Introduction
Acute lung injury (ALI) is a major cause of
morbidity and mortality, which is characterized by strong pulmonary
inflammation (1,2), leading to cell death and ultimately
to respiratory failure. Lipopolysaccharide (LPS) derived from
gram-negative bacteria is considered the principal
pathogen-associated molecular cause of ALI (3). LPS is widely used to induce pulmonary
inflammation in experimental models of ALI (4). As reported, acute lung damage is
associated with initiation of an inflammatory response that leads
to a series of pathological alterations in lung tissues, including
changes in the ultrastructure of pulmonary alveoli (5), expression of inflammatory cytokines
(6), cell damage (7) and accumulation of reactive oxygen
species (ROS) (8). Although
pharmacological therapies for ALI have been developed in recent
decades, the mortality rate remains high.
The excessive inflammatory response associated with
ALI causes cell damage, with the damaged cells being eliminated by
apoptosis, which is a mechanism that protects cells from
inflammation and necrosis (9).
However, a high degree of apoptosis overwhelms the capacity of the
immune system to clear dead cells, thus resulting in necrosis.
Necrosis is a type of cell death morphologically characterized by
cell swelling and cytomembrane rupture (10). Programmed necrosis, or necroptosis,
is a regulated form of necrosis, which is induced by various
initiators, particularly tumor necrosis factor α (TNF-α), which is
a downstream product of LPS stimulation of Toll-like receptor 4
(TLR4) and the most extensive inducer of necroptosis (11). During the process of
LPS-TLR4-TNF-α-induced necroptosis, receptor-interacting
serine/threonine-protein kinase (RIP)1 interacts with RIP3 to
induce its activation, thus leading to rupture of the cell
membrane. RIP1-RIP3 forms a complex to promote necroptosis-induced
tissue injury (12). There is a
general consensus that necroptosis is a potent inducer of
inflammation (13), whereas
necrostatin-1 (Nec-1) is an inhibitor of RIP1 that has been
confirmed to be a potent and specific allosteric inhibitor of
necroptosis. Promising results have been obtained with Nec-1 in
numerous experimental disease models, including
ischemia-reperfusion injury (14,15),
intestinal inflammation (16),
acute pancreatitis (17) and
neurodegenerative disease (18).
Therefore, inhibition of necroptosis may be a promising strategy to
improve the outcome of overloaded necroptosis-induced localized or
severe and systemic inflammatory disorders.
Under physiological conditions, ROS are regulated by
antioxidants (19), which can
either be endogenously generated or externally supplemented.
However, overproduction of ROS has been implicated in the
development of various chronic and degenerative diseases, including
cancer, and respiratory, neurodegenerative and digestive diseases
(20). ROS are likely to be among
the inducers of acute or chronic inflammation that lead to cellular
damage, since antioxidants reduce activation of inflammatory
pathways (21). The antioxidant
defense system can be overwhelmed during sustained severe
inflammation, as observed in acute lung inflammation, inflammatory
bowel disease, neurodegenerative disorders, cardiovascular diseases
and aging (22). Therefore,
certain antioxidants are essential to regulate biochemical pathways
that lead to the appropriate functioning of organs. Antioxidant
supplementation has been reported to lessen endogenous antioxidant
depletion and to alleviate oxidative damage in experimental
research (20). Therefore, the
present study evaluated the protective effects of Nec-1 on
LPS-induced ALI.
Materials and methods
Animals
All experiments using male mice (age, 8–10 weeks;
weight, 22–24 g). All animal procedures were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University. A total of 30 healthy wild-type male C57B/6 mice (age,
6–8 weeks; weight, 18–22 g) purchased from the Animal Research
Center of Wenzhou Medical University were maintained and acclimated
in a specific pathogen-free environment for 2 weeks prior to
experimentation, with free access to food and water at the Wenzhou
Medical University under a controlled temperature (25°C) with 50%
humidity and 12-h light/dark cycles.
Cells and cell culture
The rat alveolar epithelial RLE-6TN cell line, which
was derived from alveolar type II cells isolated from a 56-day-old
male F344 rat by airway perfusion with a pronase solution, was
purchased from American Type Culture Collection. Cells were
cultured in DMEM/F12 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
The cell model of ALI was induced using TNF-α (40 µM;
Sigma-Aldrich; Merck KGaA). The treatment group was co-treated with
TNF-α and Nec-1 (50 µM; Enzo Life Sciences, Inc.) for 6 h at 37°C.
The mock group was treated with saline for 6 h at 37°C. The cells
were harvested at 6 h after initiation of the experiment for
subsequent analyses.
Animal model and experimental
setup
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University. Mice were fasted for 8 h prior to the experiment.
Before induction of ALI, the mice were anesthetized by inhalation
of sevoflurane and were transtracheally injected with either Nec-1
(5 mg/kg; Enzo Life Sciences, Inc.) or saline as a control.
LPS-induced ALI was induced by transtracheal injection of LPS
(Sigma-Aldrich; Merck KGaA) at a dose of 10 mg/kg 30 min after
Nec-1 or saline treatment. Additionally, the mice of sham-operation
were transtracheally injected with saline and treated with saline
only. During the experiment, the mice were monitored every 30 min.
All mice survived until sacrifice 6 h after initiation of the
experiment. The mice were sacrificed by cervical dislocation under
3% sevoflurane inhalation, after which, the lungs were quickly
removed. Lung samples were either placed in a buffered solution for
histopathological analysis, or snap-frozen for protein isolation or
for analyzing apoptosis by flow cytometry. Prior to tissue
collection, death was confirmed after 1–2 min of cardiac and
respiratory observation.
Histopathological examination of the
lungs
The lung tissues were collected after 6 h of
induction of ALI. The tissues were fixed in 4% paraformaldehyde in
PBS (pH 7.4) for 24 h at room temperature. Paraffin-embedded
tissues from each mouse were sectioned at 5 µm and were then
stained with hematoxylin for 5 min and eosin for 10 sec at room
temperature. Each lung tissue section was observed in five fields
of view using a light microscope (magnification, ×200).
Transmission electron microscopy
(TEM)
Mouse lung tissues were minced into small pieces (1
mm3) and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate
buffer for 4 h at 4°C. The tissues were post-fixed in 1% osmium
tetroxide in 1% K4Fe(CH)6 for 1 h at 37°C, and dehydrated through
graded concentrations of alcohol (50, 75, 90 and 100%; 30 min at
room temperature per concentration), clarified in propylene oxide
for 12 h at 45°C, and embedded in a mixture of Epon 812 and
Durcupan for 3 days at 60°C (Sigma-Aldrich; Merck KGaA). The
tissues were subsequently sectioned with an ultramicrotome (100
nm). The longitudinal sections were placed onto copper grids,
stained with 7.7% uranyl acetate (50 µl) at room temperature for 30
min, and 2.6% lead nitrate (50 µl) at room temperature for 2 sec.
Subsequently, the sections were visualized under a H-600 electron
microscope (magnification, ×17000; Hitachi Ltd.).
Immunofluorescence analysis
Immunofluorescence was used to detect RIP1 and RIP3
expression. Cells were treated with TNF-α (40 µM) and Nec-1 (50
µM), washed with PBS, fixed with 4% paraformaldehyde for 15 min at
4°C, and were then permeabilized with 0.5% Triton X-100 for 10 min
at room temperature. After blocking with 5% BSA (Beyotime Institute
of Biotechnology) for 1 h at 37°C, the cells were incubated with
either anti-RIP1 (cat. no. 3493; 1:200; Cell Signaling Technology,
Inc.) or anti-RIP3 (cat. no. ab62344; 1:200; Abcam) antibodies
overnight at 4°C. The slides were then incubated with Alexa
Fluor® 488-conjugated goat anti-rabbit IgG antibody
(cat. no. ab150077; 1:200; Abcam,) for 1 h at 37°C. The nuclei were
then stained with DAPI for 5 min at room temperature. Images were
obtained under a fluorescence microscope.
Protein extraction and western
blotting
Snap-frozen lung tissues or cells were homogenized
and resuspended in lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) containing 1% phenylmethylsulfonyl
fluoride (cat. no. ST506; Beyotime Institute of Biotechnology) for
30 min at 4°C. Subsequently, the lysate was centrifuged at 12,000 ×
g for 15 min at 4°C and the supernatant was collected. Protein
concentrations were determined using a bicinchoninic acid (BCA)
assay kit (cat. no. P0012; Beyotime Institute of Biotechnology) at
37°C. The proteins (50 µg/lane) were separated by SDS-PAGE on 10%
gels and were then transferred to PVDF membranes (EMD Millipore).
The transferred membranes were blocked with 5% skim milk for 1 h at
room temperature. The membranes were incubated at 4°C overnight
with the following primary antibodies: Anti-RIP1 (cat. no. 3493;
Cell Signaling Technology, Inc.), anti-RIP3 (cat. no. ab62344;
Abcam), anti-GAPDH (cat. no. 5174; Cell Signaling Technology,
Inc.), anti-nuclear factor erythroid 2-related factor 2 (NRF2; cat.
no. ab137550; Abcam) and anti-heme oxygenase 1 (HO-1; cat. no.
ab13243; Abcam). All aforementioned primary antibodies were diluted
to 1:1,000 with WB antibody diluent (cat. no. P0023A; Beyotime
Institute of Biotechnology). Subsequently, the membranes were
incubated with HRP-conjugated anti-rabbit IgG secondary antibody
(cat. no. 7074; 1:3,000; Cell Signaling Technology, Inc.) at room
temperature for 1 h. Protein bands were detected using the
Immobilon ECL Ultra Western HRP substrate (EMD Millipore) and Image
Lab software (version 3.0; Bio-Rad Laboratories Inc.), with GAPDH
as the loading control.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
RNA was isolated from lung tissues stored in
RNAlater or from the rat alveolar epithelial cell line using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. After determining RNA
concentration, 1 µg RNA was used for cDNA synthesis using the
PrimeScript™ RT Reagent kit for RT-PCR (Takara Bio, Inc.),
according to the manufacturer's protocol. qPCR analysis was
performed on cDNA using the ABI7500 instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with TB Green® Premix Ex
Taq™ II kit (Takara Bio, Inc.), according to the manufacturer's
protocol. The thermocycling conditions were as follows:
Pre-conditioning at 95°C for 30 sec; annealing for 5 sec at 95°C
and amplification for 15 sec at 60°C for 40 cycles; final extension
for 10 sec at 60°C. The relative mRNA expression levels were
normalized the internal reference gene GAPDH using the
2−ΔΔCq cycle threshold method (23). The primer sequences for qPCR are
listed in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| A, Mouse |
|---|
|
|---|
|
| Sequence
(5′→3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCA |
| HO-1 |
AGGTCCTGAAGAAGATTGC |
TCTCCAGAGTGTTCATTCG |
| NRF-2 |
AGCATCCTCTCCACTGAT |
GGTCACAGCCTTCAATAGT |
|
| B, Rat |
|
|
| Sequence
(5′→3′) |
|
|
|
| Gene | Forward | Reverse |
|
| GAPDH |
GTGCAGTGCCAGCCTCGTC |
GGCAGCACCAGTGGATGCAG |
| HO-1 |
GGTGACAGAAGAGGCTAAG |
TAGTATCTTGAACCAGGCTAG |
| NRF-2 |
CCGAGTTACAGTGTCTTAATAC |
TGGAGAGGATGCTGCTAA |
Isolation of primary alveolar
epithelial cells
The lungs of were rapidly removed, incubated with
1.0 mg/ml collagenase I (Thermo Fisher Scientific, Inc.) for 1 h at
37°C and minced into small pieces (1 mm3) on ice. The single lung
cell suspensions were used for further ROS and apoptosis
analyses.
Quantification of ROS levels
Intracellular ROS intensity was measured using the
Reactive Oxygen Species Assay kit (cat. no. S0033; Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Fluorescence was detected by flow cytometry using
FACSAria (BD Biosciences) and data were analyzed using FlowJo
software (version 10; FlowJo, LLC).
Analysis of late apoptosis
Single lung cell suspensions were immunolabeled with
an apoptosis kit (cat. no. 640914; BioLegend, Inc.), according to
the manufacturer's protocol. Flow cytometry was performed with a
FACSAria flow cytometer after gating the living cells. Data were
analyzed using FlowJo software (version 10; FlowJo, LLC).
Isolation of bronchoalveolar lavage
fluid (BALF)
Mice were sacrificed and BALF samples were collected
by slow infusion and extraction with 0.2 ml cold PBS 4 times. The
procedure was repeated four times and 0.8 ml BALF per mouse was
collected for subsequent research. The concentration of total
proteins in BALF was estimated using a BCA protein assay kit (cat.
no. P0010; Beyotime Institute of Biotechnology). The levels of SOD
and MDA in BALF were measured using a commercial SOD assay kit
(cat. no. A001-3-2; Nanjing Jiancheng Bioengineering Institute) and
MDA activity kit (cat. no. A003-4-1; Naning Jiancheng
Bioengineering Institute), according to the manufacturer's
instructions.
Analysis of inflammatory
cytokines
The expression levels of inflammatory cytokines,
TNF-α (cat. no. F11630), interleukin (IL)-1β (cat. no. F10770) and
IL-6 (cat. no. F10830), in BALF and lung tissue homogenates were
quantified using ELISA kits, according to the manufacturer's
protocol (Westang Technology Company; www.westang.com).
Statistical analyses
Data are presented as the mean ± standard error or
standard deviation of at least three independent experiments.
One-way ANOVA was performed followed by Tukey's multiple comparison
test using GraphPad Prism software (version 5; GraphPad Software
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Nec-1 protects against LPS-induced
inflammation and lung injury in ALI
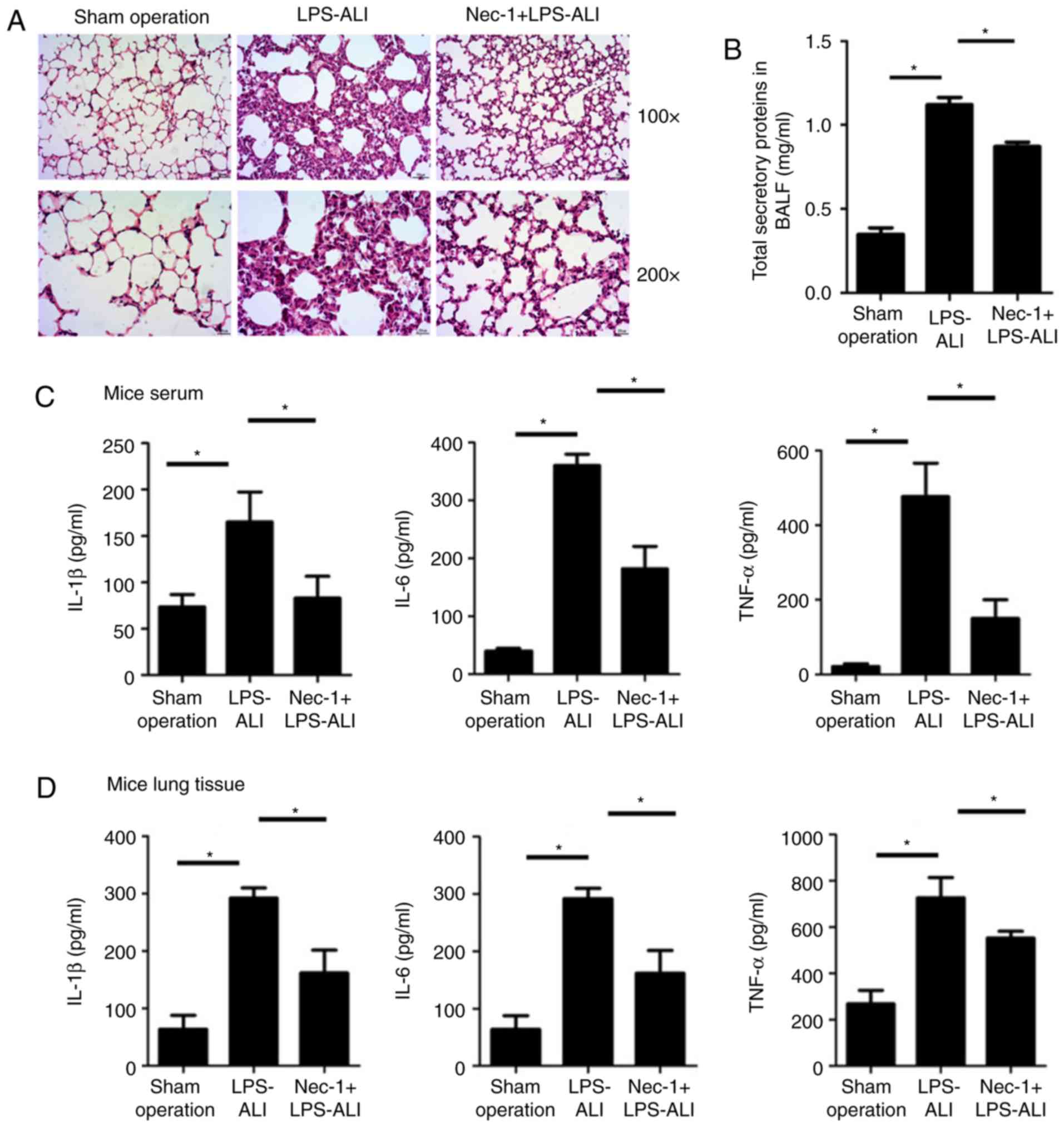
To investigate the role of Nec-1 in ALI, a mouse
model of ALI was established by transtracheal injection of LPS (10
mg/kg). Nec-1 (5 mg/kg) was administered 1 h before LPS induction.
The histopathological results revealed that Nec-1 reduced
LPS-induced lung injury compared with in mice challenged with LPS
only, although the damage was more severe than that in the
sham-operated mice (Fig. 1A).
Subsequently, pulmonary inflammatory cytokine levels were detected
by ELISA. Compared with the high level of secretory total proteins
(Fig. 1B), and inflammatory
cytokines in the BALF (Fig. 1C)
and lung tissues (Fig. 1D) of mice
in the ALI group, Nec-1-treated mice exhibited lower levels of
secretory total proteins and inflammatory cytokines, including
TNF-α, IL-1β and IL-6 (Fig. 1B-D;
Tables II and III). These findings indicated that
inflammation and injury were alleviated in the lungs of
Nec-1-treated mice.
 | Table II.Inflammatory cytokine levels in
bronchoalveolar lavage fluid. |
Table II.
Inflammatory cytokine levels in
bronchoalveolar lavage fluid.
| Group | TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) |
|---|
| Sham operation | 21.24±7.53 | 73.704±13.18 | 39.98±4.98 |
| LPS-ALI |
476.06±90.14a |
165.19±32.21a |
360.29±19.52a |
| Nec-1 +
LPS-ALI |
150.17±49.83b |
83.06±23.47b |
182.27±38.64b |
 | Table III.Inflammatory cytokines in lung
tissues. |
Table III.
Inflammatory cytokines in lung
tissues.
| Group | TNF-α (pg/ml) | IL-1β (pg/ml) | IL-6 (pg/ml) |
|---|
| Sham operation | 40.34±18.16 | 268.81±58.06 | 63.75±24.24 |
| LPS-ALI |
394.81±60.17a |
727.01±86.83a |
293.13±17.40a |
| Nec-1 +
LPS-ALI |
187.46±56.25b |
552.68±29.06b |
161.67±39.77b |
Suppressive effects of Nec-1 on
necroptosis in LPS-induced ALI
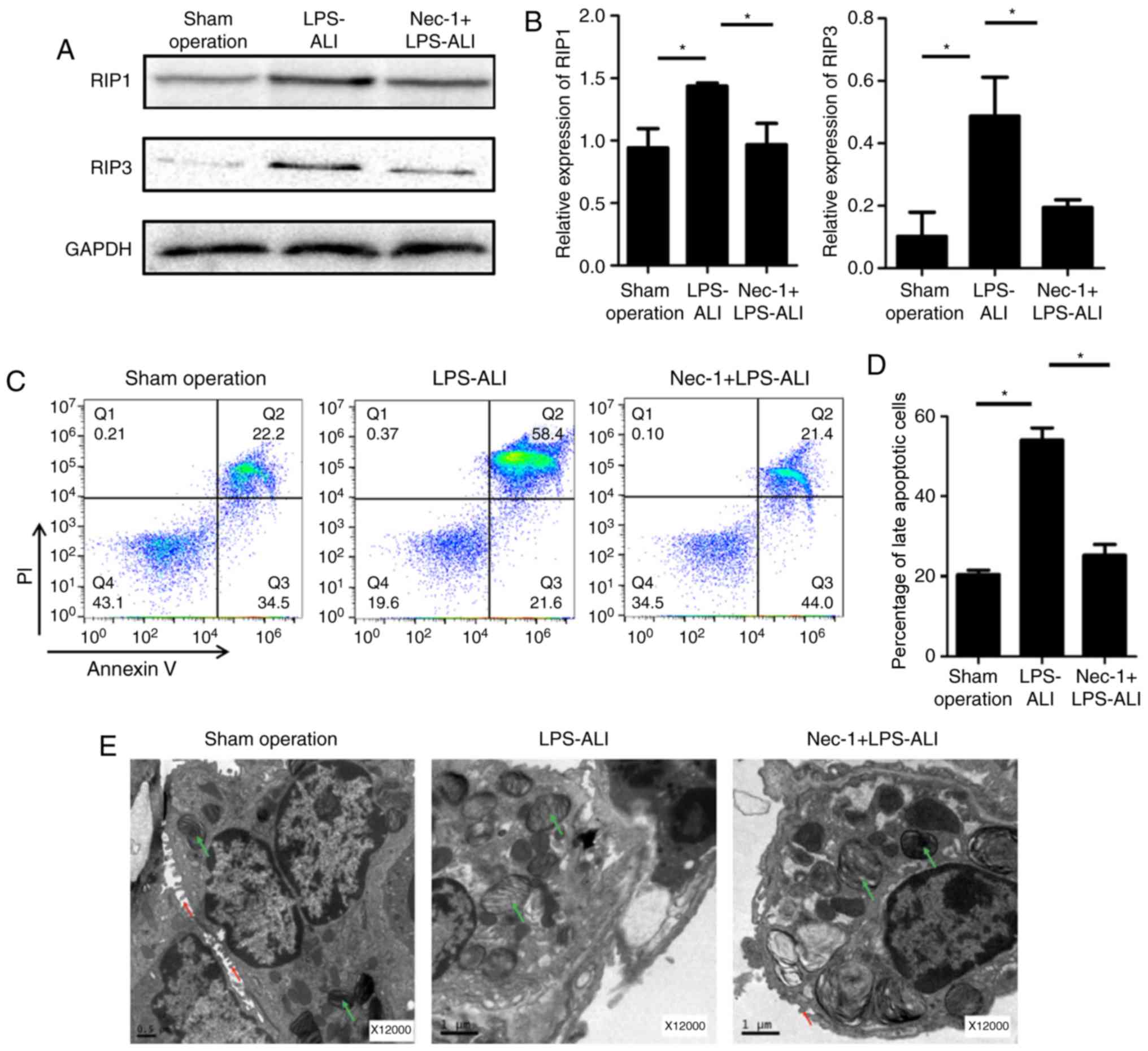
The anti-necroptotic effects of Nec-1 on LPS-induced
mice were assessed by western blotting. As shown in Fig. 2A and B, the expression levels of
RIP1 and RIP3 were significantly decreased in response to Nec-1
treatment compared with in the untreated ALI group following
exposure to LPS. Since late apoptosis also reflects the level of
necroptosis in ALI pathogenesis, flow cytometry was performed with
Annexin V and propidium iodide (PI) double-stained primary lung
cells. As shown in Fig. 2C and D,
LPS exposure significantly increased the percentage of late
apoptotic cells (right upper quadrant) compared with in the control
group, and this was effectively attenuated in lungs pretreated with
Nec-1. Furthermore, Nec-1 reduced LPS-induced alterations,
including increased number of necrosomes, lamellar body
abnormality, disappearance of microvilli, karyorrhexis, cellular
swelling and cytomembrane rupture (Fig. 2E). Taken together, these
observations strongly indicated that Nec-1 protects lungs from
necroptosis during ALI in vivo.
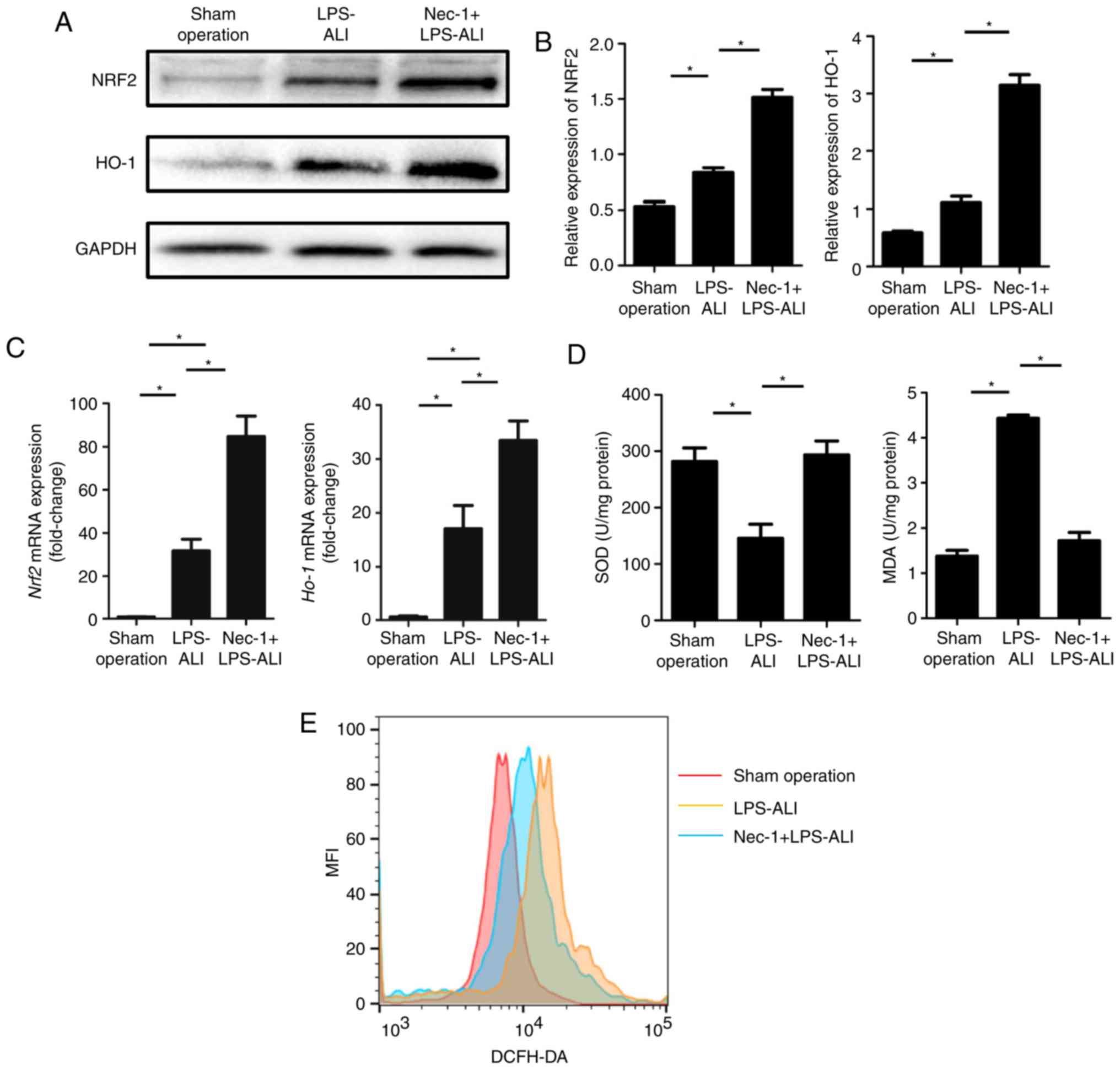
Nec-1 attenuates ROS response to
LPS-induced ALI in vivo
LPS elicits a robust ROS response and leads to the
production of ROS. To determine whether Nec-1 mediated protection
against oxidative stress dysfunction, through enhancing the
expression of antioxidant-associated proteins that mediate ROS
homeostasis, the expression levels of NRF2 and HO-1 were detected.
The expression levels of these antioxidants were increased in ALI
mice treated with Nec-1 compared with in those in the
saline-treated control group and LPS-treated ALI group (Fig. 3A and B). Nec-1 also enhanced the
induction of anti-ROS gene expression, including NRF2 and HO-1, in
the lung tissue of mice. As shown in Table IV and Fig. 3D, increased superoxide dismutase 2
(SOD2) and decreased malondialdehyde (MDA) levels were observed in
the Nec-1-treated ALI group. ROS intensity in primary lung cells
was estimated using an ROS assay kit. Nec-1-treated mice exhibited
hypoactive DCFH-DA mean fluorescence intensity (Fig. 3E), suggesting that internal ROS
production was decreased in the Nec-1 + LPS-ALI group compared with
the LPS-ALI group. These results suggested that Nec-1 may be a
mediator of ROS to maintain intrinsic homeostasis.
 | Table IV.Concentration of SOD2 and MDA in lung
tissues. |
Table IV.
Concentration of SOD2 and MDA in lung
tissues.
| Group | SOD2 (U/mg
protein) | MDA (nmol/mg
protein) |
|---|
| Sham operation | 281.87±24.1 | 1.38±0.13 |
| LPS-ALI |
133.68±24.8a |
4.01±0.07a |
| Nec-1 +
LPS-ALI |
293.47±24.6b |
1.72±0.18b |
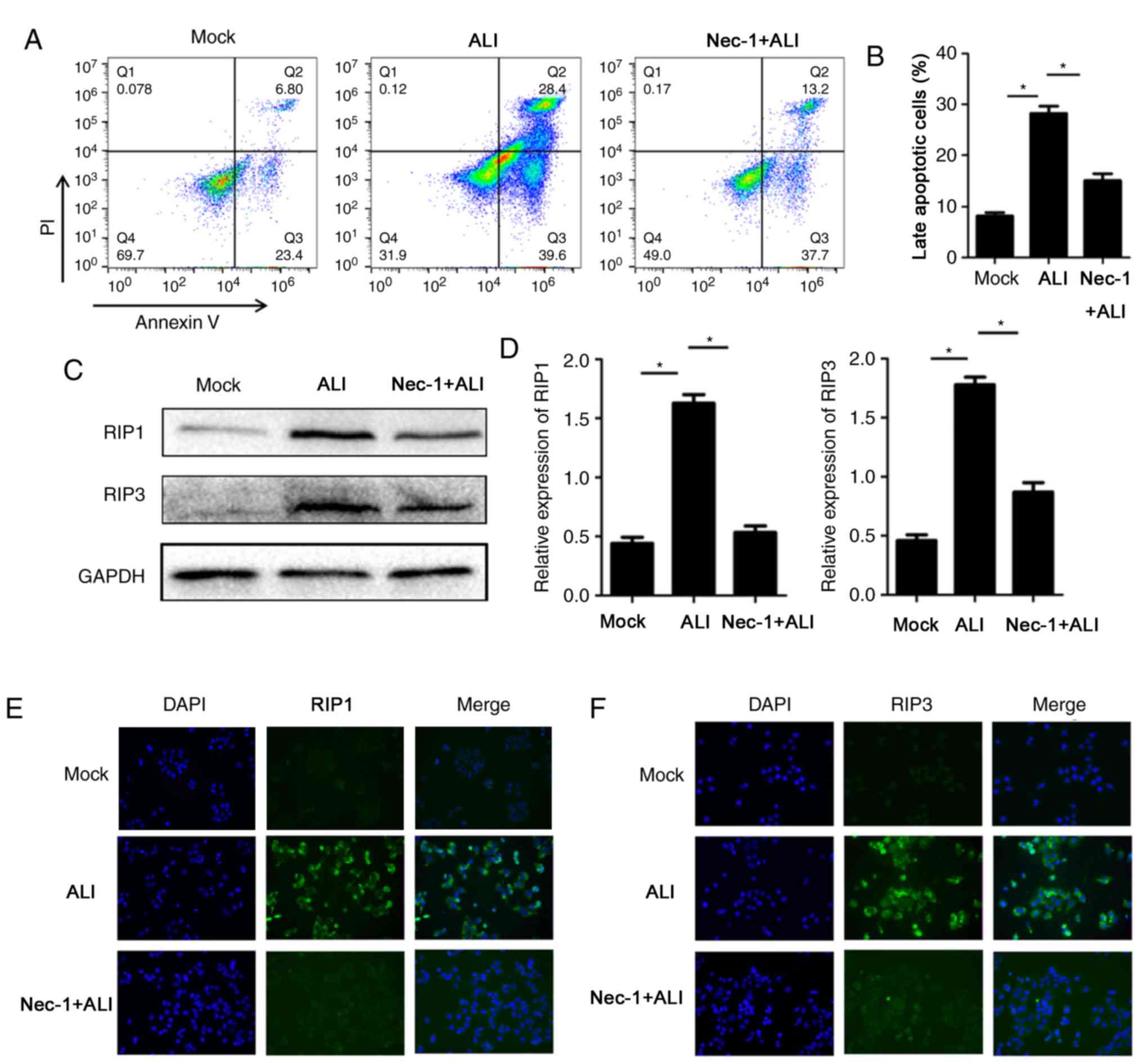
Nec-1 regulates necroptosis and ROS in
TNF-α-stimulated RLE-6TN cells
Similar to the inhibitory effects of Nec-1 on
necroptosis in vivo, Nec-1 also exerted protective effects
in vitro. Cell damage was induced by TNF-α, and necroptosis
was assessed with flow cytometry after staining with Annexin V and
PI. Nec-1 significantly reduced the percentage of late apoptotic
cells exposed to TNF-α (Fig. 4A and
B). Western blot and immunofluorescence analyses also indicated
that RIP1 and RIP3 expression was reduced in Nec-1-pretreated
damaged RLE-6TN cells (Fig. 4C-F).
Furthermore, NRF2 and HO-1 were upregulated to attenuate
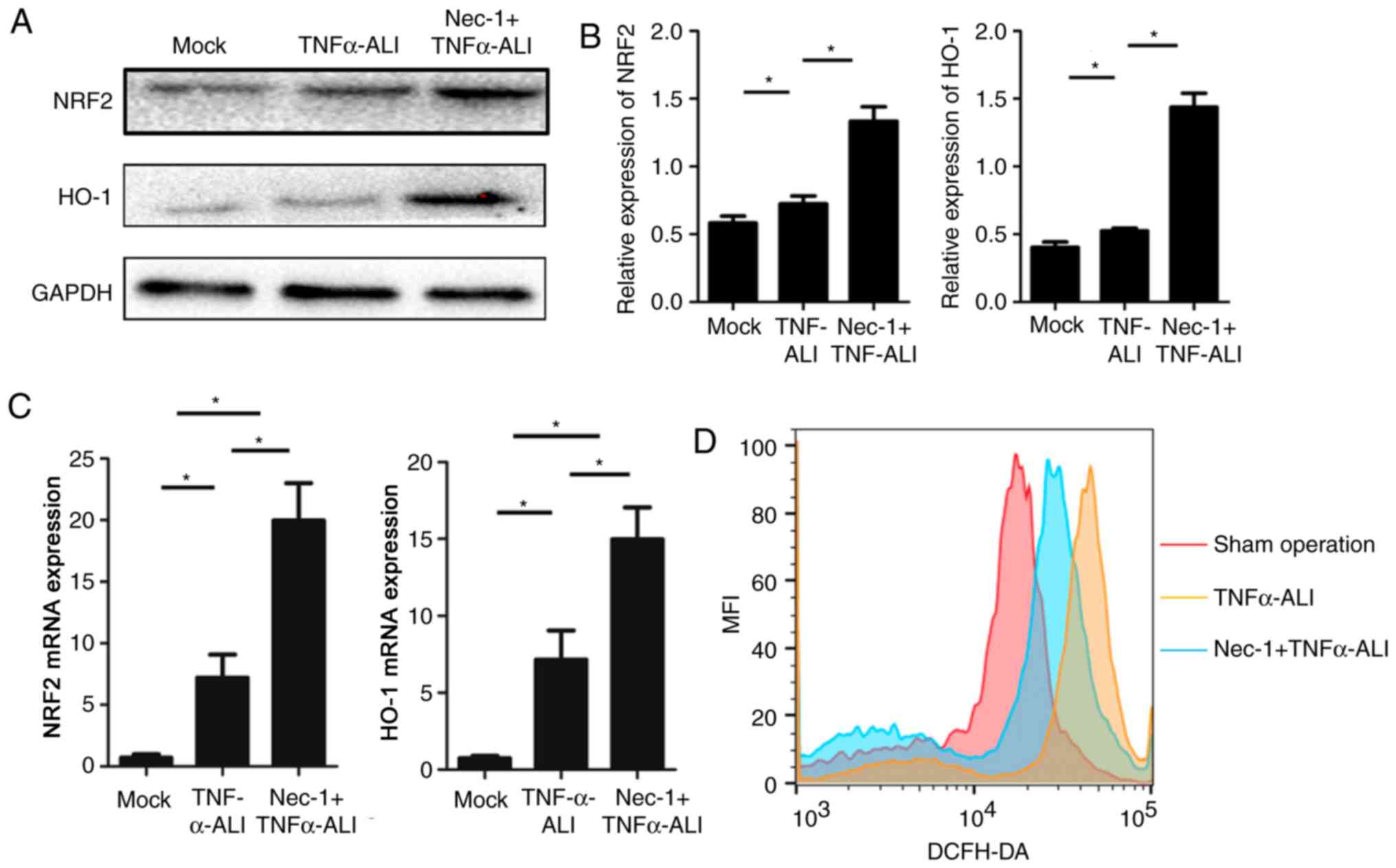
LPS-induced ROS dysfunction in the Nec-1-pretreated group (Fig. 5A and B). As expected, Nec-1 also
enhanced induction of anti-ROS gene expression, including NRF2 and
HO-1, in injured Nec-1-treated RLE-6TN cells (Fig. 5C). The intensity of the ROS
response was also evaluated by DCFH-DA probes using flow cytometry.
Nec-1 was able to downregulate the increased mean fluorescent
intensity of ROS in TNF-α-stimulated RLE-6TN cells (Fig. 5D), suggesting that internal ROS
production was decreased in the Nec-1 + TNF-α group compared with
the LPS-TNF-α group.
Discussion
ALI is characterized by a life-threatening
inflammatory response of the lungs to various insults (24). The early inflammatory phase of ALI
is characterized by alveolar epithelial and endothelial barrier
damage and dysfunction. Hemorrhage and protein-rich pulmonary edema
are followed by the proliferative phase, which involves alveolar
epithelial cell proliferation, interstitial fibrosis and air space
obliteration (25). Subsequently,
necrosis, fibrosis and emphysema are observed alongside the loss of
normal lung structure, thus resulting in acute respiratory distress
syndrome (26). Despite continuous
improvements in the medical management of ALI, it remains
associated with a high level of morbidity and mortality (1). Therefore, it is necessary to gain a
deep insight into the mechanism underlying ALI, in order to develop
a novel therapeutic strategy.
An appropriate balance among cell death,
proliferation and differentiation is critical for maintaining lung
homeostasis and retaining its vital role as an immunological
barrier against pathogens (27).
However, pathological processes, including bacterial infection,
viral infection and autoimmune disorders, jeopardize these
processes. TNF-α and its receptor (TNFR) are essential mediators of
cell death and inflammation, which have important roles in the
immune response of pulmonary alveolar cells (28). The LPS-TNF-α-TNFR axis signaling
can result in cell apoptosis, which is a means for eliminating
unhealthy cells and limiting the inflammatory cascade. Once the
degree of apoptosis exceeds the elimination capacity of immune
cells, necrosis occurs. Necroptosis is a form of cell necrosis
(29); since its discovery more
than two decades ago, it has been considered a combination of
cellular death and functional response, and its study represents an
area of ongoing research. RIP1 has been reported to shift the
balance between cell survival, apoptosis and necroptosis upon TNF-α
stimulation. It acts as a kinase in the ‘necrosome’ complex,
triggering the process of RIP3-dependent necroptosis (30). In the present study, it was
demonstrated that necroptosis was associated with the pathogenesis
of ALI, and Nec-1 exerted marked therapeutic effects by attenuating
pulmonary alveolar injury and dysfunction, thereby notably
improving the pulmonary alveolar cellular function in ALI mice.
This effect may be due to the inhibition of proteins associated
with the execution of necroptosis and reduced inflammatory cytokine
production. Notably, Nec-1 reduced the expression of necroptotic
proteins, RIP1 and RIP3. In addition, microscopic analysis
suggested that Nec-1 protected damaged alveolar epithelial cells
against cell swelling, karyorrhexis and cytomembrane rupture.
Therefore, the necroptotic inhibitor Nec-1 may be considered a
candidate for the treatment of ALI.
It has previously been reported that ROS are
essential for necroptosis-induced cellular injury (31). Cellular ROS are diverse
hyper-reactive derivatives of oxygen; cellular antioxidants
maintain ROS at physiological levels. Excessive accumulation of ROS
during cellular stress can overwhelm the antioxidant system and, if
not alleviated, this can result in oxidative stress, oxidative
damage to cellular constituents and cell death. Elevated levels of
ROS appear to be particularly important in cellular necroptosis
(32). RIP3-dependent necroptosis
can trigger inflammasome activation by inducing alterations in the
redox state, intracellular ion concentrations and cellular
metabolic status (33). RIP3
regulates the cell metabolic state and is involved in oxidative
phosphorylation. Furthermore, activated RIP3 enhances energy
metabolism, resulting in ROS accumulation (34). The NRF2/HO-1 signaling pathway
serves a vital role in oxidative stress. Under homeostatic
conditions, NRF2 is not functionally activated and exists in the
cytoplasm; however, once cells are subjected to oxidative stress,
NRF2 is translocated into the nucleus and combines with the
antioxidant response element to trigger HO-1 expression, thereby
regulating the expression of oxidant or antioxidant-related genes
and enzymes, such as SOD and MDA, and finally exerting its
antioxidant defense function (35). Therefore, strengthening the
antioxidant process may be considered a potential strategy to
reduce the level of cellular necroptosis, in order to reduce the
extent of tissue damage. This study revealed that treatment with
Nec-1 (a specific inhibitor of RIP1) attenuated LPS-induced ALI in
mice and TNF-α-induced cellular ALI by generating anti-ROS
proteins. Furthermore, blockade of ROS production by Nec-1
treatment significantly inhibited the extent of cell death. These
results strongly suggested that increased ROS levels may be
responsible for necroptosis-induced cell damage and death.
Increased cellular injury stimulates ROS generation,
which contributes to the promotion of cell swelling (36). ROS also induce cellular stress to
promote cell necroptosis. Reduced cell necroptosis results in
inhibition of ROS and increased antioxidative capacity, which can
limit the irreversible damage to the cells. Decreased ROS can also
inhibit inflammatory signaling, and contribute to the attenuation
of local and systematic inflammation (37). A physiological ROS level is
essential to induce an anti-ROS response and maintain cellular
homeostasis (37). Notably, the
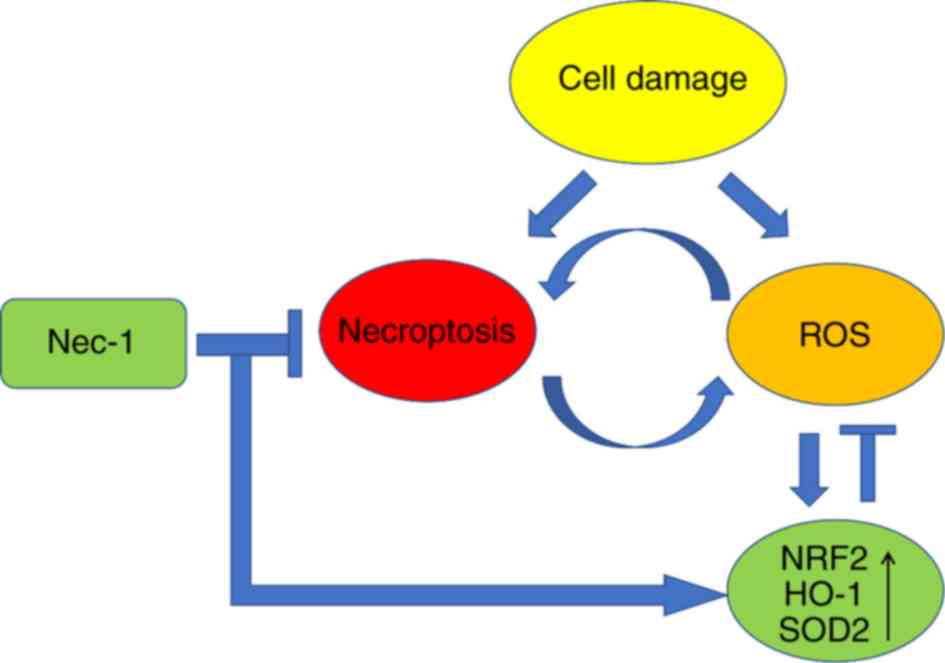
ROS pathway is a double-edged sword; it helps pulmonary alveolar
cells survive protein synthesis-associated stress; however, when
cellular injury is too severe, it leads to inflammation and changes
that ultimately damage these cells (Fig. 6). Therefore, artificially
strengthening antioxidant ability may be a promising therapeutic
strategy.
The present study treated the experimental ALI
models with Nec-1, which is a specific inhibitor of RIP1 and
demonstrated that Nec-1 exerted marked anti-necroptosis and
anti-inflammatory effects on LPS-stimulated mouse lung tissue in
vivo and TNF-α-stimulated rat alveolar epithelial cells in
vitro. Notably, inflammatory cytokines within BALF and lung
tissue were decreased in the Nec-1-treated ALI mouse model. In
addition, treatment with Nec-1 suppressed the expression of
necroptosis-associated proteins, RIP1 and RIP3. Conversely,
anti-ROS proteins were upregulated in the Nec-1-treated group
compared with in the control and non-Nec-1-treated ALI groups.
Positive anti-inflammatory and protective effects were also
observed in the TNF-α-stimulated RLE-6TN cell line. These findings
offer insights into the inhibitory effects of Nec-1 on the
progression of ALI. It may be hypothesized that Nec-1 acts as a
potential therapeutic agent for treating inflammatory lung
diseases, such as ALI.
Several time windows for Nec-1 treatment to prevent
ALI were analyzed and it was revealed that 6 h of pretreatment was
required for effective prevention in the experimental ALI mouse
model (data not shown). In addition, Nec-1 did not exert a
promising therapeutic effect on ALI mice when administered post-ALI
induction (data not shown). Typically, >6 h is required for
Nec-1 to induce the production of anti-ROS proteins during ALI
progression. Therefore, it is reasonable to propose that
pretreatment with Nec-1 is required for the induction of NRF2 and
HO-1, and to exert protective effects. Based on the current
findings, Nec-1 may be a novel ALI therapeutic agent if used in
patients with ALI at the early stage.
In conclusion, this study reported that necroptosis
was effectively induced in rat alveolar epithelial cells and in a
mouse model of LPS-induced ALI, whereas this necroptosis was
blocked by Nec-1. Further investigations revealed increased
expression of antioxidants (NRF2, HO-1 and SOD2) in response to
Nec-1. HO-1 is an anti-ROS gene that is activated upon cell injury.
Nec-1 actively enhanced the expression of HO-1 to attenuate the ROS
response to LPS-induced ALI; therefore, decreased ROS production
may be considered the most important inhibitory mechanism of
necroptosis. These results may be beneficial to understand the
detailed mechanisms of ALI caused by bacterial infections and could
provide novel prospects for further molecular targeted therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wenzhou
Science and Technology Bureau (grant no. Y20170144).
Availability of data and materials
All data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and LL conceived and designed the experiments.
BL, ZJ, XC, LZ, CW, BC and YT performed the experiments and
analyzed the data. LL wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wheeler AP and Bernard GR: Acute lung
injury and the acute respiratory distress syndrome: A clinical
review. Lancet. 369:1553–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin GS, Mannino DM, Eaton S and Moss M:
The epidemiology of sepsis in the United States from 1979 through
2000. N Engl J Med. 348:1546–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou C, Li J, Xiong S, Chen Y, Wu Q, Li Q,
Weathington NM, Han S, Snavely C, Chen BB and Mallampalli RK:
Mortality factor 4 like 1 protein mediates epithelial cell death in
a mouse model of pneumonia. Sci Transl Med. 7:311ra1712015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coon TA, McKelvey AC, Lear T, Rajbhandari
S, Dunn SR, Connelly W, Zhao JY, Han S, Liu Y, Weathington NM, et
al: The proinflammatory role of HECTD2 in innate immunity and
experimental lung injury. Sci Transl Med. 7:295ra1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayala P, Vivar R, Montalva R, Olmos P,
Meneses M and Borzone GR: Elastin degradation products in acute
lung injury induced by gastric contents aspiration. Respir Res.
19:1652018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo S, Jiang K, Wu H, Yang C, Yang Y, Yang
J, Zhao G and Deng G: Magnoflorine ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Front Pharmacol. 9:9822018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morales-Ortiz J, Deal V, Reyes F,
Maldonado-Martínez G, Ledesma N, Staback F, Croft C, Pacheco A,
Ortiz-Zuazaga H, Yost CC, et al: TLT-1 is a prognostic indicator in
ALI/ARDS and prevents tissue damage in the lungs in a mouse model.
Blood. 132:2495–2505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Yu Y, Gorshkov B, Haigh S, Bordan Z,
Weintraub D, Rudic RD, Chakraborty T, Barman SA, Verin AD, et al:
Hsp70 suppresses mitochondrial reactive oxygen species and
preserves pulmonary Microvascular barrier integrity following
exposure to bacterial toxins. Front Immunol. 9:13092018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhuriya YK and Sharma D: Necroptosis: A
regulated inflammatory mode of cell death. J Neuroinflammation.
15:1992018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He S and Wang X: RIP kinases as modulators
of inflammation and immunity. Nat Immunol. 19:912–922. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Zhang J, Yan R, Tian J, Zhang Y,
Zhang J, Chen M, Cui Q, Zhao L, Hu R, et al: Receptor-interacting
protein kinase 3 promotes platelet activation and thrombosis. Proc
Natl Acad Sci USA. 114:2964–2969. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pavlosky A, Lau A, Su Y, Lian D, Huang X,
Yin Z, Haig A, Jevnikar AM and Zhang ZX: RIPK3-mediated necroptosis
regulates cardiac allograft rejection. Am J Transplant.
14:1778–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lau A, Wang S, Jiang J, Haig A, Pavlosky
A, Linkermann A, Zhang ZX and Jevnikar AM: RIPK3-mediated
necroptosis promotes donor kidney inflammatory injury and reduces
allograft survival. Am J Transplant. 13:2805–2818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu
BQ, Li JH, Jing L, Jiang JL, Tang J and Chen ZN: Necrostatin-1
reduces intestinal inflammation and colitis-associated
tumorigenesis in mice. Am J Cancer Res. 5:3174–3185.
2015.PubMed/NCBI
|
|
17
|
Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y,
Ma J, Chen W, Zhang Y, Zhou X, et al: Mlkl knockout mice
demonstrate the indispensable role of Mlkl in necroptosis. Cell
Res. 23:994–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikseresht S, Khodagholi F, Nategh M and
Dargahi L: RIP1 inhibition rescues from LPS-induced RIP3-mediated
programmed cell death, distributed energy metabolism and spatial
memory impairment. J Mol Neurosci. 57:219–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang S and Kim JK: Effects of NADPH
oxidase inhibitors and mitochondria-targeted antioxidants on
amyloid β1-42-induced neuronal deaths in mouse mixed cortical
cultures. Chonnam Med J. 54:159–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Ren Z, Zhang J, Chuang CC,
Kandaswamy E, Zhou T and Zuo L: Role of ROS and nutritional
antioxidants in human diseases. Front Physiol. 9:4772018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang C, Chen S, Zhang T, Li D, Huang Z,
Huang J, Qin Y, Chen B, Cheng G, Ma F and Zhou M: TLR3 ligand
PolyI:C prevents acute pancreatitis through the
interferon-β/Interferon-α/β receptor signaling pathway in a
Caerulein-induced pancreatitis mouse model. Front Immunol.
10:9802019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han J, Kim YS, Lim MY, Kim HY, Kong S,
Kang M, Choo YW, Jun JH, Ryu S, Jeong HY, et al: Dual roles of
graphene oxide to attenuate inflammation and elicit timely
polarization of macrophage phenotypes for cardiac repair. ACS Nano.
12:1959–1977. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morrison TJ, Jackson MV, Cunningham EK,
Kissenpfennig A, McAuley DF, O'Kane CM and Krasnodembskaya AD:
Mesenchymal stromal cells modulate macrophages in clinically
relevant lung injury models by extracellular vesicle mitochondrial
transfer. Am J Respir Crit Care Med. 196:1275–1286. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sousse LE, Herndon DN, Andersen CR, Zovath
A, Finnerty CC, Mlcak RP, Cox RA, Traber DL and Hawkins HK:
Pulmonary histopathologic abnormalities and predictor variables in
autopsies of burned pediatric patients. Burns. 41:519–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Malaviya R, Sunil VR, Venosa A, Verissimo
VL, Cervelli JA, Vayas KN, Hall L, Laskin JD and Laskin DL:
Attenuation of nitrogen mustard-induced pulmonary injury and
fibrosis by anti-tumor necrosis factor-α antibody. Toxicol Sci.
148:71–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McConnell AM, Yao C, Yeckes AR, Wang Y,
Selvaggio AS, Tang J, Kirsch DG and Stripp BR: p53 regulates
progenitor cell quiescence and differentiation in the airway. Cell
Rep. 17:2173–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malaviya R, Laskin JD and Laskin DL:
Anti-TNFα therapy in inflammatory lung diseases. Pharmacol Ther.
180:90–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vanden Berghe T, Vanlangenakker N,
Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT,
Declercq W and Vandenabeele P: Necroptosis, necrosis and secondary
necrosis converge on similar cellular disintegration features. Cell
Death Differ. 17:922–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raju S, Whalen DM, Mengistu M, Swanson C,
Quinn JG, Taylor SS, Webster JD, Newton K and Shaw AS: Kinase
domain dimerization drives RIPK3-dependent necroptosis. Sci Signal.
11(pii): eaar21882018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen C, Wang C, Han S, Wang Z, Dong Z,
Zhao X, Wang P, Zhu H, Sun X, Ma X, et al: Aldehyde dehydrogenase 2
deficiency negates chronic low-to-moderate alcohol
consumption-induced cardioprotecion possibly via ROS-dependent
apoptosis and RIP1/RIP3/MLKL-mediated necroptosis. Biochim Biophys
Acta Mol Basis Dis. 1863:1912–1918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chauhan AK, Min KJ and Kwon TK:
RIP1-dependent reactive oxygen species production executes
artesunate-induced cell death in renal carcinoma Caki cells. Mol
Cell Biochem. 435:15–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ,
Lin SC, Dong MQ and Han J: RIP3, an energy metabolism regulator
that switches TNF-induced cell death from apoptosis to necrosis.
Science. 325:332–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu C, Qiao L, Ma L, Guo Y, Dou X, Yan S,
Zhang B and Roman A: Biogenic selenium nanoparticles synthesized by
Lactobacillus casei ATCC 393 alleviate intestinal epithelial
barrier dysfunction caused by oxidative stress via Nrf2
signaling-mediated mitochondrial pathway. Int J Nanomedicine.
14:4491–4502. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Q, Lv H, Wen Z, Ci X and Peng L:
Isoliquiritigenin activates nuclear factor erythroid-2 related
factor 2 to suppress the NOD-like receptor protein 3 inflammasome
and inhibits the NF-κB pathway in macrophages and in acute lung
injury. Front Immunol. 8:15182017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lien CF, Lee WS, Wang IC, Chen TI, Chen TL
and Yang KT: Intermittent hypoxia-generated ROS contributes to
intracellular zinc regulation that limits ischemia/reperfusion
injury in adult rat cardiomyocyte. J Mol Cell Cardiol. 118:122–132.
2018. View Article : Google Scholar : PubMed/NCBI
|