Introduction
Rheumatoid arthritis (RA) is an autoimmune disease
characterized by chronic inflammation of joints, leading to
progressive and irreversible joint destruction (1,2). The
major events in RA are the hyperplasia of the synovial tissue,
which leads to the formation of a pannus, and the release of
proinflammatory cytokines (3). The
aggressive pannus invades and destroys cartilage and bone,
therefore playing a vital role in the pathogenesis of RA (3,4). The
pannus is mainly comprised of fibroblast-like synoviocytes (FLSs)
and infiltration of lymphocytes and macrophages (5).
FLSs serve an essential role in the progression of
RA, important components of the invasive hypertrophied pannus
(6). The phenotype of FLSs in RA
patients are similar to the phenotype of tumor cells, demonstrating
pro-migratory and pro-invasive properties, which result in joint
cartilage and bone destruction (6–8).
Several cytokines destroy joints by activating proliferation and
migratory pathways of FLS; for example, tumor necrosis factor (TNF)
receptor-associated factor six can promote the migration capacity
of FLSs in patients with RA (9).
Transcription factor SRY-Box 5 (SOX5) is involved in joint
destruction in collagen-induced arthritis (CIA) animal models, as
SOX5 can increase the migration and invasion of FLSs (10). Interferon-γ can stimulate the
differentiation of FLSs into immune effector cells in Lyme
arthritis, an autoimmune disease like RA (11). Therefore, investigating the
mechanisms underlying the migration and invasion of FLSs is crucial
to understand RA pathogenesis, and for the development of
therapeutics for RA.
Accumulating evidence demonstrated that monocyte
chemoattractant protein 1 (MCP-1) is highly expressed in the joints
of patients suffering from RA (12–15).
MCP-1 is a chemokine, and has been detected in endothelial cells,
epithelial cells, fibroblasts, monocyte-macrophages and vascular
smooth muscle cells (16). MCP-1
serves a major role in inflammation by attracting monocytes and
macrophages (16). A previous
study assessing 92 subjects showed that MCP-1 was higher among
patients who developed RA compared with controls (17). A previous study using a CIA animal
model suggested that MCP-1 can destroy joints via the recruitment
of monocytes (18). Furthermore,
the inhibition of MCP-1 with P8A-MCP-1, after the onset of
arthritis, ameliorated joint inflammation and decreased macrophage
accumulation, cytokine expression and p38MAPK activation (19).
Although MCP-1 is critical to the development and
progression of arthritis, it is unclear whether MCP-1 destroys
joint by changing the function of FLSs, including their
proliferation, invasion and differentiation potential. MCP-1 has
the potential to change the phenotype of many cells, such as their
invasion capacity and differentiation potentials (20–22).
MCP-1 was found to stimulate the proliferation and migration
capacity of renal carcinoma cells (20). Additionally, MCP-1 induced in the
herniated nucleus pulposus was identified to enhance
osteoclastogenesis in the vertebral column, resulting in increased
bone erosion (22). Furthermore,
MCP-1 can mediate angiogenesis and tumor progression via vascular
endothelial growth factor (VEGF) in vitro and in vivo
(21). Targeted inhibition of
MCP-1 decreases the development and mobilization of endothelial
precursor cells, thereby blocking tumor neovascularization of
mammary tumors (23).
Since MCP-1 plays an important role in the migration
and invasion of tumor cells, in the present study it was
hypothesized that MCP-1 may affect the phenotype of FLSs and
subsequently participate in the hypertrophy and angiogenesis of the
pannus during joint destruction in RA. Therefore, the aims of the
present study were as followed: i) To investigate the effect of
MCP-1 on the proliferation and migration of FLSs; ii) to
investigate the effect of MCP-1 on the differentiation potential of
FLSs towards endothelial cells; and iii) to investigate the
signaling pathways downstream of MCP-1 affecting the cell
proliferation, migration and differentiation of FLSs.
Materials and methods
Induction of collagen-induced
arthritis (CIA) model
All experiments were carried out following the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health and all the procedures were pre-approved by
the Review Board of the First Affiliated Hospital of Medical
College of Zhejiang University. Male Sprague-Dawley rats (n=14;
age, 8 weeks; weight, 250 g) were purchased from Zhejiang
University. The rats were housed under standardized conditions with
a 12-h light-dark cycle at 25°C and 45% humidity, with free access
to sterilized water and pellet food. CIA was induced by
immunization with bovine type II collagen (cat. no. 20021;
Chondrex, Inc.). Rats were anesthetized using 1% sodium
pentobarbital (40 mg/kg) via intraperitoneal injection prior to the
establishment of CIA. Type II collagen was solubilized in 0.05 M
acetic acid to a concentration of 4 mg/ml. Using an electric
homogenizer, collagen II was emulsified with equal volume of
incomplete Freund's adjuvant (Chondrex, Inc.). CIA model rats were
established by immunization with 0.1 ml emulsion subcutaneously
injected into the tail base at day 0 and day 7. Rats injected with
vehicle (adjuvant alone) were used as controls (n=7).
Isolation and identification of
FLSs
FLSs were isolated from synovium samples obtained
from CIA rats. The rats were anesthetized using 1% sodium
pentobarbital (40 mg/kg) via intraperitoneal injection, and then
euthanatized by cervical vertebra dislocation before FLSs
isolation. FLSs were obtained by 0.2% collagenase digestion at 37°C
for 4 h. The synovium tissue was washed five times on ice with PBS
containing 1% ampicillin and streptomycin. The synovium tissue was
then sectioned into 1-mm-thick slices which were placed in a plate
containing 0.2% type II collagenase (cat. no. 17101015; Gibco;
Thermo Fisher Scientific, Inc.) and then transferred to a 37°C
incubator for 4 h. Over the process, the supernatant was collected
every 60 min and centrifuged at 290 × g for 5 min at room
temperature to collect the cell pellet. These procedures were
repeated four times. The cells were re-suspended in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) complete culture medium containing
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), and 100 U/ml of
ampicillin and streptomycin. The cells were then filtered through
200-mesh stainless steel filters and seeded in flasks at the
density of 1×105 cells/cm2 and cultured at
37°C in 5% CO2 incubator for 3–4 days. Upon reaching 90%
confluence, the cells were passaged. Cells at passage two were used
for further experiments. For ELISA, western blotting, and reverse
transcription-quantitative PCR (RT-qPCR) experiments, FLSs were
seeded in 6-well plates at 2×105 cells/well.
ELISA
The content of MCP-1 in the synovial tissue of CIA
rats was measured using a rat MCP-1 ELISA kit (cat. no. ab100778;
Abcam), according to the manufacturer's protocol. The synovial
tissue in all samples were performed in duplicate.
Cell Counting Kit-8 (CCK-8) assay
FLSs were plated at a density of 5×103
cells/well in a 96-well plate with 0.1 ml complete DMEM for 24 h,
and then starved for 24 h in serum-free DMEM medium. MCP-1 (cat.
no. 279-MC-010; R&D Systems, Inc.) was added (at final
concentration of 1, 10, 100, 200, 500 and 1,000 ng/ml) with or
without 100 ng/ml TNF-α (cat. no. 210-TA-005; R&D Systems,
Inc.). The cells were incubated for an additional 48 h at 37°C and
then counted using a CCK-8 kit (cat. no. CK04-11; Dojindo Molecular
Technologies, Inc.). A total of 10 µl kit reagent was added to each
well, and the cells were incubated for 2 h at 37°C. Cell viability
was determined by measuring the absorbance at 450 and 655 nm with a
microplate reader (Bio-Rad Laboratories, Inc.). Each experimental
condition was assessed in four wells. To confirm that P38 kinase
and PI3K play a role in MCP-1-induced proliferation, FLSs were
pretreated with PI3K inhibitor LY294002 [LY; cat. no. 9901; Cell
Signaling Technology, Inc. (CST)] and P38 inhibitor SB203580 (SB;
cat. no. 5633; CST; final concentrations, 10, 50 and 100 µM) for 1
h and then stimulated with 200 ng⁄ml MCP-1.
5-Bromo-2-deoxyuridine (BrdU)
assay
The proliferation of FLSs was also assessed using a
BrdU cell proliferation ELISA kit (cat. no. ab126556; Abcam). FLSs
were treated in the same manner as described in the CCK-8
experiments. BrdU was added to FLSs and then incubated for 2 h at
37°C to incorporate it into their DNA. Paraformaldehyde (4%) was
added for 10 min to fix the cells at 4°C and denature the DNA. The
solution was then removed and each well washed with PBS three
times. The primary detector antibody in the kit was added to each
well and incubated at room temperature for 1 h. The cells were
washed again with PBS three times. A horseradish peroxidase
(HRP)-conjugated secondary antibody included in the kit was added
to each well and incubated at room temperature for 30 min.
3,3′,5,5′-tetramethylbenzidine solution was added to each well
after washing. Stop solution was added after the detection of the
signal, and the signal intensity was recorded immediately by
measuring the absorbance at 450 and 655 nm with a microplate reader
(Bio-Rad Laboratories, Inc.). Each experimental condition was
assessed in four wells.
Transwell assay
FLSs migration ability was evaluated using 24-well
chambers (pore size, 8 mm; Corning, Inc.). MCP-1 (200 ng/ml), with
or without PI3K and P38 inhibitor (final concentrations, 50 µM),
was added to the supernatant. FLSs (1×104 cells/well)
were seeded in the upper chamber coated with Matrigel, while the
lower chamber was filled with 500 µl DMEM medium containing 10%
FBS. FLSs that remained on the top of the filters were removed with
a cotton swab after 12 h of incubation at 37°C. Cells that invaded
the Matrigel to the lower chamber of membrane were fixed with 4%
paraformaldehyde at 4°C for 20 min and then stained with crystal
violet (cat. no. C0121; Beyotime Institute of Biotechnology) at
room temperature for 20 min. The number of migrated cells were
manually counted in three random fields.
Western blotting
After MCP-1 stimulation for 0, 15, 30 and 60 min,
the total protein was extracted from each group using RIPA lysis
buffer (Beyotime Institute of Biotechnology), according to the
manufacturer's instructions (24).
For the determination of CD31 and VEGF, the cells were incubated
with MCP-1 for 48 h at 37°C. Protein concentrations were determined
using a bicinchoninic acid assay and equal amounts of protein
(20–30 µg) were resolved by SDS-PAGE (10 or 12%). Immunoblot
analysis was performed as described previously (25). PVDF membranes were incubated with
primary antibodies overnight at 4°C after blocking with 5% non-fat
milk for 2 h at room temperature. The primary antibodies used were
the following: Phosphorylated-(p-)P38 (1:1,000; cat. no. 4511;
CST), P38 (1:1,000; cat. no. 2387; CST), p-PI3K (1:2,000; cat. no.
ab182651; Abcam), PI3K (1:500; cat. no. 4249; CST), CD31 (1:1,000;
cat. no. ab134168; Abcam), VEGF (1:1,000; cat. no. ab53465; Abcam),
TNF-α (1:1,000; cat. no. ab6671; Abcam) and interleukin-1β (IL-1β;
1:2,000; cat. no. ab150777; Abcam). GAPDH (1:2,000; cat. no. 2118;
CST) was used as an internal control.
On the following day, after washing three times with
TBS/0.1% Tween-20, the membranes were incubated with an
HRP-conjugated anti-rabbit secondary antibody (cat. no. BA1054;
Boster Biological Technology) for 1 h at room temperature, then
washed another three times. The membranes were soaked in an
enhanced chemiluminescence reagent (cat. no. AR1191; Boster
Biological Technology) for 5 min at room temperature, and the bands
were visualized with X-ray films (VersaDoc imaging systems; Bio-Rad
Laboratories, Inc.). The intensity of bands was analyzed using
QuantityOne software (version 4.6; Bio-Rad Laboratories, Inc.).
Data are presented as the relative expression level, following
normalization to the internal loading control GAPDH.
RT-qPCR
For the FLSs experiments, 2×105 FLSs/well
were seeded and stimulated for 24 h with 200 ng/ml MCP-1, 100 ng/ml
TNF-α, or 200 ng/ml MCP-1 with 50 µM LY294002 or 50 µM SB203580.
Total RNA from 2 g synovial tissue and FLSs were extracted using
TRIzol® reagent (Takara Bio, Inc.). RNA (1 µg) was
reverse transcribed into cDNA using the RevertAid First Strand cDNA
Synthesis kit (Takara Bio, Inc.), according to the manufacturer's
protocol. qPCR was performed using ABI PRISM 7000 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and SYBR-Green
Real-Time PCR Master mix (cat. no. RR430A; Takara Bio, Inc.). The
following primer pairs were used for the qPCR: MCP-1 forward,
5′-AGCCACCTTCATTCCCCAAG-3′ and reverse, 5′-CTCCTTGGCCACAATGGTCT-3′;
VEGF forward, 5′-CGGTTCCAGAAGGGAGAGGA-3′ and reverse,
5′-CTGGGACCACTTGGCATGG-3′; CD31 forward, 5′-GCCTCACCAAGAGAACGGAA-3′
and reverse, 5′-AATTGGATGGCTTGGCCTGA-3′; and GAPDH forward,
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse, 5′-TTTGAGGGTGCAGCGAACTT-3′
[Sangon Biotech (Shanghai) Co., Ltd.]. The thermocycling conditions
used were as follows: Initial denaturation at 94°C for 4 min,
followed by 30–35 cycles at 95°C for 30 sec, 55°C for 30 sec and
72°C for 30 sec. Quantification of the relative expression levels
of the target genes was achieved using the formula:
2−ΔΔCq, where Cq is quantification cycle and ΔΔCq=(Cq of
the target gene-Cq of GAPDH) after treatment-(Cq of the target
gene-Cq of GAPDH) in the control (26);. Data are presented as relative
expression level, which was normalized to GAPDH and the
control.
Immunohistochemical analysis
The knee joints of rats were removed and fixed with
10% paraformaldehyde for 2 days at room temperature and then
embedded in paraffin. Paraffin-embedded sections were subsequently
cut into 5-µm sections. The sections were first incubated with 3%
peroxyl in methanol for 15 min at room temperature, then boiled
(~100°C) in citrate buffer solution for 10 min for antigen
retrieval. Following the incubation with 5% BSA (cat. no. AR1006;
Boster Biological Technology) for 20 min at room temperature,
sections were then covered with anti MCP-1 antibody (1:300; cat.
no. ab9669; Abcam) overnight at 4°C. Sections were then incubated
with the HRP-conjugated secondary antibody (1:2,000; cat. no.
ab205718; Abcam) for 30 min at room temperature and then incubated
with diaminobenzidine for 3–5 min at room temperature to visualize
the positive expression of the targeted protein. All sections were
counterstained with 5% hematoxylin for 1 min at room temperature.
Positive cells were observed using a light microscope
(magnification, ×400; Nikon Corporation).
As for the staining of FLSs with vimentin,
1×106 FLSs/cm were seeded and cultured for 3 days;
subsequently the supernatant was removed and FLSs were fixed with
4% paraformaldehyde for 20 min at 4°C. Following permeabilization
with Triton X for 20 min at room temperature, FLSs were treated
with 3% H2O2 for 15 min at room temperature.
Following incubation with 5% BSA for 20 min at room temperature,
the slices were incubated with the anti-vimentin primary antibody
(1:500; cat. no. ab92547; Abcam) for 2 h at 37°C. Then, the
procedures were the same as the immunohistochemical analysis of
sections described above.
Statistical analysis
Data are presented as the mean ± SEM and were
analyzed using GraphPad Prism (version 5.0; GraphPad Software,
Inc.). ANOVA was used when making comparisons containing multiple
groups, and Tukey's test was used as post hoc test. Student's
t-test was used to analyze MCP-1 content between the CIA group and
control group. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and identification of
synovial fibroblasts
With a seeding density of 1×105
cells/cm2, the passage two cells began to adhere and
gradually form clusters, and reached 80–90% confluence within 3–4
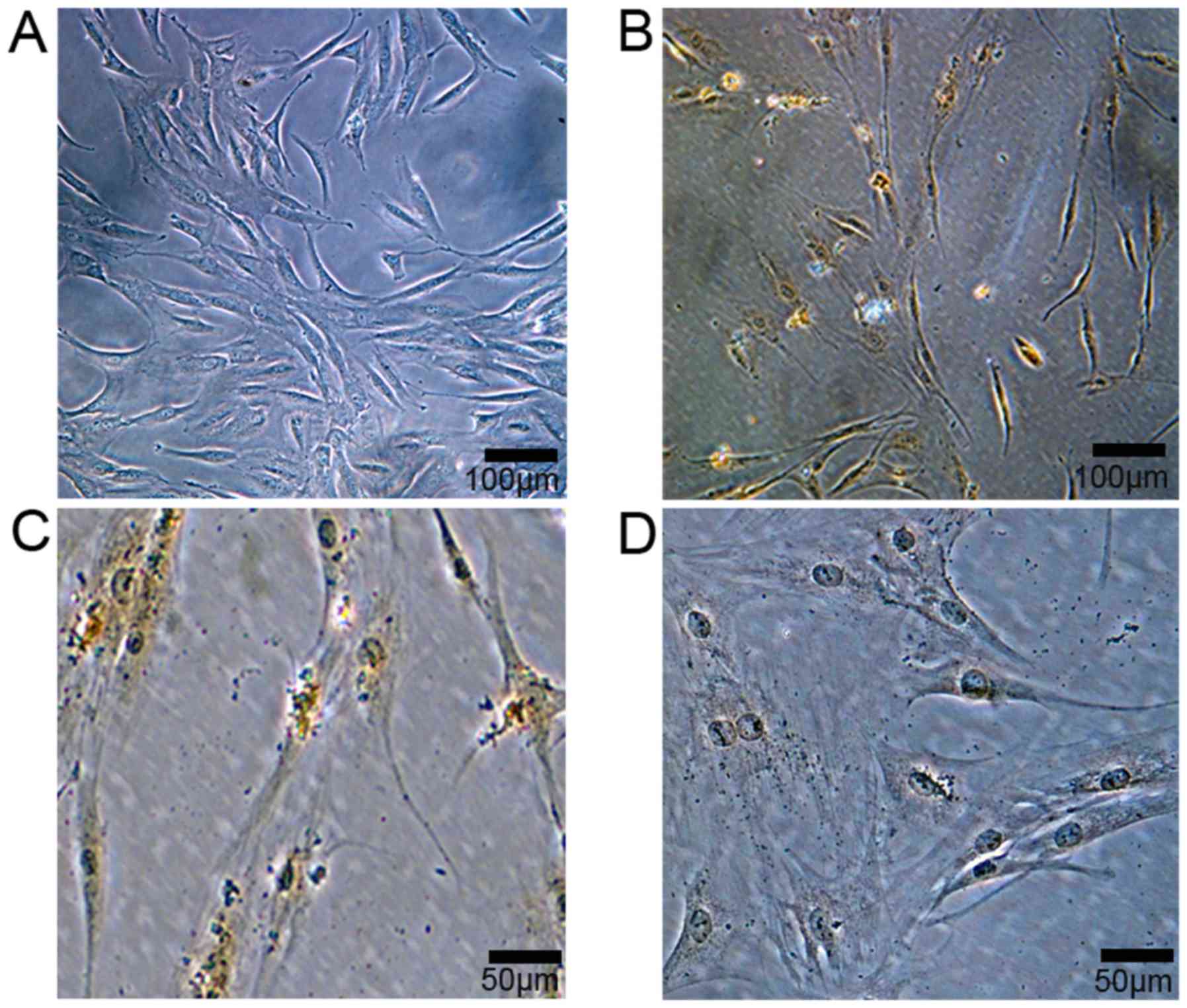
days. FLSs appeared spindle-like shaped (Fig. 1A). FLSs were identified by
immunohistology, and positive vimentin staining was observed within
FLSs (Fig. 1B and C), while no
positive staining was observed in negative controls (Fig. 1D)
Increase in MCP-1 expression in the
joints of CIA rats
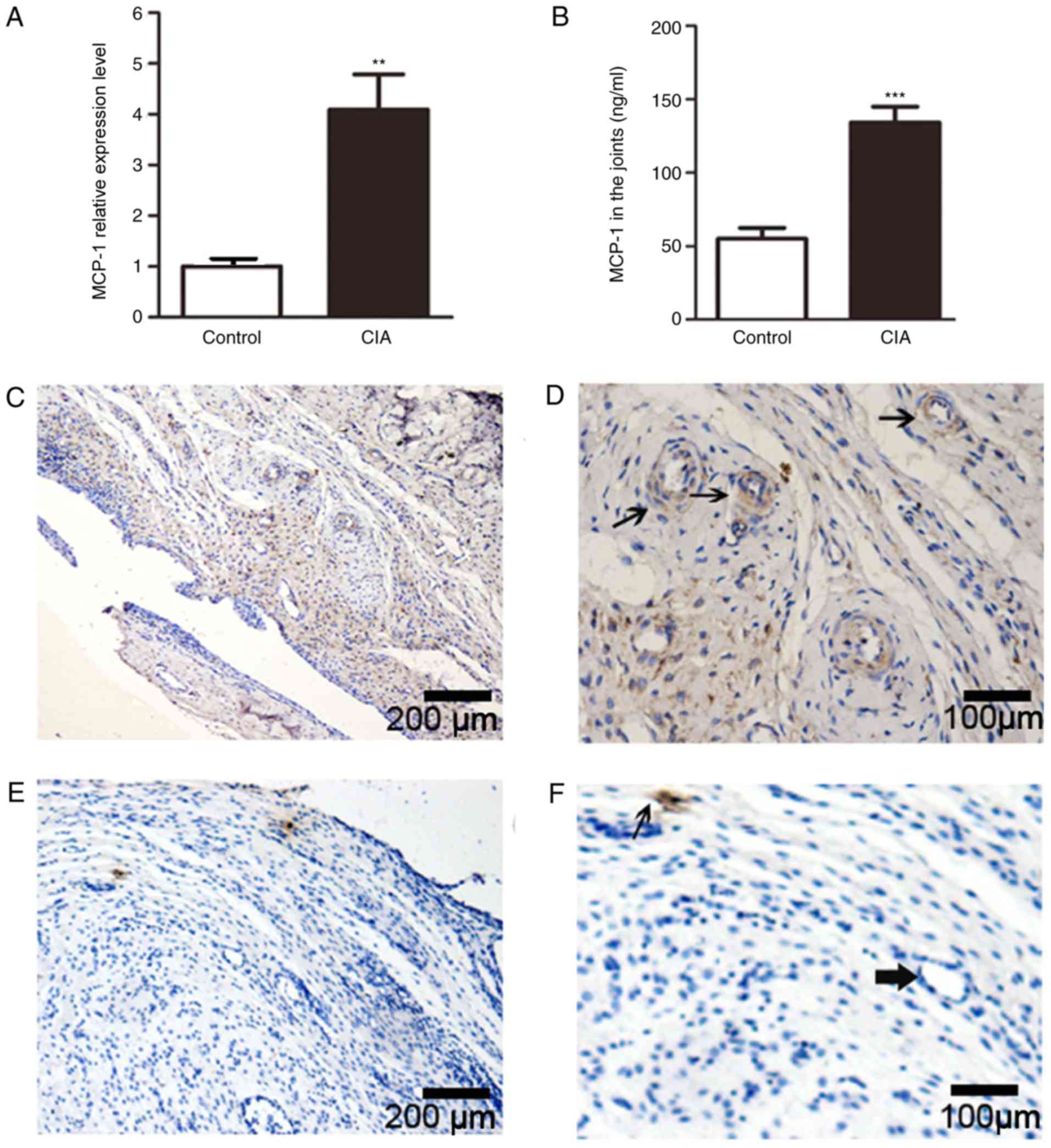
Using RT-qPCR, the present study demonstrated that
the expression levels of MCP-1 in the synovial tissue of CIA rats
were significantly increased compared with the control group
(Fig. 2A). In addition, ELISA was
used to assess the protein expression levels of MCP-1 in synovial
tissue from CIA rats, and it was found that the protein expression
levels of MCP-1 in the inflamed synovium of CIA rats were also
significantly higher compared with controls (3-fold increase;
Fig. 2B).
MCP-1 protein expression level was also examined by
immunohistology. Numerous lumen structures were observed in the
synovial tissue of CIA rats, and broad areas positive for MCP-1
staining were identified in the inflamed synovium (Fig. 2C and D), especially around lumen
structures (thin arrow in Fig.
2D). By contrast, MCP-1 staining was rarely observed in the
controls (Fig. 2E and F),
especially around lumen structures (rough arrow in Fig. 2F).
MCP-1 induces proliferation of FLSs in
CIA rats via the activation of the PI3K/P38 pathway
Cell proliferation was analyzed using CCK-8 and BrdU
assay. To investigate the effect of MCP-1 on the viability of FLS
cells, FLS cells were treated with increasing doses of MCP-1
(0–1,000 ng/ml). MCP-1 promoted the proliferation of FLSs in a
dose-dependent manner (Fig. 3A).
MCP-1 exerted the maximum effect on the proliferation of FLSs at a
concentration of 200 ng/ml (Fig.
3A), which was then used in the subsequent experiments. This
finding was also confirmed by the BrdU test results (Fig. S1). Additive effects of MCP-1 in
combination with TNF-α on FLSs proliferation were also found by
stimulating FLSs with TNF-α (100 ng/ml), MCP-1 (200 ng/ml) or the
two cytokines in combination (Fig.
3B). The present study observed that TNF-α could also promote
the proliferation of FLSs, and increase the proliferation potential
of FLSs induced by MCP-1 (Fig.
3B).
 | Figure 3.Effect of MCP-1 on the proliferation
of FLSs. (A) FLSs were incubated with MCP-1 at 1, 10, 100, 200, 500
and 1,000 ng/ml. Columns show the percentage of the values of cells
without MCP-1 treatment. MCP-1 at a concentration of 200 ng/ml
exerted the maximum effect on the proliferation of FLSs. (B) MCP-1
with TNF-α increased the proliferation of FLSs. (C) Total and
phosphorylated expression levels of PI3K and P38 at 15, 30 and 60
min following MCP-1 treatment compared with untreated samples. (D)
Phosphorylation expression levels relative to total protein
expression levels of P38 and PI3K were higher after 15, 30 and 60
min of MCP-1 stimulation compared with the control. To test whether
PI3K and P38 were involved in the proliferation of FLSs induced by
MCP-1, FLSs were pretreated with LY294002 (at 10, 50 and 100 µM)
and SB203580 (at 10, 50 and 100 µM) for 30 min prior to MCP-1
stimulation, which was performed for 48 h. The cell proliferation
induced by MCP-1 was significantly reduced when incubated with (E)
LY and (F) SB in a concentration-dependent manner. Data are
presented as the mean ± SEM. *P<0.05, **P<0.01 and
***P<0.001 vs. respective control; ##P<0.01,
###P<0.001 vs. MCP-1 group. MCP-1, monocytes
chemotactic protein 1; FLSs, fibroblast-like synoviocytes; CCK-8,
cell counting kit-8; LY, LY294002; SB, SB203580; t-, total; p-,
phosphorylated; TNF-α, tumor necrosis factor-α. |
The present study analyzed the effect of MCP-1 on
the phosphorylation levels of PI3K and P38. Phosphorylation level
of PI3K increased 2.68-fold compared to that of untreated samples
at 15 min following treatment with MCP-1 (Fig. 3C). One of the PI3K downstream
signaling molecules, P38, was analyzed in addition to PI3K in FLSs.
The phosphorylation levels of P38 were 2.45- and 4.2-fold higher
after 15 and 30 min of MCP-1 stimulation, respectively, compared
with the control groups (Fig. 3D).
To further examine whether P38/PI3K signaling was involved in the
proliferation of FLSs via MCP-1, FLSs were pretreated with SB, aP38
inhibitor (at 10, 50 and 100 µM) and LY, a PI3K inhibitor (at 10,
50 and 100 µM) for 30 min prior to MCP-1 stimulation. Cell
proliferation induced by MCP-1 was significantly reduced when
incubated with SB or LY, in a concentration-dependent manner
(Fig. 3E and F).
MCP-1 promotes the migration of FLSs
through the activation of PI3K/P38 pathway
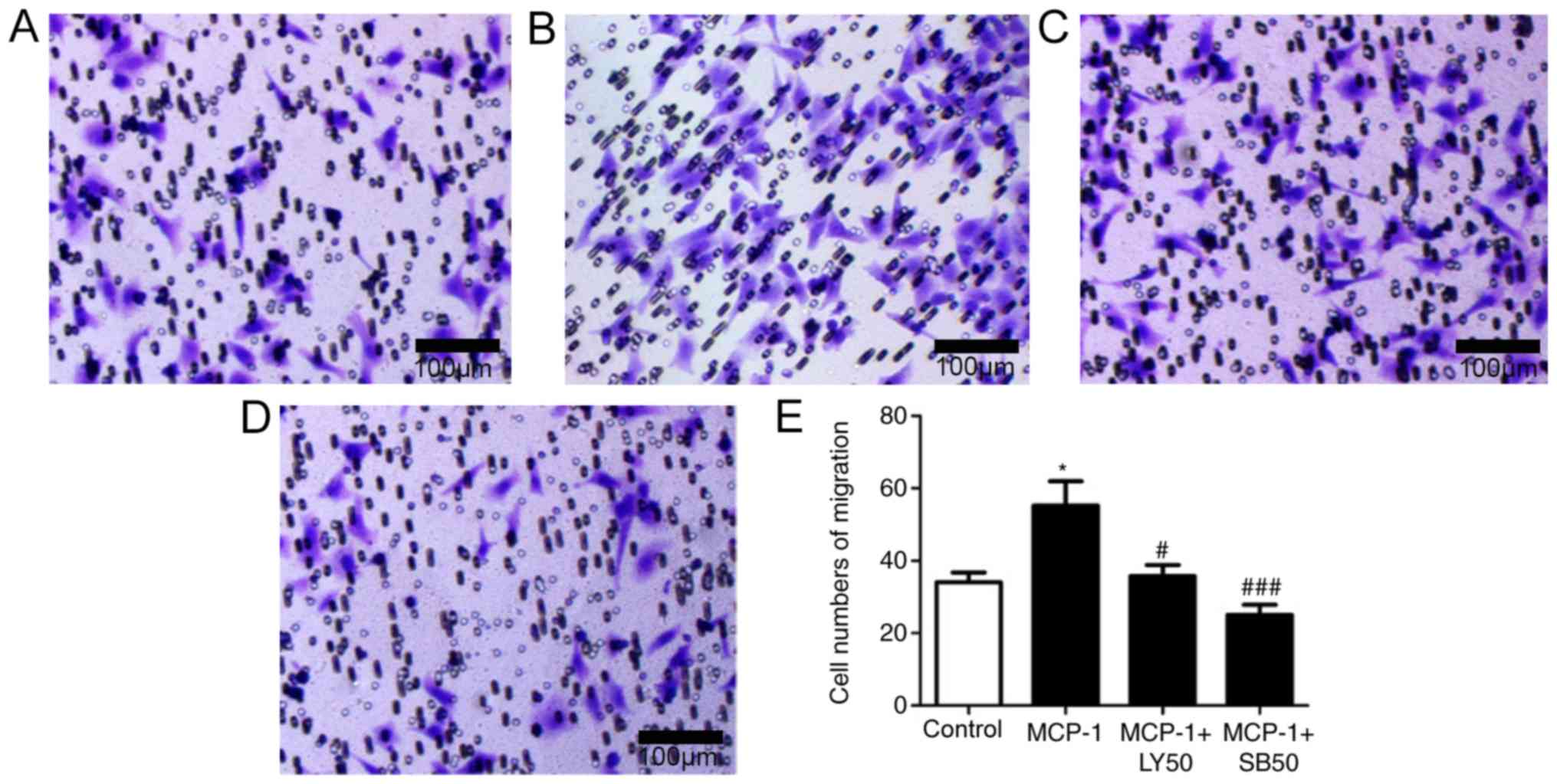
The involvement of MCP-1 in the migration and
invasion of FLSs was then investigated. MCP-1 stimulation
significantly increased the migration of FLSs (Fig. 4A and B). Additionally, the present
study investigated whether PI3K and P38 could regulate FLS
migration; the results demonstrated that MCP-1-induced migration of
FLSs was significantly blocked by LY and SB (Fig. 4C-E), suggesting that the migration
of FLSs induced by MCP-1 was activated by the PI3K and P38
pathway.
MCP-1, and not TNF-α, induces
differentiation of FLSs through the activation of P38/PI3K
pathway
MCP-1 is known to act as a regulator of cell
differentiation and proliferation, but, to the best of our
knowledge, the role of MCP-1 on the differentiation potential of
FLSs towards vascular endothelial cells remains unclear. The
results from the present study showed that the protein expression
levels of vessel formation biomarker proteins, such as VEGF and
CD31, in FLSs were significantly increased after MCP-1 treatment,
while TNF-α treatment (100 ng/ml) was unable to increase their
expression levels (Fig. 5A and B).
The expression levels of VEGF and CD31 induced by MCP-1 were also
significantly reduced by the PI3K and P38 inhibitors SB and LY
(Fig. 5C and D). Furthermore,
MCP-1 was found to upregulate the RNA expression levels of CD31 and
VEGF in FLSs (Fig. S2).
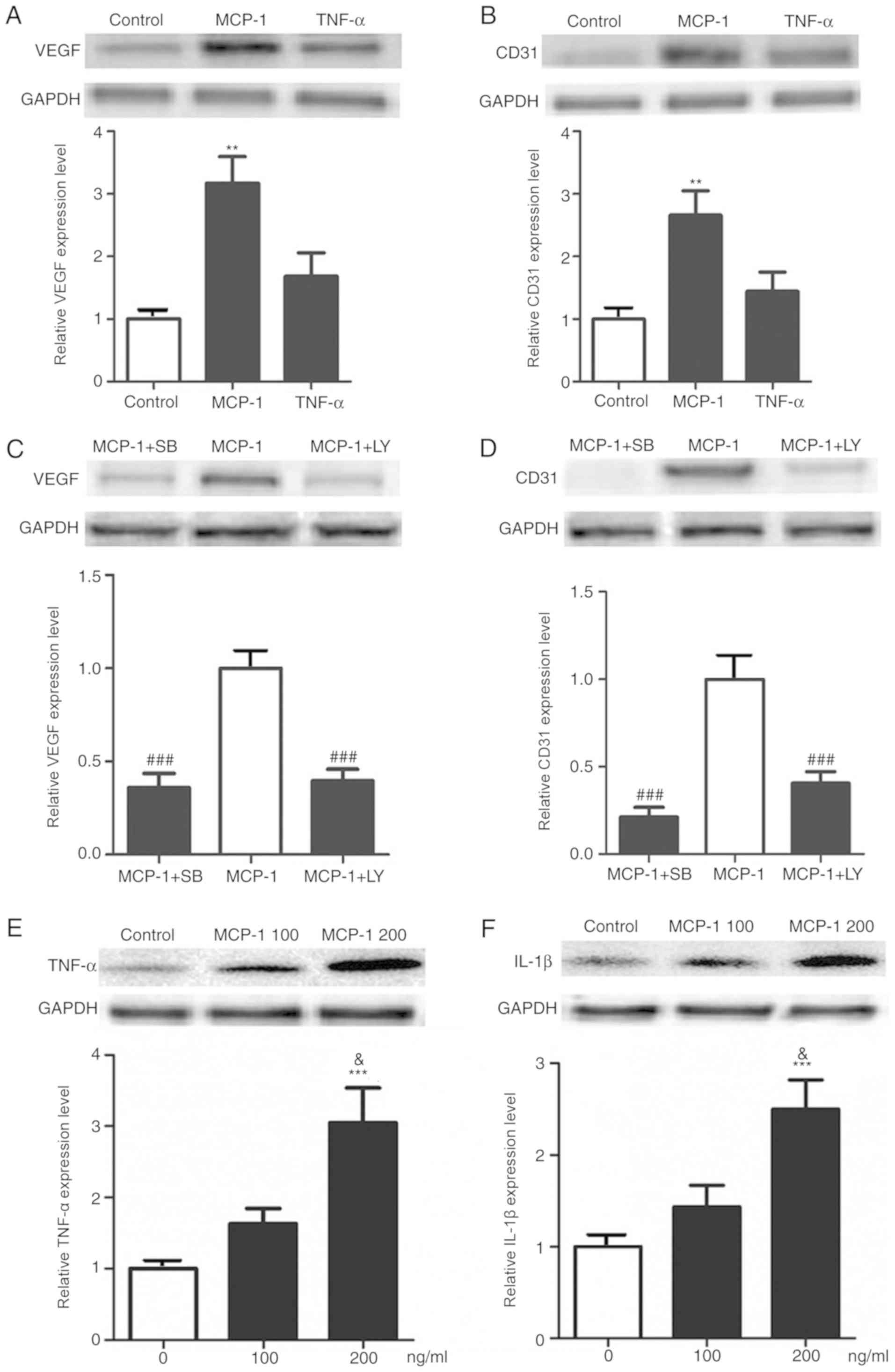 | Figure 5.Effect of MCP-1 on the expression
level of VEGF, CD31, TNF-α and IL-1β in FLSs. Expression levels of
the angiogenesis biomarkers (A) VEGF and (B) CD31 in FLSs increased
significantly after treatment with MCP-1, while their expression
after TNF-α treatment slightly increased. P38 and PI3K signaling
were examined to evaluate their effects on the expression levels of
(C) VEGF and (D) CD31. Expression levels of (E) TNF-α and (F) IL-1β
were also increased significantly after treatment with MCP-1. Data
are presented as the mean ± SEM. **P<0.01, ***P<0.001 vs.
control group; ###P<0.001 vs. MCP-1 group;
&P<0.05 vs. MCP at 100 ng/ml. MCP-1, monocytes
chemotactic protein 1; FLSs, fibroblast-like synoviocytes; SB,
SB203580; LY, LY294002; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; t-, total; p-, phosphorylated. |
MCP-1 induces the expression levels of
TNF-α and IL-1β
In the present study, it was found that the
expression levels of TNF-α and IL-1β were significantly upregulated
by treatment from MCP-1 (Fig. 5E and
F). The present results suggested that MCP-1 may be involved in
the pathogenesis of arthritis, partly through the secretion of
TNF-α and IL-1β.
Discussion
RA is a severe and debilitating disease that
ultimately results in disability (1,3). The
tumor-like proliferation of FLSs is thought to be the major cause
of hyperplasia of the synovium and the destruction of joints
(24). MCP-1 is involved in tumor
progression and metastasis in cancer through its pro-migratory and
pro-invasive effects on tumor cells (20,21,23,27,28).
Although it has been previously reported that MCP-1 plays a
critical role in the development of RA or CIA by affecting
monocytes and pro-inflammatory cytokine expression (29), the role of MCP-1 on the behavior of
FLSs remains unclear. The present study, to the best of our
knowledge, is the first to identify that MCP-1 can promote the
proliferation, migration and differentiation potential of FLSs
through the P38/PI3K signaling pathway. The results of the present
study increased the understanding of the role of MCP-1 in joint
destruction in RA and CIA animal models, which may facilitate the
development of novel therapeutic strategies.
It was previously reported that MCP-1 secreted by
FLSs is involved in RA and bacterial-mediated joint destruction.
Scian et al (30) found
increased secretion of MCP1 by FLSs in joint damage induced by
Brucella abortus infection. A previous study found that
lipopolysaccharides can enhance the expression of MCP-1 in cultured
FLSs (31). Another previous study
found that the expression levels of MCP-1 were high in the inflamed
synovium of patients with RA, but were limited in the tissue of
normal controls (32), which is
partly consistent with the present immunohistological and ELISA
results. Additional evidence indicated that MCP-1 could activate
FLSs directly, as MCP-1 receptors were found to be expressed in
FLSs in patients with RA (33).
However, the effect of MCP-1 on FLSs remains
unclear. MCP-1 is a promoter not only of inflammation, but of cell
migration and invasion in tumor cells (20,21,28).
Therefore, the present study examined the effects of MCP-1 on the
proliferation and migration capacity of FLSs, and found that both
processes were enhanced by treatment with MCP-1. The present
results indicated that MCP-1 induced proliferation and migration of
FLSs, and could therefore contribute to severe joint damage.
Therefore, the present results provided novel evidence on the
association between MCP-1 and the aggressive features of FLSs. The
present findings may have important clinical significance. Elevated
MCP-1 content in joints may lead to more severe cartilage and bone
damage. Therefore, MCP-1 may be a novel indicator of disease
severity, which is supported by the fact that blood MCP-1 levels
may be useful in monitoring RA activity (12). Therefore, targeting MCP-1 or its
receptor may facilitate the suppression of RA progression.
In addition, angiogenesis of the pannus is important
during the pathological process of RA (34). VEGF and CD31 are biomarkers of
endothelial cell differentiation, secreted by a variety of cell
types such as fibroblasts, endothelial cells and osteoblasts, and
serve important roles in inducing the differentiation of vascular
endothelial cells (35,36). It was also reported that MCP-1
could serve as a direct mediator of angiogenesis by increasing C-C
chemokine receptor type 2 expression in the endothelium (21). Consistent with these studies, the
present study showed that the expression of MCP-1 was mainly
located around the lumen structures in the synovium, and in
vitro experiments indicated that MCP-1 could induce the
expression of VEGF and CD31 in FLSs. Therefore, MCP-1 may
contribute to the formation of capillaries in the pannus through
increasing the expression of VEGF and CD31 in FLSs. Another
possible explanation is that MCP-1 may promote FLSs differentiation
into endothelial cells, as FLSs were shown to have the potential to
differentiate into osteoblasts and chondrocytes (37,38).
Nevertheless, additional studies are required to explore the role
of MCP-1 in the angiogenesis in the pannus formed during RA.
PI3K and P38 pathways have been reported to be
involved in inflammation activation and cell differentiation
induced by MCP-1 (39). MCP-1 has
been reported to promote the proliferation of smooth muscle cells
by activating PI3K (40,41) and myoblast cell proliferation by
activating P38 (42). There are
multiple crosstalk between P38 and PI3K pathways, and various
previous studies showed that proliferation and pro-inflammatory
mechanisms are activated by PI3K/P38 pathway (42,43).
In the present study, the accumulation of p-PI3K was observed
following MCP-1 treatment at 15 min, while P38 phosphorylation was
activated by MCP-1 treatment after 30 min. Collectively, the
present results suggested that MCP-1 could alter the phenotype of
FLSs through the PI3K/P38 pathway.
In conclusion, the present study suggested that
MCP-1 may promote the proliferation and migration of FLSs. In
addition, MCP-1 induced FLSs differentiation potential towards
vascular endothelial cells through the PI3K/P38 pathway, which
contributed to the formation of the pannus in CIA rats. The present
study described a novel possible mechanism of MCP-1 in joint
destruction in RA, a finding which may be beneficial to the
identification of novel disease indicators and the development of
novel therapeutic strategies.
Supplementary Material
Supporting Data
Acknowledgements
We gratefully acknowledge the help of Dr Zhiyun Feng
(Department of Orthopedic Surgery, The First Affiliated Hospital,
College of Medicine, Zhejiang University), who provided valuable
suggestions to this study.
Funding
The present study was supported by The Program
Science and Technology Department of Zhejiang Province (grant no.
LGF19H060012) and Medicine and Health Science and Technology Plan
in Zhejiang province (grant no. 2018ZD005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
XL and XT conceived and designed the study. The
experiments were performed by HZe, XT, KW and HZh. XL, XT and PG
analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were carried out following the Guide
for the Care and Use of Laboratory Animals of the National
Institutes of Health and all the procedures were pre-approved by
the Review Board of the First Affiliated Hospital of Medical
College of Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CIA
|
collagen-induced arthritis
|
|
FLS
|
fibroblast-like synoviocyte
|
|
IL-1β
|
interleukin-1β
|
|
LY
|
LY294002
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
RA
|
rheumatoid arthritis
|
|
SB
|
SB203580
|
|
TNF-α
|
tumor necrosis factor-α
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Aletaha D, Funovits J and Smolen JS:
Physical disability in rheumatoid arthritis is associated with
cartilage damage rather than bone destruction. Ann Rheum Dis.
70:733–739. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng Z, Zeng S, Wang Y, Zheng Z and Chen
Z: Bisphosphonates for the prevention and treatment of osteoporosis
in patients with rheumatic diseases: A systematic review and
meta-analysis. PLoS One. 8:57. 2013. View Article : Google Scholar
|
|
3
|
Scott DL, Smith C and Kingsley G: Joint
damage and disability in rheumatoid arthritis: An updated
systematic review. Clin Exp Rheumatol. 21((5 Suppl 31)): S20–S27.
2003.PubMed/NCBI
|
|
4
|
Feng ZY, He ZN, Zhang B, Li YQ, Guo J, Xu
YL, Han MY and Chen Z: Adenovirus-mediated osteoprotegerin
ameliorates cartilage destruction by inhibiting proteoglycan loss
and chondrocyte apoptosis in rats with collagen-induced arthritis.
Cell Tissue Res. 362:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharif K, Jumah F, Oskouian R and Tubbs
RS: Rheumatoid arthritis in review: Clinical, anatomical, cellular
and molecular points of view. Clin Anat. 31:216–223. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19:1102017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Zhao W, Cao X, Yang H, Ding J, Ding
J, Tan Z, Ma X, Hao C, Wu L, et al: SIRT1 promotes tumor-like
invasion of fibroblast-like synoviocytes in rheumatoid arthritis
via targeting TIMP1. Oncotarget. 8:88965–88973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Chen W, Wang L, Li F, Zhang C and
Xu L: Tumor necrosis factor receptor-associated factor 6 promotes
migration of rheumatoid arthritis fibroblast-like synoviocytes. Mol
Med Rep. 11:2761–2766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Wu Q, Xuan W, Feng X, Wang F, Tsao
BP, Zhang M and Tan W: Transcription factor SOX5 promotes the
migration and invasion of fibroblast-like synoviocytes in part by
regulating MMP-9 expression in collagen-induced arthritis. Front
Immunol. 9:7492018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lochhead RB, Ordonez D, Arvikar SL, Aversa
JM, Oh LS, Heyworth B, Sadreyev R, Steere AC and Strle K:
Interferon-gamma production in Lyme arthritis synovial tissue
promotes differentiation of fibroblast-like synoviocytes into
immune effector cells. Cell Microbiol. 21:e129922019.PubMed/NCBI
|
|
12
|
Liou LB, Tsai WP, Chang CJ, Chao WJ and
Chen MH: Blood monocyte chemotactic protein-1 (MCP-1) and adapted
disease activity Score28-MCP-1: Favorable indicators for rheumatoid
arthritis activity. PLoS One. 8:e553462013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Y, Sun L, Jiang T, Zhang D, He D and
Nie H: TNFα promotes Th17 cell differentiation through IL-6 and
IL-1β produced by monocytes in rheumatoid arthritis. J Immunol Res.
2014:3853522014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darrieutort-Laffite C, Boutet MA,
Chatelais M, Brion R, Blanchard F, Heymann D and Le Goff B: IL-1β
and TNFα promote monocyte viability through the induction of GM-CSF
expression by rheumatoid arthritis synovial fibroblasts. Mediators
Inflamm. 2014:2418402014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zucali JR, Dinarello CA, Oblon DJ, Gross
MA, Anderson L and Weiner RS: Interleukin 1 stimulates fibroblasts
to produce granulocyte-macrophage colony-stimulating activity and
prostaglandin E2. J Clin Invest. 77:1857–1863. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satish LD, Sergey K, Shohreh A and Bassel
ES: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rantapää-Dahlqvist S, Boman K, Tarkowski A
and Hallmans G: Up regulation of monocyte chemoattractant protein-1
expression in anti-citrulline antibody and immunoglobulin M
rheumatoid factor positive subjects precedes onset of inflammatory
response and development of overt rheumatoid arthritis. Ann Rheum
Dis. 66:121–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogata H, Takeya M, Yoshimura T, Takagi K
and Takahashi K: The role of monocyte chemoattractant protein-1
(MCP-1) in the pathogenesis of collagen-induced arthritis in rats.
J Pathol. 182:106–114. 2015. View Article : Google Scholar
|
|
19
|
Shiva S, Proudfoot AE, Park CC, Volin MV,
Haines GK, Woods JM, Aikens CH, Handel TM and Pope RM: Inhibition
of monocyte chemoattractant protein-1 ameliorates rat
adjuvant-induced arthritis. J Immunol. 180:3447–3456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuper C, Beck FX and Neuhofer W: Autocrine
MCP-1/CCR2 signaling stimulates proliferation and migration of
renal carcinoma cells. Oncol Lett. 12:2201–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salcedo R, Ponce ML, Young HA, Wasserman
K, Ward JM, Kleinman HK, Oppenheim JJ and Murphy WJ: Human
endothelial cells express CCR2 and respond to MCP-1: Direct role of
MCP-1 in angiogenesis and tumor progression. Blood. 96:34–40. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Z, Huang P, Chong Y, George SK, Wen B,
Han N, Liu Z, Kang L and Lin N: Nucleus pulposus cells derived
IGF-1 and MCP-1 enhance osteoclastogenesis and vertebrae disruption
in lumbar disc herniation. Int J Clin Exp Pathol. 7:8520–8531.
2014.PubMed/NCBI
|
|
23
|
Chen X, Wang Y, Nelson D, Tian S, Mulvey
E, Patel B, Conti I, Jaen J and Rollins BJ: CCL2/CCR2 regulates the
tumor microenvironment in HER-2/neu-driven mammary carcinomas in
mice. PLoS One. 11:e01655952016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du J, Zhang F and Guo J: miR137 decreases
proliferation, migration and invasion in rheumatoid arthritis
fibroblastlike synoviocytes. Mol Med Rep. 17:3312–3317.
2018.PubMed/NCBI
|
|
25
|
Feng ZY, He ZN, Zhang B and Chen Z:
Osteoprotegerin promotes the proliferation of chondrocytes and
affects the expression of ADAMTS-5 and TIMP-4 through MEK/ERK
signaling. Mol Med Rep. 8:1669–1679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dutta P, Sarkissyan M, Paico K, Wu Y and
Vadgama JV: MCP-1 is overexpressed in triple-negative breast
cancers and drives cancer invasiveness and metastasis. Breast
Cancer Res Treat. 170:477–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu E, Su J, Zhou Y, Zhang C and Wang Y:
CCL20/CCR6 promotes cell proliferation and metastasis in laryngeal
cancer by activating p38 pathway. Biomed Pharmacother. 85:486–492.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldbergova MP, Lipkova J, Pavek N, Pavek
N, Gatterova J, Vasku A, Soucek M and Nemec P: RANTES, MCP-1
chemokines and factors describing rheumatoid arthritis. Mol
Immunol. 52:273–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scian R, Barrionuevo P, Giambartolomei GH,
De Simone EA, Vanzulli SI, Fossati CA, Baldi PC and Delpino MV:
Potential role of fibroblast-like synoviocytes in joint damage
induced by Brucella abortus infection through production and
induction of matrix metalloproteinases. Infecti Immun.
79:3619–3632. 2011. View Article : Google Scholar
|
|
31
|
Andreassen SM, Berg LC, Nielsen SS,
Kristensen AT and Jacobsen S: mRNA expression of genes involved in
inflammation and haemostasis in equine fibroblast-like synoviocytes
following exposure to lipopolysaccharide, fibrinogen and thrombin.
BMC Vet Res. 11:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Yu M, Deng J, Lv X, Liu J, Xiao
Y, Yang W, Zhang Y and Li C: Chemokine signaling pathway involved
in CCL2 expression in patients with rheumatoid arthritis. Yonsei
Med J. 56:1134–1142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho ML, Yoon BY, Ju JH, Jung YO, Jhun JY,
Park MK, Park SH, Cho CS and Kim HY: Expression of CCR2A, an
isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and
TGF-beta in fibroblast like synoviocytes of patients with RA. Exp
Mol Med. 39:499–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paleolog EM: Angiogenesis in rheumatoid
arthritis. Arthritis Res. 4 (Suppl 3):S81–S90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamilton JL, Nagao M, Levine BR, Chen D,
Olsen BR and Im HJ: Targeting VEGF and its receptors for the
treatment of osteoarthritis and associated pain. J Bone Miner Res.
31:911–924. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choe JY, Lee SJ, Park SH and Kim SK:
Tacrolimus (FK506) inhibits interleukin-1β-induced angiopoietin-1,
Tie-2 receptor, and vascular endothelial growth factor through
down-regulation of JNK and p38 pathway in human rheumatoid
fibroblast-like synoviocytes. Joint Bone Spine. 79:137–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osta B, Roux JP, Lavocat F, Pierre M,
Ndongo-Thiam N, Boivin G and Miossec P: Differential effects of
IL-17A and TNF-α on osteoblastic differentiation of isolated
synoviocytes and on bone explants from arthritis patients. Front
Immunol. 6:1512015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hms W, Toyota K, Kim S, Fang J, Bwalya EC,
Hosoya K and Okumura M: Differentiation potential of synoviocytes
derived from joints with cranial cruciate ligament rupture and
medial patella luxation in dogs. Res Vet Sci. 114:370–377. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Biswas SK and Sodhi A: Effect of monocyte
chemoattractant protein-1 on murine bone marrow cells:
Proliferation, colony-forming ability and signal transduction
pathway involved. Int J Immunopathol Pharmacol. 15:183–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fougerat A, Smirnova NF, Gayral S, Malet
N, Hirsch E, Wymann MP, Perret B, Martinez LO, Douillon M and
Laffargue M: Key role of PI3Kγ in monocyte chemotactic
protein-1-mediated amplification of PDGF-induced aortic smooth
muscle cell migration. Br J Pharmacol. 166:1643–1653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ming Z, Yawei L, Mingmin B, Yutaka K,
Jiahuai H and Eaton JW: Vascular smooth muscle cell proliferation
requires both p38 and BMK1 MAP kinases. Arch Biochem Biophys.
400:199–207. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu S, Gao F, Wen L, Ouyang M, Wang Y,
Wang Q, Luo L and Jian Z: Osteocalcin induces proliferation via
positive activation of the PI3K/Akt, P38 MAPK pathways and promotes
differentiation through activation of the GPRC6A-ERK1/2 pathway in
C2C12 myoblast cells. Cell Physiol Biochem. 43:1100–1112. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang BP, Lin CH, Chen HM, Lin JT, Cheng
YF and Kao SH: AMPK activation inhibits expression of
proinflammatory mediators through downregulation of PI3K/p38 MAPK
and NF-κB signaling in murine macrophages. DNA Cell Biol.
34:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|