Introduction
Diffuse large B-cell lymphoma (DLBCL), a common
subtype of non-Hodgkin's lymphoma (NHL), constitutes ~40% of new
NHL cases annually in China, according to the World Health
Organization Classification (1).
As DLBCL is a highly heterogeneous disease, it has varied gene
expression and clinical manifestations, requiring different
treatment strategies (2). At
present, the standard treatment for DLBCL includes rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone (3). Bruton's tyrosine kinase (BTK) plays a
key role in B-cell development, proliferation and survival
(4). BTK exhibits abnormal
expression and mutations in X-linked agammaglobulinemia and diffuse
large B-cell lymphoma (5).
Ibrutinib, through phosphorylation of phospholipase C γ, inhibits
B-cell receptor activation, thereby affecting NF-κB signaling
(6). Moreover, ibrutinib, a
first-in-class inhibitor of BTK, has become a novel anticancer drug
that is widely used as a molecular tool to verify the role of BTK
kinase in B-cell tumors (7,8).
Despite the promising activity of ibrutinib across DLBCL cases, a
large number of patients have shown primary and secondary
resistance (9). Primary resistance
is characterized by little-to-no response during initial therapy,
whereas secondary resistance is characterized by an initial disease
response that is subsequently lost (10). Therefore, it is imperative to study
the mechanism of ibrutinib resistance in DLBCL.
Platelet-derived growth factor D (PDGFD) gene
belongs to the PDGF family of proteins, is involved in the
development and physiological processes of the body, and is also
associated with tumorigenesis, fibrosis and atherosclerosis
(11,12). An increasing number of studies have
shown that PDGFD may play a key role in the occurrence and
development of human cancer by regulating cell proliferation,
apoptosis, migration, invasion, angiogenesis and metastasis
(11,13). The expression of PDGFD has been
reported to be upregulated in prostate cancer, lung cancer, kidney
cancer, ovarian cancer, brain cancer and pancreatic cancer
(11,13–17).
In addition, PDGFD has also been reported to exhibit potential
carcinogenic activity in prostate cancer (11,14).
Wang et al (12) have
suggested that the overexpression of PDGFD is closely related to
pancreatic cancer occurrence and progression. Xu et al
(16) reported that overexpression
of PDGFD in renal cell carcinoma SN12-C cells increased cell
proliferation and migration in vitro, and increased the
coverage of perivascular cells in vivo. Epidermal growth
factor receptor (EGFR) is a receptor tyrosine kinase belonging to
the HER family, and is a key protein in epithelial cell
proliferation (18). EGFR is
highly expressed in 60–80% of colorectal cancers (19). As an oncogenic factor, EGFR is
involved in the processes of numerous cancers, including glioma,
colon cancer and pancreatic cancer (20), moreover, high EGFR levels are
associated with late-stage disease and poor prognosis (21). Due to the overexpression and
implicated functions of EGFR, EGFR is an effective therapeutic
target for various human cancers; EGFR-targeting drugs have been
used in the clinic to suppress tumor cell growth and regulate the
tumor microenvironment (22–25).
At the same time, EGFR is associated with cancer cell resistance to
chemotherapeutic agents; for example, in colon cancer, miR-20b
reduces colon cancer cell resistance to 5-FU by inhibiting
ADAM9/EGFR (26). EGFR is a target
gene for ibrutinib, and its abnormal expression leads to drug
resistance (27–29). Furthermore, PDGFD could regulate
the expression of EGFR (30).
Whether PDGFD affects the resistance of DLBCL to ibrutinib through
EGFR remains to be elucidated.
Thus, the present study analyzed differentially
expressed genes (DEGs) between ibrutinib resistance and sensitivity
in DLBCL, and identified that PDGFR was highly expressed in DLBCL
with ibrutinib resistance. Then, the effects of PDGFD and EGFR
expression on the proliferation, IC50 and apoptosis of
DLBCL/ibrutinib-resistant cells were evaluated to provide a
theoretical basis for alleviating the resistance of DLBCL cells to
ibrutinib.
Materials and methods
Data preprocessing and screening of
DEGs
The GSE93984 profile (https://www.ncbi.nlm.nih.gov/pubmed/28428442) and its
corresponding platform annotation files were downloaded from the
Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) (31). This dataset consisted of 34
samples. Differential expression of genes in ibrutinib-responsive
[complete response (CR; 10 cases) + partial response (PR; 14
cases)] and non-responsive [stable disease (SD; 10 cases)] of DLBCL
was tested with cut-off criteria of P<0.05 and fold change
(FC)|>2. Gene Ontology (GO) analysis and Kyoto Encyclopedia of
Genes and Genomes (KEGG) (https://www.genome.jp/kegg/kegg1.html) (32–34)
were performed with the Database for Annotation, Visualization and
Integrated Discovery (version 6.8; http://david.ncifcrf.gov/) (35,36).
Through The Search Tool for Interactions of Chemicals (STITCH)
database (version 4.0; http://stitch.embl.de/) (37,38),
the genes interacting with ibrutinib were extracted and visualized.
The Search Tool for the Retrieval of Interacting Genes (STRING)
(version 11.0), which provides information for experimental and
predicted interactions, is an online database (39).
Clinical tissue sample collection
Between April 2013 and March 2015, 62 patients who
were histologically diagnosed with DLBCL in the Fudan University
Shanghai Cancer Center were investigated (Table I). A total of 29 women (46.8%) and
33 men (53.2%) were included. The mean age was 50 years (range,
20–77 years). The inclusion criteria were as follows: Each patient
was diagnosed with DLBCL by pathology, received ibrutinib therapy
alone and provided informed consent. Cancer tissue samples were
then collected from these patients by means of biopsy. DLBCL
tissues were defined as showing a PR or CR following ibrutinib
treatment, and the resistant DLBCL tissues as showing
relapsed/refractory disease. This study was approved by the
Research Ethics Committee of Fudan University Shanghai Cancer
Center.
 | Table I.Clinical tissue information. |
Table I.
Clinical tissue information.
| Patient number | Age (years) | Gender | Diagnosis | Start date of
treatment |
|---|
| 1 | 56 | Female | Diffuse large
B-cell lymphoma | 2013/4/8 |
| 2 | 50 | Male | Diffuse large
B-cell lymphoma | 2013/4/12 |
| 3 | 62 | Female | Diffuse large
B-cell lymphoma | 2013/4/19 |
| 4 | 33 | Male | Diffuse large
B-cell lymphoma | 2013/5/8 |
| 5 | 63 | Female | Diffuse large
B-cell lymphoma | 2013/5/21 |
| 6 | 40 | Male | Diffuse large
B-cell lymphoma | 2013/5/24 |
| 7 | 77 | Male | Diffuse large
B-cell lymphoma | 2013/6/7 |
| 8 | 50 | Male | Diffuse large
B-cell lymphoma | 2013/6/21 |
| 9 | 53 | Male | Diffuse large
B-cell lymphoma | 2013/6/26 |
| 10 | 57 | Male | Diffuse large
B-cell lymphoma | 2013/7/12 |
| 11 | 58 | Female | Diffuse large
B-cell lymphoma | 2013/7/26 |
| 12 | 57 | Male | Diffuse large
B-cell lymphoma | 2013/7/30 |
| 13 | 50 | Male | Diffuse large
B-cell lymphoma | 2013/8/13 |
| 14 | 49 | Male | Diffuse large
B-cell lymphoma | 2013/9/3 |
| 15 | 46 | Female | Diffuse large
B-cell lymphoma | 2013/9/3 |
| 16 | 60 | Female | Diffuse large
B-cell lymphoma | 2013/9/17 |
| 17 | 74 | Female | Diffuse large
B-cell lymphoma | 2013/10/9 |
| 18 | 54 | Female | Diffuse large
B-cell lymphoma | 2013/11/5 |
| 19 | 63 | Female | Diffuse large
B-cell lymphoma | 2013/11/26 |
| 20 | 57 | Female | Diffuse large
B-cell lymphoma | 2013/12/2 |
| 21 | 57 | Female | Diffuse large
B-cell lymphoma | 2013/12/10 |
| 22 | 42 | Female | Diffuse large
B-cell lymphoma | 2013/12/10 |
| 23 | 50 | Female | Diffuse large
B-cell lymphoma | 2013/12/16 |
| 24 | 59 | Male | Diffuse large
B-cell lymphoma | 2013/12/17 |
| 25 | 49 | Male | Diffuse large
B-cell lymphoma | 2013/12/18 |
| 26 | 51 | Female | Diffuse large
B-cell lymphoma | 2013/12/19 |
| 27 | 45 | Male | Diffuse large
B-cell lymphoma | 2014/1/3 |
| 28 | 58 | Male | Diffuse large
B-cell lymphoma | 2014/1/21 |
| 29 | 45 | Male | Diffuse large
B-cell lymphoma | 2014/1/28 |
| 30 | 64 | Male | Diffuse large
B-cell lymphoma | 2014/2/21 |
| 31 | 43 | Male | Diffuse large
B-cell lymphoma | 2014/3/3 |
| 32 | 33 | Female | Diffuse large
B-cell lymphoma | 2014/3/11 |
| 33 | 59 | Female | Diffuse large
B-cell lymphoma | 2014/3/12 |
| 34 | 27 | Male | Diffuse large
B-cell lymphoma | 2014/3/14 |
| 35 | 46 | Male | Diffuse large
B-cell lymphoma | 2014/3/18 |
| 36 | 54 | Male | Diffuse large
B-cell lymphoma | 2014/5/8 |
| 37 | 33 | Male | Diffuse large
B-cell lymphoma | 2014/5/13 |
| 38 | 50 | Male | Diffuse large
B-cell lymphoma | 2014/5/19 |
| 39 | 56 | Female | Diffuse large
B-cell lymphoma | 2014/5/19 |
| 40 | 36 | Male | Diffuse large
B-cell lymphoma | 2014/5/22 |
| 41 | 56 | Female | Diffuse large
B-cell lymphoma | 2014/6/13 |
| 42 | 37 | Male | Diffuse large
B-cell lymphoma | 2014/6/16 |
| 43 | 40 | Male | Diffuse large
B-cell lymphoma | 2014/6/23 |
| 44 | 52 | Female | Diffuse large
B-cell lymphoma | 2014/7/4 |
| 45 | 45 | Male | Diffuse large
B-cell lymphoma | 2014/7/14 |
| 46 | 29 | Male | Diffuse large
B-cell lymphoma | 2014/7/23 |
| 47 | 68 | Female | Diffuse large
B-cell lymphoma | 2014/8/24 |
| 48 | 43 | Male | Diffuse large
B-cell lymphoma | 2014/9/28 |
| 49 | 64 | Female | Diffuse large
B-cell lymphoma | 2014/9/4 |
| 50 | 58 | Female | Diffuse large
B-cell lymphoma | 2014/10/5 |
| 51 | 43 | Female | Diffuse large
B-cell lymphoma | 2014/10/6 |
| 52 | 63 | Male | Diffuse large
B-cell lymphoma | 2014/10/17 |
| 53 | 60 | Female | Diffuse large
B-cell lymphoma | 2014/11/1 |
| 54 | 20 | Male | Diffuse large
B-cell lymphoma | 2014/11/9 |
| 55 | 36 | Male | Diffuse large
B-cell lymphoma | 2014/12/9 |
| 56 | 55 | Male | Diffuse large
B-cell lymphoma | 2014/12/11 |
| 57 | 33 | Female | Diffuse large
B-cell lymphoma | 2015/1/4 |
| 58 | 64 | Male | Diffuse large
B-cell lymphoma | 2015/1/17 |
| 59 | 42 | Female | Diffuse large
B-cell lymphoma | 2015/2/15 |
| 60 | 37 | Female | Diffuse large
B-cell lymphoma | 2015/2/27 |
| 61 | 49 | Female | Diffuse large
B-cell lymphoma | 2015/3/10 |
| 62 | 31 | Female | Diffuse large
B-cell lymphoma | 2015/3/26 |
Cell culture
The TMD8 and HBL1 cell lines were obtained from the
American Type Culture Collection. Cell line authentication was
performed by the Cell Check service at IDEXX Laboratories, Inc.
Cell lines in the logarithmic growth phase were cultured in RPMI
1640 medium containing 10% FBS (Atlanta Biologicals, Inc.; R&D
Systems, Inc.), 1 mM sodium pyruvate and 1% penicillin/streptomycin
(Pen/Strep); RPMI 1640 medium, sodium pyruvate and Pen/Strep were
obtained from Thermo Fisher Scientific, Inc. Ibrutinib-resistant
HBL1 and TMD8 cells were generated via in vitro culture of
the parental cell lines for prolonged periods of time with
progressively increasing concentrations of ibrutinib (0, 5, 10,
200, 500, 800 and 1,000 nM). These varying concentrations of
ibrutinib were added to ibrutinib-resistant HBL1 and TMD8 cells for
24 h prior to the MTT assay. Then, 200 nM ibrutinib was added to
ibrutinib-resistant HBL1 and TMD8 cells for 24 h prior to the flow
cytometry assay. lentiviruses Lv-EGFR was obtained from Auragene
Bioscience Corporation, Inc.
RNA isolation and quantitation
Cells were seeded in 6-well plates
(1×106/well). Total RNA was extracted using the
TaqMan® Fast Cells-to-CT™ kit (Thermo Fisher Scientific,
Inc.) and then reverse transcribed into cDNA according to the
manufacturer's instructions. quantitative PCR (qPCR) was performed
on a QuantStudio 7 Flex Real-Time PCR System (Thermo Fisher
Scientific, Inc.). Amplification was performed in a three-step
cycle procedure, 95°C (denaturation) 10 sec, 60°C (annealing) 30
sec, and 72°C (extension) 30 sec, for 40 cycles. The primers were:
PDGFD, forward 5′-GAACAGCTACCCCAGGAACC-3′, reverse
5′-CTTGTGTCCACACCATCGTC-3′; EGFR, forward
5′-CCCTCCTGAGCTCTCTGAGT-3′, reverse 5′-GTTTCCCCCTCTGGAGATGC-3′;
β-actin, forward 5′-TTGTTACAGGAAGTCCCTTGCC-3′; reverse
5′-ATGCTATCACCTCCCCTGTGTG-3′. mRNA levels were quantified using the
2−ΔΔCq method (40) and
normalized to the internal reference gene β-actin. All experiments
were repeated three times.
Construction and identification of
stable PDGFD-knockdown cell lines
The TMD8 and HBL1 ibrutinib-resistant cells in the
logarithmic growth phase were seeded in 6-well plates at a density
of 3×105 cells/well. The PDGF-D shRNA sequence was
GCGCATCCATCAAAGCTTTGC. PDGFD short hairpin RNA (shRNA; 20 pmol) and
pHBLV-U6-Puro lentiviruses (20 pmol; Auragene Bioscience
Corporation, Inc.) were added to the cells for 24 h in the presence
of Polybrene (Santa Cruz Biotechnology, Inc.; 5 µg/ml) after the
cells had adhered to the walls of the plates. Following infection
for 48 h, cells were observed under a fluorescence microscope
(IX70; Olympus Corporation), and uninfected cells were killed with
puromycin. The surviving cells were collected, and the PDGFD
protein level was analyzed using western blotting.
MTT assay
Cells were seeded into 96-well flat-bottomed tissue
culture plates (5–10×103/well in 100 µl medium). The
cells were cultured for 24–96 h at 37°C under 5% CO2
before a total of 20 µl MTT (5 mg/ml) was added to cells in the
logarithmic growth phase of each group for 4 h. Dimethyl sulfoxide
were added to dissolve purple formazan for 10 min. The optical
density (OD) value at 490 nm was measured with a microplate reader
(Bio-Rad Laboratories, Inc.). The cell survival rate was calculated
with a concentration-survival curve.
Immunohistochemistry (IHC)
Tissue samples from DLBCL and ibrutinib-resistant
DLBCL areas were formalin-fixed, dehydrated, cleared in xylene and
embedded in paraffin. Sections (5 µm) were deparaffinized,
hydrated, and 3% H2O2 solution was added for
15 min to remove endogenous catalase and antigen repair. Non-immune
normal goat serum was incubated at room temperature for 60 min at
100 µl and then stained with anti-PDGFD in 4°C overnight (1:100,
cat. no. ab181845; Abcam). Horseradish peroxidase conjugated
secondary antibodies were incubated at room temperature for 30 min
(1:1,000, Pv-80000, OriGene Technologies, Inc.), and
3,3-diaminobenzidine substrate was added for the development of
immunostaining according to the manufacturer's instructions (Dako;
Agilent Technologies, Inc.). Then the slides were counterstained
with hematoxylin and eosin at room temperature for 5 min. Positive
cells were counted in 10 randomly selected fields with a ×40
objective (Olympus CX23; Olympus Corporation).
Western blotting
Western blotting was performed as previously
described (41). In brief, cells
were harvested and lysed with RIPA buffer (cat. no. R0278;
Sigma-Aldrich; Merck KGaA) containing 1X protease/phosphatase
inhibitor. A BCA protein assay kit (Beyotime) was employed to
measure the protein concentrations. Equal amounts (20 µg/well) of
protein were separated by 10% SDS-PAGE and transferred to PVDF
membranes. The membranes were washed by 1X TBST and blocked by
Odyssey Blocking Buffer (cat. no. 927-40000; LI-COR Biosciences)
for 1 h at room temperature. Then membranes were incubated with
primary antibodies against PDGFD (1:1,000; cat. no. ab240960;
Abcam), EGFR (1:1,000; cat. no. ab52894; Abcam) and GAPDH (1:2,000;
cat. no. ab181602; Abcam) overnight at 4°C. Afterwards, membranes
were washed and incubated with secondary antibodies for 1 h at room
temperature. Signals were measured with a luminescent image
analyzer (ImageQuant LAS4000 mini) and GAPDH served as a loading
control.
Flow cytometry
The ApoDETECT Annexin V-FITC kit (Thermo Fisher
Scientific, Inc.) was used to quantify the number of apoptotic
cells in the indicated groups. Briefly, 1X binding buffer was used
to resuspend cells (5×105 cells/ml). Annexin V-FITC was
added at room temperature for 10 min in the dark. Then, the cells
were resuspended in 190 µl binding buffer containing 10 µl 20 µg/ml
propidium iodide (PI) at 4°C for 30 min and analyzed with a flow
cytometer (BD Biosciences) using ModFit LT software version 3.0
(Verity Software House, Inc.). The apoptotic rate was calculated as
early apoptotic cells + late apoptotic cells.
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS 17.0 software (SPSS, Inc.). The statistical significance
between multiple experimental groups was analyzed using one-way
ANOVA followed by Tukey's post hoc test. A Student's t-test was
used for comparisons between two groups. P<0.05 was considered
to indicate a statistically significant difference. Each experiment
was repeated at least three times.
Results
Data processing and DEG screening
Through the GEO database, the GSE93984 gene
expression profile was downloaded, and gene expression in patients
treated with ibrutinib in the CR (10 cases), PR (14 cases) and SD
(10 cases) groups were analyzed; 22,189 genes were identified. To
determine the key genes affecting DLBCL, genes with increasing
expression in the CR, PR and SD groups, and genes with FC>2.0 or
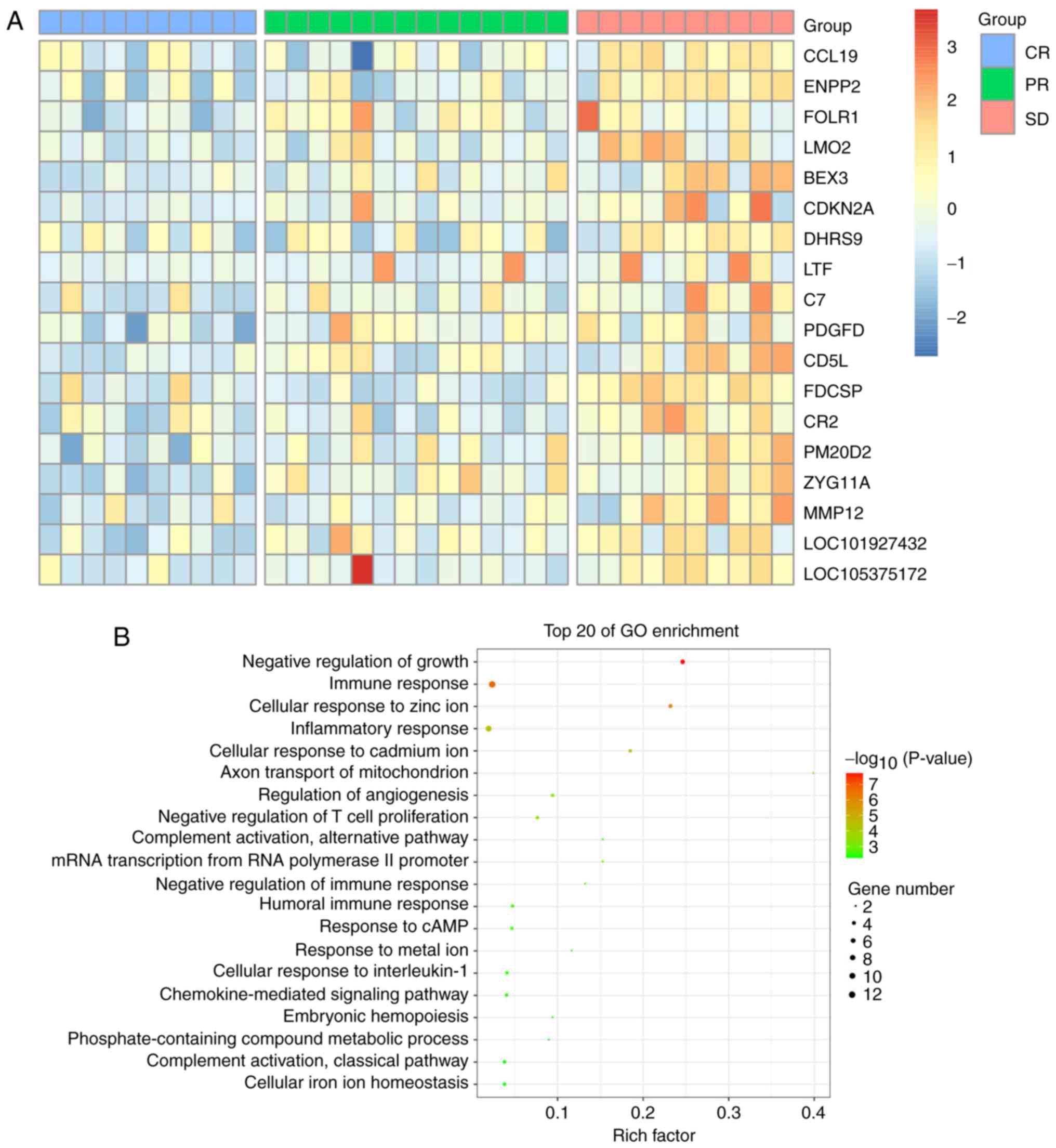
<0.5, and P<0.05 were selected (Fig. 1A). GO classification analysis
showed that DEGs were primarily involved in the negative regulation
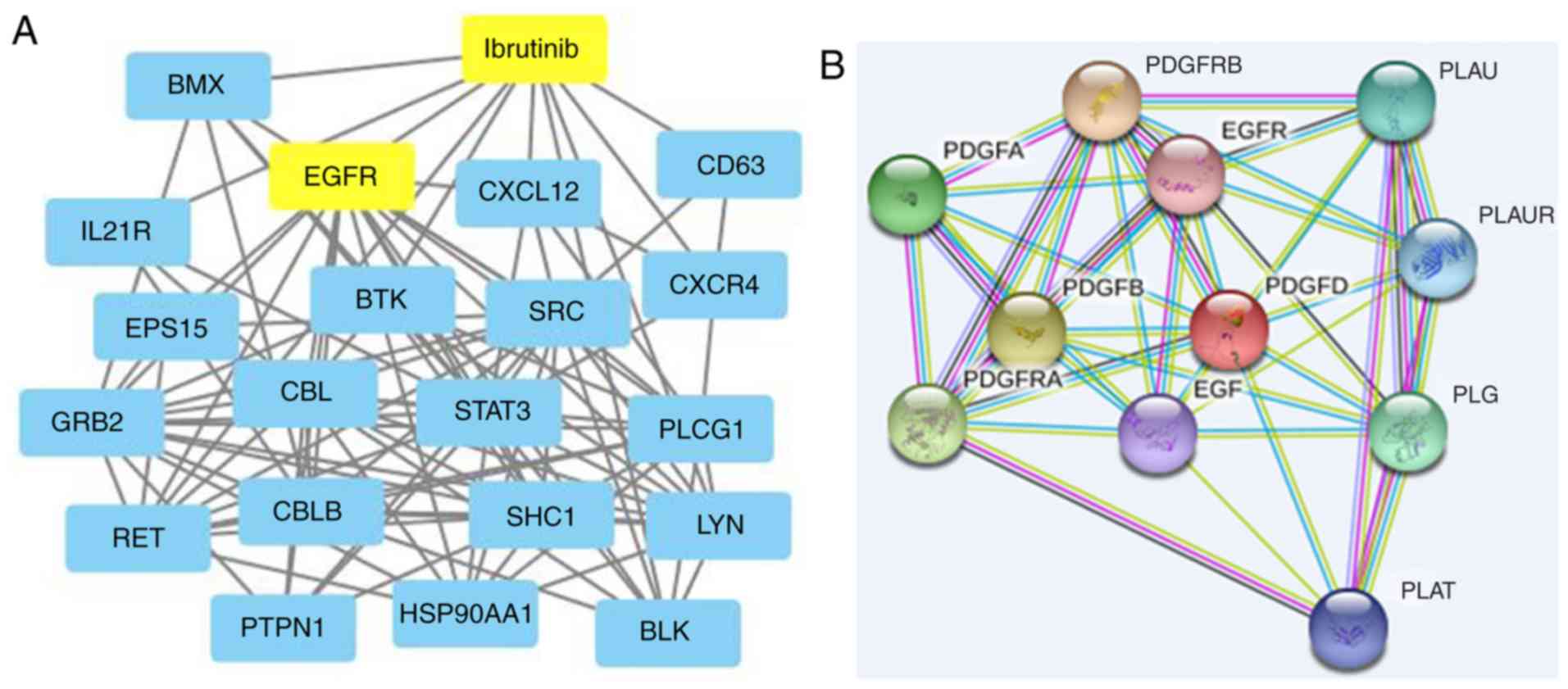
of growth and the immune response (Fig. 1B), and the gene network diagram of
the ibrutinib interaction (Fig.
2A) was analyzed with the STITCH database. It was found that
there was an interaction between EGFR and ibrutinib, and the known
phase of DLBCL was found by searching the database and related
literature. The STRING database showed that EGFR might be a
downstream target gene of PDGFD (Fig.
2B).
Ibrutinib-resistant DLBCL cells
exhibit higher PDGFD gene expression than the parental cells
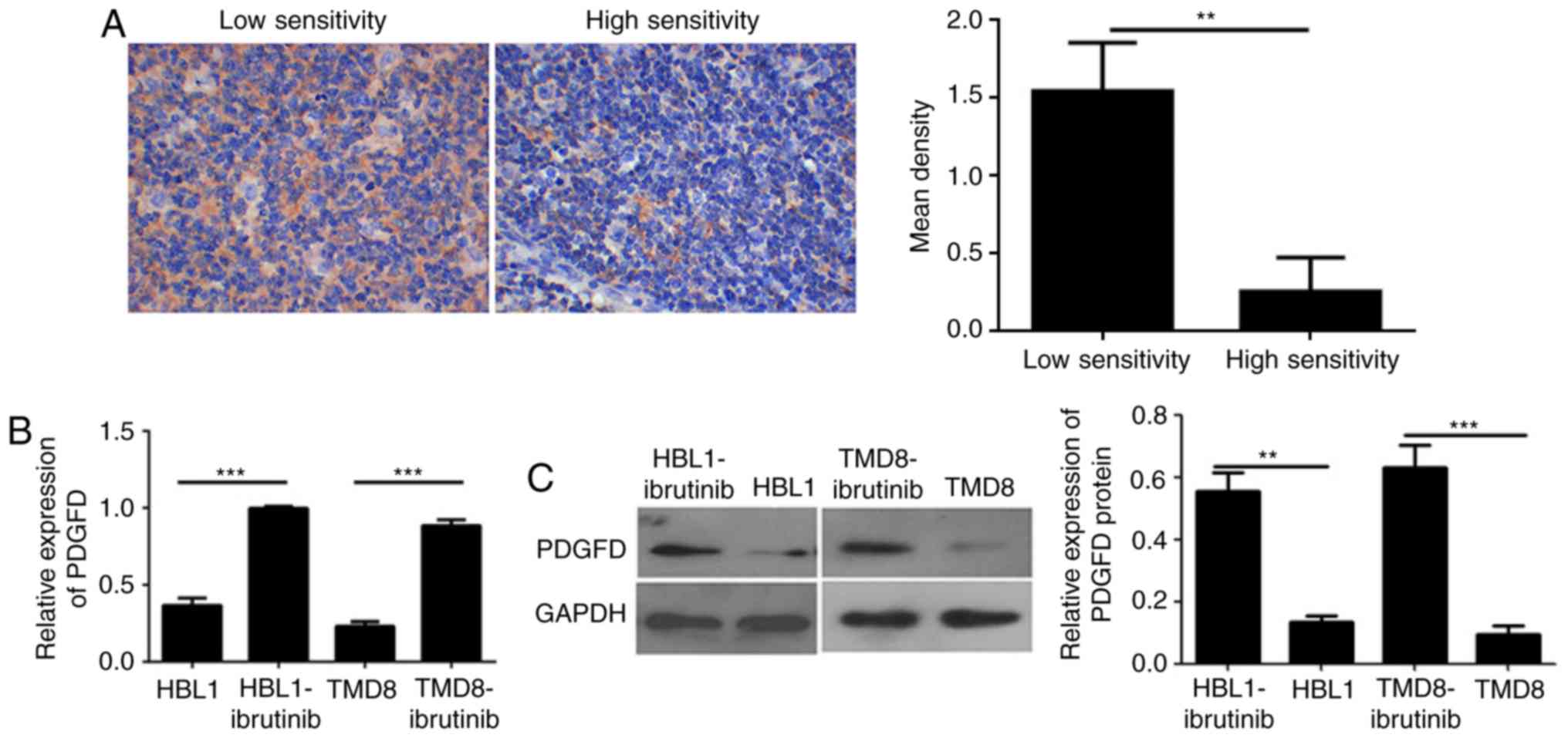
First, to verify PDGFD expression in lymphoma
drug-resistant and drug-sensitive tissues, as well as
drug-resistant cell lines and parental cell lines, IHC was
performed to detect the expression of PDGFD in these tissues. PDGFD
was expressed in low levels in the cytoplasm of cells in the
sensitive tissues. However, the expression of PDGFD in the stromal
cells of lymphoma-resistant tissues was homogeneous and high
(Fig. 3A). The expression of PDGFD
in ibrutinib-resistant cell lines was higher than in the sensitive
parental cell lines (Fig. 3B and
C), as determined via qPCR and western blotting. The results
indicated that PDGFD expression is elevated in both drug-resistant
tissues and cell lines; thus, abnormal expression of PDGFD may be
associated with ibrutinib resistance in lymphoma.
Downregulation of PDGFD reverses
ibrutinib resistance in ibrutinib-resistant DLBCL cells
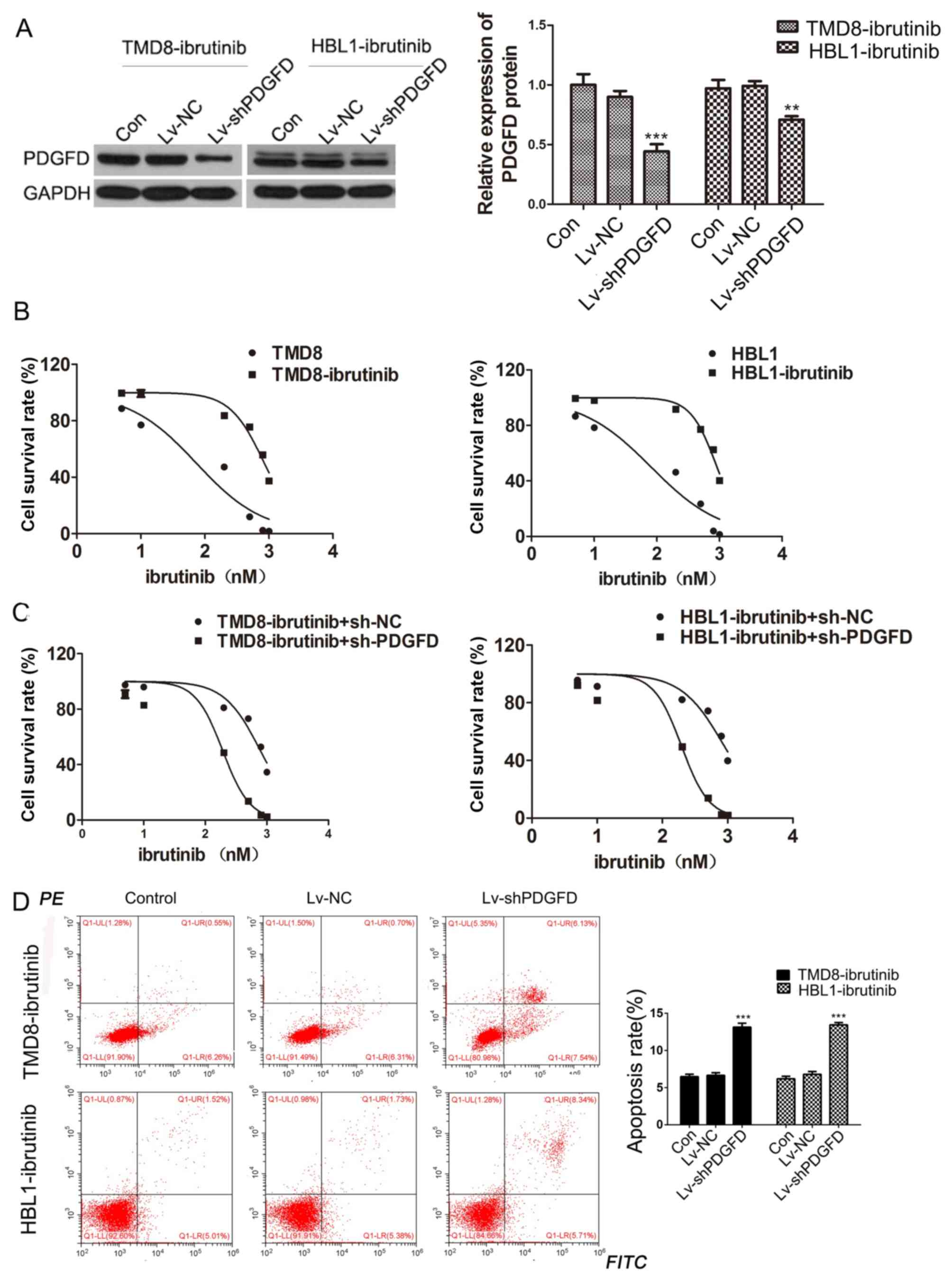
To further study the effect of PDGFD on the
resistance of DLBCL cells to ibrutinib, a lentivirus carrying
shPDGFD (Lv-shPDGFD) was constructed, and the TMD8-ibrutinib and
HBL1-ibrutinib cell lines were infected. Western blotting was
performed to verify the interference efficiency (Fig. 4A). MTT assays were then performed
to calculate the IC50 values of the resistant and the
parental strains. The resistant strains exhibited higher
IC50 values than the parental strains (Fig. 4B). Conversely, after Lv-shPDGFD was
used to infect the drug-resistant cells, it was found that
silencing of PDGFD reduced the IC50 values of ibrutinib
in drug-resistant cells (Fig. 4C).
Additionally, the apoptotic rate increased in ibrutinib-resistant
cell lines following Lv-shPDGFD infection (Fig. 4D). The data suggested that PDGFD
silencing increased the sensitivity of drug-resistant strains to
PDGFD, indicating that PDGFD is related to the resistance of DLBCL
cells to ibrutinib.
PDGFD promotes ibrutinib resistance by
activating EGFR in ibrutinib-resistant DLBCL cells
To compare EGFR expression between the
drug-resistant cell lines and parental cell lines, qPCR was used to
measure EGFR expression. Compared with the parental cell lines, the
expression of EGFR mRNA in the drug-resistant cell lines was
upregulated (Fig. 5A). In
addition, western blotting revealed that the expression of EGFR
protein in the drug-resistant cell lines was higher than that in
the parental cell lines, which was consistent with the qPCR results
(Fig. 5B). Compared with that in
the NC group, the expression of PDGFD mRNA in the Lv-shPDGFD group
was downregulated, which was accompanied by decreased EGFR
expression (Fig. 5C), suggesting
that EGFR was a downstream target of PDGFD. Finally, Lv-shPDGFD and
an EGFR overexpression lentivirus (Lv-EGFR; Fig. 5D) were coinfected into
drug-resistant cells to determine whether EGFR overexpression
reversed the IC50 changes induced by PDGFD silencing.
Compared with that in the Lv-shPDGFD-infected cells, the
IC50 for ibrutinib in the Lv-shPDGFD + Lv-EGFR-infected
cells was increased (Fig. 5E and
F), suggesting that the overexpression of EGFR could reverse
the sensitization of DLBCL to ibrutinib induced by PDGFD
interference in the drug-resistant strains. Collectively, the data
suggested that PDGFD could induce DLBCL ibrutinib resistance by
regulating EGFR expression.
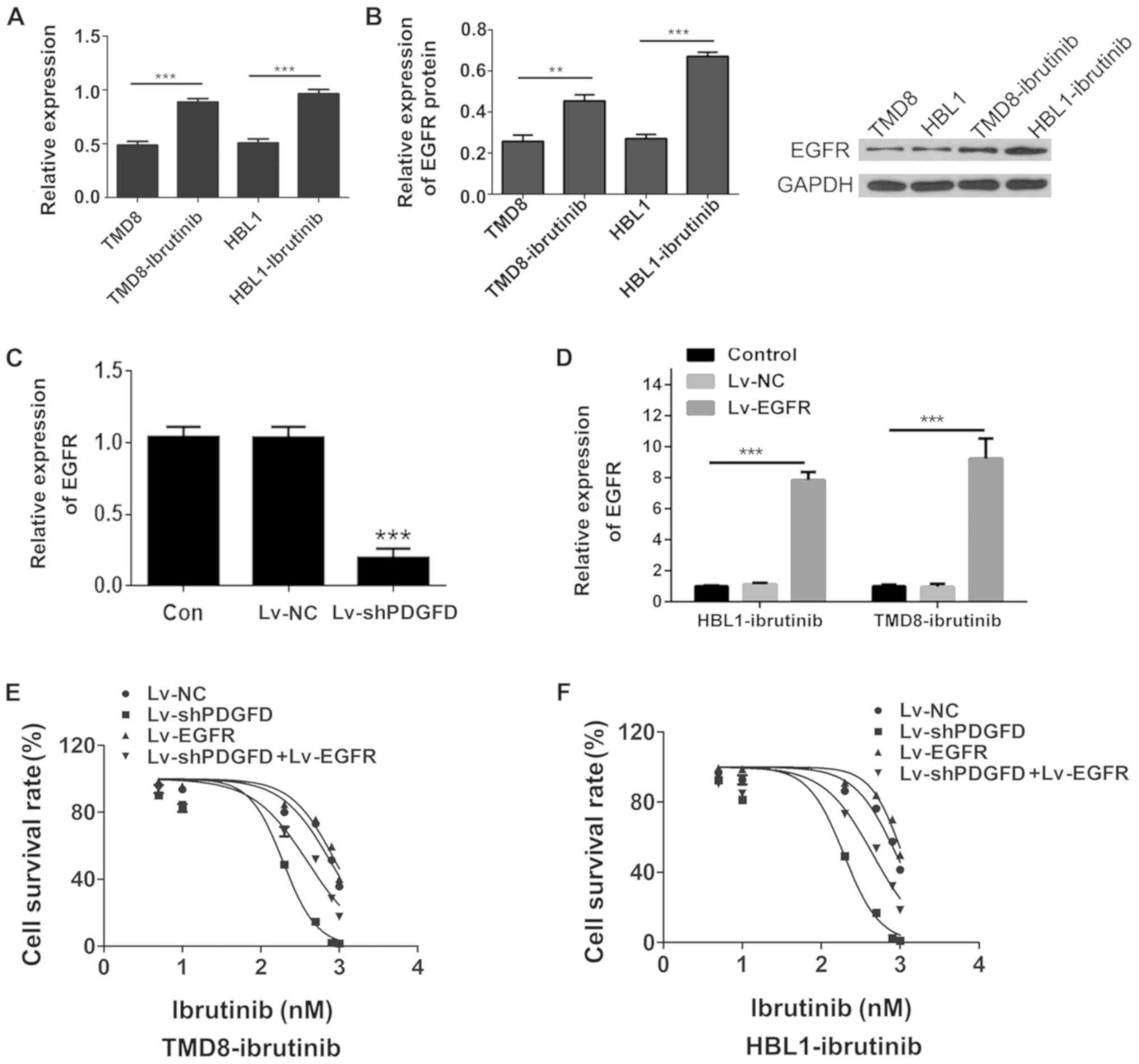 | Figure 5.Functional study of EGFR in
drug-resistant cells. (A) Expression of EGFR in TMD8-ibrutinib,
HBL1-ibrutinib, TMD8, and HBL1 cells as determined via RT-qPCR,
normalized to TMD8 or HBL1. (B) Analysis of EGFR protein expression
in TMD8-ibrutinib, HBL1-ibrutinib, TMD8, and HBL1 via western
blotting. (C) Expression of EGFR was detected by RT-qPCR in the
Lv-shPDGFD and Lv-shNC groups. (D) Efficiency of Lv-EGFR infection
as determined via RT-qPCR. MTT-based assessment of the
IC50 values in the Lv-shPDGFD and Lv-EGFR-coinfected (E)
TMD8-ibrutinib and (F) HBL1-ibrutinib cell lines. **P<0.01,
***P<0.001 vs. sh-NC. EGFR, epidermal growth factor receptor;
PDGFD, platelet-derived growth factor D; sh, short hairpin; Lv,
lentivirus; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control. |
Discussion
DLBCL is a subtype of adult non-Hodgkin's lymphoma
with significant clinical and biological heterogeneity, including
16 different clinicopathological entities (42). At present, >50% of patients with
DLBLC can be cured with the R-CHOP regimen; however, ~30–40% of
patients still die from drug-resistant or refractory disease
(43). Ibrutinib, a targeted
inhibitor of BTK, has shown promise in treating B-cell lymphoma
(44,45). Ibrutinib can disrupt the tumor
microenvironment while directly exerting cytotoxic effects on
malignant B-cells (46). Ibrutinib
has been shown to inhibit the growth of stomach, breast and colon
tumors in mouse models (47,48).
Ibrutinib overcomes mesenchymal stem cell (MSC)-mediated drug
resistance by inhibiting CXC chemokine receptor 4 expression and
inhibits MSC-induced lymphoma cell colony formation (49).
To provide a theoretical basis for the treatment and
alleviation of ibrutinib resistance in DLBCL cells, the role of
PDGFD in the resistance of DLBCL to ibrutinib was studied. The
present study revealed high expression of PDGFD in DLBCL/ibrutinib
at the tissue and cellular level. After interfering with PDGFD in
TMD8-ibrutinib and HBL1-ibrutinib cell lines, it was found that
EGFR expression decreased, apoptosis increased, the IC50
values for ibrutinib in TMD8-ibrutinib and HBL1-ibrutinib cells
decreased, and the sensitivity to ibrutinib increased. In addition,
the resistance of TMD8-ibrutinib and HBL1-ibrutinib cells to
ibrutinib induced by EGFR overexpression was reversed by PDGFD
interference. In conclusion, PDGFD may be implicated in the
resistance of DLBCL to ibrutinib. A large number of studies related
to ibrutinib resistance in DLBCL have emerged (9,50–52).
For example, ibrutinib-resistant tumors were reported to carry
mutant myeloid differentiation response 88 (MYD88) and wild-type
(WT) CD79A/B, whereas all other genotypic combinations (CD79A/B WT
+ MYD88 WT, CD79A/B mutant + MYD88 WT and CD79A/B mutant + MYD88
mutant) were responsive to ibrutinib therapy (50–52).
In addition, BTKCys481Ser drives ibrutinib resistance
via ERK1/2, and protects BTKWT MYD88-mutated Waldenström
macroglobulinemia (WM) and activated B-cell DLBCL cells via a
paracrine mechanism (9,10,52).
These studies are similar to the present study and provide clues to
explain new mechanisms of ibrutinib resistance in DLBCL.
PDGFD has been shown to be highly expressed in
various cancers (53,54), is associated with the occurrence
and development of cancer, and has been implicated in drug
resistance to numerous cancer chemotherapeutics (55,56).
Zhang et al (57) that
PDGFD overexpression is an independent predictor of platinum
chemotherapeutic resistance, and may be a potential biomarker for
targeted therapy and poor prognosis. Moreover, PDGFD plays an
important role in the epithelial-mesenchymal transition and drug
resistance of hepatocellular carcinoma cells (58). The present study for the first
time, to the authors' knowledge, reported the expression of PDGFD
in DLBCL tissues and cells, with high expression associated with
resistance to ibrutinib. In addition, in the TMD8-ibrutinib- and
HBL1-ibrutinib-resistant cells that were subjected to PDGFD
interference, a decrease in the IC50 of ibrutinib and an
increase in the apoptosis rate were observed, indicating enhanced
sensitivity of the cells to ibrutinib. These results indicated that
PDGFD plays a role in the mechanism of DLBCL cell resistance to
ibrutinib.
Bioinformatics analysis suggested that there was an
interaction between PDGFD and EGFR. It was speculated that the
effect of PDGFD on the drug resistance of DLBCL to ibrutinib may be
mediated by EGFR. It was demonstrated that EGFR was overexpressed
in drug-resistant cell lines. Other reports have shown that EGFR is
overexpressed in cancer cells, and is associated with poor efficacy
and a low survival rate (59,60).
The effect of interfering with PDGFD on the drug resistance of
TMD8-ibrutinib and HBL1-ibrutinib cells could be reversed by EGFR
overexpression, and EGFR was a target of ibrutinib treatment,
indicating that EGFR is a downstream target gene of PDGFD, and that
the regulation of EGFR leads to drug the resistance of DLBCL cells
to ibrutinib. Accordingly, ibrutinib can effectively block the
proliferation and survival of glioma cells mediated by the NF-κB
pathway activated by EGFR (61),
and promote the chemotherapy resistance of glioma cells through
Akt-independent activation of the NF-κB pathway (62). However, the relevant experiments
investigating PDGFD-regulated signaling in Lv-shPDGFD-treated or
non-treated ibrutinib-resistant TMD8 and HBL-1 cell lines were not
performed; these will be conducted in future studies. In the
pathway analysis, only DEG analysis as a whole was presented; it
would be informative to conduct pathway analysis for down- and
upregulated genes separately to further clarify how enriched
biological functions may be affected.
The present study was based on in vitro
studies of TMD8-ibrutinib and HBL1-ibrutinib, and revealed that
PDGFD induced resistance to ibrutinib in DLBCL, potentially via
effects on EGFR. The current study is the first, to the best of the
authors' knowledge, to evaluate the expression of PDGFD and its
effects on drug resistance to ibrutinib in DLBCL, to provide a
reference biological target for the targeted treatment and
prognosis of the disease, and to provide a theoretical basis for
clinical treatment. However, further studies are required to
confirm the mechanisms underlying the drug resistance of DLBCL to
ibrutinib. It is concluded that overexpression of PDGFD reduces the
sensitivity of DLBCL to ibrutinib by promoting EGFR expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and ZT preformed experiments and drafted the
manuscript; LW, JZ and FL participated in the design of the study.
JJ, LW, ZT and JZ performed statistical analysis and data
interpretation; JC and XH designed the study and revised the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Fudan University Shanghai Cancer Center. Informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun J, Yang Q, Lu Z, He M, Gao L, Zhu M,
Sun L, Wei L, Li M, Liu C, et al: Distribution of lymphoid
neoplasms in China: Analysis of 4,638 cases according to the world
health organization classification. Am J Clin Pathol. 138:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lossos IS and Morgensztern D: Prognostic
biomarkers in diffuse large B-cell lymphoma. J Clin Oncol.
24:995–1007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubuschok B, Held G and Pfreundschuh M:
Management of diffuse large B-cell lymphoma (DLBCL). Cancer Treat
Res. 165:271–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herman SE, Gordon AL, Hertlein E,
Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy
JJ, et al: Bruton tyrosine kinase represents a promising
therapeutic target for treatment of chronic lymphocytic leukemia
and is effectively targeted by PCI-32765. Blood. 117:6287–6296.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukada S, Saffran DC, Rawlings DJ,
Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T,
Quan S, et al: Deficient expression of a B cell cytoplasmic
tyrosine kinase in human X-linked agammaglobulinemia. 1993. J
Immunol. 188:2936–2947. 2012.PubMed/NCBI
|
|
6
|
Herman SE, Mustafa RZ, Gyamfi JA,
Pittaluga S, Chang S, Chang B, Farooqui M and Wiestner A: Ibrutinib
inhibits BCR and NF-kB signaling and reduces tumor proliferation in
tissue-resident cells of patients with CLL. Blood. 123:3286–3295.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendriks RW, Yuvaraj S and Kil LP:
Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev
Cancer. 14:219–232. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang ML, Rule S, Martin P, Goy A, Auer R,
Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al:
Targeting BTK with ibrutinib in relapsed or refractory mantle-cell
lymphoma. N Engl J Med. 369:507–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charalambous A, Schwarzbich MA and
Witzens-Harig M: Ibrutinib. Recent Results Cancer Res. 212:133–168.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang SQ, Smith SM, Zhang SY and Lynn Wang
Y: Mechanisms of ibrutinib resistance in chronic lymphocytic
leukaemia and non-Hodgkin lymphoma. Br J Haematol. 170:445–456.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong D, Banerjee S, Huang W, Li Y, Wang Z,
Kim HR and Sarkar FH: Mammalian target of rapamycin repression by
3,3′-diindolylmethane inhibits invasion and angiogenesis in
platelet-derived growth factor-D-overexpressing PC3 cells. Cancer
Res. 68:1927–1934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Kong D, Banerjee S, Li Y, Adsay
NV, Abbruzzese J and Sarkar FH: Down-Regulation of platelet-derived
growth factor-D inhibits cell growth and angiogenesis through
inactivation of notch-1 and nuclear factor-kappaB signaling. Cancer
Res. 67:11377–11385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lokker NA, Sullivan CM, Hollenbach SJ,
Israel MA and Giese NA: Platelet-derived growth factor (PDGF)
autocrine signaling regulates survival and mitogenic pathways in
glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D
ligands may play a role in the development of brain tumors. Cancer
Res. 62:3729–3735. 2002.PubMed/NCBI
|
|
14
|
Ustach CV, Taube ME, Hurst NJ Jr, Bhagat
S, Bonfil RD, Cher ML, Schuger L and Kim HR: A potential oncogenic
activity of platelet-derived growth factor D in prostate cancer
progression. Cancer Res. 64:1722–1729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ustach CV and Kim HR: Platelet-derived
growth factor D is activated by urokinase plasminogen activator in
prostate carcinoma cells. Mol Cell Biol. 25:6279–6288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Tong R, Cochran DM and Jain RK:
Blocking platelet-derived growth factor-D/platelet-derived growth
factor receptor beta signaling inhibits human renal cell carcinoma
progression in an orthotopic mouse model. Cancer Res. 65:5711–5719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Ahmad A, Li Y, Kong D, Azmi AS,
Banerjee S and Sarkar FH: Emerging roles of PDGF-D signaling
pathway in tumor development and progression. Biochim Biophys Acta.
1806:122–130. 2010.PubMed/NCBI
|
|
18
|
Yoshida T, Zhang G and Haura EB: Targeting
epidermal growth factor receptor: Central signaling kinase in lung
cancer. Biochem Pharmacol. 80:613–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen RB: Epidermal growth factor receptor
as a therapeutic target in colorectal cancer. Clin Colorectal
Cancer. 2:246–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hrustanovic G, Lee BJ and Bivona TG:
Mechanisms of resistance to EGFR targeted therapies. Cancer Biol
Ther. 14:304–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jänne PA, Engelman JA and Johnson BE:
Epidermal growth factor receptor mutations in non-small-cell lung
cancer: Implications for treatment and tumor biology. J Clin Oncol.
23:3227–3234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinez-Marti A, Navarro A and Felip E:
Epidermal growth factor receptor first generation tyrosine-kinase
inhibitors. Transl Lung Cancer Res. 8 (Suppl 3):S235–S246. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elbaz M, Nasser MW, Ravi J, Wani NA,
Ahirwar DK, Zhao H, Oghumu S, Satoskar AR, Shilo K, Carson WE 3rd
and Ganju RK: Modulation of the tumor microenvironment and
inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of
cannabidiol in breast cancer. Mol Oncol. 9:906–919. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arienti C, Pignatta S and Tesei A:
Epidermal growth factor receptor family and its role in gastric
cancer. Front Oncol. 9:13082019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maennling AE, Tur MK, Niebert M,
Klockenbring T, Zeppernick F, Gattenlöhner S, Meinhold-Heerlein I
and Hussain AF: Molecular targeting therapy against EGFR family in
breast cancer: Progress and future potentials. Cancers (Basel).
11:E18262019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu Q, Cheng J, Zhang J, Zhang Y, Chen X,
Luo S and Xie J: MiR-20b reduces 5-FU resistance by suppressing the
ADAM9/EGFR signaling pathway in colon cancer. Oncol Rep.
37:123–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu H, Wang A, Zhang W, Wang B, Chen C,
Wang W, Hu C, Ye Z, Zhao Z, Wang L, et al: Ibrutinib selectively
and irreversibly targets EGFR (L858R, Del19) mutant but is
moderately resistant to EGFR (T790M) mutant NSCLC cells.
Oncotarget. 6:31313–31322. 2015.PubMed/NCBI
|
|
28
|
Wang A, Yan XE, Wu H, Wang W, Hu C, Chen
C, Zhao Z, Zhao P, Li X, Wang L, et al: Ibrutinib targets
mutant-EGFR kinase with a distinct binding conformation.
Oncotarget. 7:69760–69769. 2016.PubMed/NCBI
|
|
29
|
Chen J, Kinoshita T, Sukbuntherng J, Chang
BY and Elias L: Ibrutinib inhibits ERBB receptor tyrosine kinases
and HER2-amplified breast cancer cell growth. Mol Cancer Ther.
15:2835–2844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saito Y, Haendeler J, Hojo Y, Yamamoto K
and Berk BC: Receptor heterodimerization: Essential mechanism for
platelet-derived growth factor-induced epidermal growth factor
receptor transactivation. Mol Cell Biol. 21:6387–6394. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuo HP, Ezell SA, Schweighofer KJ, Cheung
LWK, Hsieh S, Apatira M, Sirisawad M, Eckert K, Hsu SJ, Chen CT, et
al: Combination of ibrutinib and ABT-199 in diffuse large B-cell
lymphoma and follicular lymphoma. Mol Cancer Ther. 16:1246–1256.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanehisa M: Toward understanding the
origin and evolution of cellular organisms. Protein Sci.
28:1947–1951. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47:D590–D595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
The Gene Ontology Consortium, . The gene
ontology resource: 20 Years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szklarczyk D, Santos A, von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44:D380–D384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu ZH, Tao ZH, Zhang J, Li T, Ni C, Xie J,
Zhang JF and Hu XC: MiRNA-21 induces epithelial to mesenchymal
transition and gemcitabine resistance via the PTEN/AKT pathway in
breast cancer. Tumour Biol. 37:7245–7254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sukswai N, Lyapichev K, Khoury JD and
Medeiros LJ: Diffuse large B-cell lymphoma variants: An update.
Pathology. 52:53–67. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Camicia R, Winkler HC and Hassa PO: Novel
drug targets for personalized precision medicine in
relapsed/refractory diffuse large B-cell lymphoma: A comprehensive
review. Mol Cancer. 14:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Walewski J: Aggressive B-cell lymphoma:
Chasing the target. J Investig Med. 68:331–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cabanillas F and Shah B: Advances in
diagnosis and management of diffuse large B-cell lymphoma. Clin
Lymphoma Myeloma Leuk. 17:783–796. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maddocks K, Christian B, Jaglowski S,
Flynn J, Jones JA, Porcu P, Wei L, Jenkins C, Lozanski G, Byrd JC
and Blum KA: A phase 1/1b study of rituximab, bendamustine, and
ibrutinib in patients with untreated and relapsed/refractory
non-Hodgkin lymphoma. Blood. 125:242–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng
PP, Chang BY and Levy R: Therapeutic antitumor immunity by
checkpoint blockade is enhanced by ibrutinib, an inhibitor of both
BTK and ITK. Proc Natl Acad Sci USA. 112:E966–E972. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grassilli E, Pisano F, Cialdella A, Bonomo
S, Missaglia C, Cerrito MG, Masiero L, Ianzano L, Giordano F,
Cicirelli V, et al: A novel oncogenic BTK isoform is overexpressed
in colon cancers and required for RAS-mediated transformation.
Oncogene. 35:4368–4378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu L, Zhang YZ, Xia B, Li XW, Yuan T, Tian
C, Zhao HF, Yu Y and Sotomayor E: Ibrutinib inhibits mesenchymal
stem cells-mediated drug resistance in diffuse large B-cell
lymphoma. Zhonghua Xue Ye Xue Za Zhi. 38:1036–1042. 2017.(In
Chinese; Abstract available in Chinese from the publisher).
PubMed/NCBI
|
|
50
|
Erdmann T and Lenz G: Approaching
resistance to ibrutinib in diffuse large B-cell lymphoma. Leuk
Lymphoma. 57:1254–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim JH, Kim WS, Ryu K, Kim SJ and Park C:
CD79B limits response of diffuse large B cell lymphoma to
ibrutinib. Leuk Lymphoma. 57:1413–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen JG, Liu X, Munshi M, Xu L, Tsakmaklis
N, Demos MG, Kofides A, Guerrera ML, Chan GG, Patterson CJ, et al:
BTK(Cys481Ser) drives ibrutinib resistance via ERK1/2 and protects
BTK(wild-type) MYD88-mutated cells by a paracrine mechanism. Blood.
131:2047–2059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Olsen RS, Dimberg J, Geffers R and
Wågsäter D: Possible role and therapeutic target of PDGF-D
signalling in colorectal cancer. Cancer Invest. 37:99–112. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bartoschek M and Pietras K: PDGF family
function and prognostic value in tumor biology. Biochem Biophys Res
Commun. 503:984–990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang JC, Li GY, Wang B, Han SX, Sun X,
Jiang YN, Shen YW, Zhou C, Feng J, Lu SY, et al: Metformin inhibits
metastatic breast cancer progression and improves chemosensitivity
by inducing vessel normalization via PDGF-B downregulation. J Exp
Clin Cancer Res. 38:2352019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Appiah-Kubi K, Wu M, Yao X, Qian
H, Wu Y and Chen Y: The platelet-derived growth factors (PDGFs) and
their receptors (PDGFRs) are major players in oncogenesis, drug
resistance, and attractive oncologic targets in cancer. Growth
Factors. 34:64–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang M, Liu T, Xia B, Yang C, Hou S, Xie
W and Lou G: Platelet-derived growth factor D is a prognostic
biomarker and is associated with platinum resistance in epithelial
ovarian cancer. Int J Gynecol Cancer. 28:323–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang R, Li Y, Hou Y, Yang Q, Chen S, Wang
X, Wang Z, Yang Y, Chen C, Wang Z and Wu Q: The
PDGF-D/miR-106a/twist1 pathway orchestrates epithelial-mesenchymal
transition in gemcitabine resistance hepatoma cells. Oncotarget.
6:7000–7010. 2015.PubMed/NCBI
|
|
59
|
Nogi H, Kobayashi T, Suzuki M, Tabei I,
Kawase K, Toriumi Y, Fukushima H and Uchida K: EGFR as paradoxical
predictor of chemosensitivity and outcome among triple-negative
breast cancer. Oncol Rep. 21:413–417. 2009.PubMed/NCBI
|
|
60
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yue C, Niu M, Shan QQ, Zhou T, Tu Y, Xie
P, Hua L, Yu R and Liu X: High expression of bruton's tyrosine
kinase (BTK) is required for EGFR-induced NF-kB activation and
predicts poor prognosis in human glioma. J Exp Clin Cancer Res.
36:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tanaka K, Babic I, Nathanson D, Akhavan D,
Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J, et al: Oncogenic
EGFR signaling activates an mTORC2-NF-kB pathway that promotes
chemotherapy resistance. Cancer Discov. 1:524–538. 2011. View Article : Google Scholar : PubMed/NCBI
|