|
1
|
Chacko S and Samanta S: ‘Hepatocellular
carcinoma: A life-threatening disease’. Biomed Pharmacother.
84:1679–1688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sayiner M, Golabi P and Younossi ZM:
Disease burden of hepatocellular carcinoma: A global perspective.
Dig Dis Sci. 64:910–917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
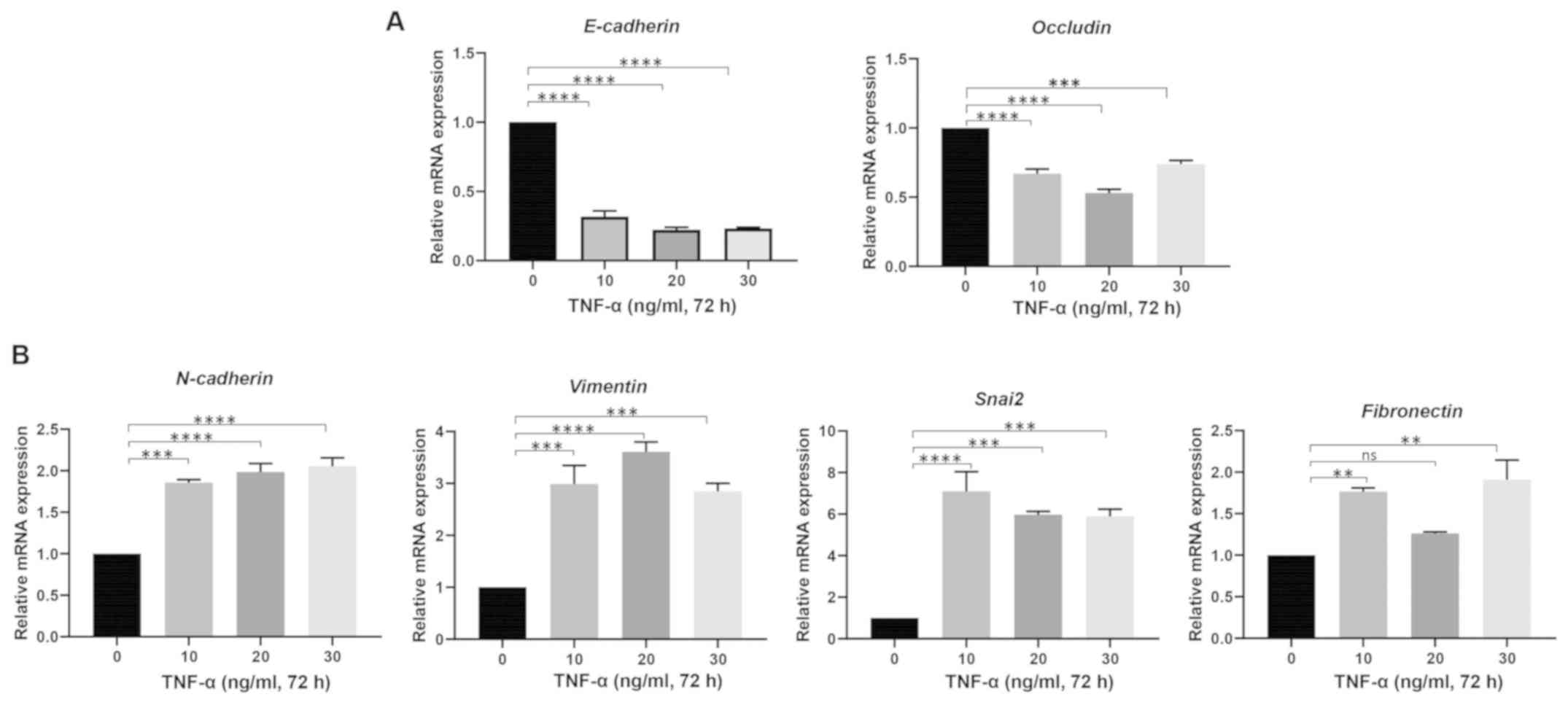
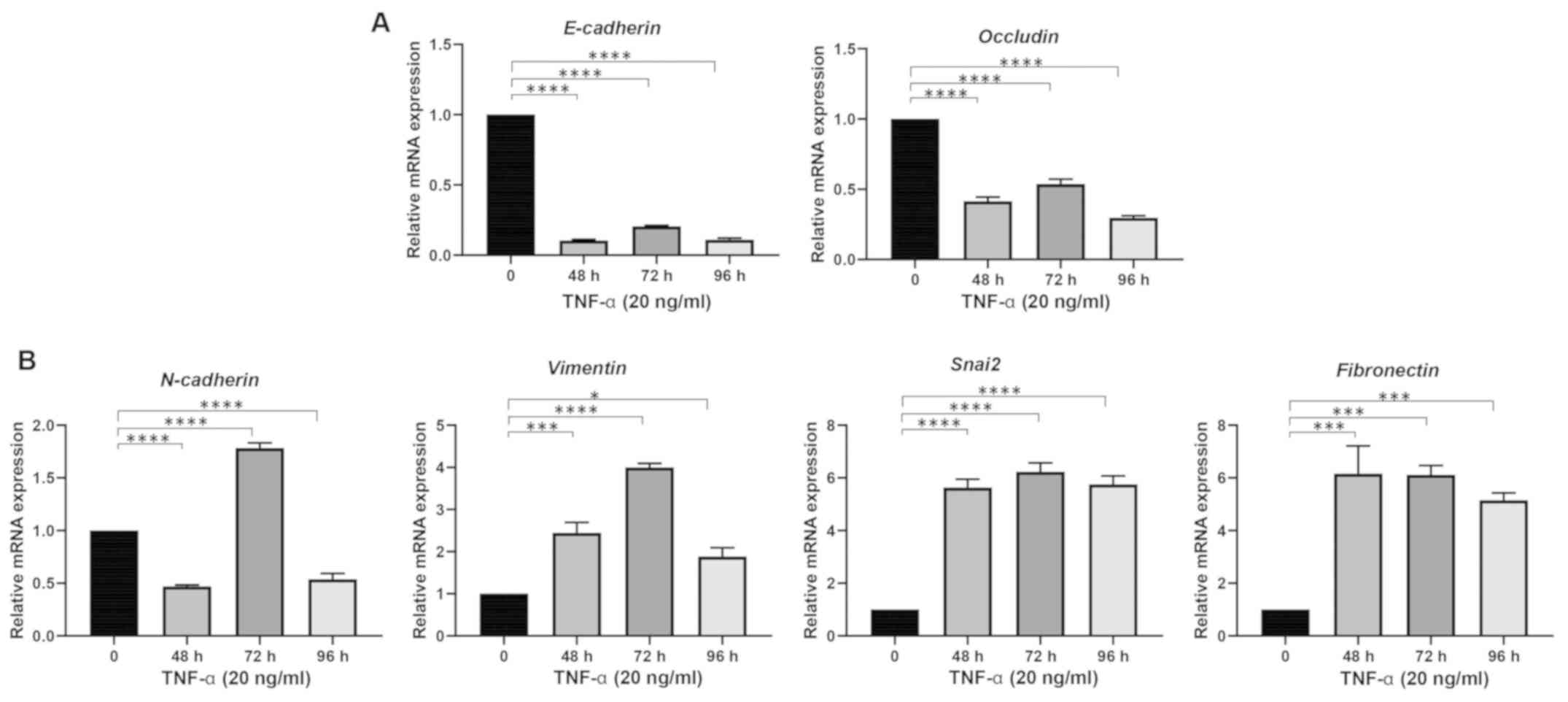
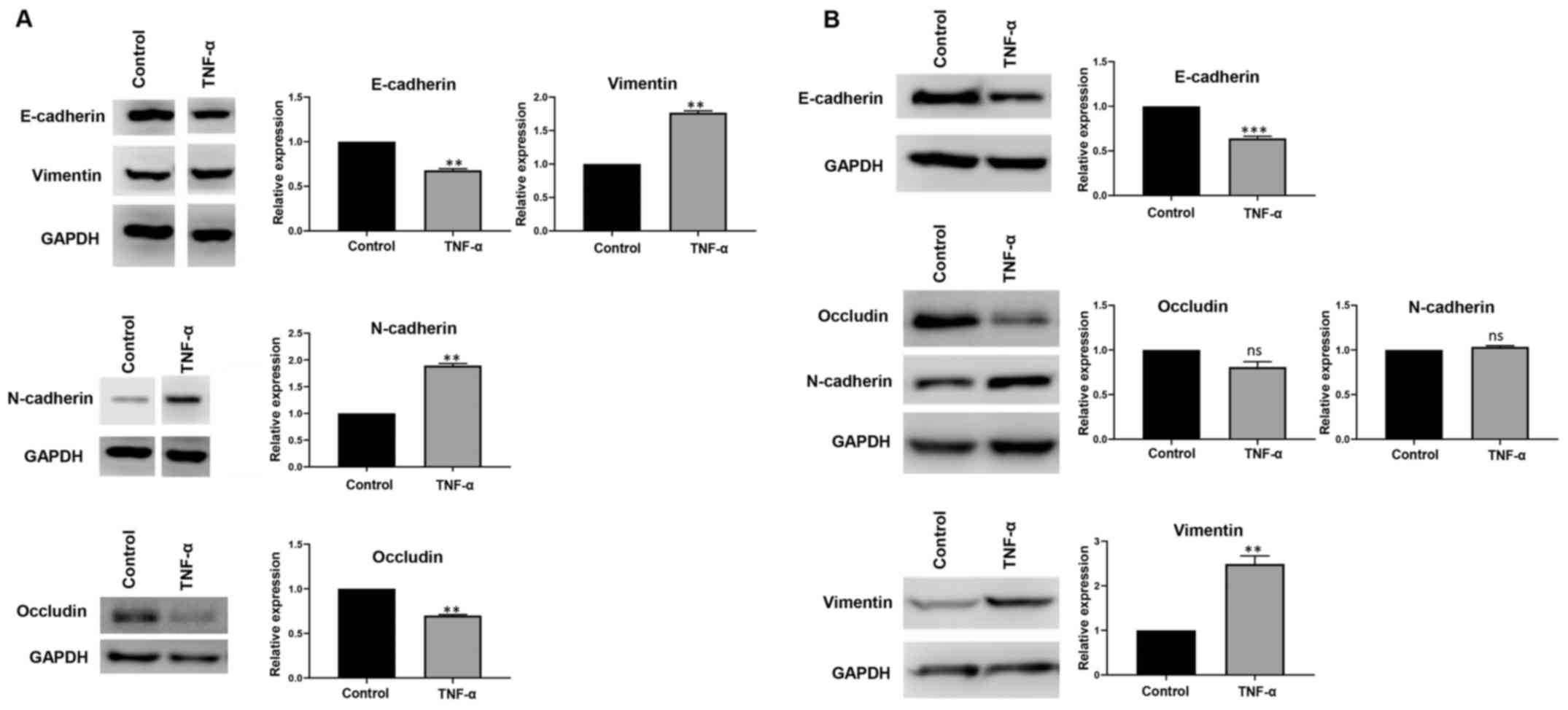
Kudo M: A new era of systemic therapy for
hepatocellular carcinoma with regorafenib and lenvatinib. Liver
Cancer. 6:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mir N, Jayachandran A, Dhungel B, Shrestha
R and Steel JC: Epithelial-to-mesenchymal transition: A Mediator of
sorafenib resistance in advanced hepatocellular carcinoma. Curr
Cancer Drug Targets. 17:698–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
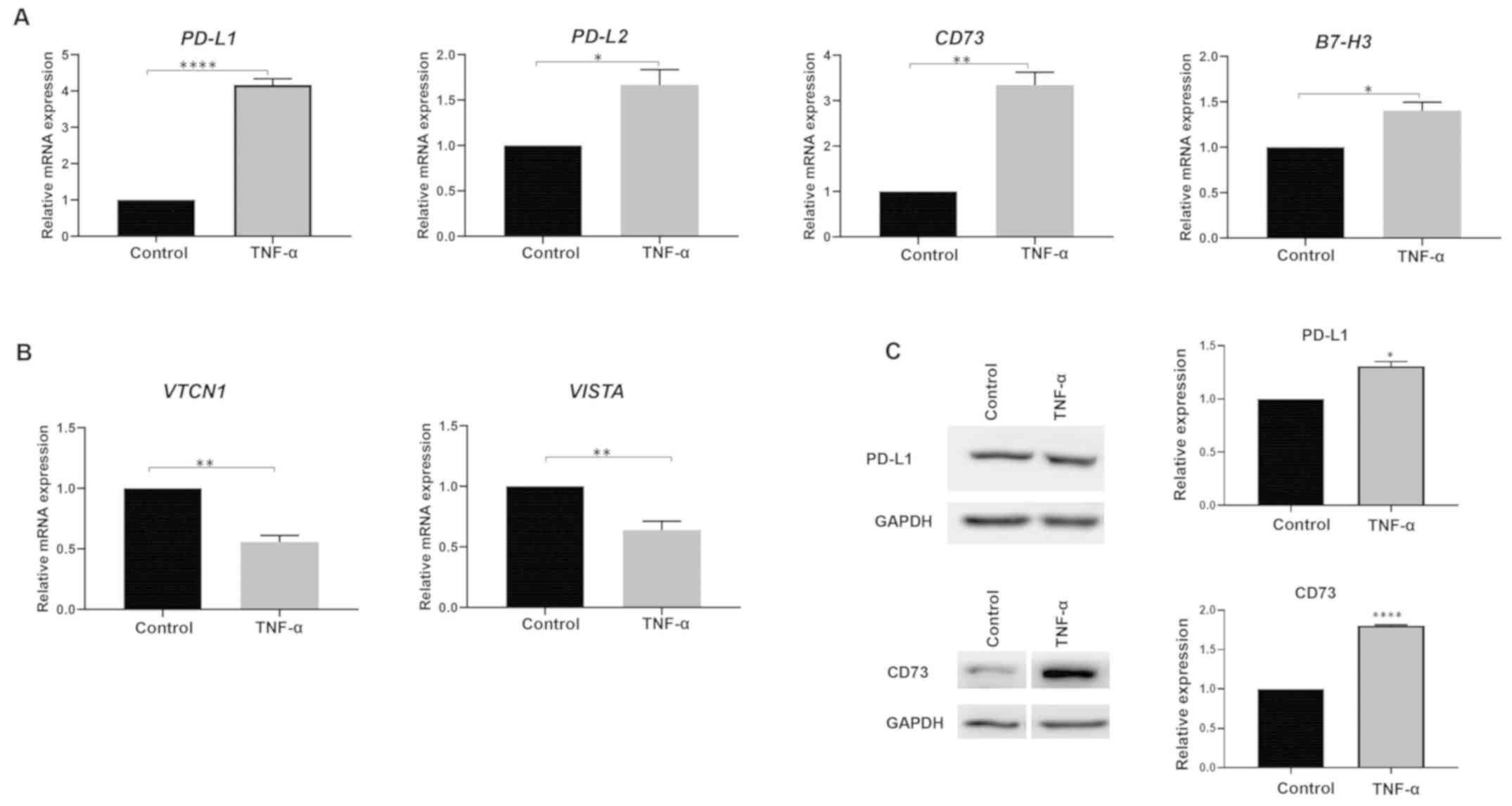
|
|
8
|
Shrestha R, Prithviraj P, Anaka M, Bridle
KR, Crawford DH, Dhungel B, Steel JC and Jayachandran A: Monitoring
immune checkpoint regulators as predictive biomarkers in
hepatocellular carcinoma. Front Oncol. 8:2692018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hato T, Goyal L, Greten TF, Duda DG and
Zhu AX: Immune checkpoint blockade in hepatocellular carcinoma:
Current progress and future directions. Hepatology. 60:1776–1782.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jindal A, Thadi A and Shailubhai K:
Hepatocellular carcinoma: Etiology and current and future drugs. J
Clin Exp Hepatol. 9:221–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudo M: Immune checkpoint inhibition in
hepatocellular carcinoma: Basics and ongoing clinical trials.
Oncology. 92 (Suppl 1):50–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Li N, Li F, Zhou Z, Sang J, Chen Y,
Han Q, Lv Y and Liu Z: Immune checkpoint proteins PD-1 and TIM-3
are both highly expressed in liver tissues and correlate with their
gene polymorphisms in patients with HBV-related hepatocellular
carcinoma. Medicine (Baltimore). 95:e57492016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayachandran A, Dhungel B and Steel JC:
Epithelial-to-mesenchymal plasticity of cancer stem cells:
Therapeutic targets in hepatocellular carcinoma. J Hematol Oncol.
9:742016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
David JM, Dominguez C, McCampbell KK,
Gulley JL, Schlom J and Palena C: A novel bifunctional
anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts
mesenchymalization of human lung cancer cells. OncoImmunology.
6:e13495892017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
David JM, Dominguez C and Palena C:
Pharmacological and immunological targeting of tumor
mesenchymalization. Pharmacol Ther. 170:212–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alsuliman A, Colak D, Al-Harazi O, Fitwi
H, Tulbah A, Al-Tweigeri T, Al-Alwan M and Ghebeh H: Bidirectional
crosstalk between PD-L1 expression and epithelial to mesenchymal
transition: Significance in claudin-low breast cancer cells. Mol
Cancer. 14:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noman MZ, Janji B, Abdou A, Hasmim M,
Terry S, Tan TZ, Mami-Chouaib F, Thiery JP and Chouaib S: The
immune checkpoint ligand PD-L1 is upregulated in EMT-activated
human breast cancer cells by a mechanism involving ZEB-1 and
miR-200. Oncoimmunology. 6:e12634122017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asgarova A, Asgarov K, Godet Y, Peixoto P,
Nadaradjane A, Boyer-Guittaut M, Galaine J, Guenat D, Mougey V,
Perrard J, et al: PD-L1 expression is regulated by both DNA
methylation and NF-κB during EMT signaling in non-small cell lung
carcinoma. Oncoimmunology. 7:e14231702018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imai D, Yoshizumi T, Okano S, Itoh S,
Ikegami T, Harada N, Aishima S, Oda Y and Maehara Y: IFN-γ promotes
epithelial-mesenchymal transition and the expression of PD-L1 in
pancreatic cancer. J Surg Res. 240:115–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Xiong Y, Li J, Zheng X, Zhou Q,
Turner A, Wu C, Lu B and Jiang J: PD-L1 expression promotes
epithelial to mesenchymal transition in human esophageal cancer.
Cell Physiol Biochem. 42:2267–2280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Hu J, Wang Y, Ye W, Zhang X, Ju H,
Xu D, Liu L, Ye D, Zhang L, et al: EGFR activation induced
Snail-dependent EMT and myc-dependent PD-L1 in human salivary
adenoid cystic carcinoma cells. Cell Cycle. 17:1457–1470. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y,
Huang Y, Qiu Q, Lin J, Huang X, et al: TNF-α derived from M2
tumor-associated macrophages promotes epithelial-mesenchymal
transition and cancer stemness through the Wnt/β-catenin pathway in
SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 378:41–50.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jayachandran A, Shrestha R, Dhungel B,
Huang IT, Vasconcelos MY, Morrison BJ, Ramlogan-Steel CA and Steel
JC: Murine hepatocellular carcinoma derived stem cells reveal
epithelial-to-mesenchymal plasticity. World J Stem Cells.
9:159–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aguirre-Gamboa R, Gomez-Rueda H,
Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R,
Rodriguez-Barrientos A, Tamez-Peña JG and Treviño V: SurvExpress:
An online biomarker validation tool and database for cancer gene
expression data using survival analysis. PLoS One. 8:e742502013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoshida Y, Nijman SM, Kobayashi M, Chan
JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K,
Hashimoto M, et al: Integrative transcriptome analysis reveals
common molecular subclasses of human hepatocellular carcinoma.
Cancer Res. 69:7385–7392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoshida Y, Villanueva A, Kobayashi M, Peix
J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuchiya M, Parker JS, Kono H, Matsuda M,
Fujii H and Rusyn I: Gene expression in nontumoral liver tissue and
recurrence-free survival in hepatitis C virus-positive
hepatocellular carcinoma. Mol Cancer. 9:742010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Awan FM, Naz A, Obaid A, Ali A, Ahmad J,
Anjum S and Janjua HA: Identification of Circulating Biomarker
Candidates for Hepatocellular Carcinoma (HCC): An Integrated
Prioritization Approach. PLoS One. 10:e01389132015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, et al: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Z, Shen MX, Ma DZ, Wang LY and Zha XL:
TGF-beta1-promoted epithelial-to-mesenchymal transformation and
cell adhesion contribute to TGF-beta1-enhanced cell migration in
SMMC-7721 cells. Cell Res. 13:343–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Y, Cheng Y, Guo Y, Chen J, Chen F, Luo
R and Li A: Protein kinase D2 contributes to TNF-α-induced
epithelial mesenchymal transition and invasion via the
PI3K/GSK-3β/β-catenin pathway in hepatocellular carcinoma.
Oncotarget. 7:5327–5341. 2016.PubMed/NCBI
|
|
36
|
Xu F, Jin T, Zhu Y and Dai C: Immune
checkpoint therapy in liver cancer. J Exp Clin Cancer Res.
37:1102018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M,
Meng YL, Yang AG and Wen WH: B7-H1 expression is associated with
poor prognosis in colorectal carcinoma and regulates the
proliferation and invasion of HCT116 colorectal cancer cells. PLoS
One. 8:e760122013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Calderaro J, Rousseau B, Amaddeo G, Mercey
M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay
D, et al: Programmed death ligand 1 expression in hepatocellular
carcinoma: Relationship with clinical and pathological features.
Hepatology. 64:2038–2046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Crane CA, Panner A, Murray JC, Wilson SP,
Xu H, Chen L, Simko JP, Waldman FM, Pieper RO and Parsa AT: PI(3)
kinase is associated with a mechanism of immunoresistance in breast
and prostate cancer. Oncogene. 28:306–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dey N, Crosswell HE, De P, Parsons R, Peng
Q, Su JD and Durden DL: The protein phosphatase activity of PTEN
regulates SRC family kinases and controls glioma migration. Cancer
Res. 68:1862–1871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghebeh H, Tulbah A, Mohammed S, Elkum N,
Bin Amer SM, Al-Tweigeri T and Dermime S: Expression of B7-H1 in
breast cancer patients is strongly associated with high
proliferative Ki-67-expressing tumor cells. Int J Cancer.
121:751–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Funaki S, Shintani Y, Kawamura T, Kanzaki
R, Minami M and Okumura M: Chemotherapy enhances programmed cell
death 1/ligand 1 expression via TGF-β induced epithelial
mesenchymal transition in non-small cell lung cancer. Oncol Rep.
38:2277–2284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jung HI, Jeong D, Ji S, Ahn TS, Bae SH,
Chin S, Chung JC, Kim HC, Lee MS and Baek MJ: Overexpression of
PD-L1 and PD-L2 is associated with poor prognosis in patients with
hepatocellular carcinoma. Cancer Res Treat. 49:246–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang H, Zhou X, Sun L and Mao Y:
Correlation Between PD-L2 expression and clinical outcome in solid
cancer patients: A meta-analysis. Front Oncol. 9:472019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv
LH, Wang H, Zhou Y, Jin AL, Sun YF, et al: CD73 promotes
hepatocellular carcinoma progression and metastasis via activating
PI3K/AKT signaling by inducing Rap1-mediated membrane localization
of P110β and predicts poor prognosis. J Hematol Oncol. 12:372019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sciarra A, Monteiro I, Ménétrier-Caux C,
Caux C, Gilbert B, Halkic N, La Rosa S, Romero P, Sempoux C and de
Leval L: CD73 expression in normal and pathological human
hepatobiliopancreatic tissues. Cancer Immunol Immunother.
68:467–478. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiao Z, Li X, Kang N, Yang Y, Chen C, Wu
T, Zhao M, Liu Y and Ji X: A Novel specific Anti-CD73 antibody
inhibits triple-negative breast cancer cell motility by regulating
autophagy. Int J Mol Sci. 20:E10572019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dong P, Xiong Y, Yue J, Hanley SJB and
Watari H: B7H3 as a promoter of metastasis and promising
therapeutic target. Front Oncol. 8:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kang FB, Wang L, Jia HC, Li D, Li HJ,
Zhang YG and Sun DX: B7-H3 promotes aggression and invasion of
hepatocellular carcinoma by targeting epithelial-to-mesenchymal
transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int.
15:452015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zheng Y, Liao N, Wu Y, Gao J, Li Z, Liu W,
Wang Y, Li M, Li X, Chen L, et al: High expression of B7 H2 or B7
H3 is associated with poor prognosis in hepatocellular carcinoma.
Mol Med Rep. 19:4315–4325. 2019.PubMed/NCBI
|
|
56
|
Xu ZL, Zhang Y, Wang L, Li F, Man HW, Li
PF and Shan BE: B7 H3 promotes malignant progression of muscle
invasive bladder cancer. Oncol Rep. 40:2722–2733. 2018.PubMed/NCBI
|
|
57
|
Jiang B, Zhang T, Liu F, Sun Z, Shi H, Hua
D and Yang C: The co-stimulatory molecule B7-H3 promotes the
epithelial-mesenchymal transition in colorectal cancer. Oncotarget.
7:31755–31771. 2016.PubMed/NCBI
|