Introduction
MicroRNAs (miRNAs/miRs) are a class of small,
non-coding single-stranded RNAs that either repress translation or
degrade mRNA by base pairing to the 3′ untranslated region (3′UTR),
thus contributing to inhibition of target gene expression (1,2).
miRNAs are involved in various physiological and pathological
processes, including carcinogenesis, cell proliferation and
differentiation (2). Moreover, it
has been reported that miRNA alterations are associated with the
initiation, progression and metastasis of human cancer (3,4).
Osteosarcoma is a common primary malignant bone tumor mainly
occurring in childhood and adolescence, which has a high mortality
rate, with a 5-year survival rate of <60%, due to robust
invasion and metastasis (5). While
treatment for osteosarcoma has progressed, the prognosis of
osteosarcoma remains poor, with a relatively unchanged 5-year
survival rate of 65% over the last 30 years (6). Furthermore, the pathogenesis of
osteosarcoma is not fully understood.
Abnormal miRNA expression may be involved in the
occurrence and development of osteosarcoma. It has been reported
that miR-144 suppressed tumor growth and metastasis in osteosarcoma
via dual-suppression of the Ras homolog family member A and
Rho-associated coiled-coil-containing protein kinase 1 signaling
pathways (7). However, miR-181a
and miR-199b-5p have been shown to promote cell proliferation,
invasion and metastasis in osteosarcoma (8,9).
Furthermore, a recent study identified the involvement of miR-744
in osteosarcoma and chemotherapy resistance (10). Sun et al (11) revealed that miR-744 promoted
osteosarcoma development and progression by suppressing large tumor
suppressor kinase 2 (LATS2) signaling. However, due to the limited
number of studies examining miR-744 in osteosarcoma, the role of
miR-744 and its underlying molecular mechanisms in the pathogenesis
of osteosarcoma are not fully understood. PTEN, a unique tumor
suppressor gene, has been identified to be a targeted gene of
miR-744 in laryngeal squamous cell carcinoma (12), and has also been shown to be
associated with the growth and metastasis of various malignant
tumors (13). Therefore, the aim
of the present study was to investigate whether PTEN is a direct
target of miR-744 in the regulation of osteosarcoma cell
proliferation.
Materials and methods
Cell culture and tissue samples
The MG63, U2OS and SaOS2 human osteosarcoma cell
lines were obtained from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences. The hFOB1.19 human fetal
osteoblastic cell line was obtained from the Institutes for
Biological Sciences, Chinese Academy of Sciences. Human
osteosarcoma cell lines were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.), and the human fetal osteoblastic cell line was
cultured in DMEM-F12 medium (Gibco; Thermo Fisher Scientific,
Inc.). The aforementioned media were supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. Cells were maintained and subcultured at
37°C in a humidified incubator containing 5% CO2. Cells
were pre-treated with 0.1% DMSO (control) or 20 µM LY294002
(dissolved in 0.1% DMSO; Sigma-Aldrich; Merck KGaA) for 8 h at
37°C.
In total, 25 osteosarcoma tissue samples and
corresponding adjacent non-tumor tissues (>2 cm from the tumor
margin) were obtained from the Department of Orthopedic Surgery,
The Second Affiliated Hospital of Wenzhou Medical University.
Tissue samples were collected from patients undergoing complete
resection surgery between July 2015 and June 2019. Informed consent
was obtained from the patients (age, ≥18 years) or their parents
(patients aged <18 years), and all patients had not received any
radiation therapy or chemotherapy prior to surgery. The samples
were obtained from 15 male patients and 10 female patients (age,
23.6±8.6 years; age range, 12–43 years) with an average tumor size
of 8.7±3.5 cm (tumor size range, 3.8–16.1 cm). With regards to
tumor subtype, 21 were high-grade osteosarcoma, one was
intermediate-grade osteosarcoma and three were low-grade
osteosarcoma. WHO classification was used to identify the
osteosarcoma subtypes based on the characteristics of tumor tissue
observed under the microscope (14). This study was approved by the
Ethics Committee of The Second Affiliated Hospital, Wenzhou Medical
University.
Analysis of miRNA expression using
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissue samples and cell
lines using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RNA
was reverse transcribed into cDNA using stem-loop primers and the
TaqMan miRNA RT kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The temperature and duration conditions of RT were as
follows: 37°C for 15 min and 85°C for 5 sec. The specific primer
sequences were as follows: miR-744, forward
5′-ACACTCCAGCTGGGTGCGGGGCTAGGGCTAAC-3′, reverse
5′-CTCAACTGGTGTCGTGGA-3′; and U6 small nuclear RNA, forward
5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′.
miR-744 expression was detected using a TaqMan human miRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. qPCR was performed using an Applied
Biosystems 7500 Sequence Detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with U6 small nuclear RNA as the
internal control. The following thermocycling conditions were used:
Initial denaturation at 95°C for 5 min; followed by 40 cycles at
95°C for 15 sec, and primer annealing and extension at 60°C for 30
sec; and final extension for 1 min at 72°C. The relative expression
of miR-744 was calculated using the 2−ΔΔCq method
(15).
Cell transfection
miR-744 precursor (5′-UGCGGGGCUAGGGCUAACAGCA-3′;
final concentration, 100 nM), miR-744 inhibitor (anti-miR-744;
5′-UGCUGUUAGCCCUAGCCCCGCA-3′; final concentration, 100 nM) and
their corresponding miR negative controls (miR-control;
5′-UUCUCCGAACGUGUCACGUTT-3′; final concentration, 100 nM; and
anti-miR-control; 5′-CAGUACUUUUGUGUAGUACAA-3′; final concentration,
100 nM) were purchased from Shanghai GenePharma Co., Ltd. At 60–70%
confluency, MG63 osteosarcoma cells were transfected for 6 h at
37°C using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were then transferred to
complete medium and incubated at 37°C for 48 h before harvesting.
Transfection efficiency was subsequently evaluated by RT-qPCR at 48
h after transfection. Subsequent experiments were performed at 24 h
post-transfection.
Bromodeoxyuridine (BrdU) assay
Cell proliferation was assessed using an ELISA BrdU
kit (cat. no. Roche-11647229001; Sigma-Aldrich; Merck KGaA).
Following successful transfection, cells were seeded in 96-well
plates at a density of 2×103 cells/well. The BrdU assay
was subsequently performed according to the manufacturer's
protocol. Absorbance was measured at a wavelength of 450 nm using
an enzyme immunoassay analyzer (Bio-Rad Laboratories, Inc.). To
determine the cell number, cells collected, centrifuged at 150 × g
for 5 min at room temperature, resuspended and counted using a
light microscope (magnification, ×400) with a Neubauer counting
chamber. The experiment was repeated ≥3 times.
Western blot analysis
To isolate proteins, cells or tissues were harvested
and lysed with ice-cold RIPA lysis buffer (Beyotime Institute of
Biotechnology). The lysates were centrifuged at 20,000 × g for 15
min at 4°C. Proteins in the supernatants were then collected and
quantified using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (40 µg/lane)
were separated by SDS-PAGE on 10% gels, and then transferred to
PVDF membranes (Bio-Rad Laboratories, Inc.). After blocking with 5%
non-fat milk in TBS-Tween (TBST; 50 mM Tris-HCl; pH 7.5; 150 mM
NaCl; 0.05% (v/v) Tween 20) for 2 h at room temperature, membranes
were incubated with specific primary antibodies overnight at 4°C,
including anti-phosphorylated (p)-PTEN (cat. no. 9554; 1:500),
anti-PTEN (cat. no. 9552; 1:500), anti-p-AKT (cat. no. 4060;
1:1,000), anti-AKT (cat. no. 9272; 1:1,000) and anti-GAPDH (cat.
no. 8884; 1:1,000), which were purchased from Cell Signaling
Technology, Inc. After washing three times with TBST, membranes
were incubated with horseradish peroxidase-linked rabbit anti-mouse
secondary antibody (cat. no. A0216; 1:2,000; Beyotime Institute of
Biotechnology) at room temperature for 2 h. The protein signals
were detected using an enhanced chemiluminescence system western
blotting kit (GE Healthcare Bio-Sciences), according to the
manufacturer's protocol. The density of protein bands was
semi-quantified by ImageJ software (version 1.6; National
Institutes of Health) and the value was normalized to that of
GAPDH.
Luciferase reporter assay
TargetScan (version 7.2; www.targetscan.org/vert_72) and miRanda (version 2010;
www.microrna.org/microrna/home.do) were used to
predict the target sites for miR-744, and subsequently identified
binding sites between PTEN and miR-744. The predicted wild-type and
mutant 3′UTRs of PTEN containing the binding sites to hsa-miR-744
were cloned by PCR amplification. Genomic DNA of MG63 cell was used
as the template. PCR was performed using the Takara™ LA
Taq DNA polymerase kit (Takara Bio, Inc.) with the following
thermocycling conditions: Denaturation at 95°C for 5 min; followed
by 30 cycles at 95°C for 15 sec; primer annealing and extension at
72°C for 30 sec; followed by 15 cycles at 95°C for 15 sec, 53°C for
30 sec, 72°C for 30 sec; and a final extension step of 10 min at
72°C. Subsequently, genes were inserted into the pMIR-REPORT
luciferase vector (Applied Biosystems; Thermo Fisher Scientific,
Inc.) at the SacI and HindIII sites. The following
primers were used to amplify specific fragments: Wild-type PTEN
forward, 5′-CCACATCCTACCCCTTTGCACTGGCAACAGATAAGTTTGCAGTTGGCTAA-3′
and reverse,
5′-AGCTTTAGCCAACTGCAAACTTATCTGTTGCCAGTGCAAAGGGGTAGGATGTGGACT-3′;
and mutant PTEN forward,
5′-CCACATCCTACCCCTTTGCACCTATCGTCGATAAGTTTGCAGTTGGCTAA-3′ and
reverse,
5′-AGCTTTAGCCAACTGCAAACTTATCGACGATAGGTGCAAAGGGGTAGGATGTGGAGCT-3′.
For the reporter assay, all cells were seeded (1×105
cells/well) in 24-well plates. At 70% confluency, pMIR-REPORT
vector (200 ng) containing the wild-type or mutant 3′UTR of PTEN,
along with miR-744 precursor (200 ng) or anti-miR-744 (200 ng) were
co-transfected into the cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After transfection at 37°C for 36 h, cells
were lysed using 0.25% trypsin-EDTA and the luciferase activity was
determined using a dual-luciferase reporter assay system (Promega
Corporation). Relative firefly luciferase activity was normalized
to Renilla luciferase activity.
Statistical analysis
Experiments were repeated ≥3 times, and data are
presented as the mean ± standard error of the mean. Differences
between groups were analyzed using a paired Student's t-test or
one-way ANOVA followed by a Tukey's multiple comparison post hoc
test. Statistical analyses were performed using GraphPad Prism 5.0
software (GraphPad Software, Inc.) and SPSS software version 18
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-744 expression is upregulated in
osteosarcoma tissues and cells
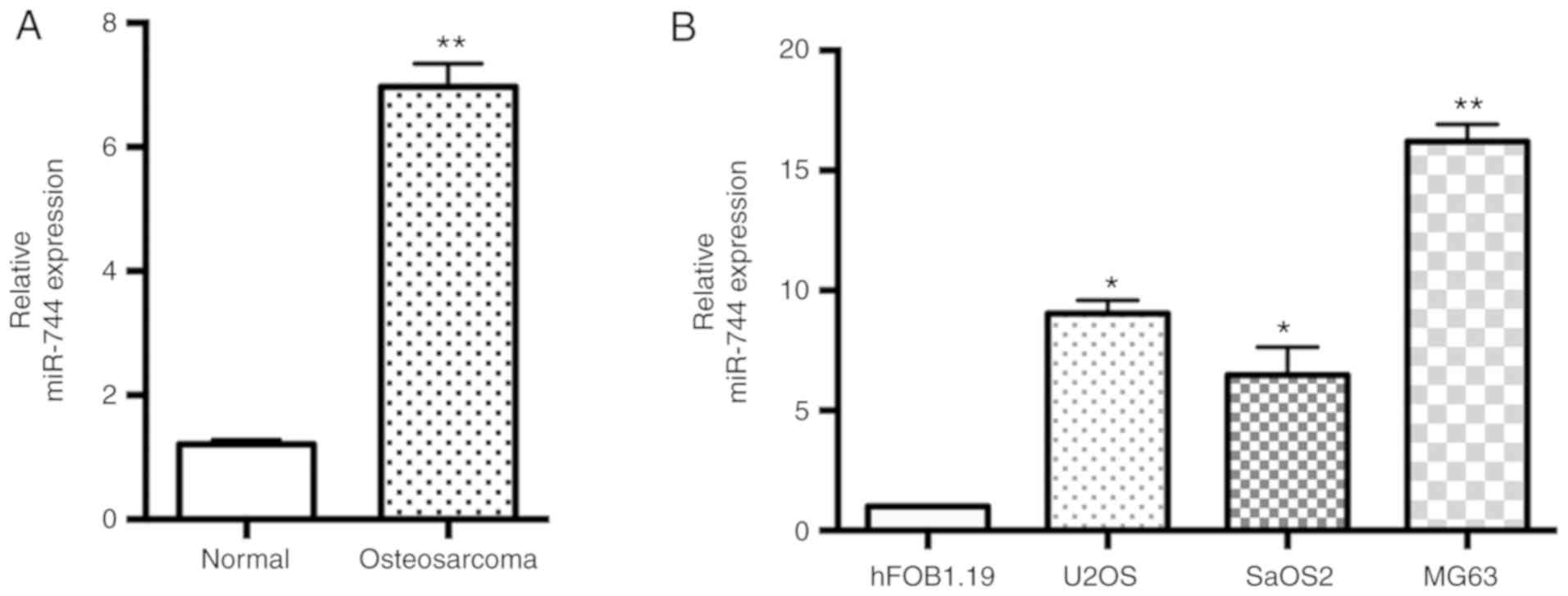
miR-744 expression in the 25 pairs of human
osteosarcoma tissues and adjacent non-malignant tissues was
assessed using the TaqMan RT-qPCR assay. It was revealed that the
expression levels of miR-744 were significantly increased in
osteosarcoma tissues compared with the adjacent non-malignant
tissues (Fig. 1A). Moreover,
osteosarcoma cell lines (U2OS, SaOS2 and MG63) expressed higher
levels of miR-744 compared with the human fetal osteoblastic cell
line hFOB1.19 (Fig. 1B). The MG63
cell line was selected for further studies as it exhibited the
highest expression of miR-744 among the three osteosarcoma cell
lines. Based on the overexpression of miR-744 in osteosarcoma
tissues and cells, it was hypothesized that miR-744 may act as a
functional miRNA in osteosarcoma, particularly in the MG63 cell
line.
miR-744 enhances proliferation of
osteosarcoma cells
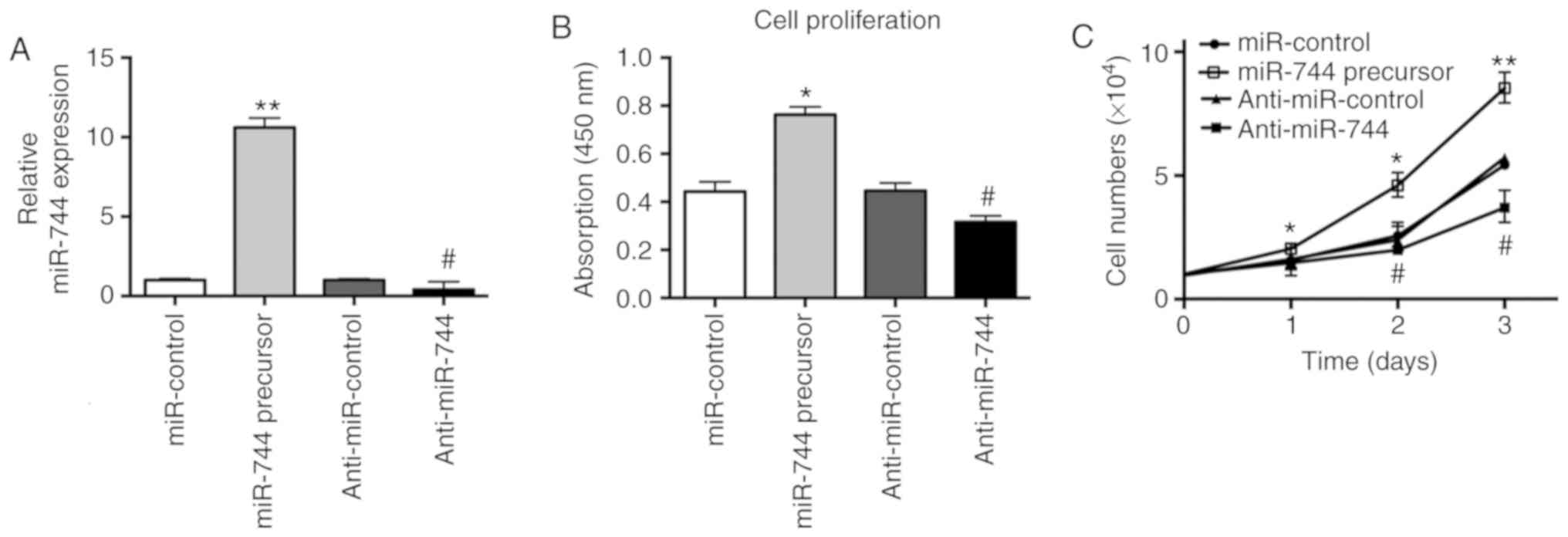
In order to investigate whether miR-744 was involved
in osteosarcoma carcinogenesis, miR-744 precursor and anti-miR-744
were transiently transfected into MG63 cells. The effects of
miR-744 on osteosarcoma cell proliferation were subsequently
assessed by BrdU assays. After 48 h, as determined by RT-qPCR,
miR-744 was significantly overexpressed or knocked down by miR-744
precursor and anti-miR-744, respectively (Fig. 2A). Furthermore, BrdU assay results
demonstrated that miR-744 overexpression significantly enhanced the
proliferation of MG63 cells. However, knockdown of miR-744
significantly inhibited the proliferation of MG63 cells (Fig. 2B). Meanwhile, cell counting
experiments indicated that miR-744 overexpression or knockdown
resulted in significance alterations to the cell numbers within 3
days (Fig. 2C). The miR-744
overexpression or knockdown-mediated effects on cell numbers were
more obvious with incubations >3 days (data not shown).
PTEN is a direct target of miR-744 in
osteosarcoma cells
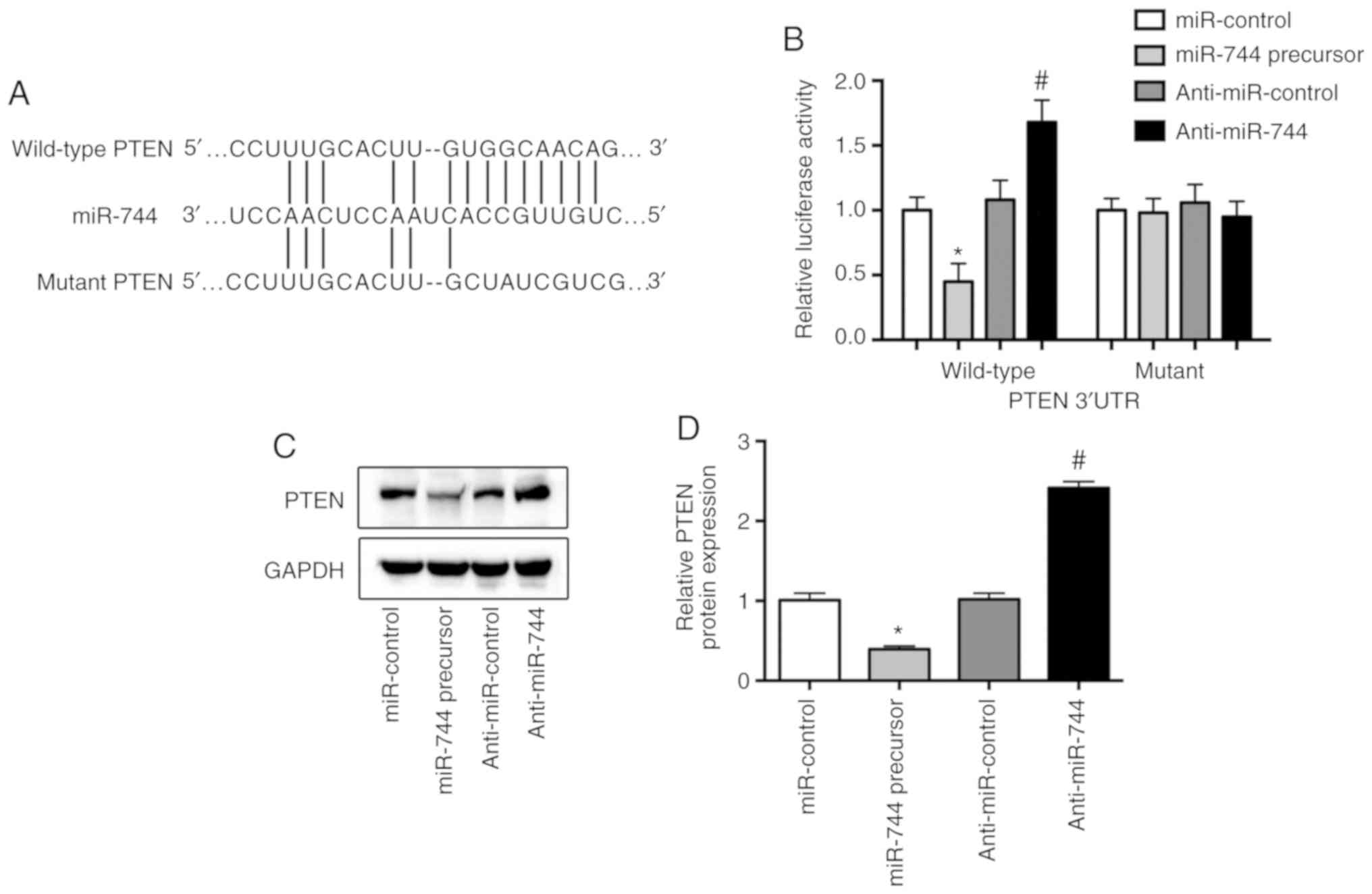
Bioinformatics analysis using TargetScan 7.2 and
miRanda suggested various potential target genes for miR-744. Of
these potential candidates, PTEN, which is a tumor suppressor gene
(13), harbored potential miR-744
binding sites within its 3′UTR (Fig.
3A). To further assess whether miR-744 directly targeted PTEN,
a vector containing the PTEN-3′UTR was constructed and transfected
into MG63 cells. It was demonstrated that the miR-744 precursor
decreased luciferase activity, whereas anti-miR-744 significantly
increased the luciferase activity of MG63 cells transfected with
the wild-type PTEN 3′UTR (Fig.
3B). Furthermore, the protein expression of PTEN was
significantly reduced by miR-744 overexpression, but significantly
increased by miR-744 inhibition in osteosarcoma cells (Fig. 3C and D).
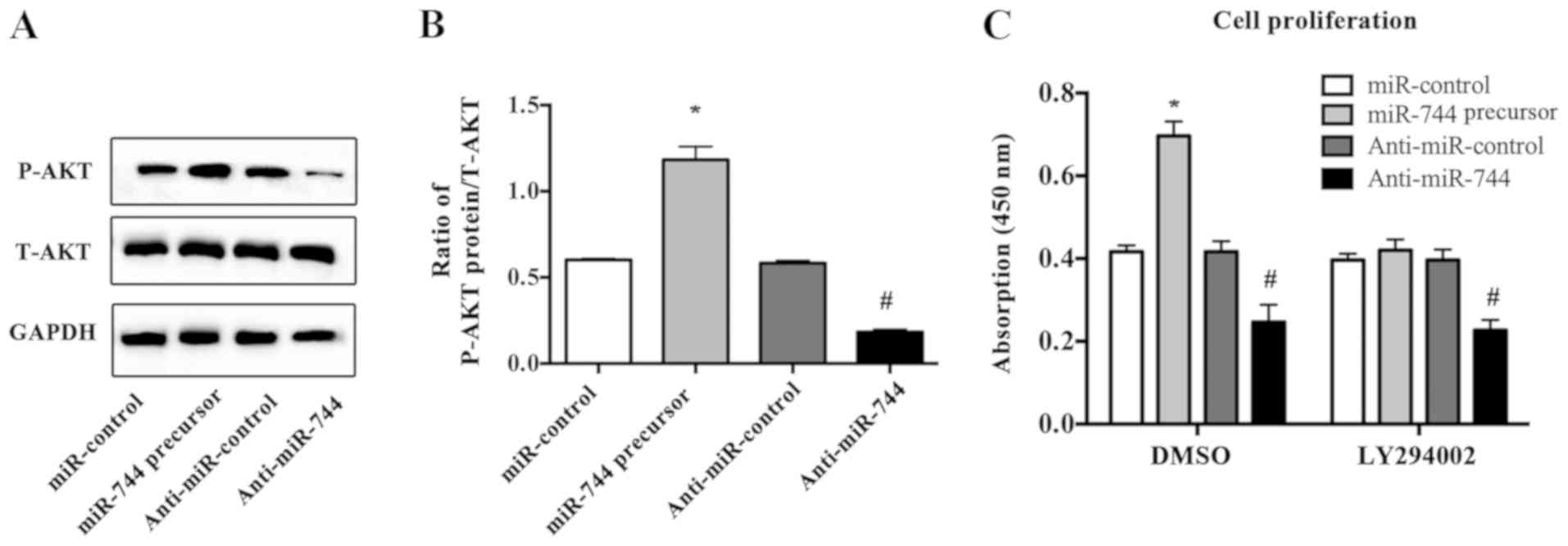
PTEN functions as a negative regulator of the
PI3K/AKT signaling pathway, which has been reported to be active in
a wide range of tumor types (16–18).
Therefore, the present study detected the protein expression of
active AKT via western blotting in osteosarcoma cells transfected
with miR-744 precursor or anti-miR-744. It was identified that
miR-744 overexpression induced AKT activation in MG63 cells,
whereas downregulation of miR-744 decreased P-AKT expression
(Fig. 4A and B). In addition,
LY294002, an AKT inhibitor, abrogated the proliferative effect
induced by miR-744 overexpression in osteosarcoma cells (Fig. 4C). Collectively, the present
results indicated that PTEN may be a direct target of miR-744 in
osteosarcoma cells.
Discussion
Osteosarcoma, one of the most malignant types of
bone cancer, is associated with rapid deterioration and metastasis,
and is a major health threat to pediatric and adolescent patients
(19,20). Previous studies have shown that
miRNAs are involved in the regulation of various biological
processes in osteosarcoma, including cell proliferation,
differentiation, migration and invasion (7–9). The
present results suggested that miR-744 expression was upregulated
in osteosarcoma tissues and cells. Moreover, it was demonstrated
that ectopic overexpression of miR-744 promoted the proliferation
of MG63 cells, whereas miR-744 inhibition using antisense
oligonucleotides inhibited cell proliferation.
It has been reported that miR-744 may function as
either an oncogene or a tumor suppressor in different cancer types.
Consistent with the present results, miR-744 was highly expressed
and acted as an oncogene in pancreatic cancer (21), prostate cancer (22) and nasopharyngeal carcinoma
(23). Furthermore, overexpression
of miR-744 promoted tumor metastasis by suppressing PTEN and
programmed cell death protein 4 pathways in laryngeal squamous cell
carcinoma (12). However, other
studies have suggested a role for miR-744 as a tumor suppressor
rather than a dominant oncogene. miR-744 was downregulated and
acted as a tumor suppressor in glioblastoma (24), gastric cancer (25) and colorectal cancer (26). Moreover, low expression of miR-744
has been implicated in the development and progression of liver
cancer, and thus may be considered a potential prognostic marker in
patients with hepatocellular carcinoma (27). Therefore, it is speculated that the
dual roles of miR-744 may be attributed to the different cellular
contexts and its interaction with target genes. Sun et al
(11) revealed that miR-744
promoted osteosarcoma carcinogenesis by inhibiting the LATS2
signaling pathway. Furthermore, Sun et al (11) conducted cell viability, migration
and invasion assays, and reported that miR-744 promoted cell
viability and metastasis of osteosarcoma by suppressing LATS2
signaling. However, the biological functions and the underlying
molecular mechanisms of miR-744 in osteosarcoma are not fully
understood. Therefore, the present study investigated whether the
oncogene miR-744 promoted osteosarcoma via a novel PTEN signaling
pathway. The present study revealed that miR-744 was overexpressed
in osteosarcoma tissues and cell lines, and also demonstrated that
miR-744 promoted osteosarcoma cell proliferation via the
suppression of PTEN expression. Thus, the present results may
increase the understanding of the functional role of miR-744 in
osteosarcoma, as it was indicated that miR-744 may primarily act as
an oncogenic miRNA in osteosarcoma. However, whether miR-744
promotes cell migration and invasion of osteosarcoma via the PTEN
pathway in vivo and in vitro requires further
investigation.
PTEN is a tumor suppressor gene, and loss of its
expression has been reported to be associated with growth and
metastasis of various malignant tumors (13). PTEN deficiency may also result in
activation of the PI3K/AKT signaling pathway, thus inhibiting
apoptosis and promoting proliferation of tumor cells (16,28).
Previous studies showed that the PTEN/PI3K/AKT signaling pathway
was critical for carcinogenesis and progression in numerous cancer
types, and AKT phosphorylation has been used as an indicator of
activation of this pathway (29–31).
Moreover, miR-744, acting as an oncogenic miRNA, has been reported
to promote metastasis of laryngeal squamous cell carcinoma by
suppressing PTEN expression and activating the PI3K/AKT signaling
pathway (12). The present results
indicated that miR-744 promoted cell proliferation in MG63 cells,
which was associated with reduced PTEN expression and increased AKT
phosphorylation. In addition, it was demonstrated that blocking the
PI3K/AKT pathway with LY294002, an AKT inhibitor, abrogated the
proliferative effect induced by miR-744. Therefore, the present
results suggested that miR-744 increased osteosarcoma cell
proliferation via the suppression of PTEN and subsequent activation
of the PI3K/AKT pathway.
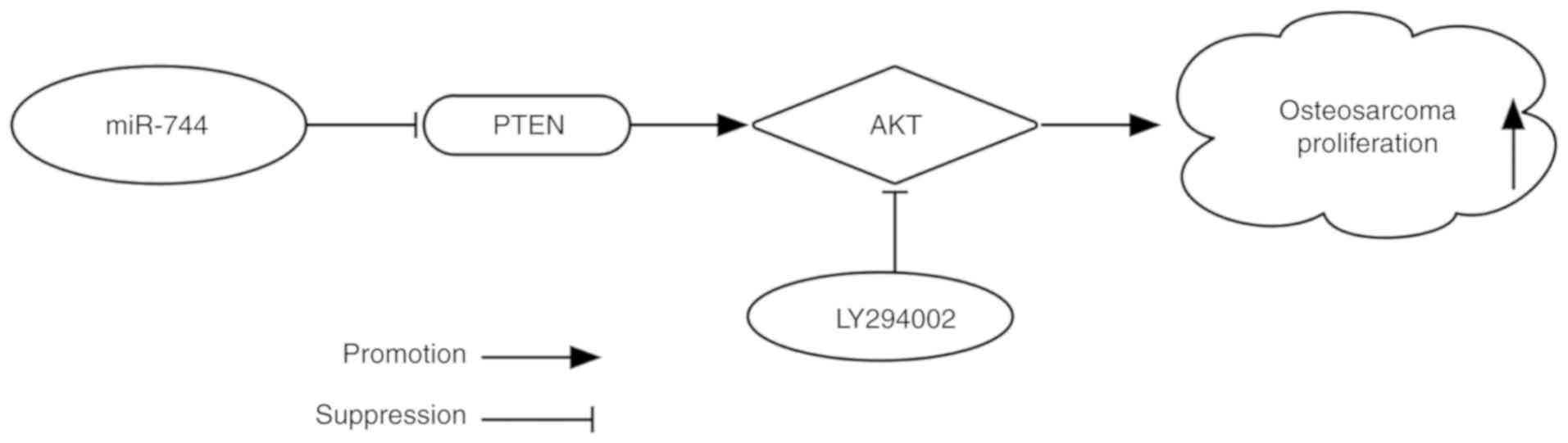
In conclusion, the present study demonstrated that
miR-744 was overexpressed in osteosarcoma tissues and cells.
miR-744 was suggested to have an active role in promoting cell
proliferation via suppression of the PTEN signaling pathway
(Fig. 5). These results may
provide novel insights into the pathophysiological mechanism of
osteosarcoma and facilitate the development of therapeutic targets
for the treatment of this cancer type. However, there are some
limitations to the present study. For example, this experiment was
primarily conducted at the cellular level, and further in
vivo studies are required to elucidate the role of miR-744 in
osteosarcoma. Moreover, further experiments are required to
investigate miR-744 regulation, as well as its underlying
mechanisms in cell migration and invasion of osteosarcoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Province Technology Project (grant no. 2015C33209) and the Wenzhou
Technology Project (grant no. Y20150243).
Availability of data and materials
The datasets used and/or analyzed through the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
WY performed the majority of experiments and drafted
the manuscript. PBC, FCC and SLD assisted with the experiments. WY
and PBC analyzed the data and drafted the manuscript. WY and XYP
conceived the study, supervised the experiments and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital, Wenzhou Medical
University. Informed consent was obtained from the patients (age,
≥18 years) or their parents (patients aged <18 years).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saraf AJ, Fenger JM and Roberts RD:
Osteosarcoma: Accelerating progress makes for a hopeful future.
Front Oncol. 8:42018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin
F, Wang Z, Zhang Y, Li S, Miao Y, et al: Inhibition of CaMKIIα
activity enhances antitumor effect of fullerene C60 nanocrystals by
suppression of autophagic degradation. Adv Sci (Weinh). 6:1801233.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu JL, Li J, Xu JJ, Xiao F, Cui PL, Qiao
ZG, Chen XD, Tao WD and Zhang XL: miR-144 inhibits tumor growth and
metastasis in osteosarcoma via dual-suppressing RhoA/ROCK1
signaling pathway. Mol Pharmacol. 95:451–461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jianwei Z, Fan L, Xiancheng L, Enzhong B,
Shuai L and Can L: MicroRNA 181a improves proliferation and
invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol.
34:3331–3337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Zhao G, Zhang Y, Ma Y, Ding Y and
Xu N: miR-199b-5p promotes malignant progression of osteosarcoma by
regulating HER2. J BUON. 23:1816–1824. 2018.PubMed/NCBI
|
|
10
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA networks in osteosarcoma chemo-resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun L, Liu M, Luan S, Shi Y and Wang Q:
MicroRNA-744 promotes carcinogenesis in osteosarcoma through
targeting LATS2. Oncol Lett. 18:2523–2529. 2019.PubMed/NCBI
|
|
12
|
Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei
WI, Ho WK and Wong TS: MicroRNA 744-3p promotes MMP-9-mediated
metastasis by simultaneously suppressing PDCD4 and PTEN in
laryngeal squamous cell carcinoma. Oncotarget. 7:58218–58233.
2016.PubMed/NCBI
|
|
13
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading,
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenberg AE, Cleton-Jansen AM, de Pineux
G, Deyrup AT, Hauben E and Squire J: Conventional osteosarcoma. WHO
Classification of Tumours of Soft Tissue and Bone. 4th. Fletcher
CDM, Bridge JA, Hogendoorn CWH and Mertens F: IARC Press; Lyon: pp.
282–288. 2013
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sansal I and Sellers WR: The biology and
clinical relevance of the PTEN tumor suppressor pathway. J Clin
Oncol. 22:2954–2963. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Wang F, Li M, Yu Z, Qi R, Ding J,
Zhang Z and Chen X: Self-Stabilized hyaluronate nanogel for
intracellular codelivery of doxorubicin and cisplatin to
osteosarcoma. Adv Sci (Weinh). 5:17008212018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Zhang T, Xu W, Ding J, Yin F, Xu J,
Sun W, Wang H, Sun M, Cai Z and Hua Y: Sarcoma-targeting
peptide-decorated polypeptide nanogel intracellularly delivers
shikonin for upregulated osteosarcoma necroptosis and diminished
pulmonary metastasis. Theranostics. 8:1361–1375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou W, Li Y, Gou S, Xiong J, Wu H, Wang
C, Yan H and Liu T: miR-744 increases tumorigenicity of pancreatic
cancer by activating Wnt/β-catenin pathway. Oncotarget.
6:37557–37569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M and Li H, Zhang Y and Li H:
Oncogenic miR-744 promotes prostate cancer growth through direct
targeting of LKB1. Oncol Lett. 17:2257–2265. 2019.PubMed/NCBI
|
|
23
|
Yu Q, Zhang F, Du Z and Xiang Y:
Up-regulation of serum miR-744 predicts poor prognosis in patients
with nasopharyngeal carcinoma. Int J Clin Exp Med. 8:13296–13302.
2015.PubMed/NCBI
|
|
24
|
Deng Y, Li Y, Fang Q, Luo H and Zhu G:
microRNA-744 is downregulated in glioblastoma and inhibits the
aggressive behaviors by directly targeting NOB1. Am J Cancer Res.
8:2238–2253. 2018.PubMed/NCBI
|
|
25
|
Xu AJ, Fu LN, Wu HX, Yao XL and Meng R:
MicroRNA744 inhibits tumor cell proliferation and invasion of
gastric cancer via targeting brainderived neurotrophic factor. Mol
Med Rep. 16:5055–5061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen J and Li M: MicroRNA-744 inhibits
cellular proliferation and invasion of colorectal cancer by
directly targeting oncogene Notch1. Oncol Res. Feb 22–2018.(Epub
ahead of print). View Article : Google Scholar
|
|
27
|
Tan YL, Bai ZG, Zou WL, Ma XM, Wang TT,
Guo W, Liu J, Li JS, Jie-Yin, Zang YJ and Zhang ZT: miR-744 is a
potential prognostic marker in patients with hepatocellular
carcinoma. Clin Res Hepatol Gastroenterol. 39:359–365. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colakoglu T, Yildirim S, Kayaselcuk F,
Nursal TZ, Ezer A, Noyan T, Karakayali H and Haberal M:
Clinicopathological significance of PTEN loss and the
phosphoinositide 3-kinase/Akt pathway in sporadic colorectal
neoplasms: Is PTEN loss predictor of local recurrence? Am J Surg.
195:719–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saal LH, Holm K, Maurer M, Memeo L, Su T,
Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, et al:
PIK3CA mutations correlate with hormone receptors, node metastasis,
and ERBB2, and are mutually exclusive with PTEN loss in human
breast carcinoma. Cancer Res. 65:2554–2559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang JM, He QY, Guo RX and Chang XJ:
Phosphorylated Akt overexpression and loss of PTEN expression in
non-small cell lung cancer confers poor prognosis. Lung Cancer.
51:181–191. 2006. View Article : Google Scholar : PubMed/NCBI
|