Introduction
Osteoarthritis (OA) is a chronic and progressive
disease that affects more than 20% of people over 45 years
(1). The pathology of knee OA
includes progressive articular cartilage loss, sclerotic changes in
subchondral bone, and osteophyte formation. The cells in the OA
joint change their phenotype as a result of aging and exposure to
mechanical stress, oxidative stress, and inflammation (2,3). It
is now well-recognized that there is a major inflammatory component
to this disease and it is thought that inflammatory mediators may
play an important role in joint degradation (4). Particularly, interleukin-1β (IL-1β)
is known as a major inducer of articular cartilage extracellular
matrix (ECM) degradation, promoting the production and activation
of different factors that act as mediators and/or effectors of
progressive cartilage loss (5).
To date, there is no treatment that cures OA. Thus,
the development of therapeutic alternatives that can prevent or
delay the destruction of cartilage and tissues involved in the knee
joint or stimulate its adequate repair is required (6). The usefulness of treatments focused
on the repair of focal lesions is very limited in OA, because more
generalized cartilage defects and joint inflammation are present.
Therefore, intra-articular treatment options seem more viable as
therapeutic alternatives for the management of knee OA (7). Some of the advantages of using
intra-articular injections are increased local bioavailability,
reduced systemic exposure, fewer adverse events, and reduced cost
(8).
The intra-articular injection of hyaluronic acid
(HA) for knee OA has been widely studied. One of the most recent
available products is non-animal stabilized HA (NASHA, Durolane
HA®; Bioventus LLC). NASHA is a type of HA that is
produced through bacterial synthesis of HA. It is subsequently
purified and stabilized in a carefully controlled process involving
molecular cross-linking (9).
Available clinical evidence supports the use of NASHA as an
effective and safe procedure for symptom relief in knee OA
(10). However, the
anti-inflammatory effect at the cellular and molecular level of
this type of HA in knee OA has not been studied.
On the other hand, results from clinical studies
suggest a clinical improvement for intra-articular injection of
mesenchymal stromal/stem cells (MSC) in knee OA (11,12).
Along with their differentiation potential into several lineages,
including chondrogenic lineage, MSC influence their
micro-environment by secreting trophic mediators (13–15).
The paracrine capacities of MSC raise the attractive approach of
possibly basing future therapies on their secreted factors rather
than on the cells themselves.
In vitro stimulation with inflammatory
factors provides an option to further use the MSC trophic effects
since they need to be activated to exert such action (16–18).
The effect of the mediators produced by stimulated MSC on
inflammation and catabolic processes involved in OA have been
tested before (16,17,19).
The aim of this study was to evaluate the anti-inflammatory and
anti-catabolic effect of the NASHA and compare it with the
conditioned media from adipose-derived MSC in an in vitro
coculture model of cartilage and synovium.
Materials and methods
Patients and samples
Cartilage and synovial tissue explants were
harvested as surgical waste from patients with OA undergoing total
knee arthroplasty (n=7) from April 2017 to December 2018. Usage of
human tissues was approved by the Research Ethics Committee of the
University Hospital and School of Medicine of Universidad Autonoma
de Nuevo Leon (approval no. OR17-00002). All patients had advanced
knee OA diagnosis (Kellgren-Lawrence grade IV), disabling knee pain
and required total knee prosthesis. The cartilage and synovial
explants for the coculture assays were obtained from seven male
patients with a median of 71 years and an interquartile range of
60–79 years. Patients with joint infection were excluded and
patients in whom the tissue sample was insufficient were
eliminated. Informed consent was obtained from all patients. To
obtain the explants, the joint tissue from the femoral condyles and
synovial capsule was recovered, from which cuts of 6–8 mm in
diameter were made by means of the Osteochondral Autograft Transfer
System (OATS®, Arthrex Inc.). Efforts were made to
obtain cartilage explants from samples without erosion or exposure
of the subcondral bone which were identified in non-weight bearing
zones and at the periphery of the femoral condyles. Most of
synovial samples from which explants were obtained were
characterized for presenting friability, redness, hyperemia and
synovial hypertrophy. The obtained explants were washed three times
with 1X sterile phosphate-buffered saline (PBS; Gibco; Thermo
Fisher Scientific, Inc.), once in 70% ethanol and once in sterile
PBS 1X with gentamicin 50 µg/ml.
Cartilage-synovial tissue
coculture
Cartilage and synovial tissue explants from the same
donor were distributed in 12-transwell plates for coculture. In
each well, an explant of cartilage and synovial tissue was placed;
such explants were divided by a polystyrene membrane transwell
insert with a pore diameter of 1.0 µm (Corning Incorporated).
Cartilage explant was placed within the insert and the synovial
tissue explant was placed at the bottom of the plate. Each
coculture was maintained under the following conditions: 1) Basal:
Complete DMEM/F12 (Gibco) [added with gentamicin 50 µg/ml and
fungizone 0.25 µg/ml (Gibco), supplemented with fetal bovine serum
(FBS) 10% (Gibco)], 2) Interleukin-1β (IL-1β): Complete DMEM/F12
with 10 ng/ml of human recombinant protein IL-1β (R&D Systems
Inc.), 3) NASHA: Complete DMEM/F12 added with NASHA (hyaluronic
acid, 1 mg/ml, Durolane HA) and 10 ng/ml of IL-1β; and 4) Stem
cell-conditioned medium (SC-CM): Complete DMEM/F12 added with
conditioned stem cell culture media (1 ml) and 10 ng/ml of IL-1β.
All the cultures were incubated at 37°C, with 5% CO2
upon 0, 48 and 72 h for further RNA isolation and supernatants were
storage to −80°C until required for additional analysis.
MSC derived from adipose tissue
Adipose tissue samples were obtained from total or
partial hip arthroplasty surgeries (n=5). The adipose tissue
samples used to isolate MSC were derived from five male patients
with a median of 70 years and an interquartile range of 67–77
years. MSC isolation was performed following a method previously
described (20). Briefly, after
the isolation, cells were plated in a 75 cm2 culture
flasks, changes of complete culture medium (DMEM/F12 added with
gentamicin 50 µg/ml and fungizone 0.25 µg/ml, supplemented with FBS
10%) were made every 72 h until the cells reached 80–90% confluence
and were ready to undergo a subculture. The cells from the
different donors received a maximum of 2 subcultures, then they
were pooled and maintained in new 75 cm2 culture flasks
until 80–90% confluency (approximately 2×106 cells), and
were stimulated with IL-1β (10 ng/ml) in 5 ml of complete DMEM/F12
medium for 24 h; the culture medium of the stimulated confluent
cells (stem-cell conditioned medium, SC-CM) was stored at −80°C and
was used to supplement the cocultures of the cartilage and synovial
tissue explants.
Immunophenotyping of the
adipose-derived MSC by flow cytometry
The immunophenotype of the MSC in culture was
evaluated within passages 2 and 3. Adherent cells cultured in 75
cm2 bottles were detached with 0.75% trypsin/0.5 mM EDTA
(Gibco). Cell pellet was obtained and resuspended in complete DMEM
medium and incubated at 37°C for 20 min. Subsequently, the cells
were centrifuged and the cell button was diluted at approximately
200,000 cells per tube in 50 µl of staining buffer (SB, Becton
Dickinson Pharmingen) or in a 0.5% bovine serum albumin (BSA,
Sigma-Aldrich; Merck KGaA) solution in PBS. The antibody was added
following the manufacture's recommendation and then the mix was
incubated for 20 min in the dark. Then the samples were washed with
0.5 ml of 0.5% BSA in PBS and centrifuged at 1,800 × g (730 × g),
for 1 min at 4°C. The button of stained cells from each tube were
resuspended in 250 µl of 0.5% BSA in PBS and analyzed for
three-color immunofluorescence by flow cytometry (FACSAria; BD
Biosciences).
Differentiation potential of the
adipose-derived MSC
To confirm the multilineage differentiation
potential of the isolated MSC, we investigated the ability of those
cells to differentiate into chondrogenic, adipogenic, and
osteogenic lineages (21).
Briefly, for adipocyte differentiation, the cells were induced with
an adipogenic differentiation medium containing dexamethasone (1
mM), indomethacin (60 mM), 3-isobutyl-1-metyl-xanthine (500 mM;
IBMX), and insulin (5 mg/ml) for 3 weeks. Differentiation into
adipocytes was demonstrated by Oil-Red-O staining for the presence
of lipid droplets. For chondrocyte differentiation, MSC were
maintained as micromass cultures; the formed pellets were induced
in the presence of a medium containing TGF-β1 (10 ng/ml),
dexamethasone (0.1 µM), ascorbic acid (50 µg/ml), and
insulin-transferrin-selenium solution (ITS, 10 µg/ml) for 3 weeks.
The cells were differentiated toward chondrocytes, as shown by
glycosaminoglycan (GAG) deposition and Alcian Blue staining. To
evaluate the osteoblastic potential, cells were stained with
Alizarin Red to detect mineralization after a cultivation period of
14 days with an osteogenic medium containing dexamethasone (0.1
µM), β-glycerophosphate (10 mM), and ascorbic acid (50 µg/ml).
Total RNA extraction and gene
expression analysis by reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from the cartilage and
synovial explants samples at the indicated culture times using the
commercial kit RNeasy Lipid Tissue Mini kit (Qiagen GmbH) following
the manufacturer's recommendations. The quality of the samples
obtained from RNA was evaluated by spectrophotometric
quantification in the NanoDrop 2000 equipment (Thermo Fisher
Scientific Inc.). From each total RNA sample obtained,
complementary DNA synthesis (cDNA) was performed by means of a
retrotranscription reaction with the GoTaq Probe 2-Step RT-qPCR
System commercial kit (Promega Corporation). From the synthesized
cDNA, the expression of different genes related to the inflammatory
response and the ECM synthesis in cartilage and synovium was
evaluated through the use of TaqMan probes (Applied Biosystems;
Thermo Fisher Scientific, Inc.) specific for each gene in each
coculture test and in each experimental group (4 groups) in
triplicate. The expression assays were performed in the 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The list of genes evaluated is shown in Table I. Data analysis was carried out by
means of the ΔΔCq method for relative expression, using
β-2-microglobulin (B2M) as endogenous gene to normalization
(22).
 | Table I.Analyzed genes by reverse
transcription-quantitative PCR. The corresponding code is presented
for each of the TaqMan probes used. |
Table I.
Analyzed genes by reverse
transcription-quantitative PCR. The corresponding code is presented
for each of the TaqMan probes used.
| Gene | Symbol | Codea |
|---|
| Interleukin-1
β | IL-1β | Hs01555410_m1 |
| Matrix
metallopeptidase 13 | MMP13 | Hs00233992_m1 |
| ADAM
metallopeptidase with thrombospondin type 1 motif 5 | ADAMTS5 | Hs00199841_m1 |
| Tissue inhibitor of
metalloproteinases 1 | TIMP1 | Hs00171558_m1 |
|
β-2-microglobulin | B2M | Hs99999907_m1 |
Release of GAG
The sulfated GAG (chondroitin sulfate, heparan
sulfate, keratan sulfate and dermatan sulfate) were quantified by
means of a spectrophotometric analysis using the metachromatic
probe dimethylmethylene blue (DMB) using the Rheumara®
commercial GAG detection kit (Astarte Biologics). The binding of
DMB to sulfated GAG induces a change in the absorption spectrum,
which is directly proportional to the amount of sulphated GAG.
Measurements were performed in 96-well plates in the Cytation3 HT
multimodal plate reader (BioTek Instruments Inc.) at a wavelength
of 525 nm. The results obtained were normalized with respect to the
wet weight of the cartilage explants. The presence of sulphated GAG
from each coculture trial (7 trials) and from each experimental
group (4 groups) was evaluated 5 times.
Nitric oxide production
Nitric oxide (NO) was determined in the culture
supernatants by using the Griess reagent (Sigma-Aldrich), which
converts available nitrate into nitrite through nitrite reductase.
Absorbance at 540 nm was determined in 96-well plates in the
Cytation3 HT multimodal plate reader (BioTek Instruments Inc.).
Sodium nitrate was used to generate a standard curve with which the
concentrations of the samples to be evaluated were determined. The
production of NO from each coculture trial (7 trials) and from each
experimental group (4 groups) was evaluated 5 times.
Statistical analysis
This study was an experimental, prospective, in
vitro study. Sample size was calculated for the comparison of
related means, based on an alpha value of 0.05 in two directions
and a β value of 0.08; resulting in 7 trials per experimental
group. The data are presented as the mean ± standard deviation (SD)
of seven independent experiments performed in triplicate unless
otherwise mentioned. The numerical variables were analyzed with the
parametric ANOVA test with the Tukey post hoc test for multiple
comparisons to observe the possible differences between the
experimental groups. P<0.05 was considered to indicate a
statistically significant difference. Data were processed with
GraphPad Prism software version 5.00 for Windows (GraphPad
Software, Inc.).
Results
Immunophenotyping of human
adipose-derived MSC
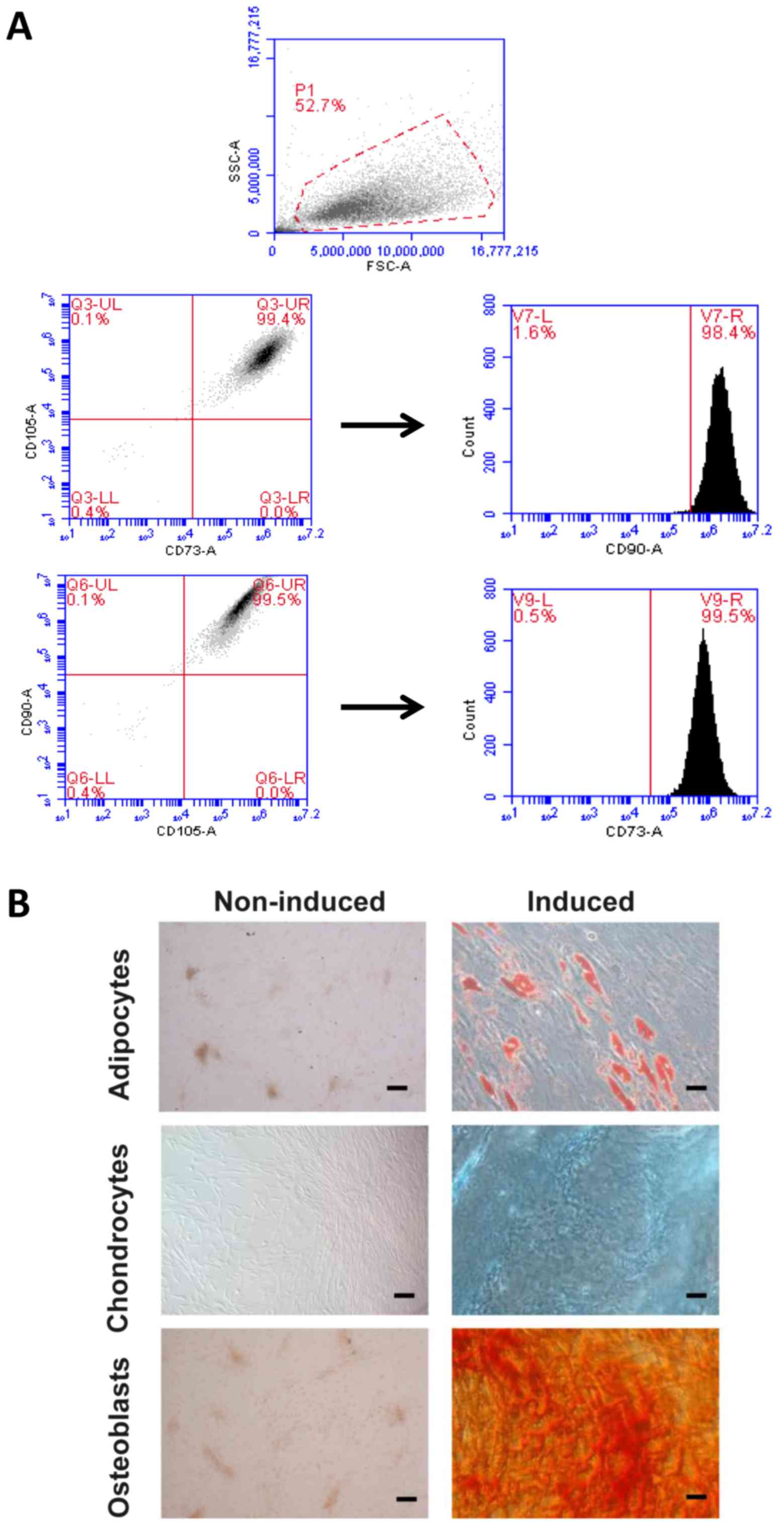
The analysis by flow cytometry of the MSC revealed
the surface markers that should be positive for this type of cells
(CD73+/CD90+/CD105+). In the
histogram of the positive markers, only a well-defined peak is
distinguished, which demonstrates the specificity of the test
(Fig. 1A). The expression of the
markers in the sample analyzed is also plotted; as expected, the
expression of the CD105 was the one that varied more between
individuals and passages. The markers CD73 and CD90 were the most
stable. In Fig. 1A, a
representative image of the analysis of the samples is shown, which
is expressed as dot-plot and shows the purity of the cell
population. Most of the cells are located in a very concentrated
area and in the quadrant corresponding to those that are double
positive.
Assessment of pluripotency in the
human adipose-derived MSC
We assess the ability of isolated human
adipose-derived MSC to differentiate into adipocytes, chondrocytes,
and osteoblasts. The differentiated MSC to adipocytes underwent
production of vacuoles containing lipid droplets detected with
Oil-Red-O staining as red areas (Fig.
1B). The induced chondrogenic differentiation was detected by
the presence of GAG within the extracellular matrix stained with
Alcian Blue (Fig. 1B). Lastly, MSC
developed a more round polygonal appearance and the mineralized
foci were detected as red spotted areas stained with Alizarin Red
after osteogenic differentiation (Fig.
1B). These data confirmed that the isolates of adipose-derived
MSC used in this study are efficiently capable to initiate a
multilineage differentiation process.
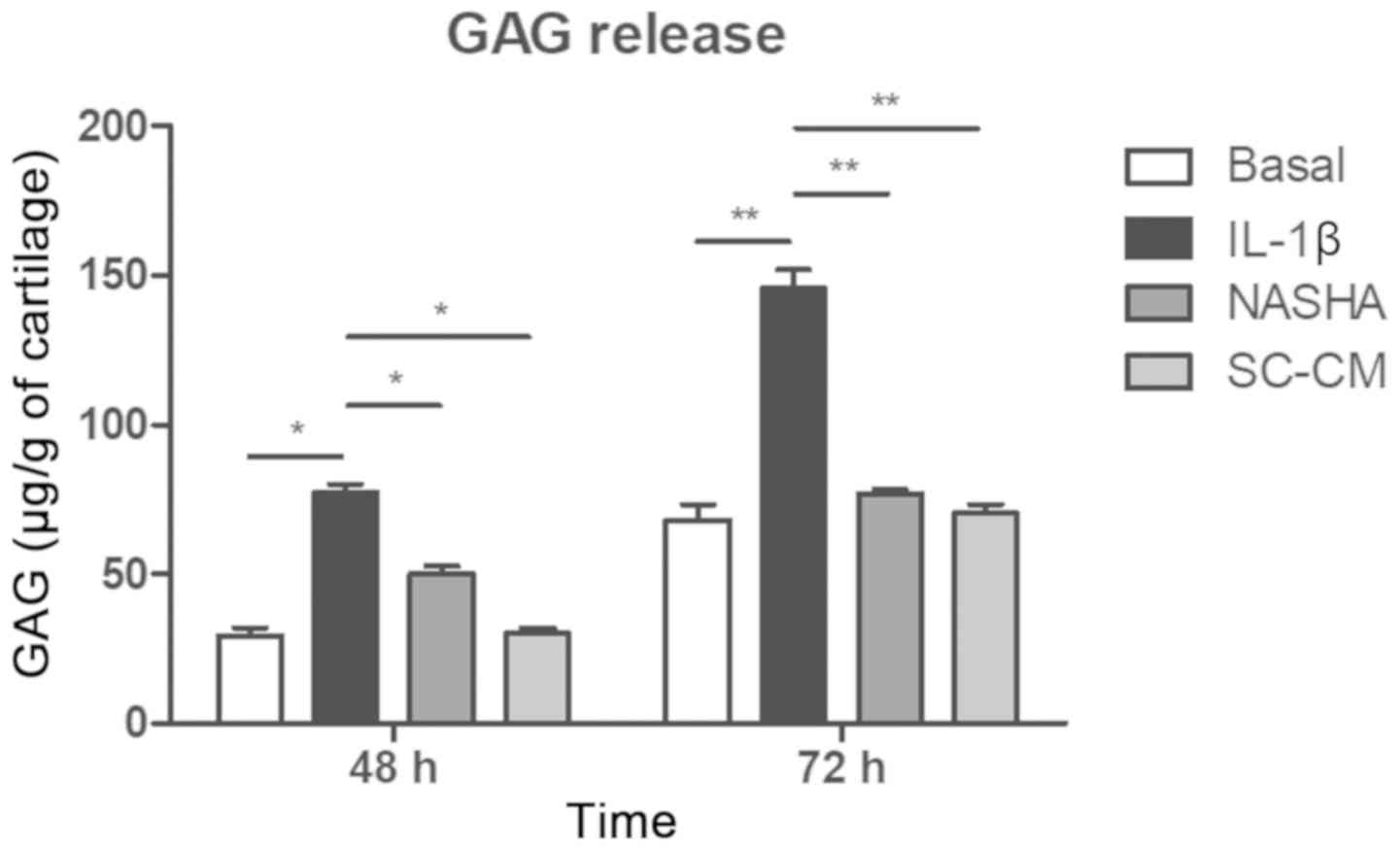
Release of GAG to the culture
medium
The presence of sulfated GAG was detected in the
culture media of all four experimental groups at 48 and 72 h
(Fig. 2). A greater release of GAG
was detected in the treatment group stimulated with IL-1β with
respect to the basal, NASHA and SC-CM group at 48 h (P<0.05) and
72 h (P<0.01). It was observed that the level of GAG released to
the culture medium was maintained at the level of the basal group
(68.3±5.1 µg/g of cartilage) after supplementation with NASHA
(76.9±1.5 µg/g of cartilage) or with SC-CM (70.6±2.9 µg/g of
cartilage) at 72 h, with no significant changes between these two
last groups.
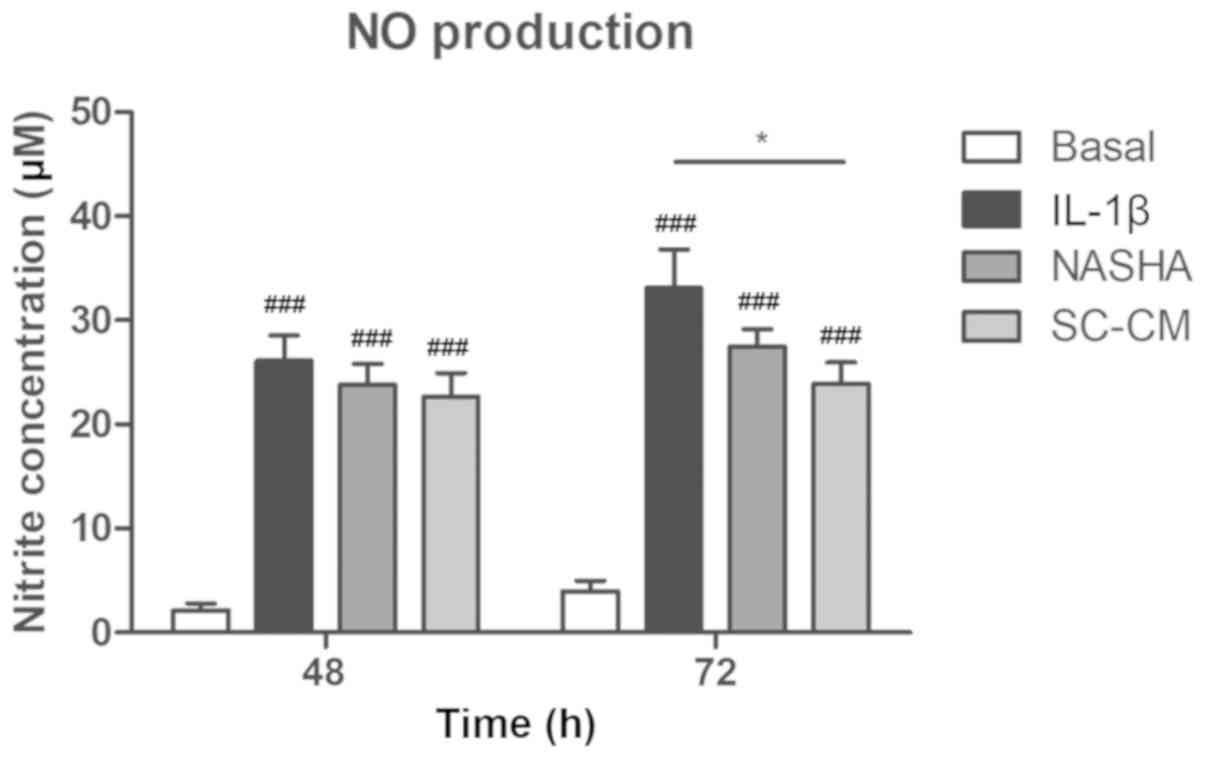
Quantification of NO
The concentrations of nitrite, as an index of NO
production, increased markedly in the groups in which inflammation
with IL-1β was induced (IL-1β, NASHA and SC-CM) compared with the
basal group at 48 and 72 h (P<0.001; Fig. 3). A decrease in the production of
NO was observed in the groups treated with NASHA and MSC with
respect to the IL-1β group at 72 h; nevertheless, only in the SC-CM
group a significant decrease in the NO levels was detected (IL-1β,
33.1±9.8 µM vs. SC-CM, 23.9±5.4 µM; P<0.05).
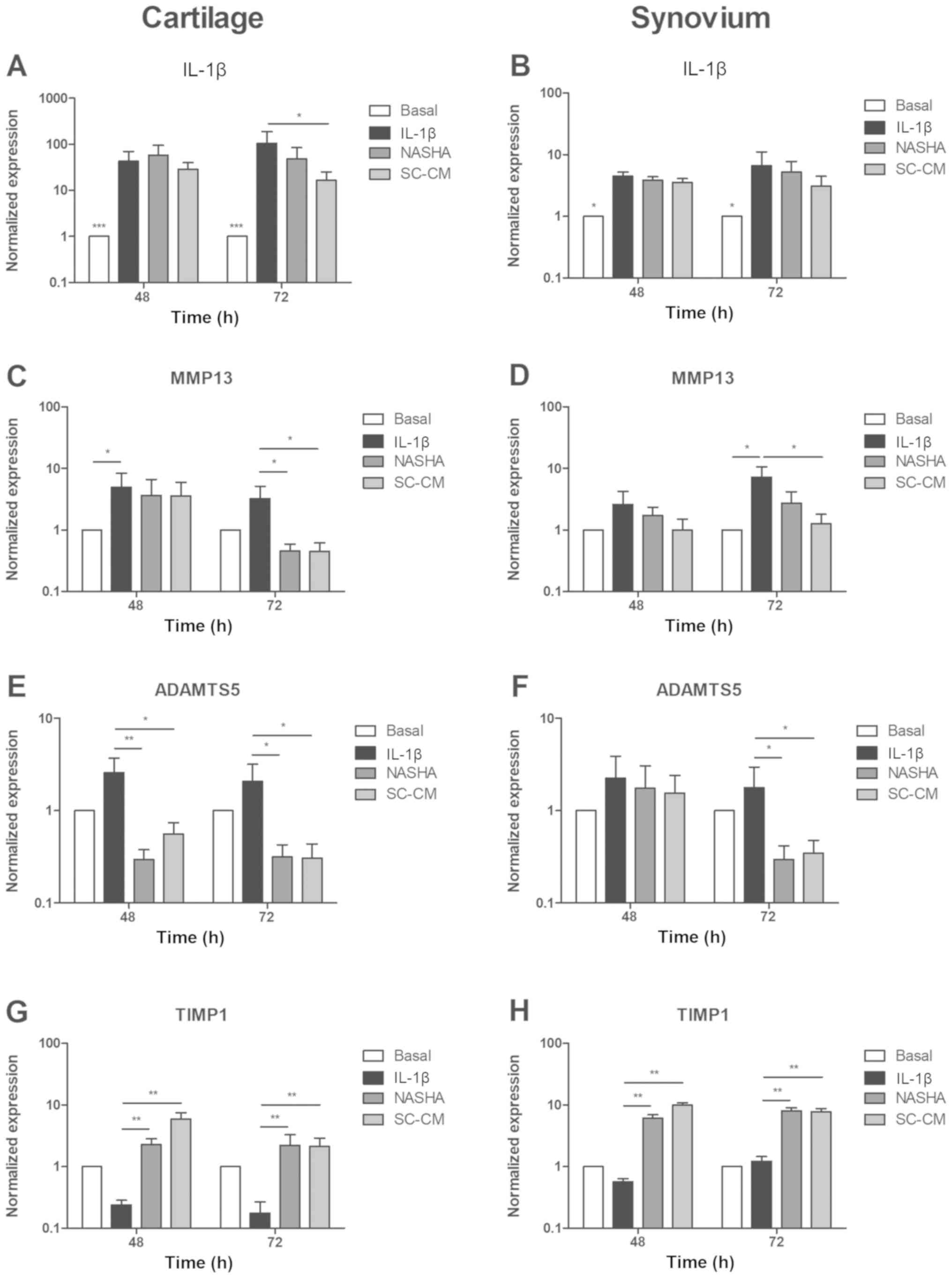
Expression of genes related to
inflammation and matrix turnover in cartilage and synovial membrane
explants
IL-1β
The recombinant IL-1β markedly increased the
expression of IL-1β in the IL-1β, NASHA, and SC-CM groups,
both in cartilage (P<0.001) and in the synovium (P<0.05)
showing a clearly inflammatory effect (Fig. 4A, B). Treatments with NASHA and
SC-CM decreased the expression of IL-1β at 72 h in both
cartilage and synovial membrane. In cartilage, only the treatment
with the conditioned culture medium of MSC significantly decreased
the expression of IL-1β (P<0.05) at 72 h. In synovial
membrane, the treatment with SC-CM also decreased the expression
level of IL-1β compared to the treatment with NASHA. Despite
this, the difference shown was not statistically significant
(NASHA, 5.2±5.7 vs. SC-CM, 3.1±3.1; P>0.05).
MMP13
The expression of MMP13 was significantly
increased in the IL-1β group compared with its basal expression
levels after exposure to recombinant IL-1β in cartilage and
synovial explants (P<0.05; Fig. 4C,
D). However, the expression of MMP13 significantly
decreased in both cartilage and synovium (P<0.05) after 72 h of
treatment with SC-CM. Treatment with NASHA exhibited a
significantly downregulation of MMP13 only in cartilage at
72 h (P<0.05).
ADAMTS5
In the cartilage explants, both treatments
significantly decreased the expression of ADAMTS5 at 48 and
72 h (P<0.05 and P<0.01, respectively) despite the
inflammatory influence of recombinant IL-1β (Fig. 4E). In explants of synovial tissue,
the same behavior was detected only at 72 h (P<0.05; Fig. 4F).
TIMP1
Conversely to the effect of IL-1β in the
aforementioned genes in cartilage explants, the expression of
TIMP1 was decreased as a consequence of the inflammatory
stimulation compared with basal level (Fig. 4G). It can be distinguished how in
both cartilage and synovial membrane explants, treatments with
NASHA and SC-CM recovered the expression level of TIMP1
(Fig. 4G, H), and the difference
was significantly higher than in the IL-1β group at 48 and 72 h
(P<0.01).
Discussion
Exposure of synovial and cartilage explants in
coculture to NASHA and SC-CM, resulted in positive changes in gene
expression profiles and release of agents consistent with an
anti-inflammatory and anti-catabolic activity in articular tissues.
However, only the mediators produced by the MSC previously
stimulated with IL-1β were able to significantly decrease a greater
number of inflammation and matrix degradation targets studied in
the in vitro model used.
It is known that the tissues involved in the OA
joint produce a wide variety of catabolic and proinflammatory
mediators that lead to the destruction of the extracellular matrix
of these structures (23);
therefore, is important to evaluate these processes in tissues
other than articular cartilage. While in vitro inflammation
models exist for some inflammatory diseases, to date, there is no
simply and readily available OA model. As earlier mentioned, we
used an ex vivo coculture system of cartilage and synovial
membrane explants using inserts that allowed the interaction of the
two types of tissue through different molecule production to better
represent the joint environment, unlike what happens in an isolated
culture of cartilage or synovium (16,24).
Additionally, models based on explant culture have the main
advantage that they can be used to examine the response of cells in
their natural extracellular matrix, without affecting the cell
phenotype (25).
The release of GAG has been reported to be useful as
a measure of ECM degradation in cultures of cartilage explants
after an inflammatory stimulus (e.g., IL-1β) (26–28).
In our model, we detected an increase of GAG release induced by
IL-1β (10 ng/ml) and both NASHA and SC-CM were able to protect
articular tissues against ECM degradation. This effect might be
directly related with the reduction of gene expression of ECM
degrading enzymes such as MMP13 and ADAMTS5.
The inflammatory effects of IL-1β include triggering
a signaling pathway that stimulates the release of NO. As
consequence, cartilage degradation is produced due to an increased
synthesis of proteolytic enzymes such as MMP13 and ADAMTS5
(29). In our study, both NASHA
and SC-CM reduced the IL-1β-induced production of the inflammatory
mediator NO but only the SC-CM showed a meaningful significantly
difference. This outcome could be a consequence of the decreased
gene expression of MMP13 and ADAMTS5 in our cartilage
and synovial explant coculture model. It has been reported that
human SC-CM might produce this protective action as a consequence
of iNOS downregulation (17), since NO inhibits ECM synthesis
(30).
In OA, anabolic and catabolic processes are
significantly affected as a result of a homeostasis imbalance.
Important markers of catabolism in OA are MMP13 and ADAMTS5, which
are commonly elevated. We found an increased IL-1β-induced
expression of MMP13 in cartilage and synovial explants. This
effect has been previously reported over MMP13 and other MMP
in similar OA explant-based culture models (16,31).
On the other hand, ADAMTS5 was not upregulated by IL-1β in
explant tissues. These is in agreement with previous investigations
who have found that ADAMTS5 is not affected by inflammatory
cytokines (i.e., IL-1β, TNF-α) especially in human chondrocytes and
cartilage (32–34). Our results showed that SC-CM
inhibited the expression of IL-1β, MMP13 and ADAMTS5
in cartilage, whereas NASHA produced a similar effect only in
MMP13 and ADAMTS5. Moreover, synovial tissues had
also different decreased expression levels of catabolic markers
after SC-CM (MMP13 and ADAMTS5) and NASHA
(ADAMTS5) treatments. We also found a significant
overexpression of TIMP1 in IL-1β-treated cartilage and
synovial explants after exposure to NASHA and SC-CM. TIMP1 is a
natural inhibitor of different MMP, including MMP13 (35), and is thought to play a role in
regulating ECM turnover by modulating the degradative capacity of
these MMP. A higher TIPM1 gene expression might indicate a
protective effect against ECM degradation in articular tissues.
Together, these results suggest that both NASHA and SC-CM exerts an
anti-inflammatory and anti-catabolic effect by partially counteract
the catabolic effect of proinflammatory cytokines through different
pathways.
Several clinical studies have shown that the use of
NASHA is well tolerated in patients in whom it has been
administered intra-articularly, and that its clinical effectiveness
remains for several weeks in the treatment of knee OA (36–38).
However, to our knowledge, the ability of NASHA to reduce the
inflammatory process associated with the development and
progression of OA has not been evaluated. On the other hand, it has
been shown that much of the chondrogenic potential of MSC is due to
the secretion of soluble factors that act in a paracrine fashion
and that play an important role in immunomodulation and tissue
regeneration (17,39). Considering this characteristic, we
could compare the anti-inflammatory effect of these two types of
therapies in the same culture system.
A systematic review about the anti-inflammatory
effect of intra-articular HA reported that high molecular weight HA
can promote an anti-inflammatory response within the cell through
binding sites of CD44, TLR-2 and TLR-4 receptors (40). The binding of the high molecular
weight HA to CD44 receptor downregulates the expression of IL-8,
IL-33, MMP, proteoglycans, and PGE2 and suppresses NF-κB
activation (40). It would be
important to determine if NASHA exerts its anti-inflammatory effect
over some of the targets reported in this study through the
aforementioned mechanism.
Our findings are in line with those of previous
research, which found that conditioned media from MSC stimulated
with pro-inflammatory mediators are able to inhibit inflammatory
processes by targeting catabolic and inflammatory factors (16,17).
One of these reports suggest that the inhibitory effects of SC-CM
could be partially supported by the fact that mediators produced by
MSC stimulated with proinflamatory agents induce a reduction of
NF-κB activation in OA chondrocytes (17).
Some of the limitations of this study include that
we only show changes in gene expression of markers related to
inflammation and matrix turnover. It would be important to confirm
these changes at the protein level and with other specific markers
of main ECM components such as collagen type II and aggrecan at
both gene and protein level. Moreover, a further characterization
of specific markers related to degradation and inflammation of
cartilage and synovium would yield more information about these
processes since only the GAG and NO were quantified.
This study evaluated the anti-inflammatory and
anti-catabolic properties of NASHA compared to those of SC-CM. Both
treatments showed similar favorable effects; however, SC-CM was
able to counteract inflammatory effects over a higher number of the
evaluated targets. The wide number of bioactive molecules probably
works in favor of the MSC to achieve the positive effects observed.
Further studies are needed to elucidate the exact mechanism by
which each therapy exerts its positive effects.
Acknowledgements
The authors would like to thank Dr Sergio Lozano
(member of the American Translators Association and the American
Medical Writers Association) for editing the English of the
manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MSM, SALS, FVC, VMPM and LFM conceived the original
idea and designed the experiments. MSM, SALS and VPS performed the
experiments. HGMR, FVC and CAAO analyzed the data. VPS, FVC and
VMPM interpreted the data. MSM and VMPM wrote the manuscript. FVC
and LFM edited the original article. FVC, LFM and VMPM critically
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
OR17-00002) by The Ethical Committee and Research Review Board
(Research Ethics Committee) of the University Hospital and School
of Medicine of Universidad Autonoma de Nuevo Leon. Informed consent
was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lawrence RC, Felson DT, Helmick CG, Arnold
LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG,
et al: Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States: Part II. Arthritis Rheum.
58:26–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen J, Abu-Amer Y, O'Keefe RJ and
McAlinden A: Inflammation and epigenetic regulation in
osteoarthritis. Connect Tissue Res. 58:49–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jørgensen AEM, Kjær M and Heinemeier KM:
The effect of aging and mechanical loading on the metabolism of
articular cartilage. J Rheumatol. 44:410–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldring MB, Otero M, Tsuchimochi K, Ijiri
K and Li Y: Defining the roles of inflammatory and anabolic
cytokines in cartilage metabolism. Ann Rheum Dis. 67 (Suppl
3):iii75–iii82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li MH, Xiao R, Li JB and Zhu Q:
Regenerative approaches for cartilage repair in the treatment of
osteoarthritis. Osteoarthritis Cartilage. 25:1577–1587. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evans CH, Kraus VB and Setton LA: Progress
in intra-articular therapy. Nat Rev Rheumatol. 10:11–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agerup B, Berg P and Akermark C:
Non-animal stabilized hyaluronic acid: A new formulation for the
treatment of osteoarthritis. BioDrugs. 19:23–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leighton R, Fitzpatrick J, Smith H,
Crandall D, Flannery CR and Conrozier T: Systematic clinical
evidence review of NASHA (Durolane hyaluronic acid) for the
treatment of knee osteoarthritis. Open access Rheumatol. 10:43–54.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta PK, Chullikana A, Rengasamy M,
Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D,
Viswanathan P, et al: Efficacy and safety of adult human bone
marrow-derived, cultured, pooled, allogeneic mesenchymal stromal
cells (Stempeucel®): Preclinical and clinical trial in
osteoarthritis of the knee joint. Arthritis Res Ther. 18:3012016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pers YM, Rackwitz L, Ferreira R, Pullig O,
Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, et
al: Adipose mesenchymal stromal Cell-based therapy for severe
osteoarthritis of the knee: A phase I dose-escalation trial. Stem
Cells Transl Med. 5:847–856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Timmers L, Lim SK, Hoefer IE, Arslan F,
Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, et
al: Human mesenchymal stem cell-conditioned medium improves cardiac
function following myocardial infarction. Stem Cell Res. 6:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Blanc K and Mougiakakos D: Multipotent
mesenchymal stromal cells and the innate immune system. Nat Rev
Immunol. 12:383–396. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caplan AI: Mesenchymal stem cells: Time to
change the Name! Stem Cells Transl Med. 6:1445–1451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Buul GM, Villafuertes E, Bos PK,
Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JA, Bernsen MR
and van Osch GJ: Mesenchymal stem cells secrete factors that
inhibit inflammatory processes in short-term osteoarthritic
synovium and cartilage explant culture. Osteoarthritis Cartilage.
20:1186–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Platas J, Guillén MI, del Caz MD, Gomar F,
Mirabet V and Alcaraz MJ: Conditioned media from
adipose-tissue-derived mesenchymal stem cells downregulate
degradative mediators induced by interleukin-1β in osteoarthritic
chondrocytes. Mediators Inflamm. 2013:3570142013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crop MJ, Baan CC, Korevaar SS, Ijzermans
JN, Pescatori M, Stubbs AP, van Ijcken WF, Dahlke MH, Eggenhofer E,
Weimar W and Hoogduijn MJ: Inflammatory conditions affect gene
expression and function of human adipose tissue-derived mesenchymal
stem cells. Clin Exp Immunol. 162:474–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khatab S, van Osch GJ, Kops N,
Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA, Bernsen MR and van
Buul GM: Mesenchymal stem cell secretome reduces pain and prevents
cartilage damage in a murine osteoarthritis model. Eur Cell Mater.
36:218–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hernandez-Hurtado AA, Borrego-Soto G,
Marino-Martinez IA, Lara-Arias J, Romero-Diaz VJ, Abrego-Guerra A,
Vilchez-Cavazos JF, Elizondo-Riojas G, Martinez-Rodriguez HG,
Espinoza-Juarez MA, et al: Implant composed of demineralized bone
and mesenchymal stem cells genetically modified with AdBMP2/AdBMP7
for the regeneration of bone fractures in ovis aries. Stem Cells
Int. 2016:74038902016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciuffreda MC, Malpasso G, Musarò P, Turco
V and Gnecchi M: Protocols for in vitro differentiation of human
mesenchymal stem cells into osteogenic, chondrogenic and adipogenic
lineages. Methods Mol Biol. 1416:149–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sellam J and Berenbaum F: The role of
synovitis in pathophysiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osterman C, McCarthy MB, Cote MP, Beitzel
K, Bradley J, Polkowski G and Mazzocca AD: Platelet-Rich plasma
increases Anti-inflammatory markers in a human coculture model for
osteoarthritis. Am J Sports Med. 43:1474–1484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson CI, Argyle DJ and Clements DN: In
vitro models for the study of osteoarthritis. Vet J. 209:40–49.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi CH, Kim TH, Sung YK, Choi CB, Na YI,
Yoo H and Jun JB: SKI306X inhibition of glycosaminoglycan
degradation in human cartilage involves down-regulation of
cytokine-induced catabolic genes. Korean J Intern Med. 29:647–655.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Behrendt P, Preusse-Prange A, Klüter T,
Haake M, Rolauffs B, Grodzinsky AJ, Lippross S and Kurz B: IL-10
reduces apoptosis and extracellular matrix degradation after
injurious compression of mature articular cartilage. Osteoarthritis
Cartilage. 24:1981–1988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gierman LM, van El B, van der Ham F,
Koudijs A, Stoop R, Verheijen JH, Kloppenburg M, van Osch GJ,
Stojanovic-Susulic V, Huizinga TW and Zuurmond AM: Profiling the
secretion of soluble mediators by end stage osteoarthritis synovial
tissue explants reveals a reduced responsiveness to an inflammatory
trigger. PLoS One. 8:e626342013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abramson SB: Osteoarthritis and nitric
oxide. Osteoarthritis Cartilage. 16 (Suppl 2):S15–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haltmayer E, Ribitsch I, Gabner S, Rosser
J, Gueltekin S, Peham J, Giese U, Dolezal M, Egerbacher M and
Jenner F: Co-culture of osteochondral explants and synovial
membrane as in vitro model for osteoarthritis. PLoS One.
14:e02147092019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koshy PJ, Lundy CJ, Rowan AD, Porter S,
Edwards DR, Hogan A, Clark IM and Cawston TE: The modulation of
matrix metalloproteinase and ADAM gene expression in human
chondrocytes by interleukin-1 and oncostatin M: A time-course study
using real-time quantitative reverse transcription-polymerase chain
reaction. Arthritis Rheum. 46:961–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pratta MA, Yao W, Decicco C, Tortorella
MD, Liu RQ, Copeland RA, Magolda R, Newton RC, Trzaskos JM and
Arner EC: Aggrecan protects cartilage collagen from proteolytic
cleavage. J Biol Chem. 278:45539–45545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Simental-Mendía M, Vilchez-Cavazos F,
García-Garza R, Lara-Arias J, Montes-de-Oca-Luna R, Said-Fernández
S and Martínez-Rodríguez HG: The matrix synthesis and
anti-inflammatory effect of autologous leukocyte-poor platelet rich
plasma in human cartilage explants. Histol Histopathol. 33:609–618.
2018.PubMed/NCBI
|
|
35
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leighton R, Akermark C, Therrien R,
Richardson JB, Andersson M, Todman MG and Arden NK; DUROLANE Study
Group, : NASHA hyaluronic acid vs methylprednisolone for knee
osteoarthritis: A prospective, multi-centre, randomized,
non-inferiority trial. Osteoarthritis Cartilage. 22:17–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altman RD, Akermark C, Beaulieu AD and
Schnitzer T; Durolane International Study Group, : Efficacy and
safety of a single intra-articular injection of non-animal
stabilized hyaluronic acid (NASHA) in patients with osteoarthritis
of the knee. Osteoarthritis Cartilage. 12:642–649. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Estades-Rubio FJ, Reyes-Martín A,
Morales-Marcos V, García-Piriz M, García-Vera JJ, Perán M, Marchal
JA and Montañez-Heredia E: Knee viscosupplementation:
Cost-effectiveness analysis between stabilized hyaluronic acid in a
single injection versus five injections of standard hyaluronic
acid. Int J Mol Sci. 18(pii): E6582017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singer NG and Caplan AI: Mesenchymal stem
cells: Mechanisms of inflammation. Annu Rev Pathol. 6:457–478.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Altman R, Bedi A, Manjoo A, Niazi F, Shaw
P and Mease P: Anti-inflammatory effects of Intra-articular
hyaluronic acid: A systematic review. Cartilage. 10:43–52. 2019.
View Article : Google Scholar : PubMed/NCBI
|