Introduction
Osteosarcoma has an annual incidence rate of
5/1,000,000 individuals and a mortality rate of >50% in patients
<20 years old worldwide (1–4).
Surgery combined with radiotherapy and chemotherapy is the primary
treatment method for osteosarcoma (5). However, ≤80% of patients have poor
prognosis due to metastatic lesions that are already present at the
time of diagnosis, and the 5-year survival rate is ~30% (6). Given this poor disease prognosis, the
development of effective therapeutic strategies and pharmaceuticals
to reduce osteosarcoma malignancy are important.
Piperine is an alkaloid found in Piper nigrum
Linn and Piper longum Linn (Piper nigrum L., family
piperaceae). Piperine is used as a food flavoring and as a
traditional Chinese medicine due to its pharmacological benefits
(7,8). Moreover, piperine is used to treat
gastrointestinal disorders such as constipation and diarrhea
(9). Furthermore, it has
well-characterized anti-inflammatory (10) and antitumor effects in numerous
types of cancer, including breast, lung and liver cancer, and
lymphoma (11–14). Piperine has been reported to
dose-dependently (15–20) regulate cell growth and
differentiation via the Akt/JNK/MAPK pathway (21), and can increase cytokine production
via the mTOR signaling pathway (22).
Tumor metastasis is a complex process involving
tumor cell dissociation, extracellular matrix degradation,
infiltration and adhesion to vascular endothelial cells (23). Notably, matrix metalloproteinases
(MMPs), such as MMP-2 and MMP-9, and collagen type IV are
significantly upregulated in osteosarcoma and metastases, and are
indices of poor prognosis (24).
Moreover, MMP-2 downregulation can inhibit osteosarcoma metastasis
and infiltration (21,25). Vascular endothelial growth factor
(VEGF) is known to promote angiogenesis, and its upregulation is
correlated with poor osteosarcoma prognosis (26,27).
Furthermore, VEGF downregulation has been shown to reduce vascular
density and inhibit metastases in osteosarcoma (28).
While the antitumor effect of piperine on U2OS cells
has been reported (21), its
underlying molecular mechanisms of action are not fully understood.
As the Wnt/β-catenin signaling pathway is known to regulate cell
proliferation and differentiation (29,30),
the present study hypothesized that it may be involved in
modulating the antitumor effects of piperine. Therefore, the aim of
the present study was to test this hypothesis; the results may
provide a novel insight into the antitumor mechanism of
piperine.
Materials and methods
Chemical reagents
DMSO and MTT were purchased from Sigma-Aldrich
(Merck KGaA). Piperine (molecular weight, 285.35 kDa; National
Institutes for Food and Drug Control) was dissolved in DMSO at the
concentration of 150 µM and stored at −20°C. An Annexin V-FITC/PI
double staining cell apoptosis detection kit was obtained from
Nanjing KeyGen Biotech Co., Ltd. NQBB FBS was obtained from Wuhan
ChunDuBio Co., Ltd. Anti-MMP-2 (cat. no. 10373-2-AP; 1:1,000) was
purchased from ProteinTech Group, Inc. Anti-VEGF (cat. no. GB11034;
1:3,000), anti-c-Myc (cat. no. GB13076; 1:500), anti-cyclin D1
(cat. no. GB11079; 1:1,000), anti-cyclooxygenase-2 (COX2; cat. no.
GB11072; 1:500), anti-β-catenin (cat. no. GB11015; 1:500) and
anti-glycogen synthase kinase-3β (GSK-3β; cat. no. GB11099;
1:1,000) were purchased from Wuhan Servicebio Technology Co.,
Ltd.
Cell culture
Human osteosarcoma U2OS and 143B cells were provided
by Cheeloo College of Medicine, Shandong University. 143B cells
were identified by STR from Shanghai Cinoasia Institute, and the
results showed that the cells were not contaminated, had homology
with HOS/KHOS-240s cells and were human osteosarcoma cells. The
cells were cultured in McCoy's 5A medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS at 37°C in a 5% CO2
humidified incubator until cells reached the logarithmic growth
phase; cells were then harvested for subsequent experiments.
MTT cell viability assay
U2OS cells (4×103 cells/well) and 143B
cells (1×103 cells/well) were seeded in 96-well plates
and incubated at 37°C in a 5% CO2 humidified incubator
with different piperine concentrations (0, 50, 100 and 150 µM) for
24, 48 and 72 h. Subsequently, cell viability was determined using
an MTT kit (Cell Titer 96AQ; Promega Corporation). The supernatant
was aspirated and 0.05% DMSO (150 µl) was added to each well, and
then shake at a low speed (3.2 g) for 10 min to fully dissolve the
formazan. The optical density (OD) values of piperine-treated cells
were measured at 490 nm using an ELISA microplate reader (Rt2100c;
Rayto Life and Analytical Sciences Co., Ltd.). Inhibition rate
%=(1-OD value of experimental group/OD value of 0 µM group) ×
100%.
Flow cytometry
U2OS cells (5.0×104 cells/well) and 143B
cells (1.0×104 cells/well) were seeded in 6-well plates and
incubated at 37°C in 5% CO2 with different
concentrations of piperine (0, 50, 100 and 150 µM) for 48 h. Cells
were then harvested and 3 ml pre-chilled PBS was added at 4°C,
which were centrifugated at 337 × g for 5 min at room temperature
and 200 µl binding buffer was then used to suspend the supernatant.
Next, double fluorescence staining was performed with 20 µg/ml PI
and 5 µl Annexin V-FITC for 15 min at room temperature before
analysis with a flow cytometer (Beckman CytoFLEX; Beckman Coulter,
Inc.) and FlowJo software version 10.0.7 (Stanford University).
Annexin V-FITC (green) and PI (red) fluorescence intensities were
detected using the FITC channel (FL1) and PI channel (FL2),
respectively. Total apoptotic rate was calculated as follows: Total
apoptotic rate=early apoptotic rate + late apoptotic rate.
Transwell invasion assay
A Matrigel Transwell 24 holes with an aperture of
3.0 µm (Corning, Inc.) assay was performed to assess cell invasion.
Matrigel matrix (50 mg/l; BD Biosciences) was diluted in serum-free
medium at a 1:3 ratio to reconstitute the basement membrane. U2OS
and 143B cells (5×104 cells/ml) were separately added to
the upper Transwell chamber, and 10% FBS-containing culture medium
containing different concentrations (50–150 µM) of piperine was
added to the lower chamber. After incubation for 48 h at 37°C in 5%
CO2, the cells in the upper chamber were fixed with 75%
ethanol at room temperature for 10 min, stained with 0.1% crystal
violet for 5 min at 37°C and examined using light microscopy
(Olympus Corporation) at ×400 magnification. In total, five random
visual fields were used to count the total number of migrating
cells, and the images were analyzed using Image-Pro Plus 6.0
software (Media Cybernetics, Inc.).
Western blot analysis
U2OS and 143B cells were treated with different
concentrations of piperine (0, 50, 100 and 150 µM) for 48 h prior
to protein extraction at 37°C. After the cells were harvested and
washed with RIPA buffer (Wuhan Servicebio Technology Co., Ltd.;
cat. no. G2002) total protein from cells was extracted using a
protein extraction kit (cat. no. KGBSP002; Nanjing KeyGen Biotech
Co., Ltd.). Protein concentration was measured by bicinchonic acid
protein kit (Wuhan Servicebio Technology Co., Ltd.) Proteins (20
µg) were then separated by SDS-PAGE with 10% gels. The separated
protein bands were transferred onto nitrocellulose membranes
blocked with 5% skimmed milk/TBST(0.1% Tween-20) at 37°C for 60
min, which were probed with the respective primary antibodies
[Anti-MMP-2 (1:1,000; ProteinTech Group, Inc.) Anti-VEGF (1:3,000),
anti-c-Myc (1:500), anti-cyclin D1 (1:1,000), anti-cyclooxygenase-2
(COX2; 1:500), anti-β-catenin (1:500) and anti-glycogen synthase
kinase-3β (GSK-3β; 1:1,000; Wuhan Servicebio Technology Co., Ltd.)]
overnight at 4°C. Subsequently, the membranes were incubated with a
corresponding horseradish peroxidase-labeled secondary antibody
(1:3,000; cat. no. 23301; Wuhan Servicebio Technology Co., Ltd.)
for 30 min at room temperature, before being developed with an
enhanced chemiluminescence reagent (Wuhan Servicebio Technology
Co., Ltd.). Protein bands were analyzed using AlphaEaseFC software
(ver 4.0.0; Alpha Innotech).
Statistical analysis
Statistical analyses were performed using SPSS
version 16.0 software (SPSS, Inc.). Data from ≤3 independent
experiments are presented as the mean ± SD. A one-way ANOVA
followed by Bonferroni post-hoc analysis was performed to compare
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Piperine inhibits cell proliferation
and induces apoptosis
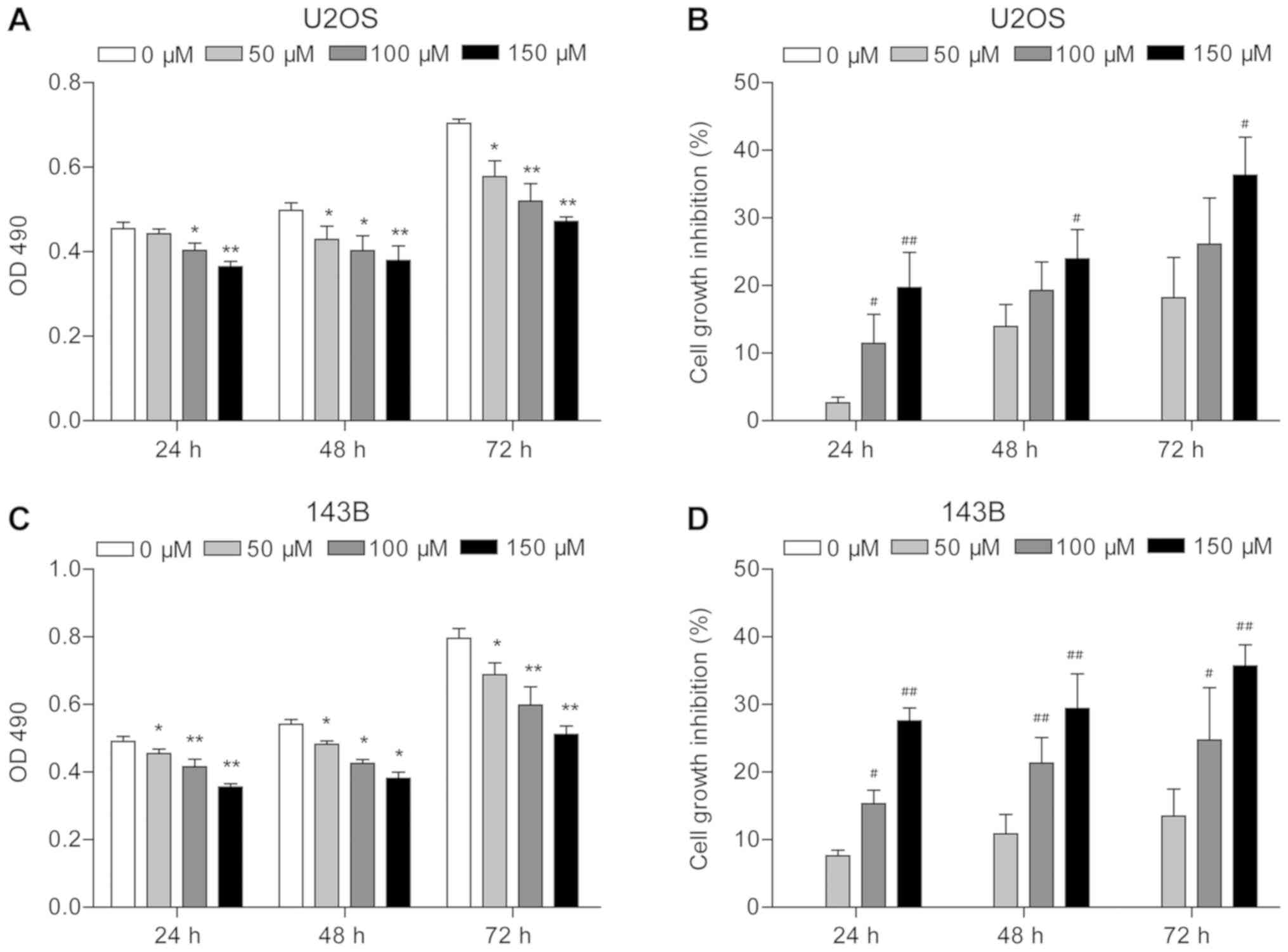
MTT assay results indicated that piperine
significantly inhibited U2OS and 143B cell proliferation
(P<0.05; Fig. 1). It was
revealed that cell density was reduced in the presence of piperine
compared with untreated cells, with the lowest OD490
obtained for cells treated for 24 h with 150 µM piperine
(P<0.01; Fig. 1A and C).
Moreover, the strongest growth inhibition was observed in response
to 150 µM piperine for 72 h (35% for U2OS and 35.7% for 143B;
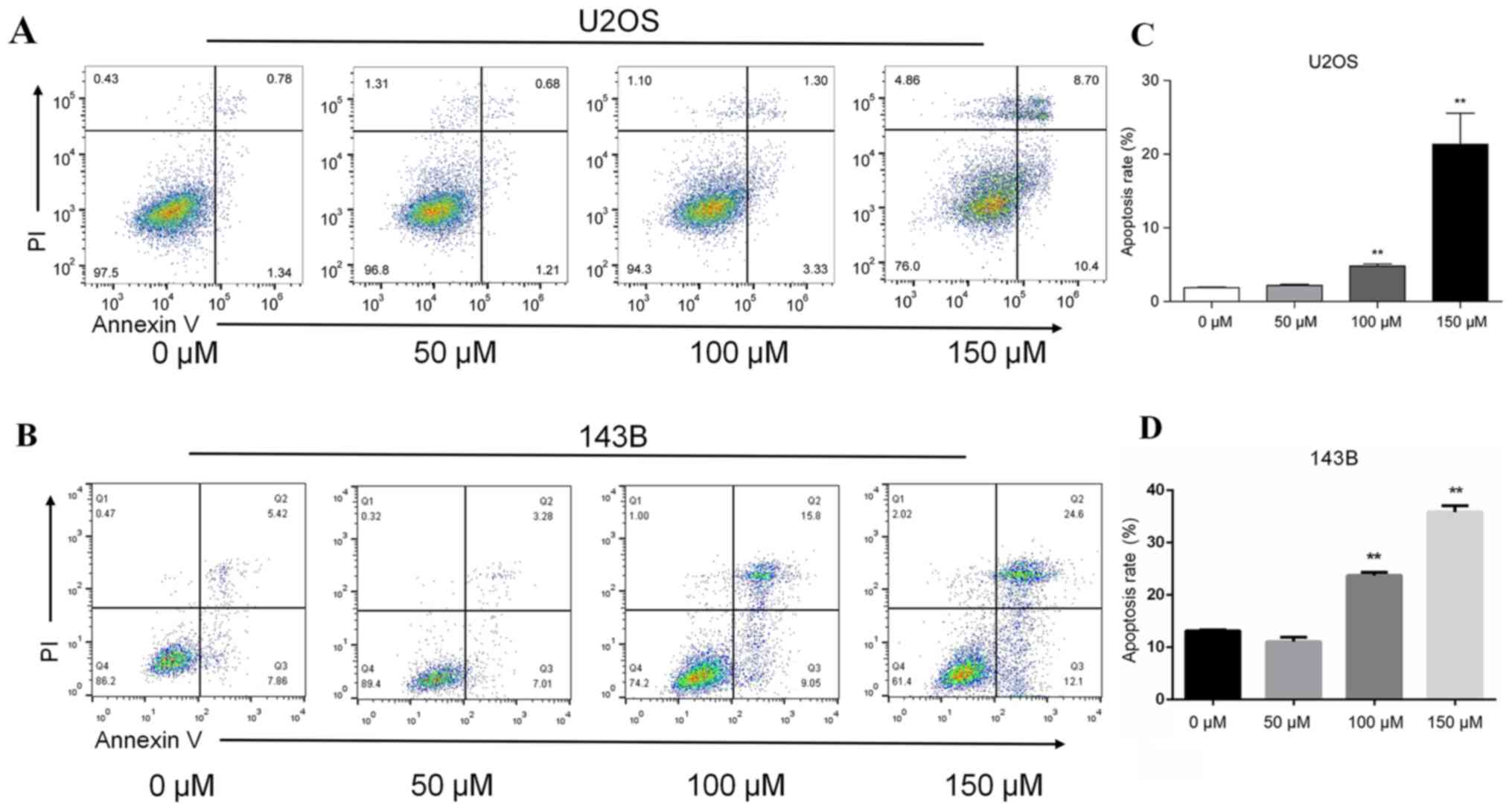
P<0.01; Fig. 1B and D). In U2OS
cells, the apoptotic rate was positively associated with piperine
concentration (Fig. 2). The
apoptotic rate in the absence of piperine was 1.89%, whereas the
apoptotic rates increased to 2.12, 4.63 and 19.1%, in the presence
of 50, 100 and 150 µM piperine, respectively (P<0.05; Fig. 2A). For 143B cell the apoptotic
rates was 10.29, 13.28, 24.85 and 36.7% in the presence of 0, 50,
100 and 150 µM piperine, respectively (P<0.05; Fig. 2B)
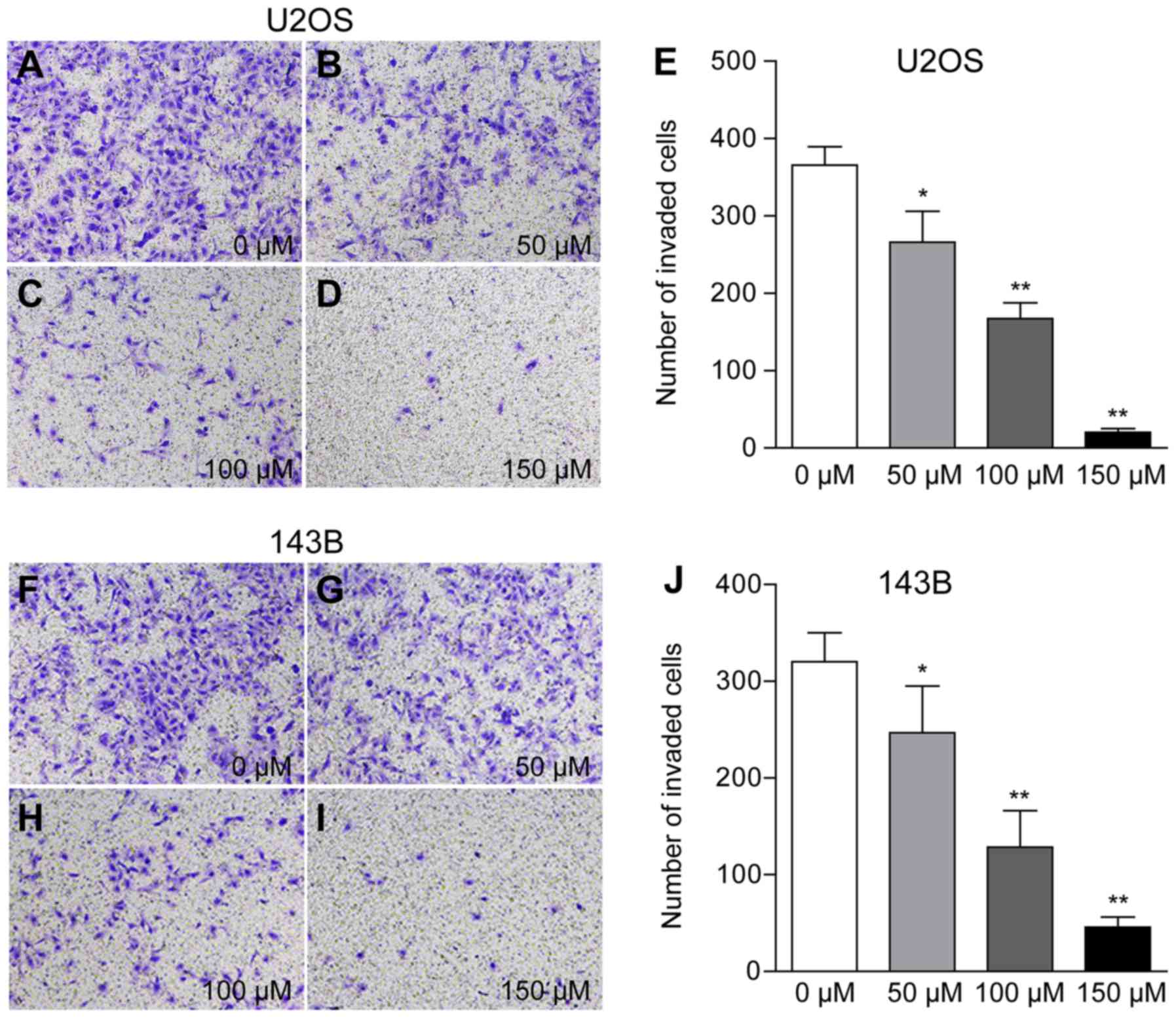
Piperine inhibits cell invasion by
downregulating MMP-2 and VEGF protein expression
The present results suggested that osteosarcoma
cells treated with 50, 100 and 150 µM piperine exhibited reduced
cell(U2OS: Number of invading cells 266, 167 and 20 per field;
143B: 246, 128 and 45 per field, respectively; P<0.01; Fig. 3; Table
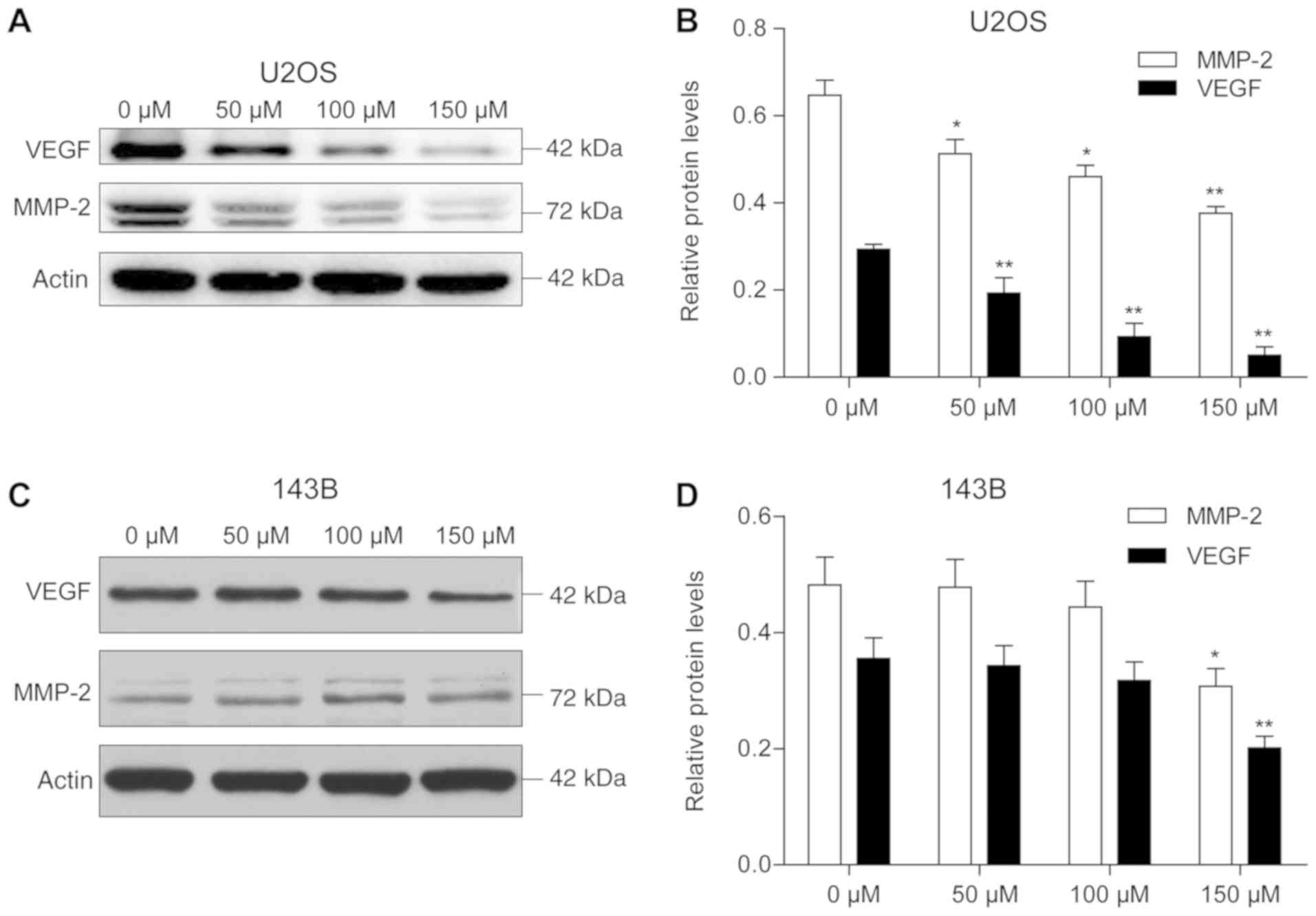
I). Furthermore, western blotting results demonstrated a
dose-dependent decrease in MMP-2 and VEGF protein expression levels
in the presence of piperine, with the most significant reduction
observed at 150 µM piperine for both proteins. However, with
increasing concentration of piperine, U2OS had a more significant
effect on MMP-2 and VEGF compared with 143B cells (Fig. 4).
 | Table I.Data from the Transwell assay |
Table I.
Data from the Transwell assay
|
| Invaded cells |
|---|
|
|
|
|---|
| Treatment group,
µM | U2OS | 143B |
|---|
| 0 | 365.6±23.8 | 320.3±30.0 |
| 50 | 266.2±39.7 | 246.9±48.3 |
| 100 | 167.2±20.6 | 128.5±37.7 |
| 150 | 20.4±4.8 | 45.8±10.2 |
Piperine suppresses cell proliferation
and invasion via the Wnt/β-catenin signaling pathway
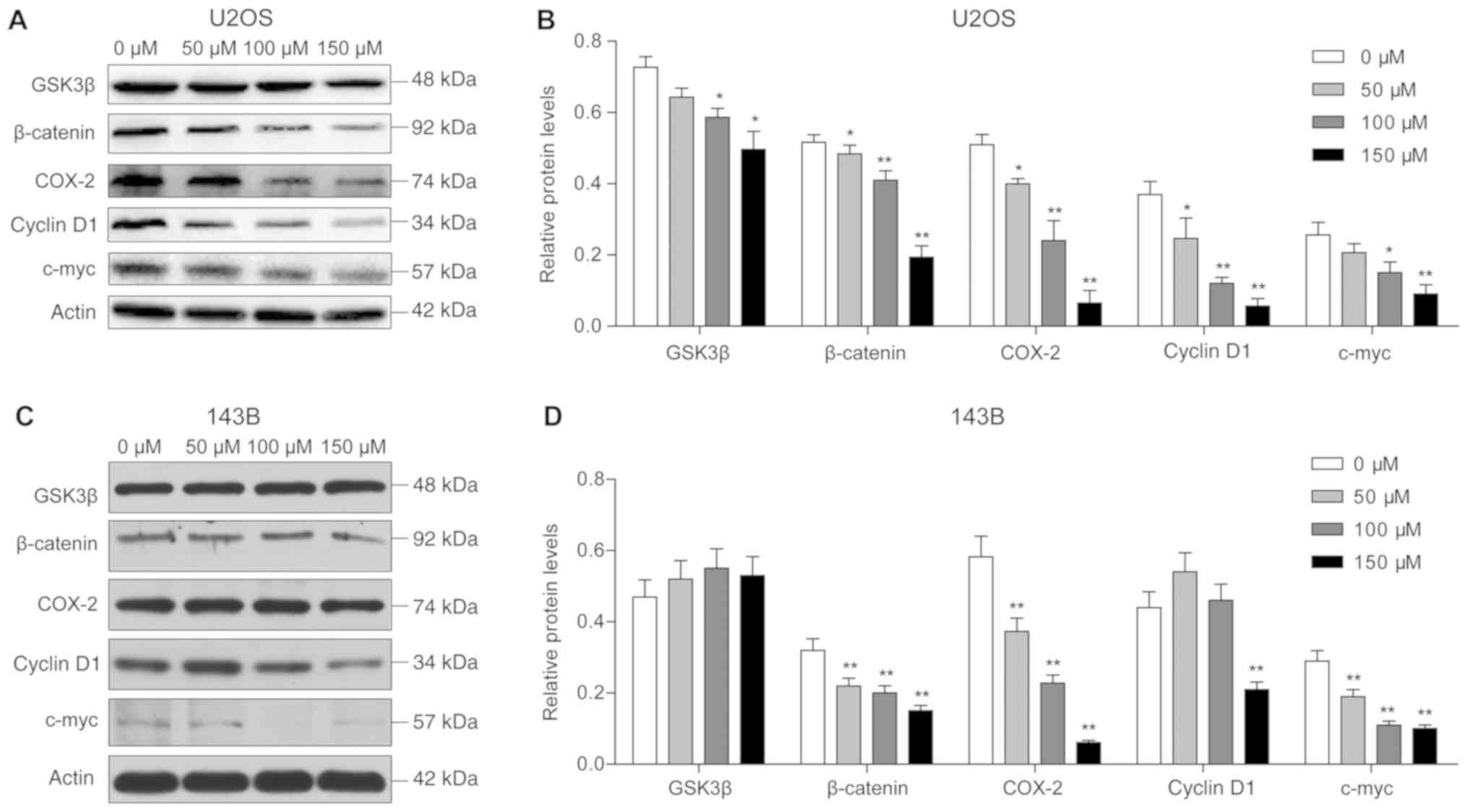
Western blot analysis results revealed that the
protein expression levels of GSK-3β and β-catenin were
significantly reduced in U2OS cells after piperine treatment, and
downregulation of these proteins was inversely associated with
piperine concentration. Notably, the lowest protein expression
levels were observed following treatment with 150 µM piperine
(P<0.05; Fig. 5). In addition,
reduced expression levels of the target proteins COX-2, cyclin D1
and c-myc were observed with increasing piperine concentrations
(P<0.01; Fig. 5). In 143B
cells, it was demonstrated that β-catenin protein expression was
significantly reduced, and the expression levels of COX-2 and c-myc
were decreased with increasing piperine concentrations.
Discussion
The present results suggested that piperine
treatment increased apoptosis, and reduced cell invasion and
proliferation of U2OS and 143B cells. Notably, cell proliferation
was most significantly inhibited in response to treatment with the
highest concentration of piperine (150 µM) for the longest
treatment duration (72 h). Furthermore, the dose-dependent decrease
observed in the protein expression levels of metastatic markers in
the presence of piperine were in line with previous studies on the
roles of these proteins in tumor metastasis and heterotopic
angiogenesis (8,14,18).
Therefore, the present results provided evidence on the role of
piperine in inhibiting osteosarcoma cell proliferation and
metastasis (21). In addition, the
present western blotting results identified that the Wnt/β-catenin
signaling pathway, which has a role in regulating osteosarcoma cell
proliferation and apoptosis (31–34),
may regulate piperine-mediated osteosarcoma apoptosis.
The core mediator of the Wnt/β-catenin signaling
pathway, β-catenin, is regulated by phosphorylation, which is
promoted by GSK-3β, and degradation via the ubiquitin/proteasome
pathway (26). β-catenin mutations
can cause abnormal activation of Wnt target genes, thus leading to
cancer development (35). In
addition, dysregulation of the Wnt signaling pathway can affect
downstream gene expression (36).
The present results suggested that piperine dose-dependently
downregulated the protein expression levels of GSK-3β and
β-catenin, as well as those of the downstream oncogenic proteins
cyclin D1, c-Myc and COX-2. Therefore, piperine may effectively
inhibit U2OS and 143B cell proliferation and metastasis, and
ectopic angiogenesis via regulation of the Wnt/β-catenin signaling
pathway. The PI3K/Akt pathway is frequently hyperactivated in
osteosarcoma, and contributes to disease initiation and development
(37). Moreover, inhibition of
this pathway via small compounds is a potential therapeutic
approach for osteosarcoma (38).
Previous studies have shown that Wilms' tumor 1 knockdown in MG-63
cells results in deregulation of cell cycle proteins and
downregulation of the PI3K/AKT pathway (39,40).
Piperine has previously been reported to inhibit cell proliferation
and metastasis of U2OS cells (21), and affect several signaling
pathways, such as the Akt/MAPK and mTOR pathways (21,22,41),
in several cancer cell types. However, to the best of our
knowledge, the present study is the first to demonstrate that the
Wnt/β-catenin signaling pathway may mediate the antitumor effects
of piperine.
In the present study, it was demonstrated that
piperine exerted a lower inhibitory effect on the 143B cell line
compared with the U2OS cell line, and therefore, it was difficult
to select a cell line to be used as control cells. Thus, the
present study did not include a negative control. Furthermore, the
cytotoxicity of piperine on healthy cells has not been thoroughly
investigated in previous studies despite the well-characterized
anticancer effects of piperine. However, it has been previously
reported that piperine administration does not increase hepatorenal
toxicity in nude mice (42,43).
Thus, piperine may not cause osteosarcoma-specific cytotoxic
effects.
In relation to the modest antitumor efficacy of
piperine shown in the present study, further in vivo and
clinical experiments are required to evaluate its efficacy on
osteosarcoma proliferation, and to clarify the underlying molecular
mechanisms. In addition, future studies will investigate whether
combination drug treatment with cisplatin increases antitumor
potency on osteosarcoma growth. The present study did not use
agonists or antagonists to confirm the involvement of the
Wnt/β-catenin pathway. However, the present study proposed that the
expression levels of upstream signaling molecules in the
Wnt/β-catenin pathway, such as β-catenin and GSK-3β, as well as the
downstream target proteins COX-2, cyclin D1 and c-myc, could
demonstrate the involvement of the Wnt/β-catenin pathway in
piperine-mediated osteosarcoma inhibition. Furthermore, future
in vivo experiments will use specific agonists and
inhibitors of the Wnt/β-catenin pathway.
In conclusion, the present results suggested that
the Wnt/β-catenin signaling pathway underlies the antitumor effects
of piperine on U2OS and 143B cell proliferation, apoptosis and
invasion. However, further research is needed to investigate the
anti-osteosarcoma mechanism of piperine.
Acknowledgements
The authors would like to thank Professor Ming Zhang
and Assistant Researcher Wenxia Li of Key Laboratory of
Cardiovascular Remolding and Function Research Chinese Ministry of
Health for their help in the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LN contributed to the conception of the study. YBQ
performed the experiments, data analysis and wrote the manuscript.
WY and MS contributed to data interpretation and analyses. All
authors have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COX-2
|
cyclooxygenase-2
|
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Petriceks AH and Salmi D: Educational
case: Primary osteosarcoma. Acad Pathol. 6:23742895188203372019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pu F, Chen F, Chen S, Wang B, Liu J and
Shao Z: Association between GSTP1 polymorphisms and prognosis of
osteosarcoma in patients treated with chemotherapy: A
meta-analysis. Onco Targets Ther. 8:1835–1842. 2015.PubMed/NCBI
|
|
3
|
Van de Luijtgaarden AC, Kapusta L,
Bellersen L, Bokkerink JP, Kaal SE, Versleijen-Jonkers YM,
Schreuder HW and van der Graaf WT: High prevalence of late adverse
events in malignant bone tumour survivors diagnosed at adult age.
Neth J Med. 72:516–522. 2014.PubMed/NCBI
|
|
4
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979–2007. Int J Cancer.
131:E508–E517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castillo-Tandazo W, Mutsaers AJ and
Walkley CR: Osteosarcoma in the post genome Era: Preclinical models
and approaches to identify tractable therapeutic targets. Curr
Osteoporos Rep. 17:343–352. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng J, Zhou Y, Li Y, Xu DP, Li S and Li
HB: Spices for prevention and treatment of cancers. Nutrients.
8:E4952016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parthasarathy N, Selwyn MA and Udayakumar
M: Tropical dry evergreen forests of 627 peninsular India: Ecology
and conservation significance. Trop Conservation Sci. 1:89–110.
2008. View Article : Google Scholar
|
|
9
|
Mehmood MH and Gilani AH: Pharmacological
basis for the medicinal use of black pepper and piperine in
gastrointestinal disorders. J Med Food. 13:1086–1096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Chen M, Wang X, Wang Y, Duan C, Gao
G, Lu L, Wu X, Wang X and Yang H: Piperine induces autophagy by
enhancing protein phosphotase 2A activity in a rotenone-induced
Parkinson's disease model. Oncotarget. 7:60823–60843.
2016.PubMed/NCBI
|
|
11
|
Aumeeruddy MZ and Mahomoodally MF:
Combating breast cancer using combination therapy with 3
phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer.
125:1600–1611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Y, Xu J, Liao H, Li L and Pan L:
Piperine induces apoptosis of lung cancer a549 cells via
p53-dependent mitochondrial signaling pathway. Tumour Biol.
35:3305–3310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhardwaj RK, Glaeser H, Becquemont L,
Klotz U, Gupta SK and Fromm MF: Piperine, a major constituent of
black pepper, inhibits human p-glycoprotein and cyp3a4. J Pharmacol
Exp Ther. 302:645–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sunila ES and Kuttan G: Immunomodulatory
and antitumor activity of piper longum linn and piperine. J
Ethnopharmacol. 90:339–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenshields AL, Doucette CD, Sutton KM,
Madera L, Annan H, Yaffe PB, Knickle AF, Dong Z and Hoskin DW:
Piperine inhibits the growth and motility of triple-negative breast
cancer cells. Cancer Lett. 357:129–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gunasekaran V, Elangovan K and Niranjali
Devaraj S: Targeting hepatocellular carcinoma with piperine by
radical-mediated mitochondrial pathway of apoptosis: An in vitro
and in vivo study. Food Chem Toxicol. 105:106–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH,
Chung YC and Jeong HG: Suppression of
phorbol-12-myristate-13-acetate-induced tumor cell invasion by
piperine via the inhibition of PKCα/ERK1/2-dependent matrix
metalloproteinase-9 expression. Toxicol Lett. 203:9–19. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pathak N and Khandelwal S: Cytoprotective
and immunomodulating properties of piperine on murine splenocytes:
An in vitro study. Eur J Pharmacol. 576:160–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taqvi SI, Shah AJ and Gilani AH: Blood
pressure lowering and vasomodulator effects of piperine. J
Cardiovasc Pharmacol. 52:452–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wattanathorn J, Chonpathompikunlert P,
Muchimapura S, Priprem A and Tankamnerdthai O: Piperine, the
potential functional food for mood and cognitive disorders. Food
Chem Toxicol. 46:3106–3110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhu X, Li H, Li B, Sun L, Xie T,
Zhu T, Zhou H and Ye Z: Piperine inhibits proliferation of human
osteosarcoma cells via G2/M phase arrest and metastasis by
suppressing MMP-2/-9 expression. Int Immunopharmacol. 24:50–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan H, Xu LH, Huang MY, Zha QB, Zhao GX,
Hou XF, Shi ZJ, Lin QR, Ouyang DY and He XH: Piperine metabolically
regulates peritoneal resident macrophages to potentiate their
functions against bacterial infection. Oncotarget. 6:32468–32483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oudin MJ and Weaver VM: Physical and
chemical gradients in the tumor microenvironment regulate tumor
cell invasion, migration, and metastasis. Cold Spring Harb Symp
Quant Biol. 81:189–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang K, Liu LH, Ouyang Y, Bu J, Guo
HB and Xiao T: A systematic review of matrix metalloproteinase 9 as
a biomarker of survival in patients with osteosarcoma. Tumour Biol.
35:5487–5491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fromigue O, Hamidouche Z and Marie PJ:
Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces
osteosarcoma cell invasion. J Biol Chem. 283:30549–30556. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling L, Nurcombe V and Cool SM: Wnt
signaling controls the fate of mesenchymal stem cells. Gene.
433:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng N, Gao S, Guo X, Wang G, Cheng C, Li
M and Liu K: Silencing of VEGF inhibits human osteosarcoma
angiogenesis and promotes cell apoptosis via VEGF/PI3K/AKT
signaling pathway. Am J Transl Res. 8:1005–1015. 2016.PubMed/NCBI
|
|
28
|
Manzano-Moreno FJ, Costela-Ruiz VJ,
Melguizo-Rodríguez L, Illescas-Montes R, García-Martínez O, Ruiz C
and Ramos-Torrecillas J: Inhibition of VEGF gene expression in
osteoblast cells by different NSAIDs. Arch Oral Biol. 92:75–78.
2008. View Article : Google Scholar
|
|
29
|
Olivares-Navarrete R, Hyzy S, Wieland M,
Boyan BD and Schwartz Z: The roles of Wnt signaling modulators
Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state
in osteogenesis on microstructured titanium surfaces. Biomaterials.
31:2015–2024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian J, He H and Lei G: Wnt/β-catenin
pathway in bone cancers. Tumour Biol. 35:9439–9445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brafman D and Willert K: Wnt/β-catenin
signaling during early vertebrate neural development. Dev
Neurobiol. 77:1239–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Fiore R, Guercio A, Puleio R, Di Marco
P, Drago-Ferrante R, D'Anneo A, De Blasio A, Carlisi D, Di Bella S,
Pentimalli F, et al: Modeling human osteosarcoma in mice through
3AB-OS cancer stem cell xenografts. J Cell Biochem. 113:3380–3392.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katoh M and Katoh M: Molecular genetics
and targeted therapy of WNT-related human diseases (Review). Int J
Mol Med. 40:587–606. 2017.PubMed/NCBI
|
|
34
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ng TL, Gown AM, Barry TS, Cheang MC, Chan
AK, Turbin DA, Hsu FD, West RB and Nielsen TO: Nuclear beta-catenin
in mesenchymal tumors. Mod Pathol. 18:68–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghosh N, Hossain U, Mandal A and Sil PC:
The wnt signaling pathway: A potential therapeutic target against
cancer. Ann N Y Acad Sci. 1443:54–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Qu P, Zhao H, Zhao T and Cao N:
Cox 2 promotes epithelial-mesenchymal transition and migration in
osteosarcoma MG 63 cells via PI3K/AKT/NF kB signaling. Mol Med Rep.
20:3811–3819. 2019.PubMed/NCBI
|
|
38
|
Liu G, Yuan D, Sun P, Liu W, Wu PF, Liu H
and Yu GY: Linc00968 functions as an oncogene in osteosarcoma by
activating the PI3K/AKT/mTOR signaling. J Cell Physiol.
233:8639–8647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Graziano AC, Cardile V, Avola R, Vicario
N, Parenti C, Salvatorelli L, Magro G and Parenti R: Wilms' tumor
gene 1 silencing inhibits proliferation of human osteosarcoma MG-63
cell line by cell cycle arrest and apoptosis activation.
Oncotarget. 8:13917–13931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoo ES, Choo GS, Kim SH, Woo JS, Kim HJ,
Park YS, Kim BS, Kim SK, Park BK, Cho SD, et al: Antitumor and
apoptosis-inducing effects of piperine on human melanoma cells.
Anticancer Res. 39:1883–1892. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lai LH, Fu QH, Liu Y, Jiang K, Guo QM,
Chen QY, Yan B, Wang QQ and Shen JG: Piperine suppresses tumor
growth and metastasis in vitro and in vivo in a 4T1 murine breast
cancer model. Acta Pharmacol Sin. 33:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|