Introduction
Acute promyelocytic leukemia (APL) is one of the
most common types of adult acute leukemia (1), in which variations in myeloid-derived
hematopoietic stem cells in the bone marrow prevent the
differentiation of myeloid cells. Primordial or naïve myeloid cells
are in a state of clonal hyperplasia, while normal hematopoiesis is
inhibited (2). Numerous studies
have shown that angiogenesis plays an important role in the
pathogenesis of APL (3–5). Bone marrow angiogenesis is a complex
process in which new blood vessels form from the original blood
vessel to create a stable vascular network in the bone marrow
microenvironment after extensive reconstruction (6). Bone marrow microvessel density is
closely associated with the occurrence, development and
chemotherapy sensitivity of APL (7,8).
Currently, the main treatments for APL are chemotherapy and stem
cell transplantation. However, drug tolerance or recurrence can
develop. In addition, chemotherapy drugs can cause serious side
effects due to their cytotoxic effects on normal cells in addition
to tumor cells (9). Therefore, in
recent years, molecularly targeted drugs for acute leukemia, such
as targeting factors related to angiogenesis, have gained attention
(9).
The bone marrow is the major microenvironment of APL
cells; various cytokines secreted by APL cells can promote the
proliferation of endothelial cells and formation of tubular
structures (10), as well as
enhance the angiogenesis of the bone marrow microvessels (11). Additionally, changes in the bone
marrow provide suitable growth conditions for APL cells (12). Vascular endothelial growth factor
(VEGF) is the most widely studied pro-angiogenic factor in acute
myeloid leukemia. VEGF not only promotes bone marrow angiogenesis,
but also stimulates APL cell proliferation (13). VEGF is highly expressed in patients
with acute leukemia, and is an effective indicator for evaluating
patient prognosis (14). Previous
research has shown that targeting VEGF is effective in treating
acute leukemia (15,16). Moreover, thalidomide and arsenic
trioxide, which have been widely used in clinical practice and
achieved successful therapeutic effects, are involved in the
inhibition of angiogenesis in APL. These agents can significantly
reduce the expression of VEGF in patients and delay the development
of the disease; however, some patients experience side effects,
such as peripheral neuritis, vascular embolism and skin damage
(17–20). Therefore, the search for
low-toxicity, safe drugs targeting angiogenesis is important for
treating APL.
7-Difluoromethyl-5,4′-dimethoxygenistein (DFMG),
designed and synthesized by our research group, is a compound
derived from legume plants, and causes few adverse reactions in the
human body compared with other molecularly targeted drugs (21). In our previous studies, DFMG was
shown to prevent human umbilical vein endothelial (HUVE-12) cells
from hydrogen peroxide-induced impairment in vitro (21), decrease the release of cell
adhesion molecules and inflammatory factors by downregulating the
expression of Toll-like receptor 4/nuclear factor κB (TLR4/NF-κB)
and decrease the adhesion of circulating monocytes to endothelial
cells (22). Additionally, DFMG
was found to inhibit angiogenesis during atherosclerotic plaque
formation (23). Based on this
information, it was predicted that DFMG may have an
anti-angiogenesis role in APL. Therefore, in the present study, it
was investigated whether DFMG affects angiogenesis induced by APL
HL-60 cells, and the underlying mechanism of DFMG was explored.
Materials and methods
Cell culture
Human promyelocytic leukemia cell line HL-60 cells
(preserved in our laboratory) and human umbilical vein endothelial
cell line HUVE-12 cells (cat. no. GDC166; China Center For Type
Culture Collection) were cultured in RPMI-1640 (Biological
Industries) with 10% heat-inactivated fetal bovine serum
(Biological Industries) and 1% penicillin-streptomycin, and
maintained at 37°C and 5% CO2. After DFMG was dissolved
in dimethyl sulfoxide, different concentrations of DFMG
(synthesized by our research group; patent no. ZL200710104389.4)
were added to the culture medium of HL-60 cells and HUVE-12 cells
for 48 h. HL-60 cells were pre-incubated with TLR4 activator
lipopolysaccharide (LPS; Beyotime Institute of Biotechnology) and
TLR4 blocker TAK-242 (MedChemExpress) for 4 h at 37°C and 5%
CO2, and then treated with 100 µM DFMG for 48 h at 37°C
and 5% CO2. The cells were divided into the following
groups: Blank control; solvent control (1% dimethyl sulfoxide);
DFMG; LPS; LPS + DFMG; TAK-242; and TAK-242 + DFMG.
CCK-8 assay
The CCK-8 assay was performed to detect cell
viability in different groups. Cells were seeded into a 96-well
culture plate at a density of 5×104/ml, 100 µl per well,
with three replicate wells per group. Cells were cultured with
different concentrations of drugs (0, 25, 50, 100 and 200 µmol/l)
for 48 h, and then 10 µl CCK-8 solution (Nanjing Jiancheng
Bioengineering Institute) was added into the well at 37°C for 2 h.
The optical density (OD) at 450 nm was measured with a microplate
reader (Elx800; BioTek Instruments, Inc.), and the relative cell
viability was calculated in terms of OD values.
Lactate dehydrogenase (LDH) assay
HL-60 cells were seeded into 24-well culture plates
at a density of 5×105 cells per well. Different
concentrations of DFMG (0, 25, 50, 100 and 200 µmol/l) were used to
treat HL-60 cells for 48 h at 37°C. The cell culture supernatant
was collected by centrifugation (300 × g for 8 min at room
temperature) to evaluate the concentration of LDH released from the
cells using an LDH kit (Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's instructions. The OD at
450 nm was measured with a microplate reader (Elx800; BioTek
Instruments, Inc.), and the concentration of LDH was calculated
according to the manufacturer's protocols.
Chorioallantoic membrane (CAM)
experiment
Fertilized chicken eggs (Lvjian Ecological
Agriculture Institute) were incubated in a hatching incubator
(model 150; Weizhen Instruments, Inc.) equipped with an automatic
rotator at 37°C and a relative humidity of 60% for 10 days. After
incubation the eggshell was disinfected with 75% ethanol (Sinopharm
Chemical Reagent Co., Ltd). The eggshell was removed along the
fracture line and one part of the CAM was exposed. The HL-60 cells
were incubated with DFMG for 48 h at 37°C and 5% CO2,
following which the cell culture supernatant (collected by
centrifugation at 300 × g for 8 min at room temperature) was
discarded in order to eliminate the influence of the drugs in the
supernatant. The cells were rinsed three times with serum-free
medium, and then incubated in 1 ml RPMI-1640 serum-free medium
(Biological Industries) for an additional 10 h at 37°C and 5%
CO2. After the second incubation, the cell culture
supernatant was collected by centrifugation (300 × g for 8 min at
room temperature). The gelatin sponge was cut into small squares of
~5 mm, and immersed in HL-60 cell culture supernatants treated with
different drugs, and then the gelatin sponges soaked with different
cell culture supernatants were added to the CAM. Eggs were
incubated at 37°C and a relative humidity of 60% for 3 days, and
then images of the CAM blood vessels were captured using a
microscope (SZX16; Olympus Corporation) at magnification, ×1. The
angiogenesis area was analyzed using Image-Pro Plus 7.0 software
(Media Cybernetics, Inc.). All experimental procedures involving
the use of animals were approved by the Animal Use and Care
Committee of Hunan Normal University (Changsha, China).
Matrigel tubule formation assay
Matrigel (BD Biosciences) was thawed at 4°C
overnight. Matrigel was diluted 1:3 with RPMI1640, and then added
to a pre-cooled 24-well culture plate at ~300 µl per well and
placed in an incubator at 37°C and 5% CO2 for 1 h. To
eliminate the influence of the drugs in the cell culture
supernatant, the HL-60 cells were incubated with DFMG for 48 h, and
the supernatant was collected by centrifugation (300 × g for 8 min
at room temperature) and then discarded. The HL-60 cells were
rinsed three times with RPMI-1640 serum-free medium (Biological
Industries) and then incubated for an additional 10 h in 1 ml
serum-free medium at 37°C and 5% CO2. After the second
incubation, the cell culture supernatant was collected by
centrifugation (300 × g for 8 min at room temperature). HUVE-12
cells were resuspended in the culture supernatant of HL-60 cells
treated with different drugs. The HUVE-12 cell density was adjusted
to 1×106/ml, and 1 ml per well cells was inoculated onto
the Matrigel surface and incubated at 37°C and 5% CO2
for 8 h. Images of tube formation of HUVE-12 cells were captured
using a microscope (IX51; Olympus Corporation) at magnification,
×200, and tube numbers were analyzed with ImageJ 1.52t software
(National Institutes of Health).
Western blot (WB) analysis
Protein was extracted from HL-60 cells lysed in RIPA
buffer (Beijing ComWin Biotech Co., Ltd.). The total protein
concentration was determined with a bicinchoninic acid protein
assay kit (Beijing Solario Science Technology Co., Ltd.). Proteins
were then mixed with protein loading buffer and denatured in
boiling water at 100°C for 10 min. Equal amounts of protein (30 µg)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes at 4°C. After blocking the membranes with 5% skimmed milk
at room temperature for 2 h, they were incubated with primary
antibodies at 4°C overnight and probed with the appropriate
secondary antibodies for 1 h at room temperature the following day.
The primary antibodies included VEGF (1:10,000; Abcam; cat. no.
ab52917), TLR4 (1:1,000; ABclonal Biotech Co., Ltd.; cat. no.
A11226), NF-κB p65 (1:1,000; Abbkine Scientific Co., Ltd.; cat. no.
Abp57495), phosphorylated (P)-NF-κB p65 (1:2,000; ABclonal Biotech
Co., Ltd.; cat. no. AP0123), IκB-α (1:2,000; ABclonal Biotech Co.,
Ltd.; cat. no. A1187), and β-actin (1:10,000; Abbkine Scientific
Co., Ltd.; cat. no. Abp50151). The secondary antibodies included
horseradish peroxidase-conjugated anti-rabbit (1:10,000; Abbkine
Scientific Co., Ltd.; cat. no. A21020) and anti-mouse (1:10,000;
Abbkine Scientific Co., Ltd.; cat. no. A25012,). Immunoreactive
bands were visualized using electrochemiluminescence reagent (New
Cell & Molecular Biotech Co., Ltd) and were scanned using a
chemiluminescence imaging analysis system (Tanon 5200
Chemiluminescent Imaging System). The relative quantitative
analysis of proteins was performed using ImageJ software version
1.52t (National Institutes of Health).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA of HL-60 cells was extracted with TRIzol™
reagent (Vazyme Biotech Co., Ltd.), and then a reverse
transcription kit (Vazyme Biotech Co., Ltd.) was used to produce
cDNA from RNA under the following conditions: 50°C for 15 min,
followed by 85°C for 5 sec. RT-qPCR was carried out using SYBR
Premix Extaq™ (Vazyme Biotech Co., Ltd.) and a 7500 fast qPCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) under
the following conditions: 95°C for 5 min, then 40 cycles of 10 sec
at 95°C and 30 sec at 60°C, with a final extension for 15 sec at
95°C and 1 min at 60°C. The sequences of the RT-qPCR primers were
as follows: VEGF, forward 5′-GCACATAGAGAGAATGAGCTTCC-3′, reverse
5′-CTCCGCTCTGAACAAGGCT-3′; TLR4, forward
5′-CCGAGGCCATTATGCTATGT-3′, reverse 5′-TCCCTTCCTCCTTTTCCCTA-3′; and
GAPDH, forward 5′-CAGGAGGCATTGCTGATGAT-3′, reverse
5;-GAAGGCTGGGGCTCATTT-3′. The relative quantitative analysis of
mRNA expression was normalized to the reference gene GAPDH and
calculated using the 2−ΔΔCq method (24).
Statistical analysis
Data from three independent repeats were analyzed
with SPSS 20.0 software (IBM Corp.), and the results are shown as
the mean ± standard deviation (SD). One-way ANOVA followed by
Tukey's post hoc test was used for the multiple-group comparison
analysis. P<0.05 was considered to indicate statistically
significant difference.
Results
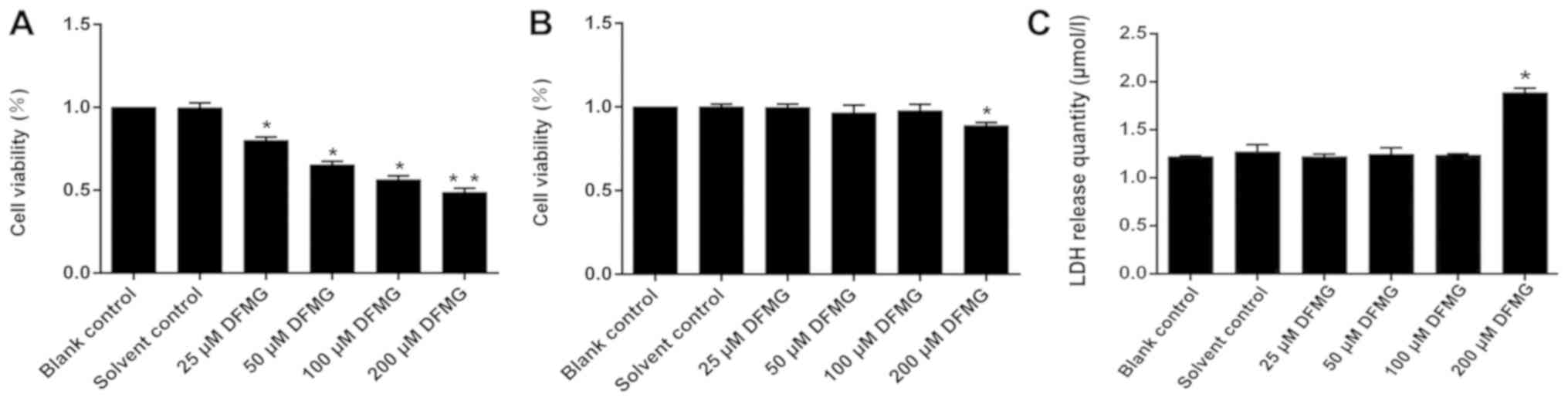
DFMG decreases viability of HL-60
cells but not HUVE-12 cells
Cells were treated with different concentrations of
DFMG (blank control, solvent control, 25, 50, 100 and 200 µM DFMG)
for 48 h, and cell viability was observed. It was found that when
the concentration of DFMG reached 50 µM, the viability of HL-60
cells was significantly decreased compared with the solvent control
(Fig. 1A). However, when DFMG was
<100 µM, the viability of HUVE-12 cells was unaffected (Fig. 1B).
High concentration of DFMG promotes
release of LDH from HL-60 cells
HL-60 cells were treated with different
concentrations of DFMG (blank control, solvent control, 25, 50,
100, and 200 µM DFMG) for 48 h, and LDH release was detected. It
was shown that 200 µM DFMG promoted the release of LDH from HL-60
cells, indicating that 200 µM DFMG is toxic towards HL-60 cells
(Fig. 1C).
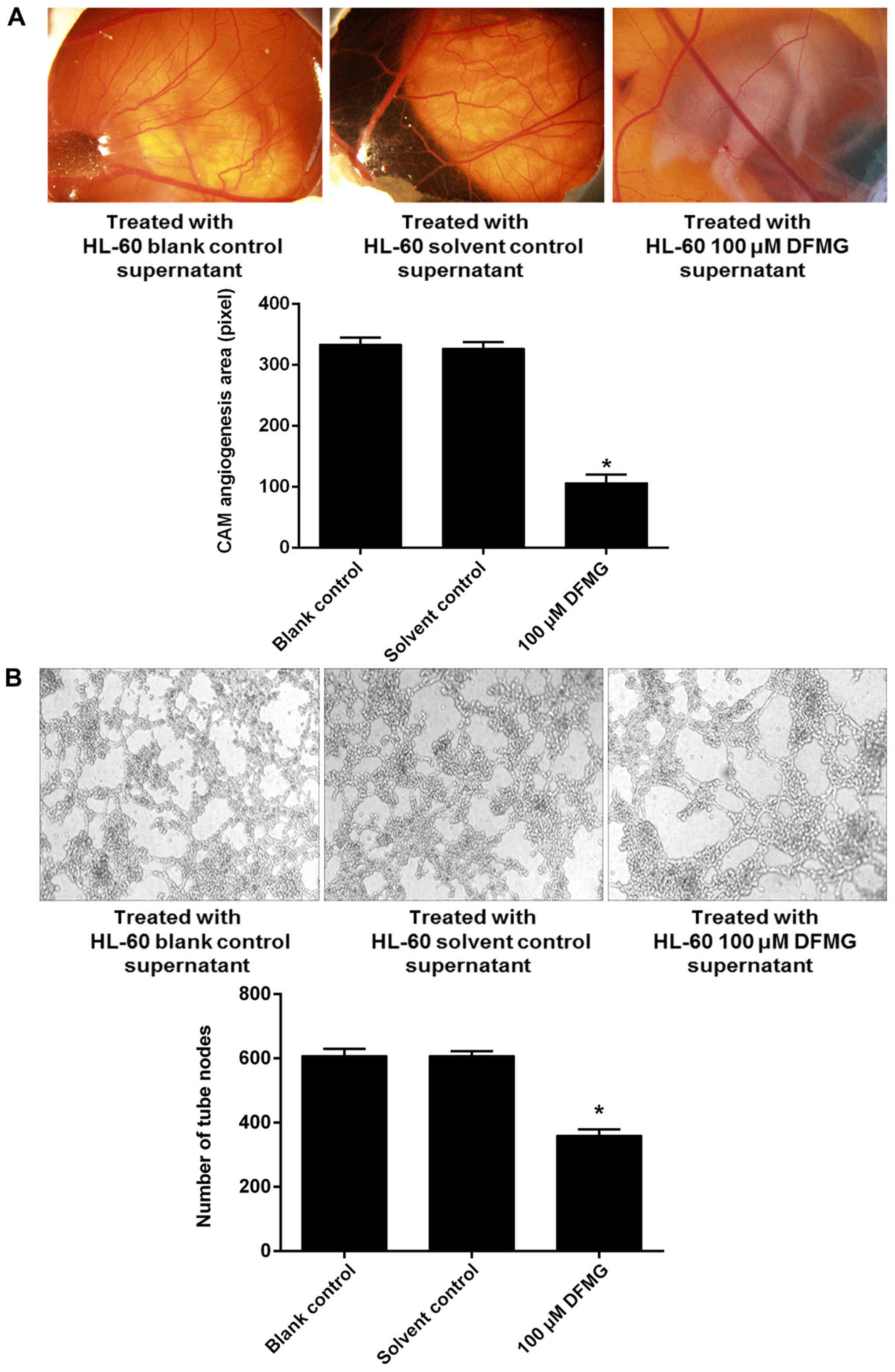
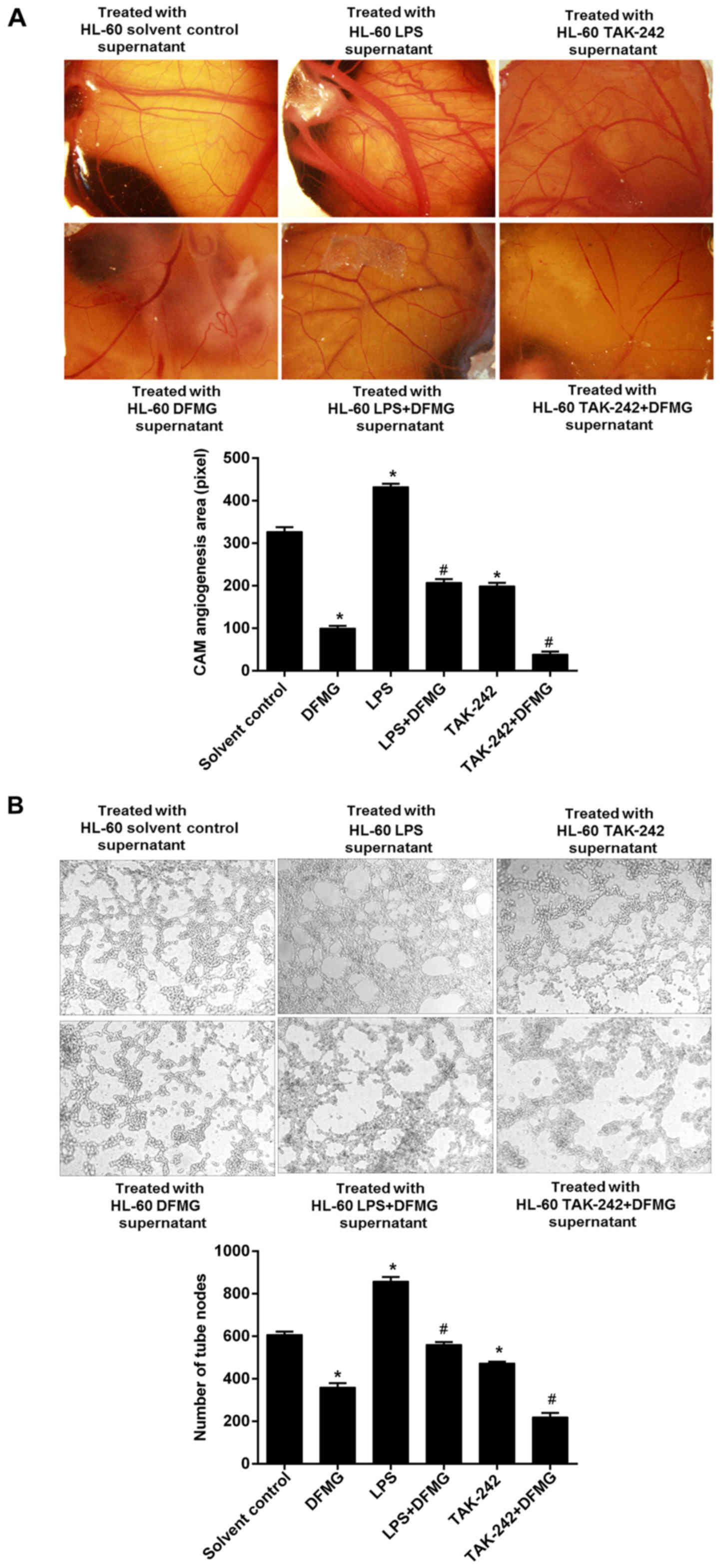
DFMG inhibits angiogenesis induced by
HL-60 cells
HL-60 cell viability was decreased by a >50 µM
dosage of DFMG and treatment with 200 µM DFMG appeared to be toxic
towards HL-60 cells, additionally <100 µM DFMG did not affect
HUVE-12 viability. Therefore, 100 µM DFMG was selected to
investigate its effects on angiogenesis induced by APL HL-60 cells
in the following experiments. CAM assays showed that the area of
angiogenesis in the membrane was reduced by the culture supernatant
of HL-60 cells treated with 100 µM DFMG (Fig. 2A). Matrigel tubule formation assays
showed that the tube formation ability of HUVE-12 cells was
significantly reduced when they were incubated in the culture
supernatant of HL-60 cells treated with 100 µM DFMG (Fig. 2B).
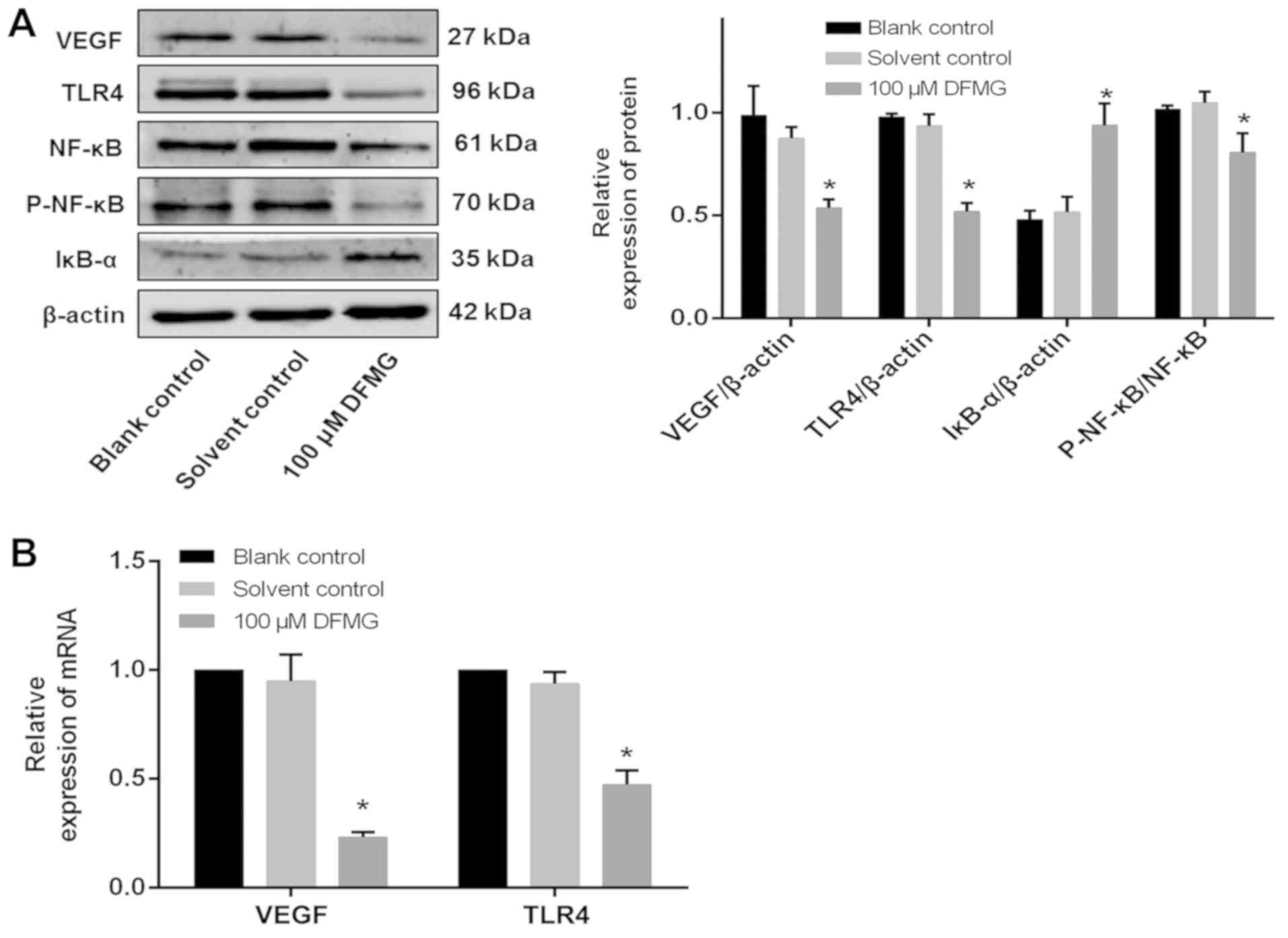
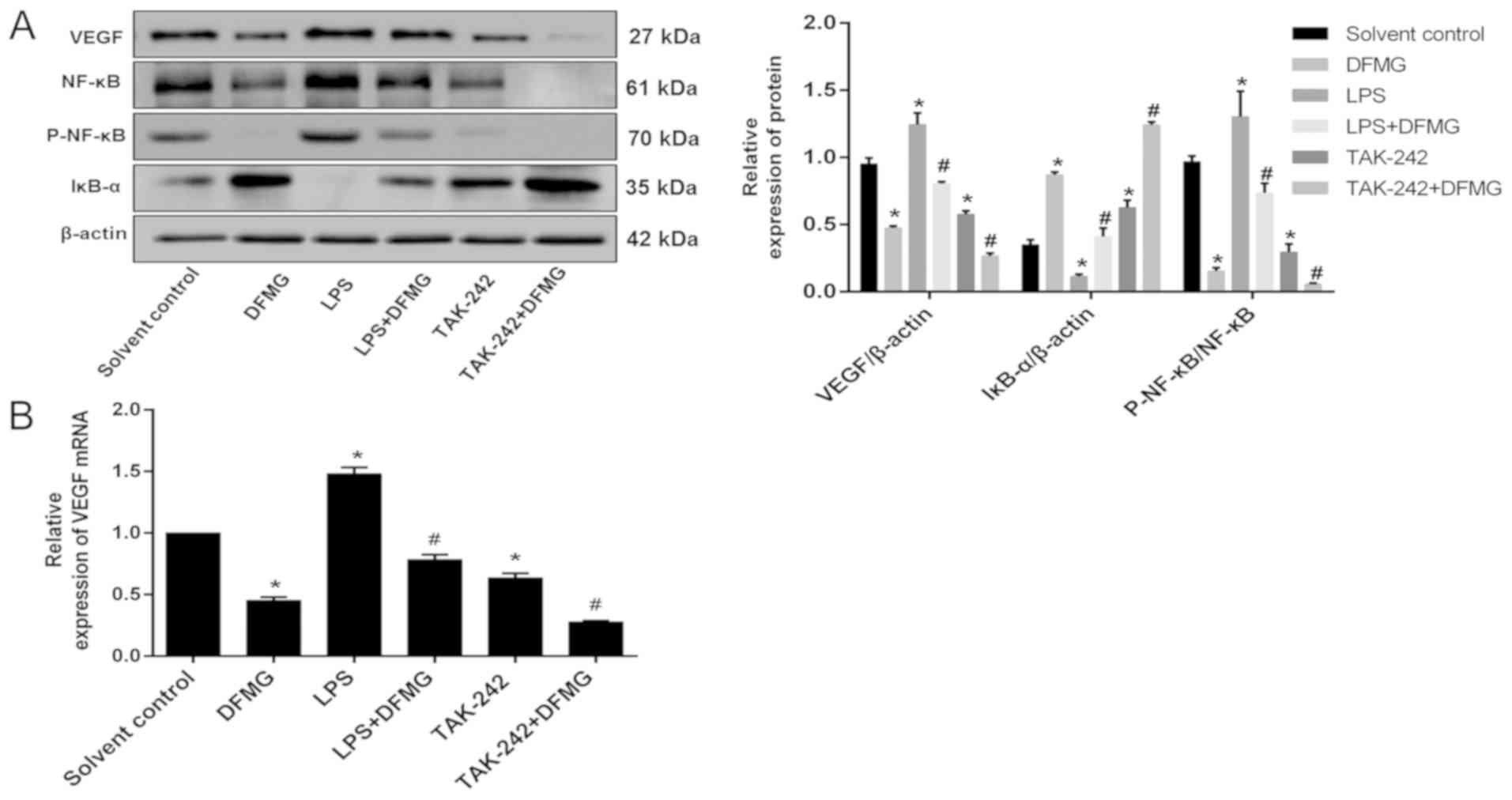
DFMG inhibits the TLR4/NF-κB signaling
pathway and reduces the protein and mRNA expression of VEGF in
HL-60 cells
VEGF is an important pro-angiogenic regulator of APL
(25). Numerous studies have shown
that the TLR4/NF-κB signaling pathway plays an important role in
regulating the activity of VEGF (26–28).
Therefore, the effects of DFMG on the TLR4/NF-κB signaling pathway
and VEGF in HL-60 cells were examined by WB and RT-qPCR. This
demonstrated that 100 µM DFMG could reduce the protein and mRNA
expression of VEGF and TLR4, as well as reduce the protein
expression of P-NF-κB and increase the protein expression of IκB-α
(Fig. 3). Together, this suggested
that DFMG could inhibit the TLR4/NF-κB signaling pathway in HL-60
cells.
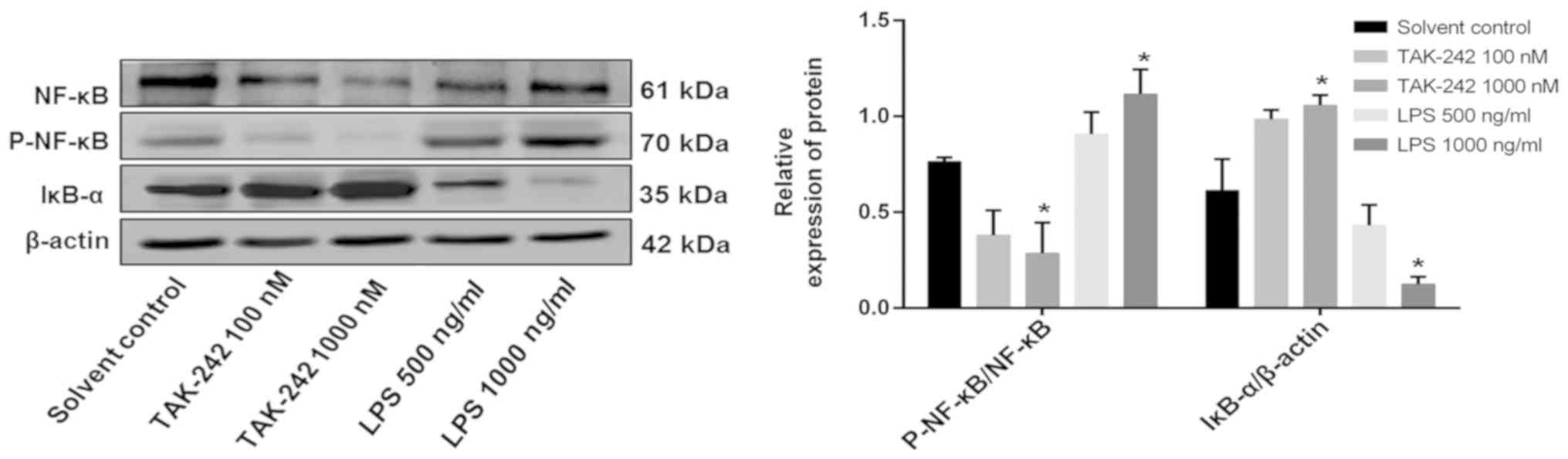
Inhibition of the TLR4/NF-κB signaling
pathway enhances the anti-angiogenic effect of DFMG and activation
of the TLR4/NF-κB signaling pathway attenuates its anti-angiogenic
effect
HL-60 cells were treated with different
concentrations of TAK-242, a selective TLR4 inhibitor (100 or 1,000
nM), or LPS (500 or 1,000 ng/ml) for 4 h. WB was then performed to
investigate the protein expression of IκB-α and P-NF-κB p65. It was
observed that TAK-242 treatment increased the protein expression of
IκB-α and decreased the protein expression of P-NF-κB in HL-60
cells. However, LPS had the opposite effect. Overall, these results
suggested that 1 µM TAK-242 and 1 µg/ml LPS significantly inhibited
or activated the TLR4/NF-κB signaling pathway, respectively
(Fig. 4).
After activation or inhibition of the TLR4/NF-κB
signaling pathway, HL-60 cells were treated with DFMG for 48 h
respectively. The angiogenesis area of the CAM was analyzed after
HL-60 cells culture supernatant was collected and added to the CAM.
Compared with the solvent control group, in the DFGM group, the
area of angiogenesis on the CAM was significantly reduced. However,
compared with the DFMG-only group, the area of angiogenesis on the
CAM decreased significantly in the TAK-242 + DFMG group, while it
increased significantly in the LPS + DFMG group (Fig. 5A).
Similar to the CAM experiment, the data from tubule
formation assay showed that compared with the solvent control
group, in the DFGM group, the numbers of tubules were significantly
reduced. However, compared with the DFMG-only group, the number of
tubules decreased significantly in the TAK-242 + DFMG group,
whereas numbers increased significantly in the LPS + DFMG group
(Fig. 5B).
The protein and mRNA expression of VEGF in HL-60
cells was assessed by WB and RT-qPCR. The data demonstrated that
compared with the solvent control group, in the DFGM group, the
protein and mRNA expression of VEGF was significantly reduced.
However, compared with the DFMG-only group, protein and mRNA
expression of VEGF decreased significantly in the TAK-242 + DFMG
group and increased significantly in the LPS + DFMG group (Fig. 6).
Discussion
In 1971, Folkman suggested that tumor growth and
metastasis depend on neovascularization (29). In tumor development, numerous new
blood vessels are formed to provide nutrition and moisture for
tumor growth while spreading tumor cells to distant locations,
forming new metastases in other parts of the body (30,31).
Physiological angiogenesis is a highly dynamic process involving
multiple pro-angiogenic and anti-angiogenic factors in the body;
during tumor development, various stimuli cause the levels of
pro-angiogenic factors to increase (30,32),
leading to an imbalance in this dynamic process (6,33).
Previous studies have shown that angiogenesis occurs in acute
myeloid leukemia, an invasive hematological malignancy
characterized by malignant proliferation of leukemia cells in the
bone marrow leading to inhibition of normal bone marrow
hematopoiesis (12,34,35).
Bone marrow angiogenesis is an important pathological process in
the changing hematopoietic microenvironment, and increased bone
marrow microvessel density can aggravate the proliferation of acute
leukemia cells (36). In studies
of APL, VEGF was found to be the most potent specific
pro-angiogenic factor (25).
Clinical studies have confirmed high expression of VEGF in the bone
marrow of patients with APL (37),
and that arsenic trioxide reduces the expression of VEGF in APL and
exerted anti-angiogenic effects (18). Therefore, treatment inhibiting bone
marrow angiogenesis and targeting pro-angiogenic factors has gained
attention in the treatment of acute leukemia (17).
DFMG is a synthesized derivative of genistein. In
our previous study, DFMG was found to inhibit angiogenesis in
atherosclerotic plaques (23). In
the present study, HL-60 cells were treated with different
concentrations of DFMG, and subsequently the viability of HL-60
cells and degree of cell damage caused by the toxicological effects
of DFMG was detected. With increasing DFMG concentrations, HL-60
cell viability was inhibited at >50 µM DFMG and release of LDH
from HL-60 cells treated with 200 µM DFMG was significantly
increased, indicating that 200 µM DFMG is toxic towards HL-60
cells. In addition, DFMG at the dosage of <100 µM did not affect
HUVE-12 viability. Therefore, 100 µM DFMG was selected to
investigate its effects on angiogenesis induced by APL HL-60 cells.
The reduction of the angiogenesis area in the CAM, as well as the
reduction in Matrigel tubule numbers, revealed that DFMG inhibited
angiogenesis induced by APL HL-60 cells compared with the solvent
control supernatant treatment. DFMG also reduced the protein and
mRNA expression of VEGF in HL-60 cells. Thus, the release of VEGF
and ability to promote angiogenesis were significantly attenuated
in HL-60 cells treated with DFMG.
Numerous studies have shown that the TLR4/NF-κB
signaling pathway plays an important role in regulating the
activity of VEGF (25,28,38).
The proteasomal degradation of IκB-α is an important event in the
canonical pathway of NF-κB activation. Subsequently, phosphorylated
NF-κB dimers enter the nucleus from the cytoplasm to promote
transcription of the gene of interest (39,40).
In our previous study, DFMG was shown to reduce the protein and
mRNA expression of TLR4 in the angiogenesis of atherosclerotic
plaques (23). In the present
study, it was shown that DFMG inhibited the TLR4/NF-κB signaling
pathway and downregulated the protein and mRNA expression of VEGF
in HL-60 cells. Next, it was demonstrated that TAK-242 and LPS
inhibited or activated the TLR4/NF-κB signaling pathway in HL-60
cells, respectively. When the TLR4/NF-κB signaling pathway was
activated, the CAM angiogenesis area, Matrigel tubule numbers and
the mRNA and protein expression of VEGF were all increased in the
HL-60 cells. The opposite results were observed in the HL-60 cells
when the TLR4/NF-κB signaling pathway was inhibited. Therefore,
when the TLR4/NF-κB signaling pathway was activated, downregulation
of the protein and mRNA expression of VEGF and inhibition of
angiogenesis induced by DFMG were attenuated. On the other hand,
when the TLR4/NF-κB signaling pathway was inhibited, downregulation
of the protein and mRNA expression of VEGF and inhibition of
angiogenesis induced by DFMG were further enhanced.
In summary, DFMG downregulates the protein and mRNA
expression of VEGF, and inhibits angiogenesis induced by APL HL-60
cells by inhibiting the TLR4/NF-κB signaling pathway. However, as
the present study used only in vitro HL-60 cells, it will be
important to verify these findings in other leukemic cell lines and
in vivo. APL is a common malignant disease in the
hematopoietic system with an increasing incidence rate. Thus,
improved treatment strategies are needed (41). The findings from the present study
extend the implications of DFMG and provide a potential new drug
candidate for the treatment of patients with APL.
Acknowledgements
Not applicable.
Funding
Financial support was provided by the Natural
Science Foundation of China (grant. no. 81370382), and Natural
Science Foundation of Hunan Province (grant. no. 14JJ2059 and
2018JJ3316).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YZ, XF, LL and XuX designed the study. XuX, PB, TK,
SL, XiX and SG performed the experiments. XuX, LL, XF and YZ
analyzed the data. XuX, PB, TK and YZ prepared the manuscript. YZ,
LL, XF and XuX revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving the use of
animals were approved by the Animal Use and Care Committee of Hunan
Normal University School of Medicine (approval no. 2013-289;
Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vardiman J and Reichard K: Acute Myeloid
leukemia with myelodysplasia-related changes. Am J Clin Pathol.
144:29–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6:e4412016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medinger M and Passweg J: Role of tumour
angiogenesis in haematological malignancies. Swiss Med Wkly.
144:W140502014.PubMed/NCBI
|
|
4
|
Lee JY and Kim HJ: (Lymph)angiogenic
influences on hematopoietic cells in acute myeloid leukemia. Exp
Mol Med. 46:e1222014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assis PA, De Figueiredo-Pontes LL, Lima
AS, Leão V, Cândido LA, Pintão CT, Garcia AB, Saggioro FP,
Panepucci RA, Chahud F, et al: Halofuginone inhibits
phosphorylation of SMAD-2 reducing angiogenesis and leukemia burden
in an acute promyelocytic leukemia mouse model. J Exp Clin Cancer
Res. 34:652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin B, Zhao K, Yang D, Bai D, Liao Y, Zhou
Y, Yu Z, Yu X, Guo Q and Lu N: Wogonoside impedes the progression
of acute myeloid leukemia through inhibiting bone marrow
angiogenesis. J Cell Physiol. 234:1913–1924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lichtenegger FS, Krupka C, Haubner S,
Kohnke T and Subklewe M: Recent developments in immunotherapy of
acute myeloid leukemia. J Hematol Oncol. 10:1422017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Y, Tan Y, Liu L, Wang Q, Zhu J and
Liu M: Levels of bone marrow microvessel density are crucial for
evaluating the status of acute myeloid leukemia. Oncol Lett.
10:211–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadia TM, Ravandi F, Cortes J and
Kantarjian H: New drugs in acute myeloid leukemia. Ann Oncol.
27:770–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pizzo RJ, Azadniv M, Guo N, Acklin J,
Lacagnina K, Coppage M and Liesveld JL: Phenotypic, genotypic, and
functional characterization of normal and acute myeloid
leukemia-derived marrow endothelial cells. Exp Hematol. 44:378–389.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drusbosky L, Gars E, Trujillo A, McGee C,
Meacham A, Wise E, Scott EW and Cogle CR: Endothelial cell derived
angiocrine support of acute myeloid leukemia targeted by receptor
tyrosine kinase inhibition. Leuk Res. 39:984–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haouas H: Angiogenesis and acute myeloid
leukemia. Hematology. 19:311–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Song K, Zhou L, Xie Z, Zhou P,
Zhao Y, Han Y, Xu X and Li P: Heparan sulfate D-glucosaminyl
3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and
proliferation by induction of VEGF in acute myeloid leukemia cells.
J Cell Biochem. 116:1101–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SE, Lee JY, Han AR, Hwang HS, Min WS
and Kim HJ: Effect of high VEGF-C mRNA expression on achievement of
complete remission in adult acute myeloid leukemia. Transl Oncol.
11:567–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez-Ariza A, Lopez-Pedrera C, Aranda
E and Barbarroja N: VEGF targeted therapy in acute myeloid
leukemia. Crit Rev Oncol Hematol. 80:241–256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zahiragic L, Schliemann C, Bieker R,
Thoennissen NH, Burow K, Kramer C, Zühlsdorf M, Berdel WE and
Mesters RM: Bevacizumab reduces VEGF expression in patients with
relapsed and refractory acute myeloid leukemia without clinical
antileukemic activity. Leukemia. 21:1310–1312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bose P, Vachhani P and Cortes JE:
Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat
Options Oncol. 18:172017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohammadi Kian M, Mohammadi S, Tavallaei
M, Chahardouli B, Rostami S, Zahedpanah M, Ghavamzadeh A and
Nikbakht M: Inhibitory effects of arsenic trioxide and thalidomide
on angiogenesis and vascular endothelial growth factor expression
in leukemia cells. Asian Pac J Cancer Prev. 19:1127–1134.
2018.PubMed/NCBI
|
|
19
|
Salemi M, Mohammadi S, Ghavamzadeh A and
Nikbakht M: Anti-vascular endothelial growth factor targeting by
curcumin and thalidomide in acute myeloid leukemia cells. Asian Pac
J Cancer Prev. 18:3055–3061. 2017.PubMed/NCBI
|
|
20
|
Chen C, Yang J and Xu W: Thalidomide in
combination with chemotherapy in treating elderly patients with
acute myeloid leukemia. Oncol Res Treat. 41:461–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu XH, Wang L, Zhao H, Xiang HL and Cao
JG: Synthesis of genistein derivatives and determination of their
protective effects against vascular endothelial cell damages caused
by hydrogen peroxide. Bioorg Med Chem Lett. 18:513–517. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Zheng X, Xiang HL, Fu XH and Cao
JG: 7-Difluoromethyl-5,4′-dimethoxygenistein inhibits oxidative
stress induced adhesion between endothelial cells and monocytes via
NF-kappaB. Eur J Pharmacol. 605:31–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bo P, Wang R, Zeng F, Xiang L, Xiang X, Fu
X and Zhang Y: The effect of DFMG on the angiogenesis and plaque
stability in the atherosclerosis model of the ApoE-/-. J Third
Military Med Univ. 17:1554–1560. 2018.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kampen KR, Ter Elst A and de Bont ES:
Vascular endothelial growth factor signaling in acute myeloid
leukemia. Cell Mol Life Sci. 70:1307–1317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dey G, Bharti R, Ojha PK, Pal I, Rajesh Y,
Banerjee I, Banik P, Parida S, Parekh A, Sen R and Mandal M:
Therapeutic implication of ‘Iturin A’ for targeting MD-2/TLR4
complex to overcome angiogenesis and invasion. Cell Signal.
35:24–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pei Z, Li H, Guo Y, Jin Y and Lin D:
Sodium selenite inhibits the expression of VEGF, TGFbeta(1) and
IL-6 induced by LPS in human PC3 cells via TLR4-NF-κB signaling
blockage. Int Immunopharmacol. 10:50–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho JS, Kang JH, Han IH, Um JY and Lee HM:
Activation of TLR4 induces VEGF expression via Akt pathway in nasal
polyps. Clin Exp Allergy. 43:1038–1047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (6 Suppl 16):S15–S18. 2002.
View Article : Google Scholar
|
|
30
|
Hida K, Maishi N, Torii C and Hida Y:
Tumor angiogenesis-characteristics of tumor endothelial cells. Int
J Clin Oncol. 21:206–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rajabi M and Mousa SA: The role of
angiogenesis in cancer treatment. Biomedicines. 5:E342017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Kang G, Wang T and Huang H: Tumor
angiogenesis and anti-angiogenic gene therapy for cancer. Oncol
Lett. 16:687–702. 2018.PubMed/NCBI
|
|
33
|
Mittal K, Ebos J and Rini B: Angiogenesis
and the tumor microenvironment: Vascular endothelial growth factor
and beyond. Semin Oncol. 41:235–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Padro T, Ruiz S, Bieker R, Bürger H,
Steins M, Kienast J, Büchner T, Berdel WE and Mesters RM: Increased
angiogenesis in the bone marrow of patients with acute myeloid
leukemia. Blood. 95:2637–2644. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Norén-Nyström U, Roos G, Bergh A and
Forestier E: Prognostic impact of vascular density and fibrosis in
the bone marrow of children with high-risk acute lymphoblastic
leukemia. Leukemia. 19:1998–2001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hatfield K, Ryningen A, Corbascio M and
Bruserud O: Microvascular endothelial cells increase proliferation
and inhibit apoptosis of native human acute myelogenous leukemia
blasts. Int J Cancer. 119:2313–2321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu J, Fu J, Chen X, Zhang Y, Gu H and Bai
Y: CD147 and VEGF co-expression predicts prognosis in patients with
acute myeloid leukemia. Jpn J Clin Oncol. 40:1046–1052. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tino AB, Chitcholtan K, Sykes PH and
Garrill A: Resveratrol and acetyl-resveratrol modulate activity of
VEGF and IL-8 in ovarian cancer cell aggregates via attenuation of
the NF-κB protein. J Ovarian Res. 9:842016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meshram SN, Paul D, Manne R, Choppara S,
Sankaran G, Agrawal Y and Santra MK: FBXO32 activates NF-κB through
IκBα degradation in inflammatory and genotoxic stress. Int J
Biochem Cell Biol. 92:134–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Sui H, Jiang C, Li S, Han Y, Huang
P, Du X, Du J and Bai Y: Dihydroartemisinin increases the
sensitivity of photodynamic therapy Via NF-κB/HIF-1α/VEGF pathway
in esophageal cancer cell in vitro and in vivo. Cell Physiol
Biochem. 48:2035–2045. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Webster JA and Pratz KW: Acute myeloid
leukemia in the elderly: Therapeutic options and choice. Leuk
Lymphoma. 59:274–287. 2018. View Article : Google Scholar : PubMed/NCBI
|