris, 137 mM NaCl, 0.1% Tween-20, pH 8.0) containing
5% BSA (Sangon Biotech Co., Ltd.). The membranes were incubated
with the following primary antibodies (all 1:1,000; Cell Signaling
Technology, Inc.) overnight at 4°C: anti-SP1(5931), anti-VEGF
(2463), anti-SMAD2 (5339), anti-phosphorylated (p)-SMAD2 (18338),
anti-TGF-β1 (3711) and anti-GAPDH (5174). Membranes were washed 3
times (7 min each) with TBST, and incubated with 1:5,000 diluted
horseradish peroxidase-conjugated secondary antibodies (anti-mouse:
G-21040 and anti-rabbit: G-21234, both from Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Protein bands were
visualized using the Enhanced Chemiluminescence Western Blotting
Detection system (GE HealthCare Bio-Sciences), quantified using
ImageJ software (version 1.47, National Institutes of Health) and
normalized to GAPDH expression levels.
ELISA
Cells were analyzed for VEGF expression using the
VEGF Human ELISA kit (cat. no. KHG0111; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Briefly,
samples were mixed with diluent buffer supplied by the kit in a
ratio of 1:1 and 100 µl was used to in each well of the 96-well
plate overnight at 4°C. Following incubation, the plates were
rinsed and incubated with biotin conjugate at room temperature for
1 h. Streptavidin-HRP (100 µl; supplied by kit) was then incubated
in the plate at room temperature in dark. Plates were subsequently
rinsed three times with wash buffer and incubated with 100 µl of
stabilized chromogen (supplied by the kit) for 30 min at room
temperature in the dark. The reaction was stopped by adding 100 µl
stop solution to each well and the optical density was determined
using an Epoch-256600 plate reader at a wavelength of 450 nm
(BioTek Instruments, Inc.).
MTT assay
An MTT assay was used to analyze cell viability.
Cells (2,000 cells/well) were cultured in 96-well plates with 100
µl of growth medium. Following the various treatments, cells were
centrifuged (300 × g, 5 min, 4°C) and the supernatants discarded.
The pellet was rinsed with PBS once and 20 µl 5 mg/ml MTT was added
to each well and incubated for 4 h at 37°C. Following the
incubation, the culture medium was replaced by 150 µl DMSO and
subsequently vibrated gently for 10 min to dissolve the purple
formazan crystals. Cell viability was analyzed by assessing the
optical density (OD) using a microplate reader at a wavelength of
490 nm (BioTek Instruments, Inc.). The cell viability was
calculated with the following formula: Cell viability (%)=[OD490 nm
of treated group)/(OD490 of control group)] ×100. All experiments
were performed in triplicate.
Transwell migration assay
Transwell assay equipment (the chamber) was fitted
with 24-well plates to form a two-compartment system (an inner
chamber and an outer well). Cells in the logarithmic growth phase
were collected by centrifugation (300 × g, 5 min, 4°C), rinsed with
PBS and centrifuged again (300 g, 5 min, 4°C). Cells were washed
twice and resuspended in serum-free medium at a density of
1×106 cells/ml. A total of 100 µl cell suspension was
plated in the upper chambers of Transwell plates and 0.8 ml medium
supplemented with 5% fetal calf serum (Hyclone, GE Healthcare Life
Sciences) was plated in the lower chambers as a chemoattractant.
Following incubation at 37°C for 24 hours, the porous membrane was
isolated, fixed with 10% methyl alcohol for 30 sec, stained with
0.1% crystal violet for 20 min at room temperature and held between
histological slides. Migratory cells found in ≥20% of the filter
area were counted using the bright field optics of an microscope
(Leica Microsystems, Inc.) at magnification of ×100.
Angiogenesis assay
An angiogenesis assay was performed to detect the
angiogenic ability of HUVECs co-cultured with MC3T3-E1 cells using
an in vitro Angiogenesis Assay kit (cat. no. ab204726;
Abcam), according to the manufacturers' protocol. Briefly, 50 µl
thawed extracellular matrix (ECM) solution was added to each well
of a pre-chilled (on ice) white 96-well sterile cell culture plate
and incubated for 1 h at 37°C to allow the solution to form a gel.
A total of 2×104 cells/well were plated in 100 µl media
onto the solidified ECM gel or control wells (no ECM gel or ECM
wells with Suramin). Angiogenesis factors/regulators (such as TGF-β
inhibitor, bevacizumab and supernatants of MC3T3-E1) were
subsequently added to the desired wells as indicated in each
figure, and cells were incubated cells for 18 h in a 37°C incubator
containing 5% CO2. Media was removed and wells were
washed with 100 µl wash buffer to remove the serum. A total of 100
µl staining dye working dilution (1:200) was added to each well and
incubated for 30 min at 37°C. Endothelial tube formation was
analyzed using a Leica DMI6000B light and fluorescence microscope
(Leica Microsystems, Inc.) using a green filter (magnification
×100).
Dual-luciferase reporter assay
The firefly luciferase reporter plasmid (pG5 luc)
and constitutively active Renilla luciferase control plasmid
(pRL-Renilla) were purchased from Promega Corporation.
TGF-β1 Promoter was cloned onto the firefly luciferase reporter
plasmid (pG5-TGF-β1-luc). 293T cells (obtained from American Type
Culture Collection) were seeded in a 24 well plate
(2×105 cells/well) and transfected with the plasmids
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, at 50%
confluency. Following incubation at 37°C for 48 h, cells were
collected and the firefly and Renilla luciferase activities
were detected using a Dual-Luciferase Reporter assay system
(Promega Corporation), according to the manufacturer's protocol.
Briefly, cells were lysed with lysis buffer for 20 min at room
temperature and 100 µl supernatant was subsequently transferred
into luminometer tubes, mixed with 20 µl luciferase assay reagent
and signals were detected on a GloMax20/20 luminometer (Promega
Corporation). Relative luciferase intensity was normalized to the
Renilla signal.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7 (GraphPad Software, Inc.) and Microsoft Excel 2003
(Microsoft Corporation); data are presented as the mean ± SD.
Statistical significance between groups was determined using a
one-way ANOVA with Tukey's post hoc analysis or a Student's t-test.
Experiments were repeated ≥3 times and all experiments were
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
SP1 promotes angiogenesis in
preosteoblasts
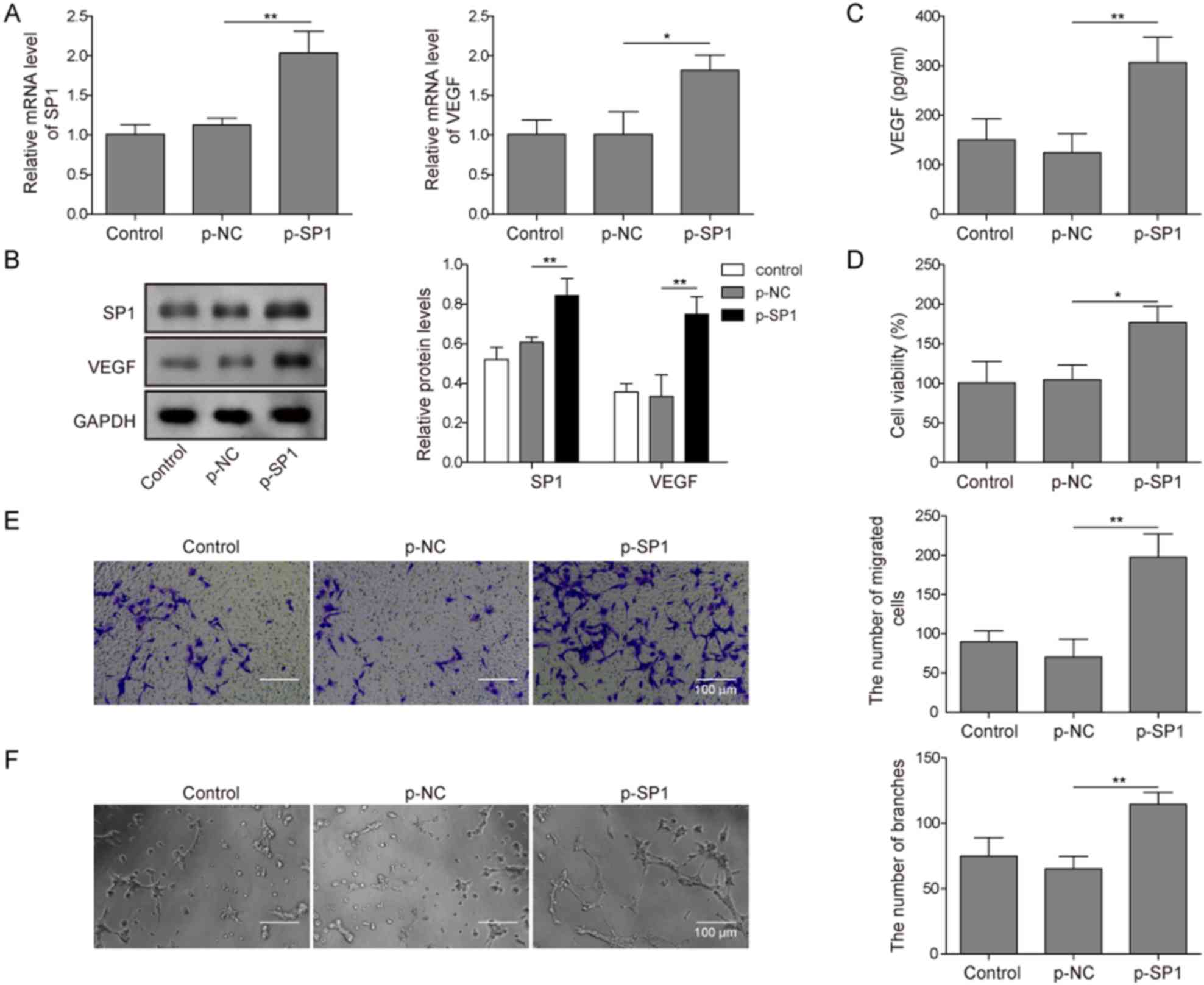
SP1 was overexpressed in MC3T3-E1 cells to detect
its effect on angiogenesis-related processes, such as migration and
proliferation. In addition, due to angiogenesis being regulated by
VEGF, the function of SP1 on VEGF expression was also assessed.
Successful p-SP1 transfection of MC3T3-E1 cells was determined by
RT-qPCR, which demonstrated that p-SP1-transfected cells expressed
significantly higher levels of SP1 compared with the
p-NC-transfected cells (Fig. 1A).
In addition, p-SP1-transfeced cells had significantly higher mRNA
expression levels of VEGF compared with the p-NC-transfected cells
(Fig. 1A). Similar results were
observed in the protein expression levels of SP1 and VEGF following
transfection; the expression levels of SP1 and VEGF were
significantly higher in p-SP1-transfected MC3T3-E1 cells compared
with p-NC-transfected cells (Fig.
1B). The concentration of secreted VEGF was also significantly
higher in p-SP1-transfected cells compared with p-NC-transfected
cells (Fig. 1C). HUVECs indirectly
co-cultured with p-SP1-transfected MC3T3-E1 cells demonstrated
significantly higher cell viability compared with HUVECs
co-cultured with p-NC-transfected MC3T3-E1 cells (Fig. 1D). A Transwell assay was used to
detect the migratory ability HUVECs co-cultured with MC3T3-E1
cells. HUVECs co-cultured with p-SP1-transfected MC3T3-E1 cells had
significantly higher migratory ability compared with HUVECs
co-cultured with p-NC-transfected MC3T3-E1 cells (Fig. 1E). A similar trend was observed in
the angiogenic ability of HUVECs co-cultured with p-SP1-transfected
MC3T3-E1 cells, which was significantly higher compared with
p-NC-transfected cells (Fig. 1F).
These findings suggested that SP1 overexpression may enhance VEGF
expression and secretion of MC3T3-E1, and promote the migration,
proliferation and angiogenesis of HUVEC cells.
TGF-β1 promotes the angiogenesis of
preosteoblasts by activating SMAD2 phosphorylation
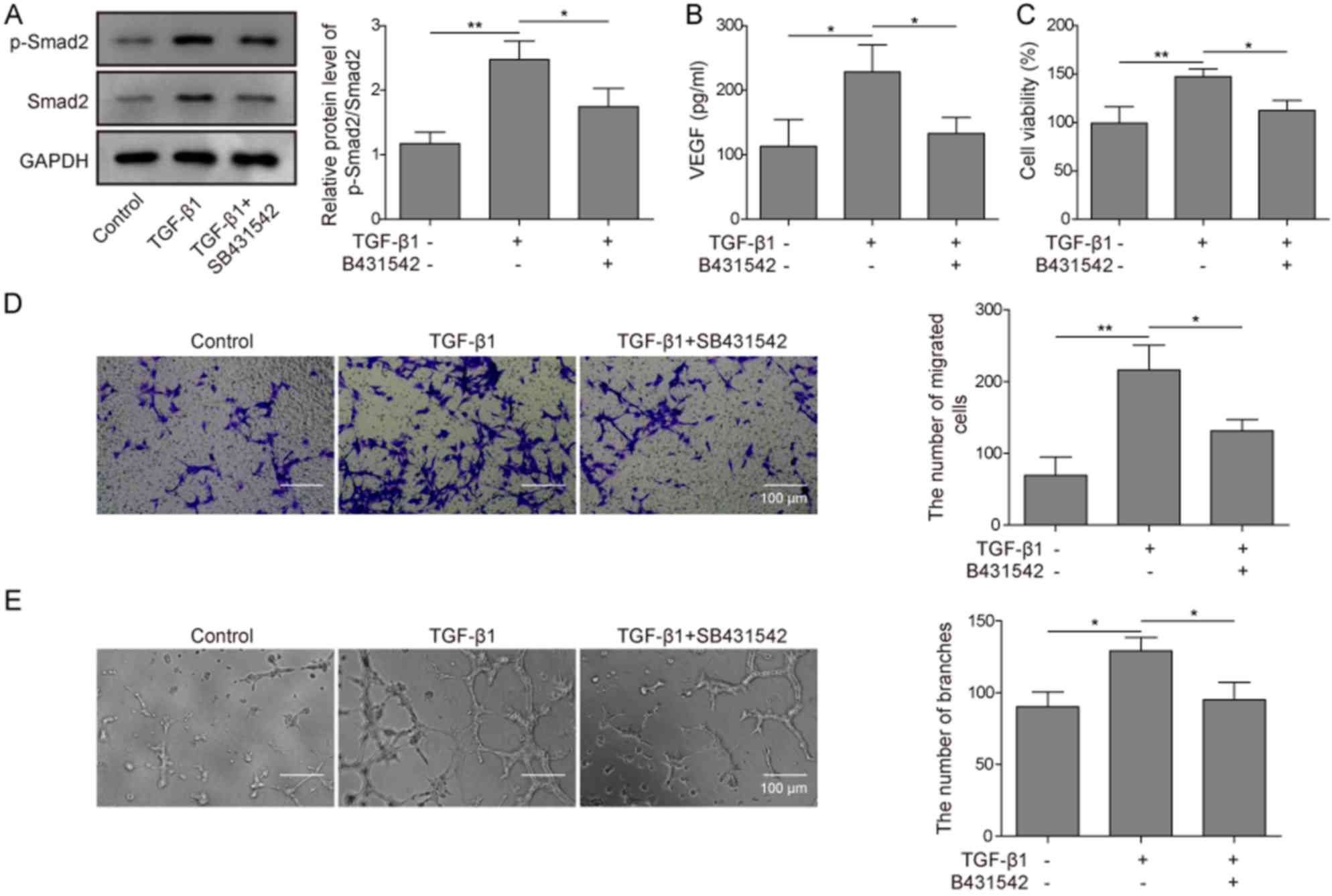
Whether TGF-β1 could affect angiogenesis in
preosteoblasts was investigated by determining whether TGF-β1 could
activate SMAD2 signaling in MC3T3-E1 cells. In MC3T3-E1 cells,
TGF-β1 treatment significantly increased the ratio of
phosphorylated vs. total SMAD2 protein levels compared with the
control (Fig. 2A). This effect was
significantly reversed in MC3T3-E1 cells pretreated with the TGF-β1
inhibitor SB431542. To evaluate whether TGF-β1 could affect VEGF
secretion, an ELISA assay was used. TGF-β1 treatment significantly
increased the VEGF concentration in the medium compared with the
control, and such function of TGF-β1 was significantly abolished
through pretreating cells with SB431542 (Fig. 2B). TGF-β1 treatment of HUVECs
co-cultured with MC3T3-E1 cells significantly increased viability,
migration and angiogenic potential of HUVECs compared with the
control, whereas SB431542 pretreatment significantly inhibited such
functions compared with TGF-β1-treated cells (Fig. 2C-E). These findings suggested that
TGF-β1 may activate the SMAD2 signaling pathway and promote VEGF
secretion of preosteoblasts, which promoted the angiogenesis of
co-cultured HUVECs.
SP1 promotes TGF-β1 expression
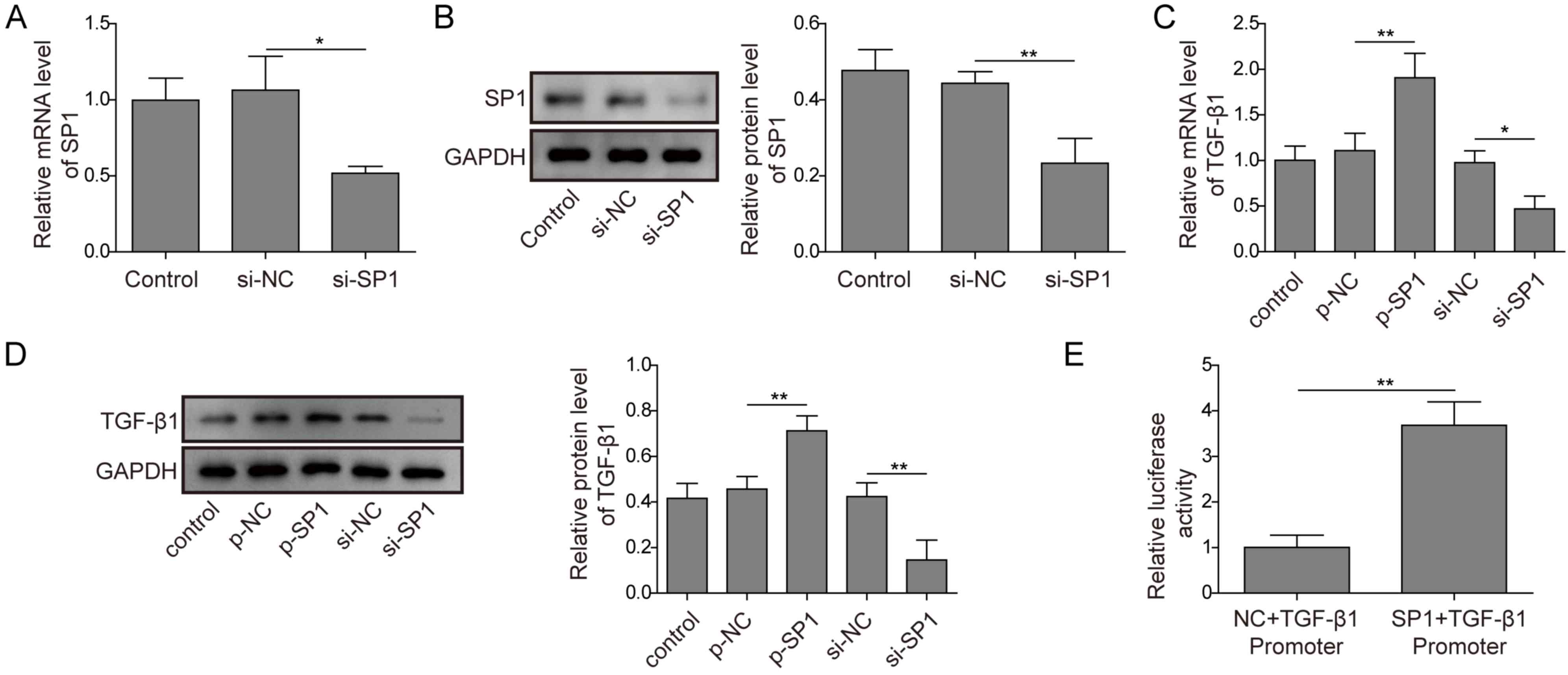
The regulatory network between SP1 and TGF-β1 in
preosteoblasts is unclear. RT-qPCR and western blotting analysis
was used to detect the silencing efficiency of si-SP; cells
transfected with si-SP1 demonstrated significantly decreased mRNA
and protein expression levels of SP1 compared with
si-NC-transfected cells (Fig. 3A and
B), which indicated that the siRNA vector was successfully
transfected. The mRNA and protein expression levels of TGF-β1 in
MC3T3-E1 cells with transfected with p-SP1 or si-SP1 were
significantly increased and decreased, respectively, compared with
their respective controls (Fig. 3C and
D). A dual-luciferase reporter assay was used to validate the
regulatory relationship between SP1 and TGF-β1. SP1 overexpression
significantly enhanced TGF-β1 luciferase activity compared with the
NC (Fig. 3E). These findings
demonstrated that SP1 may directly interact with the TGF-β1
enhancer and promote TGF-β1 expression.
SP1 promotes angiogenesis in
preosteoblasts through activating the TGF-β1/SMAD2 signaling
pathway
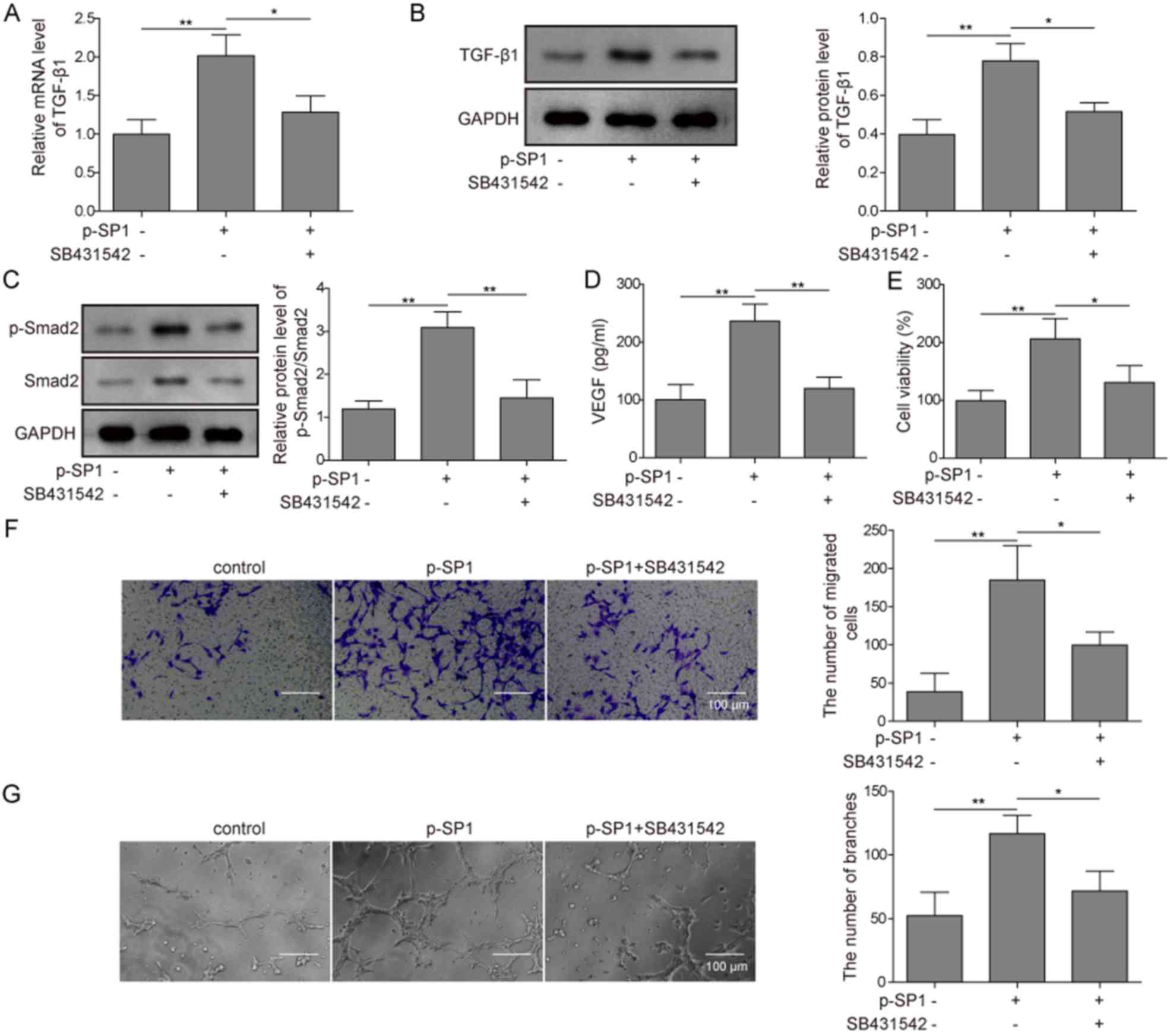
The transcription factor SP1 can regulate the
expression of numerous proteins, thus it was investigated whether
SP1 could promote angiogenesis exclusively through the TGF-β1/SMAD2
signaling pathway. In MC3T3-E1 cells, p-SP1-transfected cells
demonstrated significantly increased mRNA expression levels of
TGF-β1 compared with the control group; however, the addition of
the TGF-β1 inhibitor SB431542 significantly attenuated such effect,
with expression levels remaining non-significant from the control
group (Fig. 4A). Similar results
were observed by western blotting; protein expression levels of
TGF-β1 were significantly increased in p-SP1-transfected cells
compared with the control group, while no significant difference
compared with the control was observed in p-SP1-transfected cells
pretreated with SB431542 (Fig.
4B). The overexpression of SP1 also significantly increased
both p-SMAD and total SMAD2 protein expression levels (and the
ratio of p-SMAD vs. SMAD2) compared with the control group, whereas
the cells also pretreated with SB431542 demonstrated significantly
reduced expression levels of phosphorylated and total SMAD2 (and
the ratio of p-SMAD2 vs. SMAD2) to similar levels as the control
(Fig. 4C). p-SP1-transfected cells
significantly increased the secretion of VEGF compared with the
control, while pretreating cells with SB431542 significantly
inhibited such function (Fig. 4D).
HUVECs subsequently co-cultured with MC3T3-E1 cells overexpressing
SP1 significantly increased the viability, migratory and angiogenic
potential of HUVECs compared with the control group. These
functions were all significantly inhibited by SB431542 (Fig. 4E-G). These results indicated that
SP1 may promote the angiogenesis of preosteoblasts through
targeting the TGF-β1/SMAD2 signaling pathway.
SP1/TGF-β1/SMAD2 signaling pathway
promotes preosteoblast angiogenesis through regulating VEGF
expression
The TGF-β1/SMAD2 signaling pathway regulates the
expression of numerous proteins. Given that angiogenesis is
stimulated by VEGF, the present study aimed to investigate whether
the TGF-β1/SMAD2 pathway promoted the angiogenesis of
preosteoblasts through VEGF. HUVECs were co-cultured with
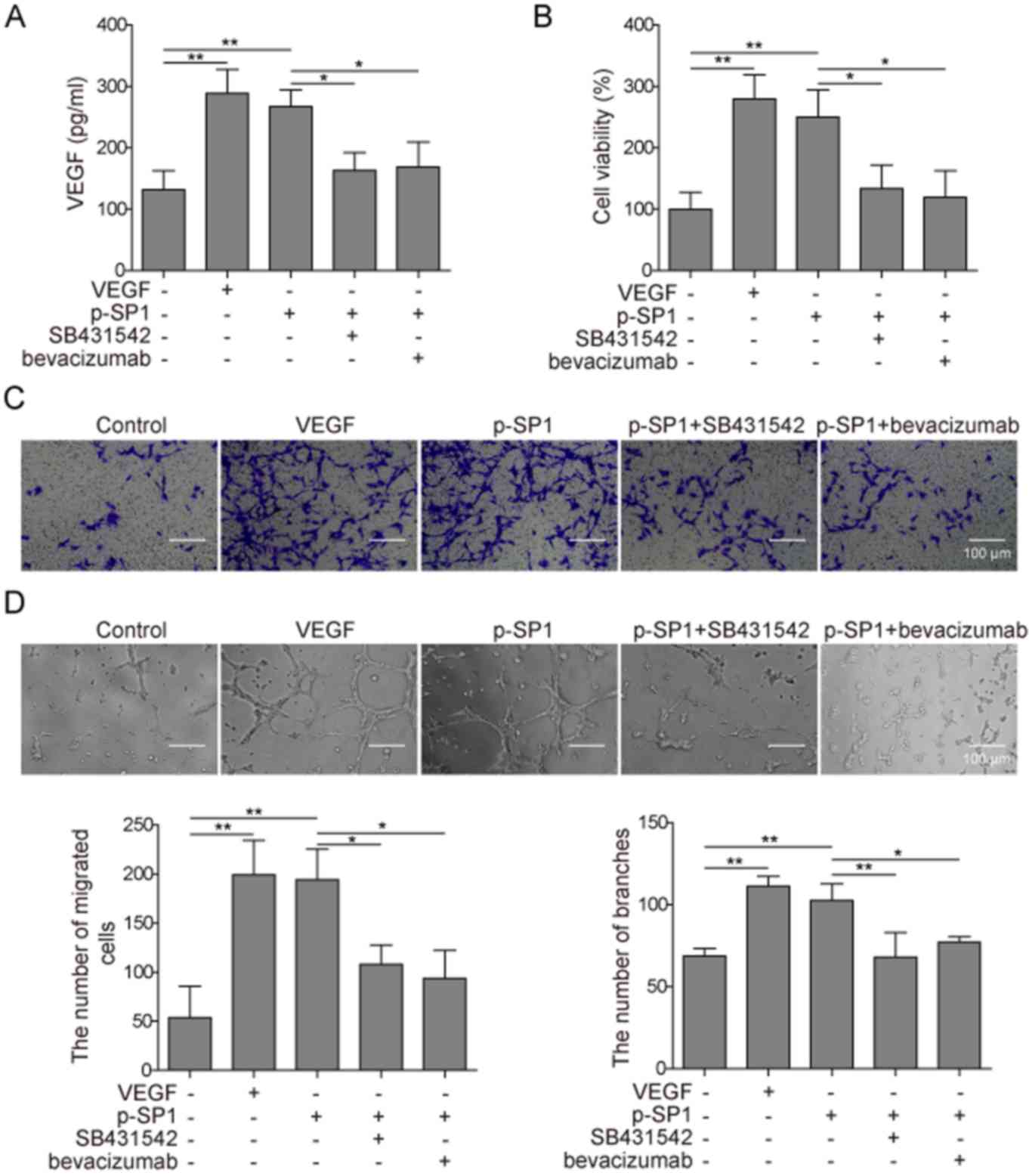
p-SP1-transfected MC3T3-E1 cells and treated with SB431542 or
bevacizumab. Compared with the control group, the expression levels
of secreted VEGF were increased in p-SP1-transfected and
VEGF-treated cells; however, pretreatment of cells with SB431542 or
bevacizumab inhibited the increased VEGF expression induced by
p-SP1 (Fig. 5A). These results
indicated that the SP1 signaling pathway in MC3T3-E1 cells could
promote VEGF expression in HUVECs. p-SP1-transfected and
VEGF-positive cells significantly increased the cell viability
compared with control group, whereas the pretreatment of cells with
SB431542 or bevacizumab inhibited such function (Fig. 5B). The migratory ability of cells
was detected by the Transwell assay; compared with the control
group, p-SP1-transfected and VEGF-positive MC3T3-E1 cells
significantly increased the migratory ability of HUVECs compared
with the control group, whereas SB431542 or bevacizumab
pretreatment significantly inhibited such function (Fig. 5C). Finally, with regards to
angiogenesis, p-SP1-transfected and VEGF-positive MC3T3-E1 cells
significantly increased the process of angiogenesis in HUVECs
compared with the control group, whereas pretreatment with SB431542
or bevacizumab exerted similar rates of angiogenesis as the
control, with no significant differences observed (Fig. 5D). These findings indicated that
SP1 may promote preosteoblast angiogenesis through regulating VEGF
expression.
Discussion
Angiogenesis serves an important role in bone
regeneration (5); however, the
regulatory mechanism is largely unknown, particularly the
relationship between VEGF and TGF signaling pathways, both of which
are associated with angiogenesis (10,11).
The present study revealed that SP1 may activate the TGF-β1/SMAD2
signaling pathway and promote VEGF secretion through TGF-β1,
facilitating angiogenesis in preosteoblasts. The function of SP1
varies according to the differential genetic and expression
profiles among different cell types. To the best of our knowledge,
this study is the first to demonstrate that SP1 may promote
angiogenesis in preosteoblasts, which is consistent with previous
findings in ovarian and pancreatic cancer (23,24).
In these studies, SP1 mainly promoted angiogenesis through the
EGFR/p38 signaling pathway; however, the present study demonstrated
that SP1 promotes angiogenesis in preosteoblasts through the
activation of the TGF-β/SMAD2 signaling pathway. This difference
may largely due to the variety in genetic and expressional profiles
among tissue and cell types. In addition, both pathways might
contribute to angiogenesis. This result also extended our
understanding of the regulation mechanism of angiogenesis.
SP1 may directly target and regulate TGF-β1 in
preosteoblasts; the association between SP1 and TGF-β1 in the
present study agreed with previous reports in other cell types,
such as chondrocytes, lung cancer cells and gastric carcinoma cells
(17,18), where SP1 genetic knockdown affected
TGF-β1 expression. However, whether there was a direct relationship
between SP1 and TGF-β1 signaling was not explored. To the best of
our knowledge, the present study is the first confirmatory study of
the direct regulatory effect of SP1 on TGF-β1 in preosteoblasts, as
demonstrated by the dual-luciferase reporter assay. Through the
TGF-β1 signaling pathway, SP1 promoted the secretion of VEGF and
increased levels of VEGF in the supernatant could promote HUVECs to
undergo angiogenesis. This notion is supported by previous studies
in multiple other cell types that the signals of the VEGF pathway
were closely associated with angiogenesis, migration and
proliferation (11,28). In the current study, it was
revealed that VEGF is under the regulation of the TGF-β1/SMAD
signaling pathway in preosteoblasts, which is similar to findings
reported in osteoblasts (11,14).
Upon considering previous studies citing association of VEGF and
TGF-β1 with angiogenesis in other cell types (7,13),
it was concluded from this study that SP1 may directly promote
TGF-β1 expression, activate the SMAD2 pathway and promote VEGF
expression and secretion, with secreted VEGF directly promoting the
angiogenesis of preosteoblasts. It was also noted that although
SB431542 should not affect the mRNA level of TGF-b1, under the
combined effect of SP1 overexpression vector and SB431542, the mRNA
level of TGF-b1 might be decreased. This could be a feed-back
regulation of the influence of osteoblast angiogenesis and other
changes in the microenvironment.
In conclusion, the present study revealed a direct
regulatory relationship between SP1 and TGF-β1, and uncovered its
effect on angiogenesis in preosteoblasts. SP1 may stimulate the
TGF-β1/SMAD2 signaling pathway to promote VEGF secretion and
facilitate angiogenesis. These effects are closely related with
bone regeneration and repair. Thus, the results from this study may
provide a theoretical basis for understanding the regulatory
mechanisms of angiogenesis in preosteoblast cells and bone repair,
and provide support for the development of novel treatment options
for bone regeneration and repair in the future.
Acknowledgements
Not applicable.
Funding
This study was supported by The Anhui Provincial
Natural Science Foundation (grant no. 1608085MH237).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
AD and ZHZ designed the study. AD and YYB acquired
and interpreted the data. AD prepared the manuscript and supervised
the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Okoturo E, Ogunbanjo OV and Arotiba GT:
Spontaneous regeneration of the mandible: An institutional audit of
regenerated bone and osteocompetent periosteum. J Oral Maxillofac
Surg. 74:1660–1667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gornitsky J, Azzi AJ and Cugno S:
Spontaneous osteogenesis of a traumatic mandibular defect in the
pediatric population. J Craniofac Surg. 30:1999–2000. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grosso A, Burger MG, Lunger A, Schaefer
DJ, Banfi A and Di Maggio N: It takes two to tango: Coupling of
angiogenesis and osteogenesis for bone regeneration. Front Bioeng
Biotechnol. 5:682017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dickson K, Katzman S, Delgado E and
Contreras D: Delayed unions and nonunions of open tibial fractures.
Correlation with arteriography results. Clin Orthop Relat Res.
302:189–193. 1994.
|
|
5
|
Hankenson KD, Dishowitz M, Gray C and
Schenker M: Angiogenesis in bone regeneration. Injury. 42:556–561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramasamy SK, Kusumbe AP, Itkin T,
Gur-Cohen S, Lapidot T and Adams RH: Regulation of hematopoiesis
and osteogenesis by blood vessel-derived signals. Annu Rev Cell Dev
Biol. 32:649–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frohlich LF: Micrornas at the interface
between osteogenesis and angiogenesis as targets for bone
regeneration. Cells. 8:E1212019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang B, Wang W, Li Q, Wang Z, Yan B,
Zhang Z, Wang L, Huang M, Jia C, Lu J, et al: Osteoblasts secrete
Cxcl9 to regulate angiogenesis in bone. Nat Commun. 7:138852016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qu J, Lu D, Guo H, Miao W, Wu G and Zhou
M: MicroRNA-9 regulates osteoblast differentiation and angiogenesis
via the AMPK signaling pathway. Mol Cell Biochem. 411:23–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CY, Su CM, Hsu CJ, Huang CC, Wang SW,
Liu SC, Chen WC, Fuh LJ and Tang CH: CCN1 promotes VEGF production
in osteoblasts and induces endothelial progenitor cell angiogenesis
by inhibiting miR-126 expression in rheumatoid arthritis. J Bone
Miner Res. 32:34–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jarad M, Kuczynski EA, Morrison J,
Viloria-Petit AM and Coomber BL: Release of endothelial cell
associated VEGFR2 during TGF-β modulated angiogenesis in vitro. BMC
Cell Biol. 18:102017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Abraham S, McKenzie JAG, Jeffs N,
Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, et
al: LRG1 promotes angiogenesis by modulating endothelial TGF-β
signalling. Nature. 499:306–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muppala S, Xiao R, Krukovets I,
Verbovetsky D, Yendamuri R, Habib N, Raman P, Plow E and
Stenina-Adognravi O: Thrombospondin-4 mediates TGF-β -induced
angiogenesis. Oncogene. 36:5189–5198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zhang X, Zhao D, Liu B, Wang B,
Yu W, Li J, Yu X, Cao F, Zheng G, et al: TGF -β1 promotes the
osteoinduction of human osteoblasts via the PI3K/AKT/mTOR/S6K1
signalling pathway. Mol Med Rep. 19:3505–3518. 2019.PubMed/NCBI
|
|
15
|
Asparuhova MB, Caballé-Serrano J, Buser D
and Chappuis V: Bone-Conditioned medium contributes to initiation
and progression of osteogenesis by exhibiting synergistic
TGF-β1/BMP-2 activity. Int J Oral Sci. 10:202018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Xu B and Xu H: TGF-β1 promoted
chondrocyte proliferation by regulating Sp1 through MSC-exosomes
derived miR-135b. Cell Cycle. 11:172018.
|
|
17
|
Zhang HW, Wang EW, Li LX, Yi SH, Li LC, Xu
FL, Wang DL, Wu YZ and Nian WQ: A regulatory loop involving miR-29c
and Sp1 elevates the TGF-β 1 mediated epithelial-to-mesenchymal
transition in lung cancer. Oncotarget. 7:85905–85916.
2016.PubMed/NCBI
|
|
18
|
Hu J, Shan Z, Hu K, Ren F, Zhang W, Han M,
Li Y, Feng K, Lei L and Feng Y: MiRNA-223 inhibits
epithelial-mesenchymal transition in gastric carcinoma cells via
Sp1. Int J Oncol. 49:325–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Yerges-Armstrong LM, Chu Y, Zmuda JM
and Zhang Y: Transcriptional regulation of frizzled-1 in human
osteoblasts by Sp1. PLoS One. 11:e01632772016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duttenhoefer F, Biswas SK, Igwe JC,
Sauerbier S and Bierhaus A: Sp1-Dependent regulation of PPARα in
bone metabolism. Int J Oral Maxillofac Implants. 29:e107–e116.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim J, Lee HW, Rhee DK, Paton JC and Pyo
S: Pneumolysin-induced autophagy contributes to inhibition of
osteoblast differentiation through downregulation of Sp1 in human
osteosarcoma cells. Biochim Biophys Acta Gen Subj. 1861:2663–2673.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su F, Geng J, Li X, Qiao C, Luo L, Feng J,
Dong X and Lv M: Bsp1 promotes tumor angiogenesis and invasion by
activating VEGF expression in an acquired trastuzumabresistant
ovarian cancer model. Oncol Rep. 38:2677–2684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu H, Han T, Zhuo M, Wu LL, Yuan C, Wu L,
Lei W, Jiao F and Wang LW: Elevated COX-2 expression promotes
angiogenesis through EGFR/p38-MAPK/Sp1-dependent signalling in
pancreatic cancer. Sci Rep. 7:4702017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Fan F, Shi P, Tu M, Yu C, Yu C and
Du M: Lactoferrin promotes MC3T3-E1 osteoblast cells proliferation
via MAPK signaling pathways. Int J Biol Macromol. 107:137–143.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhai F, Song N, Ma J, Gong W, Tian H, Li
X, Jiang C and Wang H: FGF18 inhibits MC3T3E1 cell osteogenic
differentiation via the ERK signaling pathway. Mol Med Rep.
16:4127–4132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Zhang Q, Charles EB, Wu Y, Zhang
L, Dong J and Han Y: VEGF overespression promotes the proliferation
and differentiation of human adipose-derived stem cells. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 33:352–356. 2017.(In Chinese).
PubMed/NCBI
|
|
28
|
Bhattacharya R, Fan F, Wang R, Ye X, Xia
L, Boulbes D and Ellis LM: Intracrine VEGF signalling mediates
colorectal cancer cell migration and invasion. Br J Cancer.
117:848–855. 2017. View Article : Google Scholar : PubMed/NCBI
|