Introduction
Colorectal cancer (CRC) is one of the most common
cancer types worldwide, being the third most frequently diagnosed
malignancy in males and second most in females (1). Currently, radical surgical resection
is the most effective treatment for CRC; however, most patients, in
particular elderly patients, suffer from high local recurrence and
distant metastasis (2,3). Therefore, it is important to identify
prognostic markers to facilitate the clinical treatment of CRC.
The HER family, which encodes for tyrosine kinase
receptors, includes four members, HER1 [also known as epidermal
growth factor receptor (EGFR)], HER2, HER3 and HER4 (4). Previous studies have shown the
relationship between the survival of patients and the expression of
HER2, HER3 and EGFR (5–7). Rego et al (5) reported that EGFR overexpression was
detected in 80–90% of CRC, and was associated with poor
disease-free survival and overall survival. Kapitanović et
al (6) showed that HER2
expression was correlated with the stage of disease and survival in
CRC. Mitsui et al (7)
reported that the expression of HER3 led to metastases in the lymph
node and liver, and poorer patient prognosis in CRC. However, to
the best of our knowledge, few studies have investigated whether
HER4 may serve a role in the process of epithelial-mesenchymal
transition (EMT) in CRC. Therefore, the present study examined the
role of HER4 in CRC. Previous studies have shown that HER4 has four
receptor isoforms generated by alternative splicing, extracellular
juxtamembrane domains (JM-a and JM-b) and cytoplasmic domains
(CYT-1 and CYT-2) (8). HER4
regulates cell proliferation, survival and differentiation, and is
expressed both in fetal and normal tissues (4). An increasing number of studies have
demonstrated that HER4 may be involved in tumorigenesis (9–11).
Nielsen et al (10) found
that HER4 expression is associated with short progression-free
survival in malignant melanoma. Wang et al (11) showed that HER4 expression promotes
osteosarcoma cell proliferation and tumorigenesis, and reduced
apoptosis both in vitro and in vivo. In our previous
study, CYT-2, but not CYT-1, could significantly promote CRC
progression (12).
EMT initiates the process of metastasis in tumor
progression (13–15). Through EMT, tumor cells often lose
their epithelial cell phenotype, downregulating E-cadherin
expression, and acquire a mesenchymal cell phenotype, expressing
N-cadherin and Vimentin. Vu et al (16) identified that the progression of
EMT is regulated by WNT/β-catenin and that EMT transcription
factors affect the metastasis of CRC. However, to the best of our
knowledge, few studies have evaluated whether HER4 may serve a role
in the process of EMT in CRC.
The present study investigated the expression of HER
family members in patients with CRC and the effects of HER4 on CRC
cell proliferation, survival, migration and EMT.
Materials and methods
Patients and tumor specimens
All procedures involving human participants were
approved by the ethical standards of the institutional research
committee of The Fourth Hospital of Hebei Medical University and
according to the 1964 Helsinki Declaration and its later amendments
or comparable ethical standards. The participants signed an
informed consent form after being informed about the benefits and
risks of the procedure in this study. In total, 73
paraffin-embedded primary colorectal cancer specimens were
collected from the Department of Surgery, Fourth Hospital of Hebei
Medical University, from January 2008 to February 2012. All
patients (36 males and 37 females, 24–83 years old) were confirmed
to have adenocarcinoma, and no patient had been administered
chemotherapy or radiation therapy before surgery.
Immunohistochemistry (IHC) and
antibodies
IHC staining was performed using tissues (thickness,
5 µm) from patients with CRC after surgery. The tissues were fixed
with 10% formalin at room temperature for 24 h and embedded in
paraffin at 62°C for 45 min. The sections were first incubated with
10% normal goat serum for 10 min at 37°C to block nonspecific
immunoglobulin binding. Then the sections were incubated with
anti-EGFR antibody (1:50; cat. no. GTX121919; Genetex, Inc.),
anti-Her2 antibody (1:100; cat. no. GTX117480; Genetex, Inc.),
anti-c-HER3 antibody (1:100; cat. no. ARG52855; Arigo
Biolaboratories Corp.), and anti-c-HER4 antibody (1:50; cat. no.
ARG52859; Arigo Biolaboratories Corp.) overnight at 4°C. The
sections were washed with PBS buffer. Secondary antibodies (Rabbit
SP kit; cat. no. SP-9000; OriGene Technologies, Inc.) were added to
the tissue sections and incubated at 37°C for 30 min. All
procedures were conducted according to the manufacturer's
instructions provided with the Rabbit SP Kit (OriGene Technologies,
Inc.). All tumor slides were examined under a light microscope.
According to the scoring system approved by the US
Food and Drug Administration (17), samples were scored
semi-quantitatively for EGFR, HER2, HER3 and HER4 staining as
follows: i) 0, no immunostaining or staining in <10% of CRC
cells, ii) 1+, incomplete staining of >10% of CRC cells, iii)
2+, weak-to-moderately complete staining of >10% of CRC cells
and iv) 3+, moderate-to-strongly complete staining of >10% of
CRC cells. Scores of 0 or 1+ were regarded as negative for EGFR,
HER2, HER3 and HER4 expression, while scores of 2+ and 3+ were
regarded as positive for EGFR, HER2, HER3 and HER4 expression.
HER4 knockdown cell lines and cell
culture
In total, four hairpin RNAs were constructed to
specifically target HER4 mRNA by using short hairpin RNA (shRNA)
design tools, GenBank (https://www.ncbi.nlm.nih.gov/genbank/). Using Basic
Local Alignment Search Tool (BLAST+ 2.3.0; http://blast.ncbi.nlm.nih.Gov/Blast.cgi), only the
selected gene was targeted by the designed shRNAs. The sequences of
the four designed shRNAs are shown in Table I. HER4 was previously reported to
be overexpressed in the HCT116 CRC cell line (18); therefore, this cell line was used
in the present study. The lentivirus (Shanghai GenePharma, Co.,
Ltd.) was infected (400 µl lentiviral fluid per 2 ml medium) with
HCT116 CRC cells by polybrene (cat. no. H9268; Sigma-Aldrich; Merck
KGaA). The expression levels of HER4 were determined by
quantitative PCR (qPCR) and western blotting (shown in Figs. S1 and S2). qPCR was performed by FTC-3000
(Funglyn Biotech, Inc.), SYBR Real-Time PCR kit (cat. no. E22001;
Shanghai GenePharma, Co., Ltd.) was used according to the
manufacture's protocols. qPCR thermocycling conditions were: 95°C
for 3 min; 40 cycles were performed at 95°C for 30 sec and 62°C for
40 sec. The primer sequences used were as follows: HER4 forward,
5′-CCGAGGATGAGTATGTGAATGA-3′ and reverse, 5′-AGGTGGCAGGCTGTGGTT-3′;
β-actin forward, 5′-CGTGGACATCCGCAAAGA and reverse,
5′-GAAGGTGGACAGCGAGGC-3′. Sense and antisense sequences of the
stem-loop of shRNA HER4-4 and nc-shRNA are shown in Table II. All the procedures above were
performed without any treatment. Three groups were evaluated in the
experiment: i) HCT116 group; ii) negative control (nc-shRNA) group;
and iii) silenced HER4 (shRNA-HER4) group.
 | Table I.Sequences of shRNAs targeting
HER4. |
Table I.
Sequences of shRNAs targeting
HER4.
| Number | Name | Sequences
(5′-3′) |
|---|
| shRNA HER4-1 | HER4-homo-1105 |
GCATTGGCACAGGATCATTGA |
| shRNA HER4-2 | HER4-homo-1815 |
GGTCCTGACAACTGTACAAAG |
| shRNA HER4-3 | HER4-homo-2078 |
GCTCTTCATTCTGGTCATTGT |
| shRNA HER4-4 | HER4-homo-2760 |
GCTCTGGAGTGTATACATTAC |
 | Table II.Sense and antisense sequences of the
stem-loop of shRNA-HER4-4 and nc-shRNA. |
Table II.
Sense and antisense sequences of the
stem-loop of shRNA-HER4-4 and nc-shRNA.
| Name | Sequences |
|---|
| shRNA-HER4-4 | Sense:
5′-GATCCGCTCTGGAGTGTATACATTACTTCAAGAGAGTAATGTATACACTCCAGAGCTTTTTTG-3′ |
|
| Antisense:
5′-AATTCAAAAAAGCTCTGGAGTGTATACATTACTCTCTTGAAGTAATGTATACACTCCCAGAGCG-3′ |
| nc-shRNA | Sense:
5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACTTTTTTG-3′ |
|
| Antisense:
5′-AATTCAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3′ |
The human CRC cell line HCT116 was obtained from the
Type Culture Collection of the Chinese Academy of Sciences. Cells
were cultured for 24–48 h in McCoy's 5A (cat. no. 16600082; Gibco;
Thermo Fisher Scientific, Inc.) medium supplemented with 10% FBS
(cat. no. 10099141; Gibco; Thermo Fisher Scientific, Inc.) and
penicillin (100 U/ml)-streptomycin (100 µg/ml) at 37°C in 5%
CO2.
Cell proliferation assay
Cell proliferation was measured using a CCK-8
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's protocols (19).
Cells were seeded into 96-well plates at a density of
3×103 cells/100 µl and incubated at 37°C in 5%
CO2 for 24, 48 and 72 h. Cells were incubated with 10 µl
CCK-8 reagent for another 2 h. The optical density (OD) at 450 nm
of cells was measured using the BioTek elx800 (BioTek Instruments,
Inc.).
Flow cytometry assay
To detect the apoptosis of CRC cells, cells were
stained with FITC and propidium iodide (PI) at room temperature for
15 min in the dark. A cell apoptosis assay was performed by flow
cytometry (BD FACSVerse™; BD Biosciences), and the flow cytometry
data were analyzed using BD FACSuite™ Software (BD
Biosciences).
Cell adhesive assay
To evaluate adhesive ability of CRC cells,
6×104 cells/well were seeded into six-well plates
containing Matrigel (cat. no. 356234, Corning, Inc.) and incubated
at 37°C in 5% CO2 for 1 or 2 h, and then washed with PBS
three times. The OD of cells was determined using the BioTek
elx800. The wavelength of measurement was 490 nm.
Wound healing assay
In total, 5×106 cells/well were seeded in
six-well plates. A scratch was made with a 200-µl tip on the
monolayer of CRC cells. Then, cells were washed with PBS to remove
detached cells. CRC cells were cultured in MyCoy's 5A medium (cat.
no. 16600082; Gibco; Thermo Fisher Scientific, Inc.) with 2% FBS
(cat. no. 10099141; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin in 37°C. The images were captured using an
inverted microscope (magnification, ×50) at 0, 12, 24 and 48 h.
Wound healing area was measured by calculating the wound area in
each period. The area of the wound was calculated using Image J
1.49p software (20), and
calculated by the equation: The percentage of wound healing area =
[1-(wound area at Tt/wound area at T0)] ×100, where Tt is the time
passed since wounding (12, 24 and 48 h) and T0 is the time of
initial wounding.
Western blotting
Cells were lysed in RIPA buffer (cat. no. BB-3201;
BestBio). BCA protein assay kit (cat. no. P0012S; Beyotime
Institute of Biotechnology) was used for the determination of total
protein concentration. A total of 40 µg of protein lysate was
separated by 9% SDS-PAGE and transferred onto a PVDF membrane (EMD
Millipore). The membranes were blocked with 5% non-fat dry milk for
2 h at room temperature. The membranes were incubated with a
primary antibody at 4°C overnight, followed by incubation with a
secondary antibody (HRP-conjugated goat anti-Rabbit IgG; 1:2,000;
cat. no. ZB2301; OriGene Technologies, Inc.) for 1 h at 37°C. The
protein signals were detected by DAB (OriGene Technologies, Inc.).
Primary antibodies included, anti-E cadherin antibody (1:300; cat.
no. PB0583; Wuhan Boster Biological Technology, Ltd.), anti-N
cadherin antibody (1:200; cat. no. BM-1573; Wuhan Boster Biological
Technology, Ltd.), vimentin (1:100; cat. no. BM0135; Wuhan Boster
Biological Technology, Ltd.) and β-actin (1:1,000, cat. no. TA-09;
OriGene Technologies, Inc.). The intensity of bands were calculated
using Tanon 1600 Gel Imaging system (Tanon Sciences and Technology
Co., Ltd.).
Immunofluorescence double
staining
CRC cells were fixed in 4% paraformaldehyde for 15
min at room temperature. CRC cells were incubated with 10% normal
goat serum (OriGene Technologies, Inc.) for 2 h at room
temperature. Then, CRC cells were incubated with primary antibodies
overnight at 4°C. CRC cells were double-stained (60 min at room
temperature) with a mixture of primary antibodies against
E-cadherin (1:100; cat. no. PB0583; Wuhan Boster Biological
Technology, Ltd.) and Vimentin (1:50; cat. no. BM4029; Wuhan Boster
Biological Technology, Ltd.), and cultured with a mixture of the
fluorescent secondary antibodies FITC (1:75; cat. no. ZF-0311; goat
anti-rabbit; OriGene Technologies, Inc.) and tetramethylrhodamine
(1:90; cat. no. ZF-0313; goat anti-mouse; OriGene Technologies,
Inc.). The cells were then washed with PBS and observed using a
fluorescence-inverted microscope (magnification, ×400). Image-Pro
Plus 6.0 (Media Cybernetics, Inc.) was used to evaluate the
densitometry.
Statistical analysis
Pearson's χ2 test was used to determine
the correlations of clinicopathological parameters. All data are
representative of ≥3 independent experiments. Kaplan-Meier plots
and log-rank tests were used to analyze 5-year overall survival
rate. Student's t-test was used to detect differences between two
groups, and one-way ANOVA was used to determine the differences
among multiple groups followed by a Student-Newman-Keuls-q post hoc
test. Kendall rank correlation coefficient was used to compare
correlations among HER family members. Statistical analysis was
performed using SPSS v.21.0 software (IBM Corp.). Data are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HER family members in 73
patients with CRC
A total of 90 patients were recorded; however, 17
patients were not followed up. IHC was used to examine the
expression of HER family members in primary tumor tissues. The
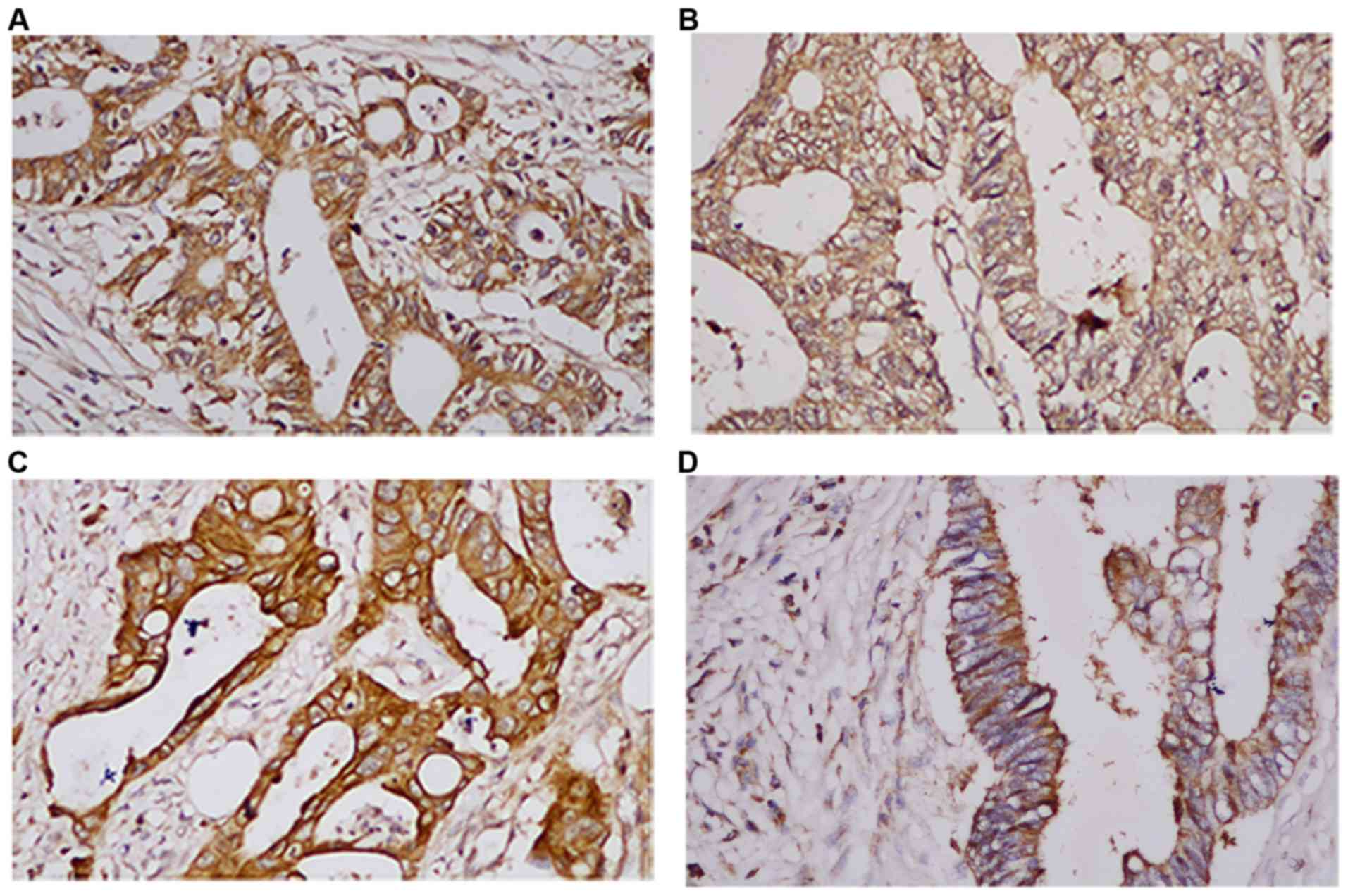
positive expression rates of EGFR, HER2, HER3 and HER4 were 72.1,
45.2, 43.8 and 34.2%, respectively (Fig. 1). The associations among EGFR,
HER2, HER3 and HER4 are summarized in Table III. The present results suggested
that HER4 expression was not associated with EGFR, HER2 or HER3
expression.
 | Table III.Correlation among the expression of
EGFR, HER2, HER3 and HER4 in CRC (n=73). |
Table III.
Correlation among the expression of
EGFR, HER2, HER3 and HER4 in CRC (n=73).
|
| EGFR | HER2 | HER3 |
|---|
| HER2 | 0.273
(0.020)a | – | – |
| HER3 | 0.439
(0.001)b | 0.362
(0.002)b | – |
| HER4 | −0.052 (0.662) | 0.099 (0.403) | −0.056 (0.636) |
Positive expression of HER4 is
positively associated with lymph node metastasis in patients with
CRC
The present study investigated the relationship
between HER4 expression and clinicopathological features, including
gender, age, tumor site, T stage, TNM stage and lymph node
metastasis (Table IV). The
present results suggested that positive rates of HER4 expression
were higher in patients with lymph node metastasis than those
without lymph node metastasis (Table
IV; 60.0 vs. 40.0%; P=0.039). Additionally, staining
experiments showed that HER4 can be found the in cell membrane,
cytoplasm and nucleus of CRC (Fig.
S3).
 | Table IV.Association between
clinicopathological characteristics and HER4 expression in 73
patients with CRC. |
Table IV.
Association between
clinicopathological characteristics and HER4 expression in 73
patients with CRC.
|
|
| HER4 expression in
CRC |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Number of patients
(%) | Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Sex |
|
|
| 0.680 | 0.282 |
|
Male | 36 (49.3) | 22 (45.8) | 14 (56.0) |
|
|
|
Female | 37 (50.7) | 26 (54.2) | 11 (44.0) |
|
|
| Age |
|
|
| 0.00 | 0.594 |
| ≤60
years | 38 (52.1) | 25 (52.1) | 13 (52.0) |
|
|
| >60
years | 35 (47.9) | 23 (47.9) | 12 (48.0) |
|
|
| Tumor site |
|
|
| 0.089 | 0.485 |
|
Colon | 45 (61.6) | 29 (60.4) | 16 (64.0) |
|
|
|
Rectum | 28 (38.4) | 19 (39.6) | 9
(36.0) |
|
|
| T stage |
|
|
| 0.978 | 0.807 |
|
T1 | 2
(2.7) | 1
(2.1) | 1
(4.0) |
|
|
|
T2 | 14 (19.2) | 10 (20.8) | 4
(16.0) |
|
|
|
T3 | 22 (30.1) | 13 (27.1) | 9
(36.0) |
|
|
|
T4 | 35 (47.9) | 24 (50.0) | 11 (44.0) |
|
|
| TNM stage |
|
|
| 1.029 | 0.222 |
|
I+II | 41 (56.2) | 29 (60.4) | 12 (48.0) |
|
|
|
III | 32 (43.8) | 19 (39.6) | 13 (52.0) |
|
|
| Lymph node
metastasis |
|
|
| 4.035 | 0.039a |
|
Positive | 41 (56.2) | 31 (64.6) | 10 (40.0) |
|
|
|
Negative | 32 (43.8) | 17 (35.4) | 15 (60.0) |
|
|
Positive expression of HER4 is
associated with a poor 5-year survival rate
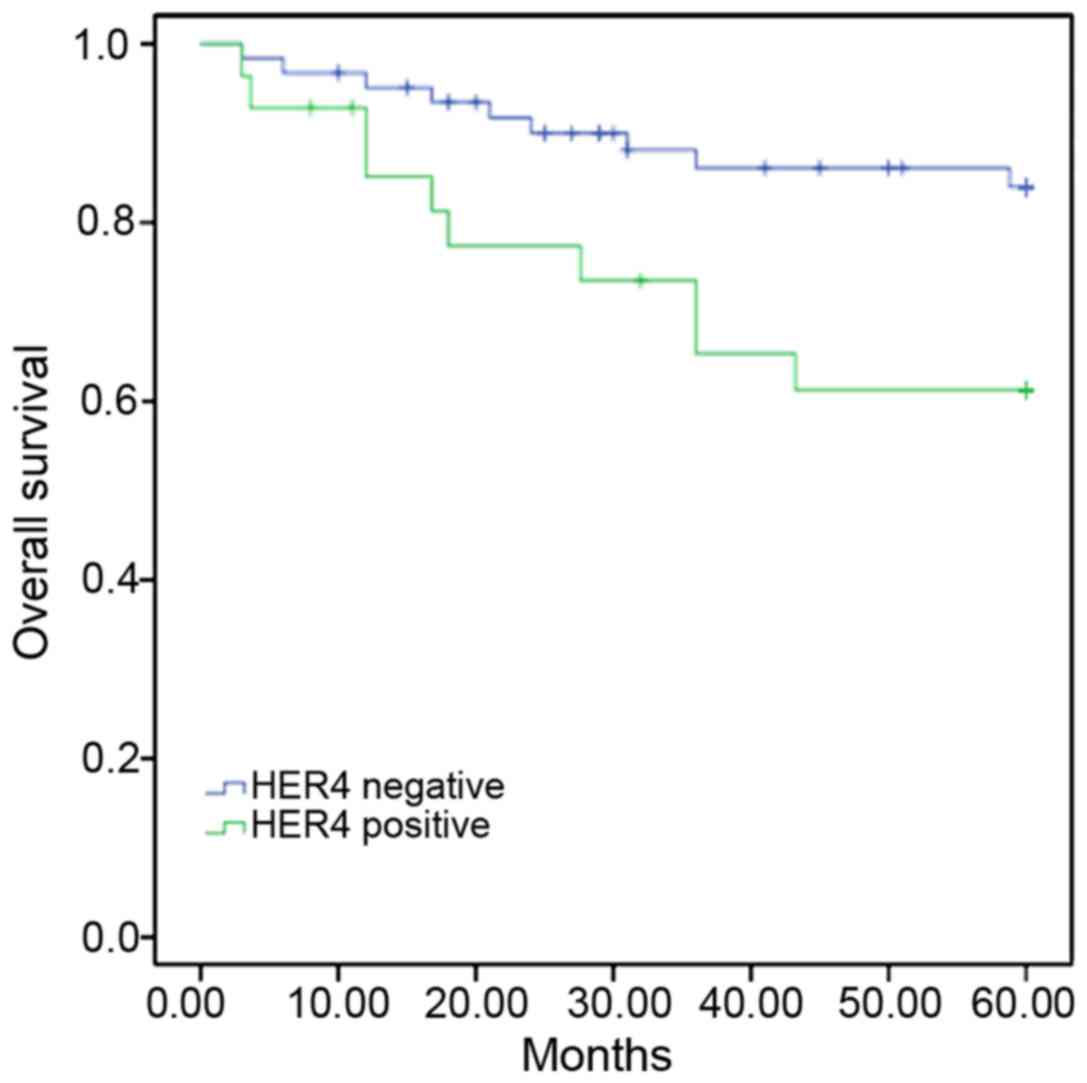
Kaplan-Meier survival analysis showed that the
5-year survival rate for patients with positive HER4 expression was
64.3%, whilst for patients with negative HER4 expression the 5-year
survival rate was 85.5% (P=0.02). The present results suggested
that the positive expression of HER4 indicated an unfavorable
prognosis in patients with CRC (Fig.
2).
HER4 knockdown suppresses CRC cell
proliferation
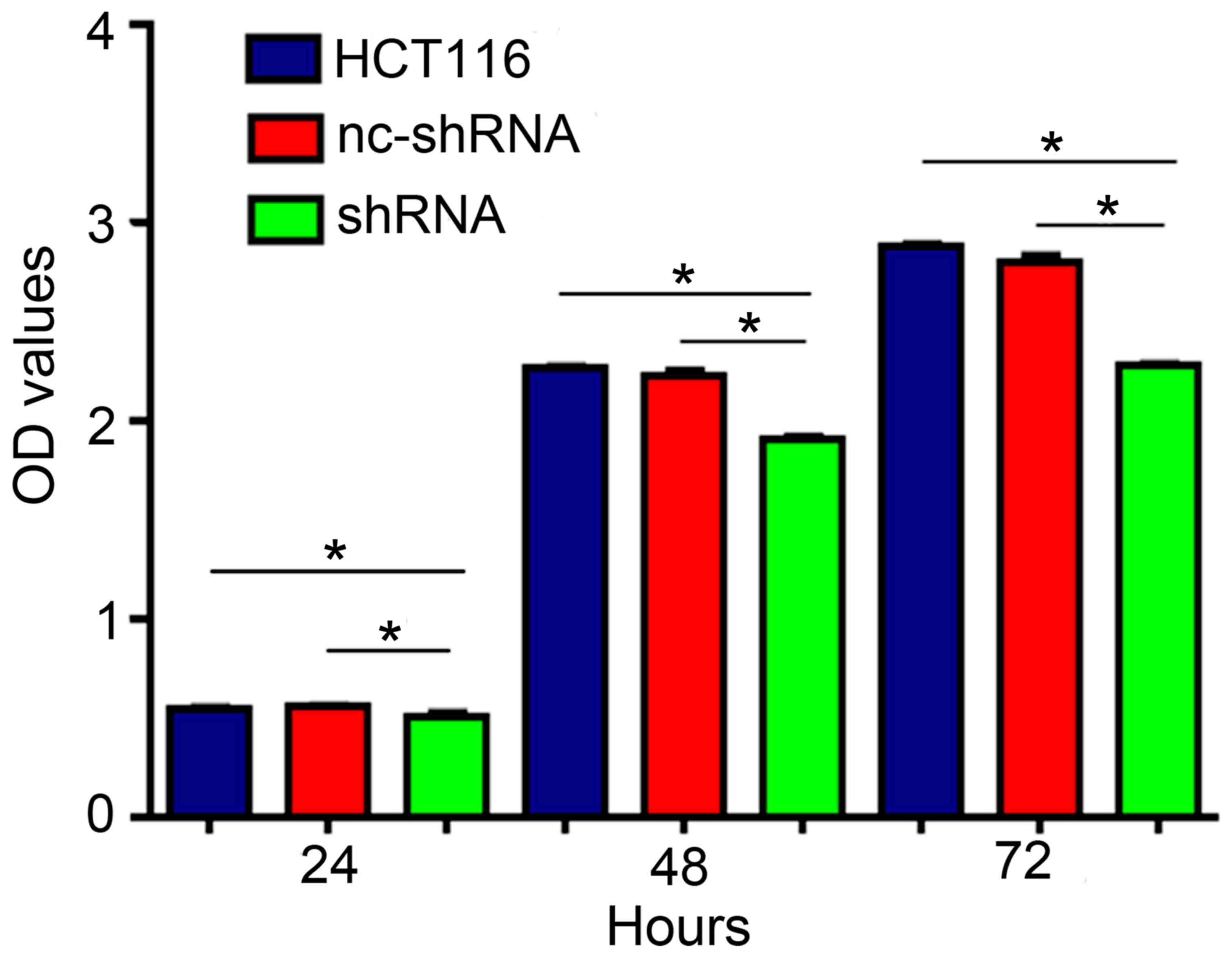
CCK-8 assay was used to determine the OD in HCT116
transfected with shRNA-HER4 and nc-shRNA, and untransfected. After
24 h the OD values were 0.552±0.006, 0.497±0.040 and 0.555±0.016,
respectively. At 48 h, the OD values were 2.268±0.014, 1.901±0.027
and 2.221±0.057. At 72 h the values were 2.875±0.031, 2.276±0.021
and 2.221±0.061. The present results indicated that knockdown of
HER4 significantly inhibited CRC cell proliferation (Fig. 3).
HER4 knockdown increases apoptosis and
the adhesive ability of CRC cells
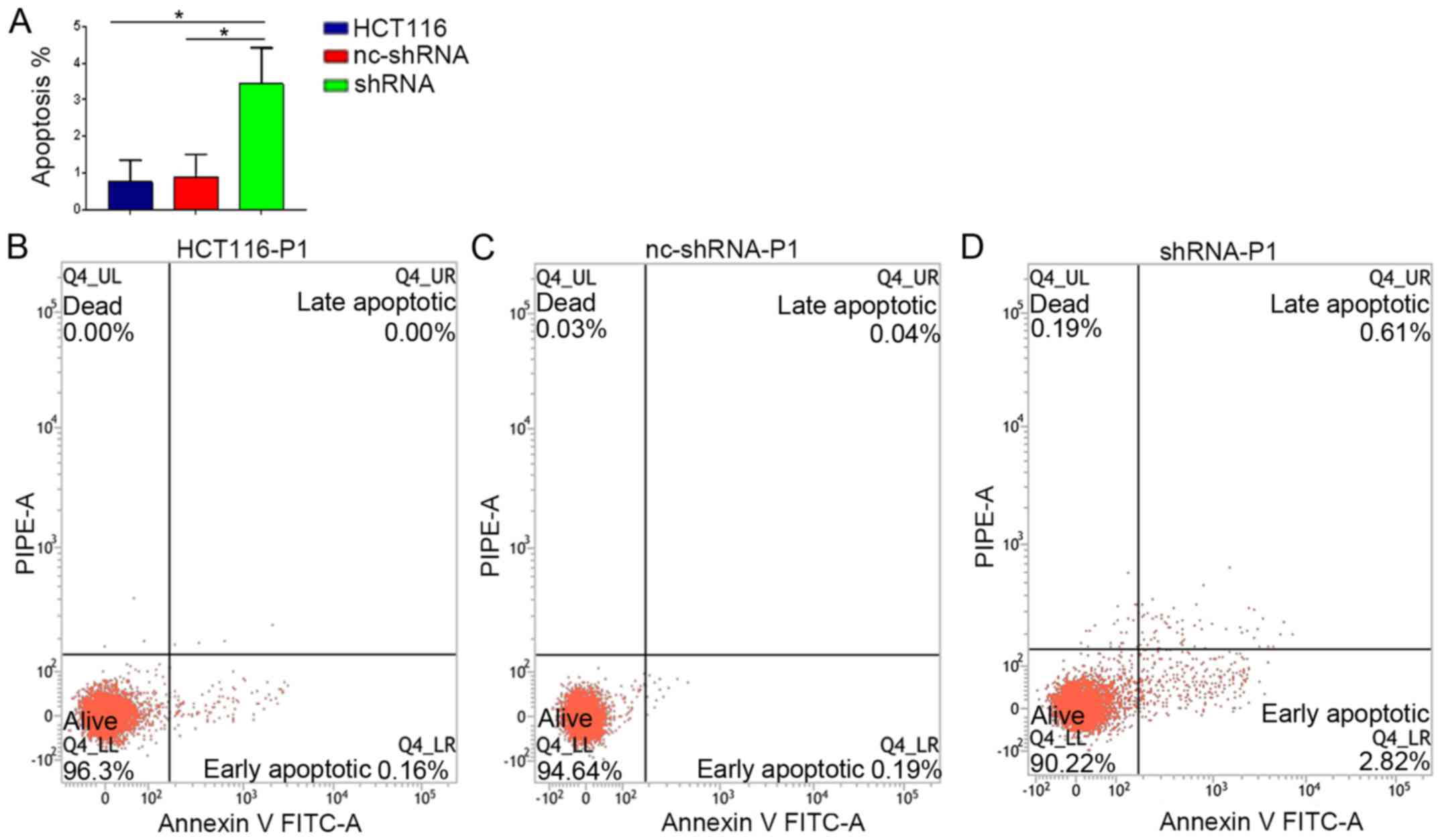
Flow cytometry showed that the rate of apoptosis in
shRNA-HER4 was 3.43±0.67%, which was significantly higher compared
with the other experimental groups (Fig. 4; P<0.05). The present results
suggested that HER4 knockdown enhanced apoptosis in CRC cells.
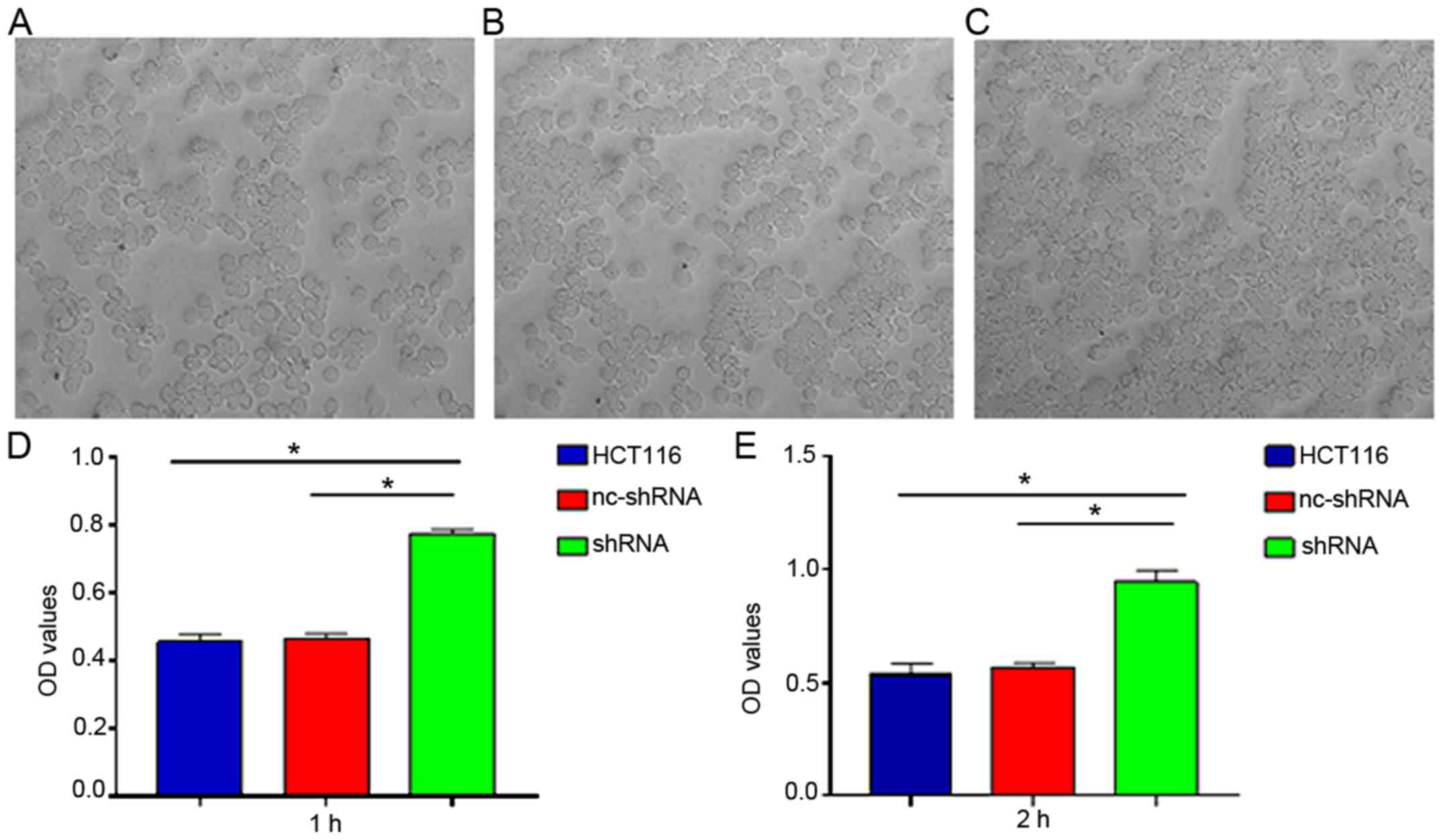
Cell adhesion assays indicated that cell adhesion in
cells transfected with shRNA-HER4 was enhanced compared to that in
the HCT116 and nc-shRNA groups (Fig.
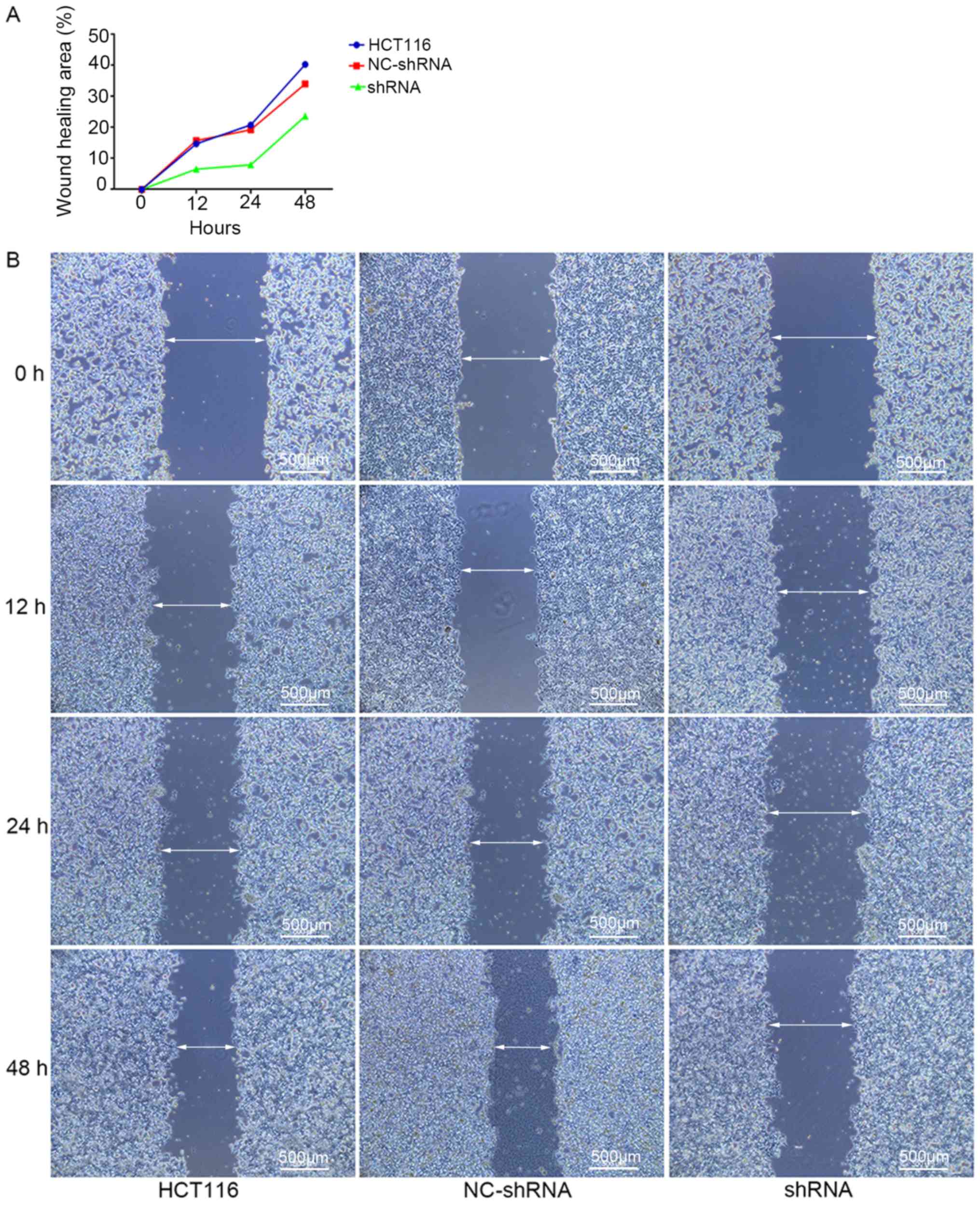
5; P<0.05). A wound healing assay demonstrated that cell
migration in shRNA-HER4 was inhibited compared with the other
experimental groups (Fig. 6;
P<0.05). The present results suggested that HER4 increased
cellular migration and inhibited adhesion activity, which may
increase migration in malignant tumors.
HER4 knockdown inhibits the protein
expression levels of EMT associated factors
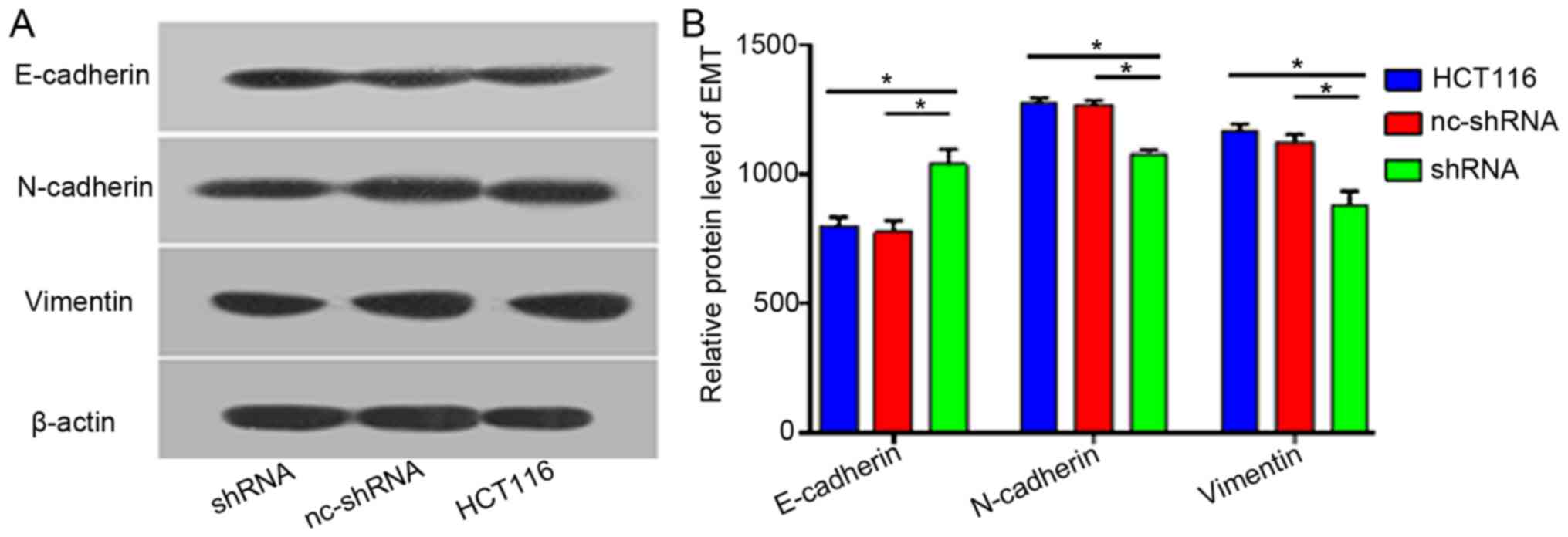
Western blotting was performed to examine
EMT-related proteins. The present study found that vimentin and
N-cadherin expression was decreased, while E-cadherin expression
was increased after HER4 knockdown compared with the other
experimental groups (Fig. 7).
However, no significant differences were found between the HCT116
group and nc-shRNA group (Fig. 7).
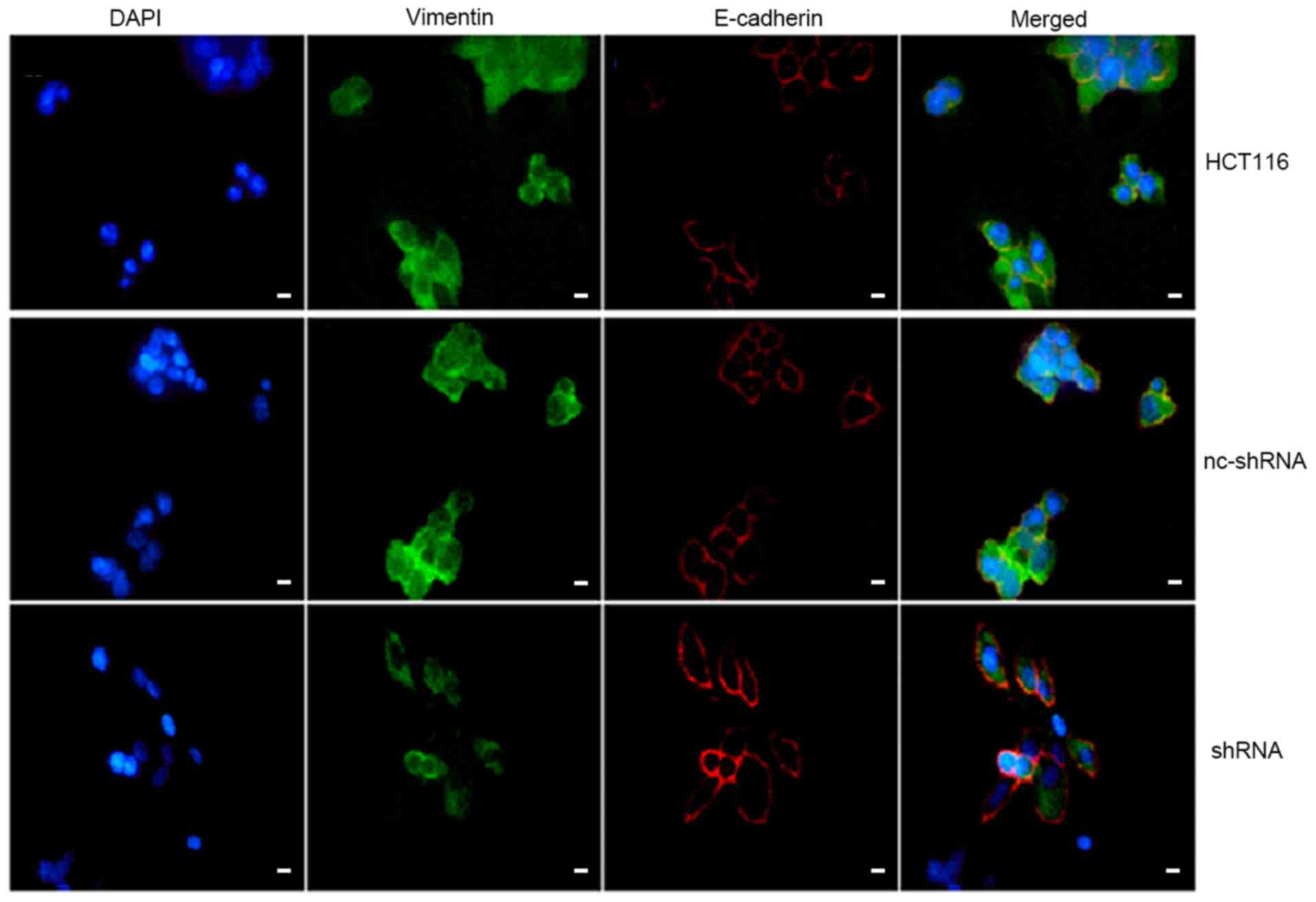
Immunofluorescent double-staining was conducted to identify and
measure E-cadherin and vimentin expression in CRC cells. HER4
knockdown led to increased E-cadherin expression levels and
decreased vimentin expression levels (Fig. 8). These results suggested that
knockdown of HER4 suppressed EMT in CRC cells.
Discussion
In the present study, although EGFR, HER2, HER3 and
HER4 showed varying expression in CRC, positive expression of HER4
was not associated with EGFR, HER2 and HER3. The positive rate of
HER4 expression was higher in patients with lymph node metastasis,
suggesting that positive HER4 expression may indicate an
unfavorable outcome in patients with CRC. The present analyses
suggested that positive HER4 expression enhanced proliferation,
reduced apoptosis and the adhesive ability of CRC cells, and
promoted the expression of biomarkers considered to be associated
with EMT.
In the present study, the positive expression rates
of the HER family members EGFR, HER2, HER3 and HER4 were 72.1,
45.2, 43.8 and 34.2%, respectively. The present results are
consistent with those of previous studies showing that the
expression of HER family members in CRC varies widely (7,18,21–23).
These discrepancies can be explained by differences in the mouse
model experiment of methodology, mouse model age, antibodies and
subject ethnicity in the studies (21). Expression of HER1 is known to be
closely associated with HER2 expression (24–27).
In breast cancer, Smad3, which can convert transforming growth
factor-β into a carcinogenic factor, is activated when HER1 and
HER2 are co-expressed, resulting in tumor progression (24). The functional tyrosine domain of
HER2 activates HER3 or HER1, promoting tumor development via
activation of signaling pathways such as STAT3,
RAS-mitogen-activated protein kinase and PI3K (25–27).
Previous studies showed that the expression of HER1, HER2 and HER3
exhibits complex interactions (24–27).
The present results suggested that HER4 had no significant
association with HER1, HER2 or HER3 in the tissue collected from
patients with CRC. Although Lee et al (28) showed that co-expression of HER2 and
HER4 resulted in a shorter prognosis in CRC, they did not observe a
relationship between HER2 and HER4 expression; which is consistent
with the present results. Therefore, further experimentation is
required to test and validate the relationship among HER family
members in CRC. The present results revealed that HER4 expression
indicated an unfavorable prognosis in patients with CRC, and the
positive rate of HER4 expression was higher in cases with lymph
node metastasis. Recent studies showed that HER family members play
a negative role in tumorigenesis (29,30).
Kountourakis et al (23)
suggested that positive HER4 expression on membranes could promote
lymph node metastasis. Wang et al (31) found that upregulation of HER4
indicated a poorer prognosis and higher risk for recurrence in
osteosarcoma. The present results showed that the 5-year survival
rate for patients with positive HER4 expression was 64.3%, while
that for patients with negative HER4 expression was 85.5%;
therefore, HER4 expression could indicate an unfavorable prognosis
in patients with CRC. Furthermore, high expression of HER4
increased the risk of lymph node metastasis. However, the
underlying mechanism remains unclear.
The present study found that HER4 can be found in
cell membrane, cytoplasm and nucleus of CRC, which is consistent
with previous results from Yun et al (32). Baiocchi et al (22) found that HER4 can be expressed in
cytoplasm and cell membrane of cancer cells. The discrepancy in
results between the studies could be due to the use of different
antibodies, the present study used anti-c-HER4 antibody (cat. no.
ARG52859, arigo Biolaboratories Corp.), Yun et al (32) used HER4 (Thermo Fisher Scientific,
Inc.), while Baiocchi et al (22) used ErbB4 from Lab Vision Corp.
(cat. no. RB-9045). Furthermore, the expression of different HER4
isoforms (CYT1 and CYT2) could impact HER4 expression localization,
as the CYT2 isoform can enter the nucleus easily, but the CYT1
isoform cannot translocate into the nucleus (32).
Metastasis is a critical factor in the prognosis of
patients with tumors (33). In
osteosarcoma, knockdown of HER4 inhibits proliferation and
tumorigenesis, induces apoptosis both in vitro and in
vivo, and enhances the sensitivity to the chemotherapeutics
methotrexate and doxorubicin (11). The present results showed that
silencing of HER4 expression decreased proliferation and increased
apoptosis of HCT116 cells, indicating that HER4 may be a valuable
biomarker and potential target for CRC therapy. Therefore, HER4 may
function as a carcinogenic factor in tumorigenesis.
EMT is a key step in tumor metastasis (13,14,34).
Previous studies have reported that microRNA-551b binds to the 3′
untranslated region of HER4 and inhibits HER4 expression, reduces
EMT and decreases distal metastasis in gastric cancer (35). A previous study has also confirmed
that activated fatty acid synthase increases the expression of
HER1, HER2 and HER4, and promotes EMT, resulting in invasion and
migration induction in breast cancer (36). The present results were consistent
with those of previous studies (35,36).
In the present study, E-cadherin expression was upregulated after
knockdown of HER4, while N-cadherin and vimentin expression levels
were downregulated, suggesting that HER4 expression promotes the
metastasis of CRC through EMT. However, this mechanism has not yet
been fully understood and requires further analysis.
The main limitation of the present study was the
small clinical sample size. In addition, no relationship between
the survival of patients and the expression of EGFR, HER2 and HER3
was detected. Positive HER4 expression was found in tissues of
patients with CRC using IHC; however, the present study did not
describe the results of the immunostaining in detail. Furthermore,
the metastasis-free survival time was not recorded, thus the
relationship between HER4 expression and metastasis-free survival
was not examined.
With regards to the techniques used in the present
study, no MTT assay was performed to detect cell proliferation.
Furthermore, cells used in the wound healing assay were
supplemented with 2% FBS rather than being serum-starved, and the
wound healing assay was used solely to detect cell migration. In
addition, the present study only investigated the effects of
positive HER4 expression on cell proliferation, apoptosis, adhesion
and EMT in CRC cells, but did not evaluate metastasis.
In conclusion, positive expression of HER4 was found
to be associated with lymph node metastasis and could indicate an
unfavorable prognosis in patients with CRC. HER4 knockdown may
inhibit the growth, survival and migration of CRC cells through
EMT. Therefore, the modulation of HER4 expression may be an
effective therapeutic strategy for patients with CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Hebei Province of China (grant. no. H2016307010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ, HW and YJ conceived and designed the
experiments. XJ, JY, LG and YG conducted experiments. XJ, BW, ZL,
WZ, LG and YJ performed data analysis and wrote the paper. All
authors discussed the results and commented on the manuscript.
Ethics approval and consent to
participate
All procedures in studies involving human
participants were performed in accordance with the ethical
standards of the institutional research committee (the Fourth
Hospital of Hebei Medical University) and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards. The participants signed an extensive informed consent
form after being informed about the benefits and risks of the
procedure in the present study.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Lau JY, Goh KL and Leung WK; Asia
Pacific Working Group on Colorectal Cancer, : Increasing incidence
of colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dawson H and Lugli A: Molecular and
pathogenetic aspects of tumor budding in colorectal cancer. Front
Med (Lausanne). 2:112015.PubMed/NCBI
|
|
4
|
Roskoski R: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rego RL, Foster NR, Smyrk TC, Le M,
O'Connell MJ, Sargent DJ, Windschitl H and Sinicrope FA: Prognostic
effect of activated EGFR expression in human colon carcinomas:
Comparison with EGFR status. Br J Cancer. 102:165–172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapitanović S, Radosević S, Kapitanović M,
Andelinović S, Ferencić Z, Tavassoli M, Primorać D, Sonicki Z,
Spaventi S, Pavelic K and Spaventi R: The expression of
p185(HER-2/neu) correlates with the stage of disease and survival
in colorectal cancer. Gastroenterology. 112:1103–1113. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsui K, Yonezawa M, Tatsuguchi A, Shinji
S, Gudis K, Tanaka S, Fujimori S and Sakamoto C: Localization of
phosphorylated ErbB1-4 and heregulin in colorectal cancer. BMC
Cancer. 14:8632014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machleidt A, Buchholz S, Diermeier-Daucher
S, Zeman F, Ortmann O and Brockhoff G: The prognostic value of Her4
receptor isoform expression in triple-negative and Her2 positive
breast cancer patients. BMC Cancer. 13:4372013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Göthlin EA, Tina E, Wegman P, Stål O,
Fransén K, Fornander T and Wingren S: HER4 tumor expression in
breast cancer patients randomized to treatment with or without
tamoxifen. Int J Oncol. 47:1311–1120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nielsen TO, Poulsen SS, Journe F, Ghanem G
and Sorensen BS: HER4 and its cytoplasmic isoforms are associated
with progression-free survival of malignant melanoma. Melanoma Res.
24:88–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Sun W, Sun M, Fu Z, Zhou C, Wang
C, Zuo D, Zhou Z, Wang G, Zhang T, et al: HER4 promotes cell
survival and chemoresistance in osteosarcoma via interaction with
NDRG1. Biochim Biophys Acta Mol Basis Dis. 1864:1839–1849. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Duan Z, Jia Y, Ren C, Lv J, Guo P,
Zhao W, Wang B, Zhang S, Li Y and Li Z: HER4 isoform CYT2 and its
ligand NRG1III are expressed at high levels in human colorectal
cancer. Oncol Lett. 15:6629–6635. 2018.PubMed/NCBI
|
|
13
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savagner P, Boyer B, Valles AM, Jouanneau
J and Thiery JP: Modulations of the epithelial phenotype during
embryogenesis and cancer progression. Cancer Treat Res. 71:229–249.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vu T and Datta PK: Regulation of EMT in
colorectal cancer: A culprit in metastasis. Cancers (Basel).
9(pii): E1712017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobs TW, Gown AM, Yaziji H, Barnes MJ
and Schnitt SJ: Specificity of HercepTest in determining HER-2/neu
status of breast cancers using the United States Food and Drug
Administration-approved scoring system. J Clin Oncol. 17:1983–1987.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams CS, Bernard JK, Demory Beckler M,
Almohazey D, Washington MK, Smith JJ and Frey MR: ERBB4 is
over-expressed in human colon cancer and enhances cellular
transformation. Carcinogenesis. 36:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang KK, Ramnarayanan K, Zhu F,
Srivastava S, Xu C, Tan ALK, Lee M, Tay S, Das K, Xing M, et al:
Genomic and epigenomic profiling of high-risk intestinal metaplasia
reveals molecular determinants of progression to gastric cancer.
Cancer Cell. 33:137–150.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tiwari A, Mukherjee B, Hassan MK,
Pattanaik N, Jaiswal AM and Dixit M: Reduced FRG1 expression
promotes prostate cancer progression and affects prostate cancer
cell migration and invasion. BMC Cancer. 19:3462019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ljuslinder I, Malmer B,
Isaksson-Mettävainio M, Oberg A, Henriksson R, Stenling R and
Palmqvist R: ErbB 1–4 expression alterations in primary colorectal
cancers and their corresponding metastases. Anticancer Res.
29:1489–1494. 2009.PubMed/NCBI
|
|
22
|
Baiocchi G, Lopes A, Coudry RA, Rossi BM,
Soares FA, Aguiar S, Guimarães GC, Ferreira FO and Nakagawa WT:
ErbB family immunohistochemical expression in colorectal cancer
patients with higher risk of recurrence after radical surgery. Int
J Colorectal Dis. 24:1059–1068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kountourakis P, Pavlakis K, Psyrri A,
Rontogianni D, Xiros N, Patsouris E, Pectasides D and Economopoulos
T: Prognostic significance of HER3 and HER4 protein expression in
colorectal adenocarcinomas. BMC Cancer. 6:462006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang F, Shi Q, Li Y, Xu L, Xu C, Chen F,
Wang H, Liao H, Chang Z, Liu F, et al: HER2/EGFR-AKT signaling
switches TGF-β from inhibiting cell proliferation to promoting cell
migration in breast cancer. Cancer Res. 78:6073–6085. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddiqa A, Long LM, Li L, Marciniak RA and
Kazhdan I: Expression of HER-2 in MCF-7 breast cancer cells
modulates anti-apoptotic proteins Survivin and Bcl-2 via the
extracellular signal-related kinase (ERK) and phosphoinositide-3
kinase (PI3K) signalling pathways. BMC Cancer. 8:1292008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Béguelin W, Díaz Flaqué MC, Proietti CJ,
Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH,
Schillaci R and Elizalde PV: Progesterone receptor induces ErbB-2
nuclear translocation to promote breast cancer growth via a novel
transcriptional effect: ErbB-2 function as a coactivator of Stat3.
Mol Cell Biol. 30:5456–5472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lo HW: Targeting Ras-RAF-ERK and its
interactive pathways as a novel therapy for malignant gliomas. Curr
Cancer Drug Targets. 10:840–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JC, Wang ST, Chow NH and Yang HB:
Investigation of the prognostic value of coexpressed erbB family
members for the survival of colorectal cancer patients after
curative surgery. Eur J Cancer. 38:1065–1071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donoghue JF, Kerr LT, Alexander NW,
Greenall SA, Longano AB, Gottardo NG, Wang R, Tabar V, Adams TE,
Mischel PS and Johns TG: Activation of ERBB4 in glioblastoma can
contribute to increased tumorigenicity and influence therapeutic
response. Cancers (Basel). 10(pii): E2432018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi J, Li F, Yao X, Mou T, Xu Z, Han Z,
Chen S, Li W, Yu J, Qi X, et al: The HER4-YAP1 axis promotes
trastuzumab resistance in HER2-positive gastric cancer by inducing
epithelial and mesenchymal transition. Oncogene. 37:3022–3038.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Zhao HF, Yao TF and Gong H:
Advanced development of ErbB family-targeted therapies in
osteosarcoma treatment. Invest New Drugs. 37:175–183. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yun S, Kwak Y, Nam SK, Seo AN, Oh HK, Kim
DW, Kang SB and Lee HS: Ligand-independent epidermal growth factor
receptor overexpression correlates with poor prognosis in
colorectal cancer. Cancer Res Treat. 50:1351–1361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sulaiman A, Li L and Wang L: E-cadherin
adhesion-mediated Wnt activation for mesoderm specification in
human embryonic stem cells needs a soft mattress. Stem Cell
Investig. 3:772016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song G, Zhang H, Chen C, Gong L, Chen B,
Zhao S, Shi J, Xu J and Ye Z: MiR-551b regulates
epithelial-mesenchymal transition and metastasis of gastric cancer
by inhibiting ERBB4 expression. Oncotarget. 8:45725–45735.
2017.PubMed/NCBI
|
|
36
|
Chen T, Zhou L, Li H, Tian Y, Li J, Dong
L, Zhao Y and Wei D: Fatty acid synthase affects expression of ErbB
receptors in epithelial to mesenchymal transition of breast cancer
cells and invasive ductal carcinoma. Oncol Lett. 14:5934–5946.
2017.PubMed/NCBI
|