Introduction
Age-related macular degeneration (AMD) is the
leading cause of vision loss among individuals aged >50 years in
industrialized nations (1). Wet
AMD is characterized by choroidal neovascularization (CNV), wherein
new blood vessels disrupt Bruch's membrane and grow towards the
outer retina from the underlying choroid (2). These immature blood vessels are more
prone to leaking and bleeding, thus causing severe impairment of
visual acuity (3). CNV is a
process involving both inflammation and angiogenesis (3,4).
Vascular endothelial growth factor (VEGF) inhibitors (ranibizumab,
bevacizumab and aflibercept) are the current standard of care for
AMD (5). Although anti-VEGF
treatment generally stabilizes and improves visual acuity, not all
patients benefit from this type of therapy (5). There is evidence suggesting that the
inhibition of transforming growth factor β (TGFβ) may be a novel
therapeutic approach in the treatment of CNV (6,7).
TGFβ is part of a superfamily of peptide growth
factors, regulating a wide range of cellular functions during
development and maintaining adult tissue homeostasis (8). High expression of TGFβ is associated
with the expression of angiogenic factors and increased new vessel
formation (9). Three separate TGFβ
isoforms (TGFβ1, TGFβ2 and TGFβ3)
have been identified in mammals, and share 70–82% homology at the
amino acid level (10). Among
them, TGFβ2 is predominant in normal aqueous and
vitreous humor (11,12). VEGF is found in numerous ocular
tissues of both healthy and diseased eyes, and it plays a critical
role in the development of CNV (13). In AMD, there is evidence that
TGFβ2 induces the upregulation of VEGF mRNA expression
and protein secretion of VEGF by the retinal pigment epithelium
(RPE) and choroid cells (14).
Pirfenidone
[5-methyl-1-phenyl-2-(1H)-pyridone; PFD] is a non-peptide,
low-molecular weight compound. It was initially evaluated as an
anti-inflammatory agent, and subsequently developed as an
anti-fibrotic drug (15). Its
anti-inflammatory effect is attributed to tumor necrosis factor-α
(TNFα) (16). Notably, it reduces
the proliferation and differentiation of fibroblasts into
myofibroblasts by inhibiting key factors in the TGFβ pathway
(17). The safety and efficacy of
PFD have been clinically evaluated in numerous disorders. In
multinational phase 3 trials involving patients with idiopathic
pulmonary fibrosis, the administration of PFD significantly reduced
disease progression with an acceptable safety profile (18,19).
In a randomized, placebo-controlled trial that included 77 patients
with diabetic kidney disease, treatment with PFD led to an
improvement in the estimated glomerular filtration rate and was not
associated with severe adverse effects (20). PFD is applied to the eye in the
following clinical situations: Ocular surface diseases; glaucoma
filtration surgery; posterior capsular opacification; and
post-traumatic proliferative vitreoretinopathy (21).
Nevertheless, the relationship between PFD and CNV
has not been investigated. Based on the peak time of CNV
development (22,23), day 7 (after laser photocoagulation)
was selected to demonstrate the influence of PFD on the formation
of CNV and its effect on VEGF.
Materials and methods
Animals
A total of 63 male C57BL/6J mice (age: 6–8 weeks;
weight: 20–25 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. The animals were housed in specific cages with
ad libitum access to food and water in a room with a 12/12-h
light/dark cycle. The humidity and temperature were maintained at
50±5% and 23±1°C, respectively. All experimental procedures were
performed in accordance with the requirements of the Animal Welfare
Committee of Nantong University [permit nos. SCXK(Su)2014-0001 and
SYXK(Su)2017-0046]. This study adhered to the Association for
Research in Vision and Ophthalmology Statement for the Use of
Animals in Ophthalmic and Vision Research (24). The research protocol for the use of
animals was approved by the Center for Laboratory Animals of
Nantong University.
Intravitreal injection
In this experiment, 54 mice were randomly divided
into three groups (n=18/group): Control, vehicle and PFD. The
control group and the remaining 9 mice (normal group) received no
treatment. The injury induced by CNV and the potential toxicity of
PFD in the control, vehicle and PFD groups were compared to the
normal group (4 mice were used in choroidal flat mount experiment
and 5 mice were used in the histopathological examination,
respectively). As described in a previous experiment (25), an intravitreal injection of 1 µl
0.5% PFD (Beijing Kangdini Pharmaceutical Co., Ltd.), or vehicle
(0.01 M PBS solution: Sodium chloride, 137 mM; disodium phosphate
dodecahydrate, 9 mM; and sodium dihydrogen phosphate dehydrate, 2.9
mM) was administered on day 0 to the PFD and vehicle group,
respectively. Mice were decapitated at day 7 and 28 following
anesthesia (5% isoflurane).
Laser-induced CNV
The induction of CNV was carried out immediately
after drug application. Anesthesia was induced in 54 mice (control,
vehicle and PFD group) through inhalation of isoflurane (induction:
5%, maintenance: 1%), and the pupils were dilated with topical
administration of tropicamide phenylephrine eye drops (Santen
Pharmaceutical Co., Ltd.). Mice in the normal group (n=9) were not
induced. Following mydriasis, the mice were placed on a platform
under the slit lamp and a laser-induced CNV model was established
due to rupture of the Brunch's membrane, as previously described
(26). Laser photocoagulation
(532-nm laser, 200-mW, 100-ms duration, 50-µm spot size) was
performed bilaterally in each mouse. Laser spots were performed in
a standard manner around the optic nerve using a slit lamp delivery
system (Vision One; Lumenis), with a handheld cover slip used as
contact lens. Photocoagulation lesions were performed in a
peripapillary distribution at a distance of 1–2 disc diameters from
the optic nerve, avoiding major vessels. The appearance of a bubble
following laser treatment, which indicates a rupture of the Bruch's
membrane, is an important factor in the induction of CNV.
Therefore, only burns in which a bubble was produced were included
in subsequent experiments. Spots with hemorrhage or absence of a
bubble at the laser site were excluded from the analysis. The eye
was subsequently coated with an antibiotic eye ointment.
Afterwards, the CNV grade was evaluated, as previously described
(25). The control group
represented laser-induced CNV without an injection of PFD or
vehicle
Immunofluorescence
Eyes were enucleated, fixed in 4% paraformaldehyde
for 24 h at 4°C, and sectioned into cryosections (5 µm) at −20°C to
determine the localization of TGFβ2 using a specific
antibody (27,28). The cryosections were blocked with
5% BSA (Sigma-Aldrich; Merck KGaA) for 2 h at room temperature and
incubated with mouse monoclonal anti-TGFβ2 antibody
(1:50; cat. no. ab36495; Abcam) at 4°C overnight. The slides were
incubated with the secondary antibody, Alexa Fluor® 488
donkey anti-mouse IgG H+L (1:200; cat. no. A-21202; Thermo Fisher
Scientific, Inc.) for 2 h, and then DAPI for 5 min, both at room
temperature. The sections were imaged using a fluorescence
microscope (magnification, ×200; Olympus Corporation).
Western blotting
The RPE-choroid-sclera complex was extracted from 5
mice in each group on day 7 after intravitreal injection to detect
the protein levels of molecules. The tissues were homogenized and
solubilized in RIPA lysis buffer (cat. no. R0278; Sigma-Aldrich;
Merck KGaA), containing 1% protease inhibitors and 1% phosphatase
inhibitors (Thermo Fisher Scientific, Inc.). Protein concentration
in the supernatant was quantified using a spectrophotometer
(NanoDrop™ 1000; NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). The proteins (25 µg), according to their concentration and a
molecular weight marker, were loaded on a 10% SDS gel and subjected
to SDS-PAGE. Proteins were subsequently transferred to a
polyvinylidene difluoride membrane and blocked with 5% skim milk
for 2 h at room temperature. The membrane was incubated with mouse
monoclonal anti-TGFβ2 antibody (1:1,000; cat. no.
ab36495; Abcam), VEGF rabbit polyclonal antibody (1:5,000; cat. no.
19003-1-AP; ProteinTech Group, Inc.) and mouse monoclonal antibody
against GAPDH (1:2,000; cat. no. AT0002; CMCTAG; Engibody
Biotechnology, Inc.) at 4°C overnight. Then, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000; cat nos. SA00001-1 and SA00001-2; ProteinTech
Group, Inc.) at 37°C for 2 h, and washed in Tris-buffered saline
and Tween-20 (20 mM Tris-chloride, pH 7.5; 137 mM sodium chloride;
and 0.1% Tween-20). The blots were then incubated with
WesternBright ECL (APG Bio, Ltd.) and exposed to an image analysis
system (Tanon 5200 Multi; Tanon Science and Technology Co., Ltd.).
The intensity of GAPDH was used as the control, and the optical
density of bands was quantified using Image J version 1.47 software
(National Institutes of Health).
Fundus fluorescein angiography
(FFA)
At 1 week after laser photocoagulation or
intravitreal injection, fundus examinations were performed on 18
mice under general anesthesia. Thereafter, the mice were euthanized
as previously described. Mice had their pupils dilated, and a
digital fundus camera was used to image the back of the eye
(Heidelberg Retina Angiograph II; Heidelberg Engineering, Inc.).
The laser-induced lesions were studied using FFA to evaluate the
development of CNV. FFA images were captured 2–5 min after
intraperitoneal injection of 0.3 ml 2% fluorescein sodium (Guangxi
Yinzhou People's Pharmaceutical Co., Ltd.) (29). Angiograms were graded as follows:
Not stained, score 0; slightly stained, score 1; moderately
stained, score 2; strongly stained, score 3 (30,31).
Choroidal flat mount
At 1 week after the CNV-inducing laser procedure or
intravitreal treatment, 15 mice (10 eyes/group) were euthanized;
the eyes were enucleated and fixed in 4% paraformaldehyde solution
for 1 h at room temperature. The RPE-choroid-sclera complexes were
obtained by removing the anterior segments and the neural retina,
using an operation microscope (Olympus Corporation). Subsequently,
the complexes were washed in PBS containing 0.5% bovine serum
albumin, 0.2% Tween-20 and 0.1% Triton-X 100. Eyecups were
incubated with fluorescein Griffonia simplicifolia lectin I
isolectin B4 (1:100; cat. no. FL-1201; Vector Laboratories Inc.)
overnight at 4°C to label invading choroid vessels and washed three
times in PBS. After staining, the eyecups were flattened through
four to six radial cuts from the edge to the equator, and flat
mounted with the scleral side facing down onto a microscope slide.
The flat mounts were analyzed using a fluorescence microscope
(magnification, ×100; Olympus Corporation). The size of isolectin
B4-positive CNV areas was quantified with Image J software
(National Institutes of Health).
Histopathological examination
After the animals were sacrificed, the eyeballs were
enucleated, fixed in 4% paraformaldehyde solution overnight at 4°C,
conventionally dehydrated and embedded in optimal cutting
temperature compound to produce cryosections at −20°C. The optic
nerve parallel to the sagittal plane at the laser photocoagulation
position was selected, and slices (thickness, 5.0 µm) were
continuously prepared. The sections were stained with
hematoxylin-eosin (H&E) for 3 min at room temperature,
observed, and photographed using a light microscope (magnification,
×200; Olympus Corporation).
TUNEL
Cryosections (−20°C; 5 µm) were permeabilized with
10% proteinase K, after fixing in 4% paraformaldehyde solution at
room temperature for ~30 min according to the manufacturer's
protocol (One Step TUNEL Apoptosis Assay Kit; Nanjing KeyGen
Biotech Co., Ltd.). Apoptotic cells were treated with terminal
deoxynucleotidyl transferase (TdT) enzyme reaction mixture (1 µl
TdT enzyme and 4 µl biotin-11-2′-deoxyuridine 5′-triphosphate
diluted in 1 ml equilibration buffer) at 37°C for 45 min and
detected using streptavidin-fluorescein. Subsequently, the slides
were washed with PBS three times (15 min each) and sealed with
mounting medium (cat. no. 4112APG; Richard-Allan Scientific™;
Thermo Fisher Scientific, Inc.). Positive and negative controls
were included by adding deoxyribonuclease I reaction mixture and
omitting the TdT enzyme reaction mixture, respectively.
TUNEL-positive cells were observed in >4 randomly selected
fields under a fluorescent microscope (magnification, ×200).
Statistical analysis
All values are presented as the mean ± SD. One-way
ANOVA was used for statistical comparisons between multiple groups.
All pairwise multiple comparisons were performed using the
Bonferroni test. Descriptive statistics were performed using the
SPSS version 22.0 software (IBM Corp.). P<0.05 was used to
indicate statistical significance. Each experiment was performed in
triplicate.
Results
Formation of CNV in the mouse retina
after laser injury
At 1 week after laser photocoagulation, FFA was
performed in normal (without CNV or treatment) and CNV model mice
to examine the formation of CNV (Fig.
1A). The laser-induced spots showed hyperfluorescent leakage,
indicating rupture of Bruch's membrane and the formation of CNV.
Histological analysis of the retina stained with H&E (Fig. 1B) also confirmed the formation of
CNV on day 7 after laser-induced rupture of Bruch's membrane.
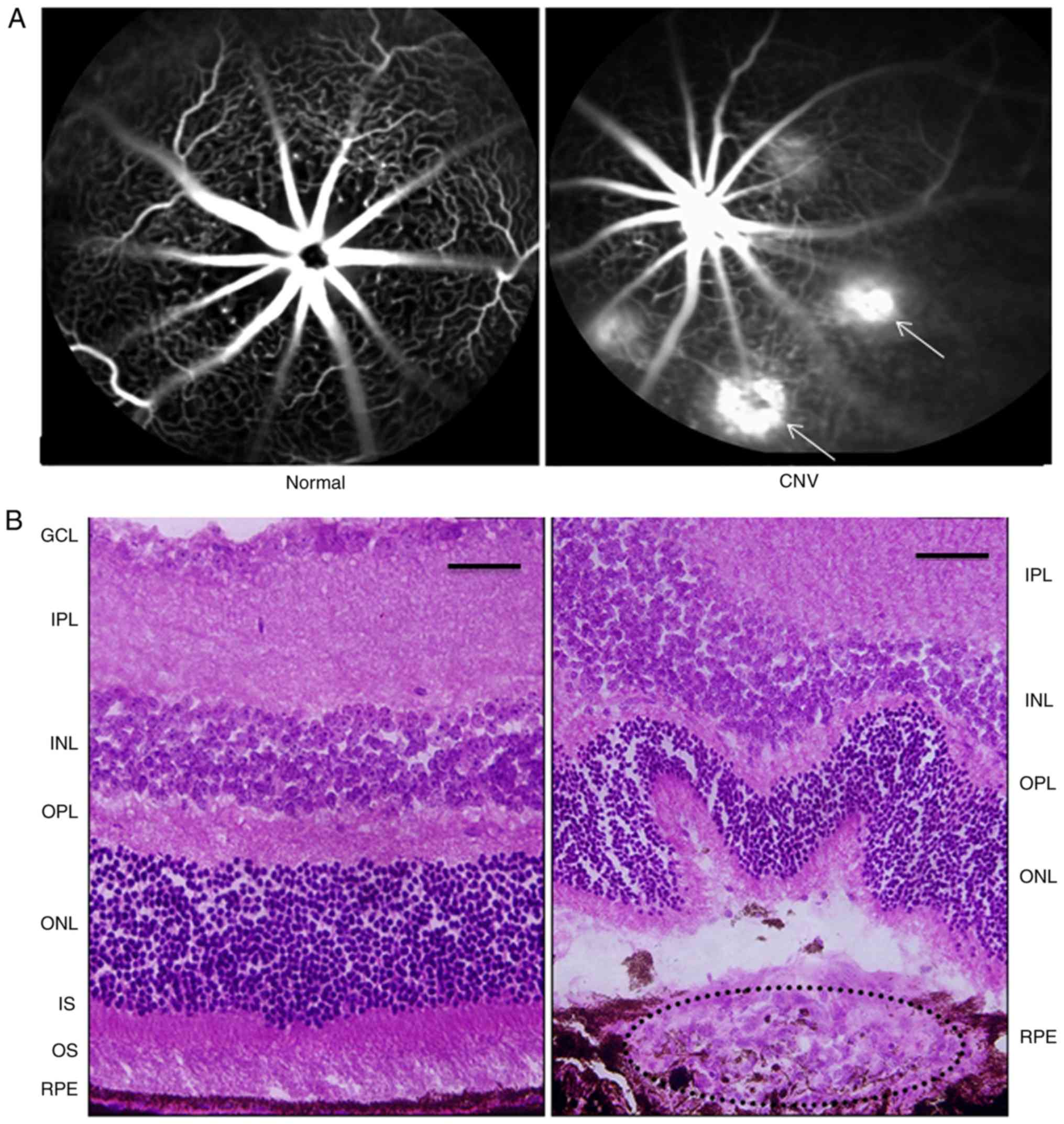 | Figure 1.Changes in the mouse retina 1 week
after laser photocoagulation. (A) FFA revealed the presence of
hyperfluorescence leakage in the laser-induced spots (white arrows)
compared with control (magnification, ×100). (B) H&E staining
of the mouse retina showed that the fibrovascular complex broke the
RPE into the subretinal space after laser photocoagulation compared
with control. Scale bar, 50 µm. RPE, retinal pigment epithelium;
OS, outer segment; IS, inner segment; ONL, outer nuclear layer;
OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner
plexiform layer; GCL, ganglion cell layer; CNV, choroidal
neovascularization; FFA, fundus fluorescein angiography. |
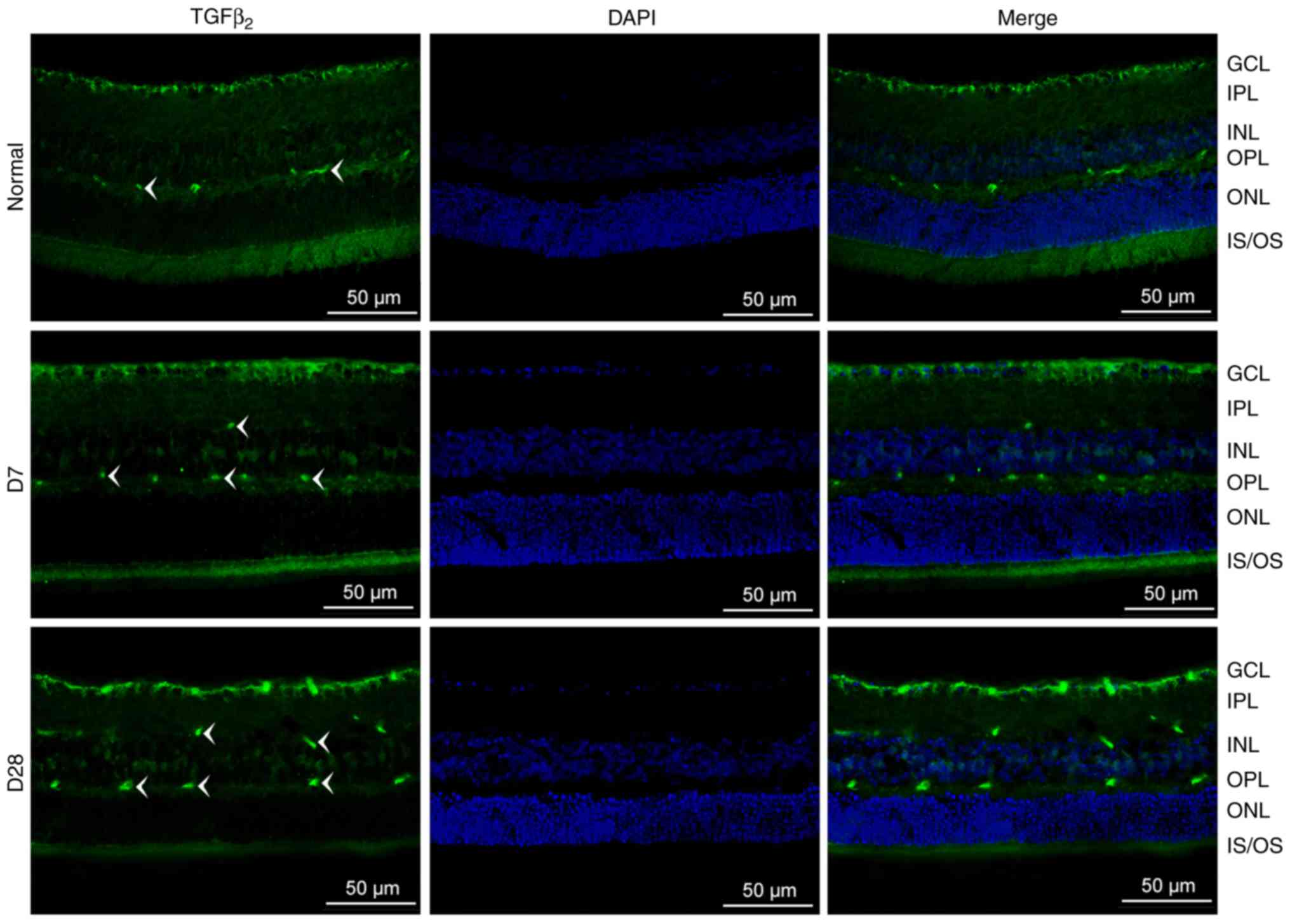
Localization of TGFβ2
changes in the mouse retina after laser injury
As shown in Fig. 2,
TGFβ2 is expressed in both the normal and injured
retina, and this expression was significantly increased on day 28.
In the normal mouse retina, TGFβ2 was localized to the
ganglion cell layer (GCL) and outer plexiform layer (OPL). After
laser photocoagulation (days 7 and 28), TGFβ2 could also
be observed in the inner plexiform layer (IPL). The green labels
were more intense on day 28 than on day 7.
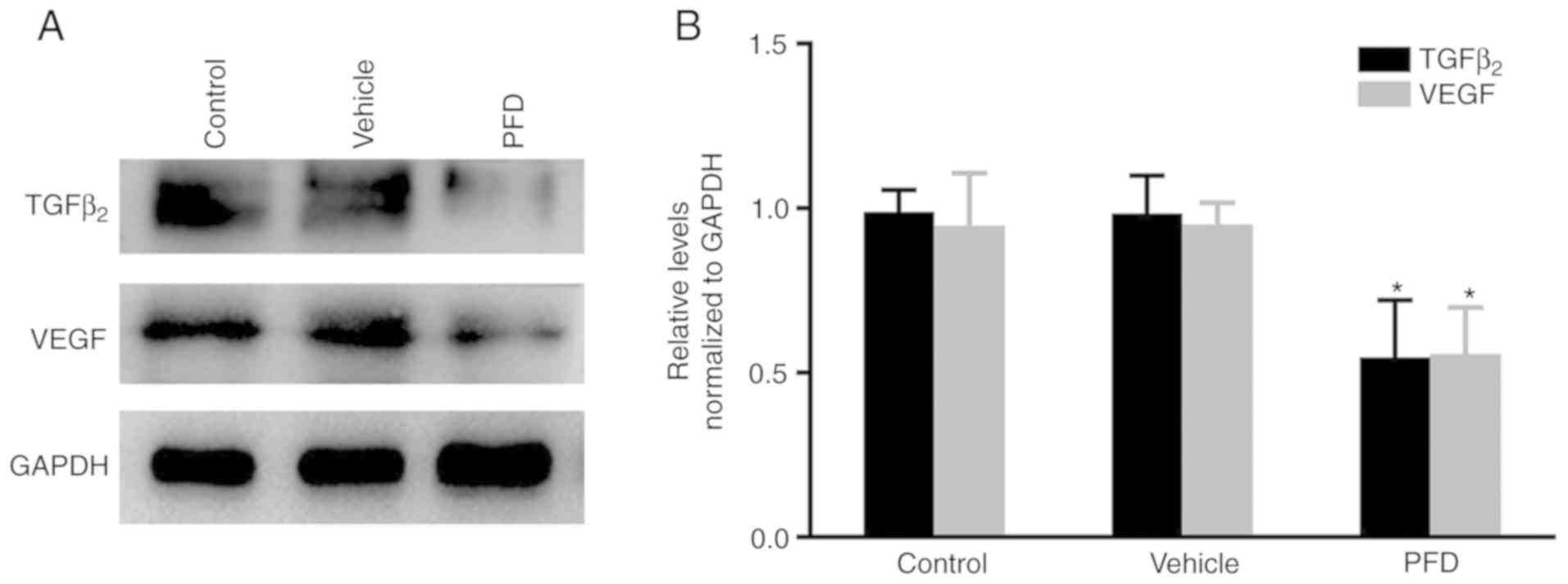
PFD suppresses the expression of
TGFβ2 and VEGF
The level of TGFβ2 protein in the
RPE-choroid complex was found to be significantly reduced in the
PFD injection group, compared with that measured in the control and
vehicle injection groups. This was also observed for VEGF (Fig. 3A and B). This suggested that PFD
may inhibit the formation of CNV by downregulating the expression
levels of TGFβ2 and VEGF.
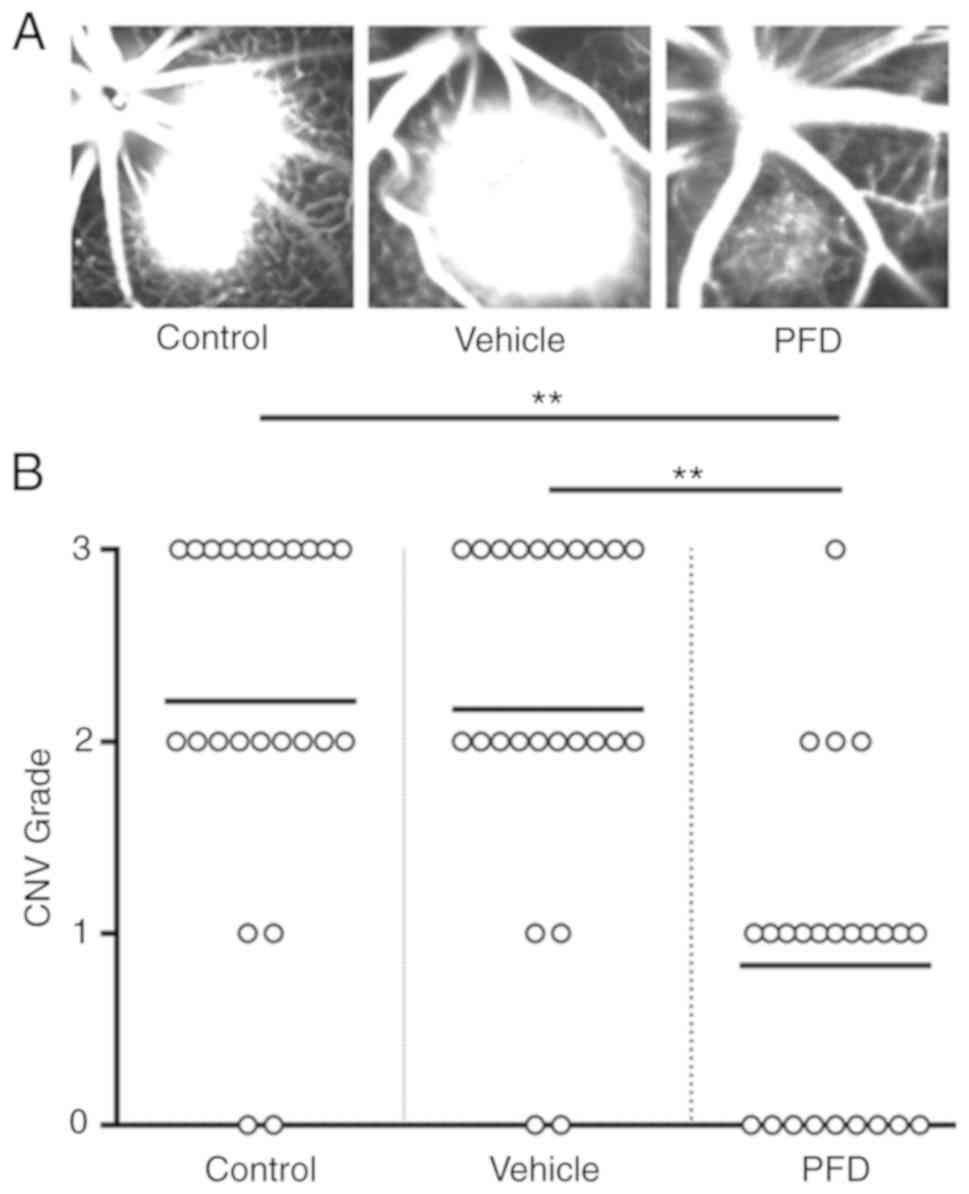
PFD alleviates the leakage of CNV
Notably, in the control and vehicle injection
groups, the brightness and size of the spot were almost identical
(Fig. 4A). Fluorescein angiography
showed that the leakage area of CNV was diminished in the PFD
injection group (Fig. 4A).
Meanwhile, PFD decreased the number of spots with extensive leakage
(score ≥2), whereas it increased the number of spots with limited
leakage (score 0 or 1; Fig.
4B).
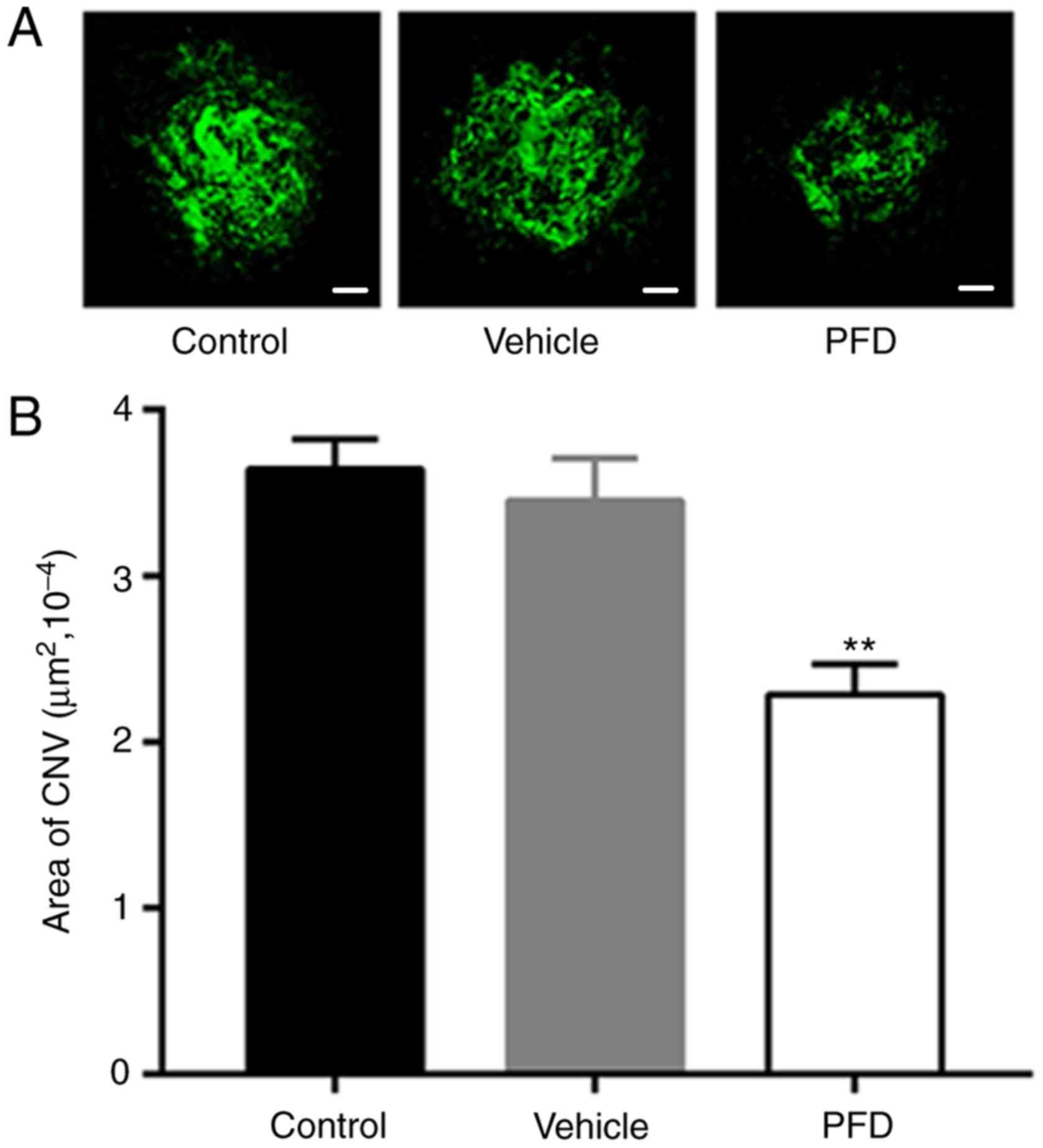
PFD reduces the area of CNV
The areas of isolectin B4 staining, marked with the
green fluorescent mass in the image, were notably smaller in the
PFD injection group than those observed in the control and vehicle
injection groups (Fig. 5A). The
difference in the quantitative measurement of the area of CNV
formation was also statistically significant (Fig. 5B). Combined with the
hypofluorescent leakage of the mouse retina shown on FFA, it was
demonstrated that an injection of 0.5% PFD into the vitreous body
of mice after laser photocoagulation could block the formation of
CNV and prevent the development of new blood vessels.
Toxicity of PFD on the mouse
retina
To confirm the safety of systemic PFD application,
the retina in the region without CNV injury was collected from the
PFD group and the histological changes were compared with the
tissue from normal animals. Similar to the normal mice, the layers
of the neural retina in the region without CNV in the PFD group
were closely organized, well-defined, and the cells were neatly
arranged; there were no obvious morphological abnormalities
(Fig. 6). Cryosections obtained
from the normal, vehicle and PFD groups on day 28 were used for the
TUNEL assay to analyze the effect of treatment with PFD on cell
death in the mouse retina. There was no obvious difference in the
apoptosis rate of the PFD group compared with the vehicle group
(Fig. 7). This confirmed that the
systemic application of PFD was safe to use on retinal tissue.
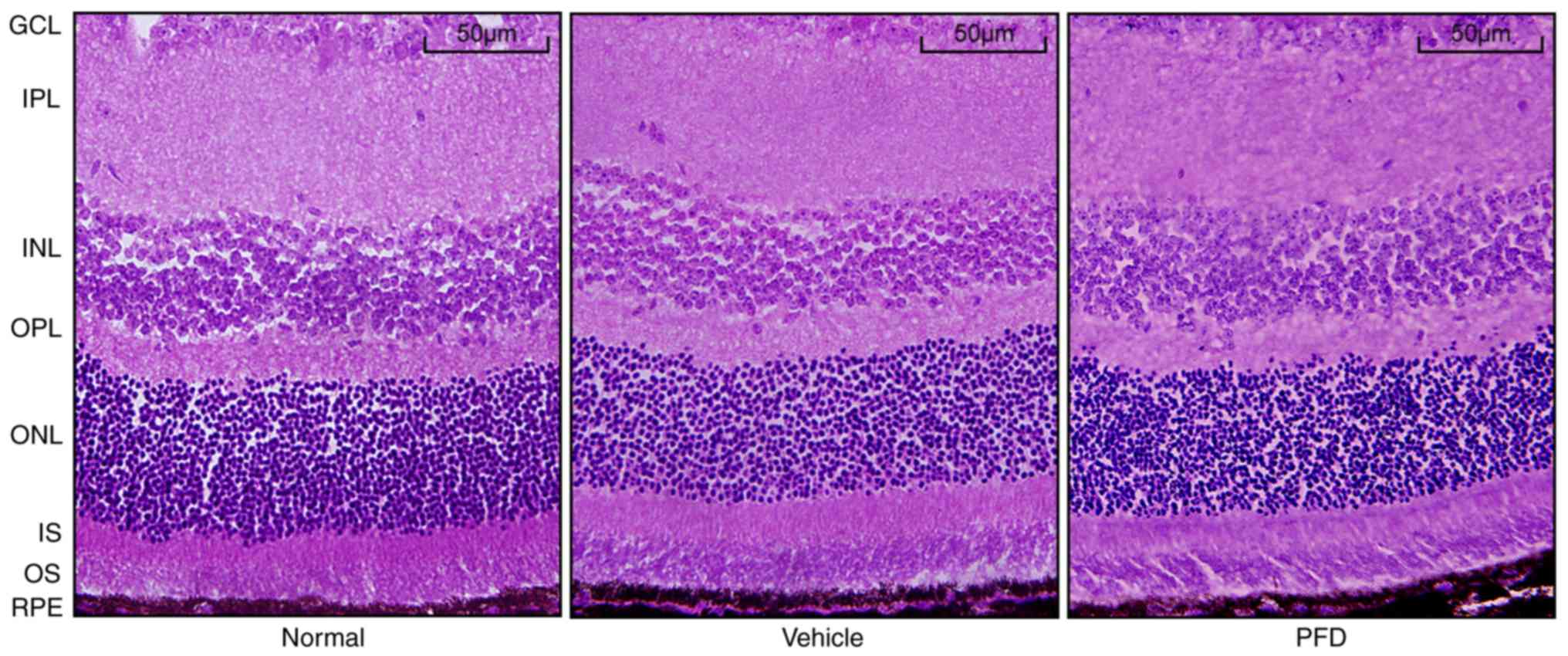 | Figure 6.Intravitreal injection of PFD has no
effect on the mouse retina. H&E staining images of choroidal
neovascularization. Retinal and choroidal structure in the normal,
vehicle and PFD groups. Scale bar, 50 µm. RPE, retinal pigment
epithelium; OS, outer segment; IS, inner segment; ONL, outer
nuclear layer; OPL, outer plexiform layer; INL, inner nuclear
layer; IPL, inner plexiform layer; GCL, ganglion cell layer; PFD,
pirfenidone. |
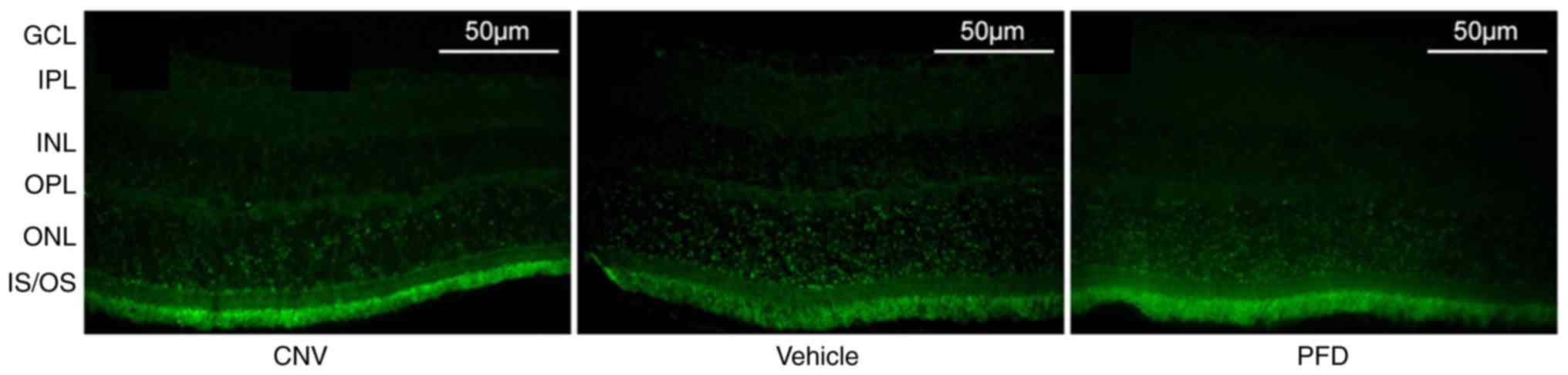 | Figure 7.Intravitreal injection of PFD does
not cause apoptosis. TUNEL labeling of the retinas obtained from
the control, vehicle and PFD groups. Scale bar, 50 µm. OS, outer
segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer
plexiform layer; INL, inner nuclear layer; IPL, inner plexiform
layer; GCL, ganglion cell layer; PFD, pirfenidone; CNV, choroidal
neovascularization. |
Discussion
In the present study, laser photocoagulation was
used to induce a CNV model in male C57BL/6J mice. The
immunofluorescent analysis of retinal cryosections showed that the
localization of TGFβ2 in the mouse retina changes from
the GCL and OPL in the normal retina to the GCL, OPL and IPL in the
injured retina. Regarding the effect of PFD on CNV, three
dimensions were analyzed to demonstrate that PFD could inhibit the
formation of CNV by downregulating the expression of VEGF. Finally,
H&E staining and a TUNEL assay showed that the administration
of PFD did not cause damage to the mouse retina.
It is well established that numerous cytokines, such
as VEGF, matrix metalloproteinases (MMPs), tissue inhibitor for
MMP3 (TIMP3) and TGFβ, are involved in different stages of CNV
(32). Specifically, VEGF plays a
key role as an inciting stimulus involved in the development of
CNV; it triggers the growth of vascular endothelial cells, enhances
microvascular permeability and promotes monocyte chemotaxis
(3,13). In elderly individuals, the
expression levels of MMPs are increased, and responsible for the
degradation of the extracellular matrix (ECM) (33). Meanwhile, TIMP3 can inhibit the
expression of these MMPs in order to remodel the ECM. As a result,
the ratio of MMPs to TIMP3 is essential for ECM turnover, and
controls pathology in the Bruch's membrane (34,35).
TGFβ is upregulated during the development of CNV, stimulates the
secretion of VEGF, and exerts a strong effect on the process of
collagen remodeling and scar contraction (36). In addition, TGF blockages could
inhibit angiogenesis and the formation of tissue fibrosis (7,29).
Based on the present results, treatment with the TGFβ2
inhibitor PFD hindered the formation and development of CNV.
In the posterior segment of the human eye, TGFβ
isoforms are distributed heterogeneously. TGFβ2 is
localized in the connective tissue of large choroidal vessels,
outer segment of photoreceptors, microglia, smooth muscle cells and
pericytes of superficial retinal blood vessels (37). Ogata et al (38) and Yamamoto et al (39) reported that TGFβ2 at
both mRNA and protein levels were detected in the GCL of normal and
photocoagulated retinas of rats. In the present study, it was
observed that TGFβ2 was mainly expressed in the GCL
prior to laser injury, while limited expression was detected in the
OPL. During the formation and development of CNV, the total
expression of TGFβ2 increased. Interestingly, on days 7
and 28, the immunolocalization of TGFβ2 was noted in the
GCL, OPL and IPL. Moreover, the immunoreaction was stronger in the
mouse retina on day 28 compared with day 7. This finding suggested
that TGFβ2 could affect CNV from the early to late
stages.
As mentioned in the introduction, PFD exerts its
pharmacokinetic effect by modulating the TNFα and TGFβ pathways,
and inhibiting the differentiation of fibroblasts into
myofibroblasts (15). There is a
body of evidence related to the anti-fibrotic effects of PFD in
vitro and in vivo. Kim et al (40) found that non-toxic concentrations
of PFD exert significant anti-fibrotic effects on orbital
fibroblasts from patients with thyroid-associated ophthalmopathy.
Chowdhury et al (41)
indicated that PFD decreased collagen synthesis, prevented
myofibroblast formation and improved corneal wound healing. After
trabeculectomy surgery, Zhong et al (42) used 0.5% PFD eye drops to improve
postoperative bleb survival. Previously, investigations on human
Tenon's fibroblasts demonstrated that PFD could inhibit cell
proliferation and migration (43,44).
Yang et al (45) revealed
that PFD inhibited the TGFβ2-induced proliferation,
migration and epithelial-mesenchymal transition of human lens
epithelial cells by downregulating the TGFβ/SMAD signaling pathway.
Using a proliferative vitreoretinopathy model, Khanum et al
(25) confirmed that PFD prevented
fibrotic changes involved in proliferative vitreoretinopathy.
Collectively, the present and previous results
indicate that PFD could be a potential treatment of wet AMD,
providing an alternative to the current methods used in the
clinical setting. Both the present study and a previous study
(46) have demonstrated that the
local application of PFD can offer protection to the eye. It is
proposed that intravenous injection should be carried out to assess
the clinical application of the drug. This study used the
laser-induced CNV model, which has been shown to be repeatable and
stable (26). However, there are a
number of limitations of the present study. Firstly, the timeline
of CNV inhibition, as well as the effect of PFD when new blood
vessels are not formed or after the formation of CNV remains
uncertain. Secondly, only the anti-neovascular function of PFD was
examined (assays were only performed on day 7 post-induction). In
future investigations, its anti-fibrotic functions and effects on
the relationship between TGFβ2 and VEGF will be
observed. In addition, this study mainly focused on the mechanisms
involved in the protective effects of PFD, which were observed
after the induction of CNV.
To conclude, the local administration of PFD reduced
the formation of CNV by downregulating the expression of VEGF. This
indicated that the use of a TGFβ inhibitor may be a promising
therapy for wet AMD.
Acknowledgements
Not applicable.
Funding
This study was supported by Nantong Science and
Technology Project (grant no. MS22015085).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YB, LH, XH, CG, YC, LW and SZ performed the
experiments and analyzed the data; YB and YS designed the study and
wrote the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the requirements of the Animal Welfare Committee of
Nantong University [permit nos. SCXK(Su) 2014-0001 and
SYXK(Su)2017-0046]. This study adhered to the Association for
Research in Vision and Ophthalmology Statement for the Use of
Animals in Ophthalmic and Vision Research. The research protocol
for the use of animals was approved by the Center for Laboratory
Animals of Nantong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gehrs KM, Anderson DH, Johnson LV and
Hageman GS: Age-related macular degeneration-emerging pathogenetic
and therapeutic concepts. Ann Med. 38:450–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambati J and Fowler BJ: Mechanisms of
age-related macular degeneration. Neuron. 75:26–39. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campa C, Costagliola C, Incorvaia C,
Sheridan C, Semeraro F, De Nadai K, Sebastiani A and Parmeggiani F:
Inflammatory mediators and angiogenic factors in choroidal
neovascularization: Pathogenetic interactions and therapeutic
implications. Mediators Inflamm. 2010:2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambati J, Atkinson JP and Gelfand BD:
Immunology of age-related macular degeneration. Nat Rev Immunol.
13:438–451. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Lookeren Campagne M, LeCouter J,
Yaspan BL and Ye W: Mechanisms of age-related macular degeneration
and therapeutic opportunities. J Pathol. 232:151–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Recalde S, Zarranz-Ventura J,
Fernández-Robredo P, García-Gómez PJ, Salinas-Alamán A,
Borrás-Cuesta F, Dotor J and García-Layana A: Transforming growth
factor-β inhibition decreases diode laser-induced choroidal
neovascularization development in rats: P17 and P144 peptides.
Invest Ophthalmol Vis Sci. 52:7090–7097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zarranz-Ventura J, Fernández-Robredo P,
Recalde S, Salinas-Alamán A, Borrás-Cuesta F, Dotor J and
García-Layana A: Transforming growth factor-beta inhibition reduces
progression of early choroidal neovascularization lesions in rats:
P17 and P144 peptides. PLoS One. 8:e654342013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding D, Li C, Zhao T, Li D, Yang L and
Zhang B: LncRNA H19/miR-29b-3p/PGRN axis promoted
epithelial-mesenchymal transition of colorectal cancer cells by
acting on Wnt signaling. Mol Cells. 41:423–435. 2018.PubMed/NCBI
|
|
9
|
Verrecchia F and Rédini F: Transforming
growth factor-β signaling plays a pivotal role in the interplay
between osteosarcoma cells and their microenvironment. Front Oncol.
8:1332018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stewart AG, Thomas B and Koff J: TGF-β:
Master regulator of inflammation and fibrosis. Respirology.
23:1096–1097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kita T, Hata Y, Arita R, Kawahara S, Miura
M, Nakao S, Mochizuki Y, Enaida H, Goto Y, Shimokawa H, et al: Role
of TGF-beta in proliferative vitreoretinal diseases and ROCK as a
therapeutic target. Proc Natl Acad Sci USA. 105:17504–17509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saika S: TGFbeta pathobiology in the eye.
Lab Invest. 86:106–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bressler SB: Introduction: Understanding
the role of angiogenesis and antiangiogenic agents in age-related
macular degeneration. Ophthalmology. 116:S1–S7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagineni CN, Samuel W, Nagineni S,
Pardhasaradhi K, Wiggert B, Detrick B and Hooks JJ: Transforming
growth factor-beta induces expression of vascular endothelial
growth factor in human retinal pigment epithelial cells:
Involvement of mitogen-activated protein kinases. J Cell Physiol.
197:453–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagami K, Oka T, Wang Q, Ishizu T, Lee
JK, Miwa K, Akazawa H, Naito AT, Sakata Y and Komuro I: Pirfenidone
exhibits cardioprotective effects by regulating myocardial fibrosis
and vascular permeability in pressure-overloaded hearts. Am J
Physiol Heart Circ Physiol. 309:H512–H522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iyer SN, Hyde DM and Giri SN:
Anti-inflammatory effect of pirfenidone in the bleomycin-hamster
model of lung inflammation. Inflammation. 24:477–491. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Selvaggio AS and Noble PW: Pirfenidone
initiates a new era in the treatment of idiopathic pulmonary
fibrosis. Annu Rev Med. 67:487–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
King TE Jr, Bradford WZ, Castro-Bernardini
S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM,
Kardatzke D, Lancaster L, et al: A phase 3 trial of pirfenidone in
patients with idiopathic pulmonary fibrosis. N Engl J Med.
370:2083–2092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noble PW, Albera C, Bradford WZ, Costabel
U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg MK,
Lancaster L, et al: Pirfenidone for idiopathic pulmonary fibrosis:
Analysis of pooled data from three multinational phase 3 trials.
Eur Respir J. 47:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma K, Ix JH, Mathew AV, Cho M,
Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, et
al: Pirfenidone for diabetic nephropathy. J Am Soc Nephrol.
22:1144–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lopez-de la Mora DA, Sanchez-Roque C,
Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S,
Macias-Barragan J and Armendariz-Borunda J: Role and new insights
of pirfenidone in fibrotic diseases. Int J Med Sci. 12:840–847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishikawa K, Kannan R and Hinton DR:
Molecular mechanisms of subretinal fibrosis in age-related macular
degeneration. Exp Eye Res. 142:19–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Zhu M, Yang X, Wang Y, Qin B, Cui
C, Chen H and Sang A: Inhibition of RACK1 ameliorates choroidal
neovascularization formation in vitro and in vivo. Exp Mol Pathol.
100:451–459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Samuels BC, Siegwart JT, Zhan W, Hethcox
L, Chimento M, Whitley R, Downs JC and Girkin CA: A novel tree
shrew (Tupaia belangeri) model of glaucoma. Invest Ophthalmol Vis
Sci. 59:3136–3143. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khanum BNMK, Guha R, Sur VP, Nandi S,
Basak SK, Konar A and Hazra S: Pirfenidone inhibits post-traumatic
proliferative vitreoretinopathy. Eye (Lond). 31:1317–1328. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah RS, Soetikno BT, Lajko M and Fawzi
AA: A mouse model for laser-induced choroidal neovascularization. J
Vis Exp. e535022015.PubMed/NCBI
|
|
27
|
Liu B, Gao J, Lyu BC, Du SS, Pei C, Zhu ZQ
and Ma B: Expressions of TGF-β2, bFGF and ICAM-1 in lens epithelial
cells of complicated cataract with silicone oil tamponade. Int J
Ophthalmol. 10:1034–1039. 2017.PubMed/NCBI
|
|
28
|
Hulin A, Deroanne CF, Lambert CA, Dumont
B, Castronovo V, Defraigne JO, Nusgens BV, Radermecker MA and
Colige AC: Metallothionein-dependent up-regulation of TGF-β2
participates in the remodelling of the myxomatous mitral valve.
Cardiovasc Res. 93:480–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Ma W, Han S, Meng Z, Zhao L, Yin
Y, Wang Y and Li J: TGF-β participates choroid neovascularization
through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet
age-related macular degeneration. Sci Rep. 7:96722017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Y, Li X, Wang YS, Shi YY, Ye Z, Yang
GD, Dou GR, Hou HY, Yang N, Cao XR and Lu ZF: Hyperglycemia
promotes vasculogenesis in choroidal neovascularization in diabetic
mice by stimulating VEGF and SDF-1 expression in retinal pigment
epithelial cells. Exp Eye Res. 123:87–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ozone D, Mizutani T, Nozaki M, Ohbayashi
M, Hasegawa N, Kato A, Yasukawa T and Ogura Y: Tissue plasminogen
activator as an antiangiogenic agent in experimental laser-induced
choroidal neovascularization in mice. Invest Ophthalmol Vis Sci.
57:5348–5354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci. 17:2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ardeljan D and Chan CC: Aging is not a
disease: Distinguishing age-related macular degeneration from
aging. Prog Retin Eye Res. 37:68–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I,
Wong TY and Cheung CMG: Age-related macular Degeneration and
polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res.
53:107–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bai Y, Liang S, Yu W, Zhao M, Huang L,
Zhao M and Li X: Semaphorin 3A blocks the formation of pathologic
choroidal neovascularization induced by transforming growth factor
beta. Mol Vis. 20:1258–1270. 2014.PubMed/NCBI
|
|
37
|
Tosi GM, Orlandini M and Galvagni F: The
controversial role of TGF-β in neovascular age-related macular
degeneration pathogenesis. Int J Mol Sci. 19:2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogata N, Yamamoto C, Miyashiro M, Yamada
H, Matsushima M and Uyama M: Expression of transforming growth
factor-beta mRNA in experimental choroidal neovascularization. Curr
Eye Res. 16:9–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto C, Ogata N, Yi X, Takahashi K,
Miyashiro M, Yamada H, Uyama M and Matsuzaki K: Immunolocalization
of transforming growth factor beta during wound repair in rat
retina after laser photocoagulation. Graefes Arch Clin Exp
Ophthalmol. 236:41–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim H, Choi YH, Park SJ, Lee SY, Kim SJ,
Jou I and Kook KH: Antifibrotic effect of Pirfenidone on orbital
fibroblasts of patients with thyroid-associated ophthalmopathy by
decreasing TIMP-1 and collagen levels. Invest Ophthalmol Vis Sci.
51:3061–3066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chowdhury S, Guha R, Trivedi R, Kompella
UB, Konar A and Hazra S: Pirfenidone nanoparticles improve corneal
wound healing and prevent scarring following alkali burn. PLoS One.
8:e705282013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong H, Sun G, Lin X, Wu K and Yu M:
Evaluation of pirfenidone as a new postoperative antiscarring agent
in experimental glaucoma surgery. Invest Ophthalmol Vis Sci.
52:3136–3142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo X, Yang Y, Liu L, Liu X, Xu J, Wu K
and Yu M: Pirfenidone induces G1 arrest in human Tenon's
fibroblasts in vitro involving AKT and MAPK signaling pathways. J
Ocul Pharmacol Ther. 33:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Na JH, Sung KR, Shin JA and Moon JI:
Antifibrotic effects of pirfenidone on Tenon's fibroblasts in
glaucomatous eyes: Comparison with mitomycin C and 5-fluorouracil.
Graefes Arch Clin Exp Ophthalmol. 253:1537–1545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Ye Y, Lin X, Wu K and Yu M:
Inhibition of pirfenidone on TGF-beta2 induced proliferation,
migration and epithlial-mesenchymal transition of human lens
epithelial cells line SRA01/04. PLoS One. 8:e568372013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang N, Ma M, Li Y, Su T, Zhou XZ, Ye L,
Yuan Q, Zhu P, Min Y, Shi W, et al: The role of pirfenidone in
alkali burn rat cornea. Int Immunopharmacol. 64:78–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|