Introduction
Osteoarthritis is a very common degenerative joint
disease involving highly diseased connective tissue and is a
leading cause of disability. Furthermore, the damaged articular
cartilage has limited self-repairing ability, making it difficult
to cure (1,2). With the development of cell therapy,
attempts have been made to expand chondrocytes in vitro to
repair cartilage defects; however, the expansion of chondrocytes
in vitro causes decreased proliferative ability and
dedifferentiation (3). Mesenchymal
stem cells (MSCs) exhibit the potential for self-renewal and
multi-directional differentiation and serve an important role in
tissue regeneration (4,5). Therefore, inducing the
differentiation of MSCs into chondrocytes has become an important
approach to repair damaged cartilage (6,7). An
inflammatory milieu may induce chondrocyte apoptosis, resulting in
cartilage destruction in patients with cartilage degenerative
diseases, such as rheumatoid arthritis or osteoarthritis (8). Notably, MSCs can differentiate into
chondrocytes under inflammatory conditions to repair damaged tissue
(9). This indicates the potential
of MSCs in the treatment of cartilage diseases. Human amniotic
mesenchymal stem cells (hAMSCs) exhibit the characteristics of MSCs
and differentiate into adipocyte-, osteoblast- and chondrocyte-like
cells, exhibiting low immunogenicity and immunoregulatory function.
Therefore, they are an ideal cell resource for stem cell therapy
and tissue engineering (10,11).
Furthermore, transplantation of hAMSCs may enhance bone strength
and decrease the incidence of fractures in mice (12). They exhibited stronger cartilage
repair capability compared with human bone marrow mesenchymal stem
cells and chondrocytes (13).
However, the regulatory mechanism underlying the differentiation of
hAMSCs into chondrocytes remains to be elucidated.
CD44 antigen (CD44) is cell surface protein that can
bind to various ligands, including extracellular matrix components,
growth factors and cytokines, and regulates cell signaling
(14). CD44 is essential for
maintaining cartilage homeostasis (15). A previous study demonstrated that
hyaluronic acid promotes the differentiation of adipose-derived
mesenchymal stem cells into chondrocytes by promoting CD44
clustering (16). In addition,
chondrocytes with high expression of CD44 exhibited stronger
chondrogenic capacity (17).
However, the specific role of CD44 in the differentiation of MSCs,
including hAMSCs, into chondrocytes remains to be elucidated.
The mitogen-activated protein kinase (MAPK)
signaling pathway serves an important role in cell proliferation,
differentiation, migration, senescence and apoptosis (18). A previous study reported that the
MAPK signaling pathway, including ERK1/2, JNK and p38 MAPK
molecules, is activated in chondrocytes (19). Activation of ERK signaling pathway
can enhance the ability of MSCs to differentiate into osteoblasts
and cardiomyocytes (20,21). In addition, the ERK1/2 signaling
pathway regulates chondrocyte differentiation and cartilage
formation in in vitro (22). However, the inhibition of ERK
signaling activation delays the maturation of hypertrophic
chondrocytes and leads to a slower rate of fracture healing
(23,24).
In the present study, it was hypothesized that
hAMSCs would differentiate into chondrocytes by enhancing CD44
expression. Therefore, the effect of CD44 on chondrogenic
differentiation of hAMSCs was evaluated. In addition, ERK
phosphorylation and Sox-9 levels in hAMSCs chondrogenesis were
examined.
Materials and methods
Cell culture
The study and use of the human amniotic membrane
were approved by the Ethics Committee of Affiliated Hospital of
Zunyi Medical University. A total of 20 pregnant women were
recruited for the study and these healthy pregnant women met the
following eligibility criteria: Between 18 and 35 years of age; and
without hepatitis B or C, or HIV infection. The sample was
collected after obtaining written informed consent from the women
and the samples were collected between May 2018 and March 2019. The
primary hAMSCs were isolated from term placental amnion of healthy
pregnant women according to the method previously described by
Zhang et al (25) and
hAMSCs were cultured at 37°C in low glucose-Dulbecco's modified
Eagle's medium (LG-DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 1% non-essential amino acids (NEAA; Gibco;
Thermo Fisher Scientific, Inc.) and 10 ng/ml basic fibroblast
growth factor (PeproTech, Inc.), and 1% L-GlutaMAX. Culture medium
was replaced by fresh medium after every 3 days. When the cells
reached 80% confluency, the harvested cells were then passaged.
Cells at passage 2 (P2) were used for the present study.
In vitro chondrocyte differentiation
of hAMSCs
The P2 hAMSCs at the logarithmic growth phase were
inoculated into 6-well plates at a dose of 2×105
cells/well. The cells were divided into 2 experimental groups; the
negative control group (NC) and the positive drug group (PG). The
NC was cultured in complete high-glucose (HG)-DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 1% GlutaMAX, 1%
NEAA and 55 µmol/l β-mercaptoethanol. The PG was cultured in
chondrogenic induction medium, which consisted of complete HG-DMEM,
10 ng/l transforming growth factor (TGF)β-3 (PeproTech, Inc.),
1×10−7 mol/l dexamethasone (Sigma-Aldrich; Merck KGaA)
and 50 mg/l vitamin C (Beijing Solarbio Science & Technology
Co., Ltd.). The medium was changed after every 3 days and
chondrocyte-associated markers, including type II collagen and
aggrecan, were detected on day 7 following induction.
Immunocytochemistry staining
Immunocytochemistry staining was used to detect the
expression of vimentin (MSCs surface marker protein) and type II
collagen (chondrocytes marker) in hAMSCs. When the P2 hAMSCs
reached 80% confluency, the cells were digested with trypsin and
washed 3 times with Dulbecco's PBS (D-PBS) for 5 min each time and
then treated with 0.3% Triton X-100 for 15 min at room temperature
to increase cell membrane permeability, and then washed 3 times
with D-PBS. The harvested cells were incubated with 5% BSA (Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature to
block non-specific antigen interaction. Following blocking for 30
min, primary antibodies, including anti-vimentin (cat. no.
GM072504; Gene Tech Co., Ltd.), anti-cytokeratin 19 (cat. no.
GM088804; Gene Tech Co., Ltd.) and anti-collagen type II (cat. no.
ab34712; Abcam) were added to the cells. Then, the cells were
incubated at 4°C overnight and washed 3 times with D-PBS for 5 min
each time. The negative control was treated with the same amount of
D-PBS but no primary antibody. Finally, the labeled cells were
treated with the secondary antibody (1:500; cat. no. SA00001-1;
ProteinTech Group, Inc.) and incubated at 37°C for 30 min.
Following washing 3 times with D-PBS, the color reaction was
developed using diaminobenzidine (at room temperature for 30 sec)
under a light microscope (magnification, ×100) and the reaction was
stopped using distilled water. Nuclei were counterstained with
hematoxylin for 1 min at room temperature.
Flow cytometry analysis
For the phenotypic characterization of hAMSCs, the
third passage hAMSCs at the logarithmic growth phase were harvested
and labeled with different antibodies for hMSC-specific markers
[5′-nucleotidase (CD73), thy-1 membrane glycoprotein (CD90) and
endoglin (CD105)] using BD stemflow Human MSC analysis kit (cat.
no. 562245; BD Biosciences) for flow cytometry analysis (26). In brief, the 3rd-passage hAMSCs
were collected following trypsin digestion, washed twice with D-PBS
containing 0.1% BSA, resuspended in D-PBS containing 0.1% BSA,
adjusted to a density of 1×106 cells/ml and then
incubated with the corresponding antibody for 1 h in the dark.
Following washing again with D-PBS containing 0.1% BSA, the cell
suspension was centrifuged at 100 × g at room temperature for 5 min
and the supernatant was discarded. Finally, the labeled cells were
analyzed by flow cytometry using Cell Quest software version 5.1
after fixation at 4°C overnight with 1% paraformaldehyde (PFA).
Toluidine blue staining
Toluidine blue staining was used to analyze the
secretion of aggrecan during the chondrogenic differentiation of
hAMSCs. Briefly, the cells were washed 3 times with D-PBS for 5 min
each time on day 7 after chondrogenic induction and then were fixed
with 4% PFA for 20 min at room temperature. After washing 3 times
for 5 min each time with D-PBS, 0.1% toluidine blue dye solution
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
the cells. The cells were stained at room temperature for 30 min,
washed with D-PBS again and observed under an inverted microscope
(magnification, ×100).
Inhibition of CD44
The P2 hAMSCs in the logarithmic growth phase were
inoculated into 6-well plates at a dose of 2×105
cells/well. The cells were divided into 4 experimental groups: The
NC group; the positive drug group (PG); anti-CD44 antibody (cat.
no. A3D8; GeneTex, Inc.) inhibition group NC + A3D8; and PG + A3D8.
After 24 h of inoculation, the anti-CD44 antibody (2 µg/ml) was
used to inhibit the expression of CD44 molecules in hAMSCs in the
incubator at 37°C for 3 or 7 days. The medium was replaced with
fresh medium after every 3 days. The expression of CD44 and
chondrocyte-associated markers was detected on day 7.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The P2 hAMSCs at the logarithmic growth phase were
seeded into 6-well plates at 2×105 cells/well. On day 7
of chondrogenic differentiation of hAMSCs, hAMSCs were collected
from the 6-well plate in each experimental group. RNAiso Plus
(Takara Biotechnology Co., Ltd.) was used to extract total RNA from
the cells, following the manufacturer's instructions. Total RNA (1
µg per 20 µl reaction volume) was reverse transcribed into cDNA
using PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocols. qPCR was performed and
monitored using SYBR Premix Ex Taq II (Takara Biotechnology Co.,
Ltd.) and a quantitative real-time PCR system (Bio-Rad Laboratories
Inc.). The cDNA samples (3 µl samples in a total volume of 15 µl
per reaction) were analyzed for the genes of interest, including
CD44, collagen type II α 1 chain (Col2a1), SRY-box
transcription factor 9 (Sox9), aggrecan (Acan),
ERK1, ERK2 and β-actin. The relative mRNA
transcriptional level of each target gene was calculated from the
threshold cycle (Ct) value of each PCR product and normalized to
the expression of β-actin using the comparative
2−ΔΔCq method (27).
Thermocycling conditions were as follows: i) Initial denaturation
at 95°C for 30 sec; ii) Denaturation at 95°C for 30 sec; iii)
Annealing at 60°C for 30 sec; iv) Elongation at 60°C for 30 sec; v)
Final extension for 39 cycles. Each experiment was repeated at
least 3 times. The primer sequences used are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) | Length of product
(bp) |
|---|
| Col2a1 | F:
CAACACTGCCAACGTCCAGAT | 121 |
|
| R:
TCTTGCAGTGGGCTGCCTTAT |
|
| Sox9 | F:
GCGGAGGAAGTCCGTGAAGA | 82 |
|
| R:
GAAGATGGCGTTGGGGGAGA |
|
| Acan | F:
GTGCCTATCAGGACAAGGTCT | 167 |
|
| R:
GATGCCTTTCACGACGACTTC |
|
| CD44 | F:
CTGCCGCTTTGCAGGTGTA | 109 |
|
| R:
CATTGTGGGCAAGGTGCTATT |
|
| ERK1 | F:
CTACACGCAGTTGCAGTACAT | 157 |
|
| R:
CAGCAGGATCTGGATCTCCC |
|
| ERK2 | F:
TCTGGAGCAGTATTACGACCC | 134 |
|
| R:
CTGGCTGGAATCTAGCAGTCT |
|
Western blot analysis
On day 7 of chondrogenic differentiation of hAMSCs,
total proteins were extracted from the cells using RIPA lysis
buffer (cat. no. R0020; Beijing Solarbio Science & Technology
Co., Ltd.) and protein concentration was determined by the BCA
method. Then, total proteins (10 µl/lane) were separated by
SDS-PAGE gel (10%) and transferred to the PVDF membranes, which
were blocked with 5% BSA for 1 h at room temperature. Following
this, PVDF membranes were incubated with overnight at 4°C with
primary antibodies, including ERK1+ERK2 antibody (1:1,000; cat. no.
ab17942; Abcam), phosphorylated (p)-ERK1/2 antibody (1:1,000; cat.
no. 9101S; Cell Signaling Technology, Inc.), Smad2/3 antibody
(1:1,000; cat. no. 3102S; Cell Signaling Technology, Inc.) and
p-Smad2/3 antibody (1:1,000; cat. no. 8828S; Cell Signaling
Technology, Inc.). Then, membranes were incubated with horseradish
peroxidase conjugated-secondary antibody (1:5,000; cat. no.
SA00001-2; ProteinTech Group, Inc.) for 2 h at room temperature.
Absin ECL hypersensitive luminescent solution (cat. no. abs920;
Absin) was used for exposure. The gray value of the strips was
analyzed by ImageJ version 1.8.0 (National Institutes of Health)
and the relative expression of the proteins was calculated. Protein
phosphorylation was calculated as the ratio of phosphorylated to
total protein expression. These data was normalized to a basal
value of 1.0.
Statistical analysis
The data were expressed as the means ± standard
errors of the mean. Statistical significance was evaluated by
unpaired t-tests in GraphPad Prism 8.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphological characteristics and
phenotypic identification of hAMSCs
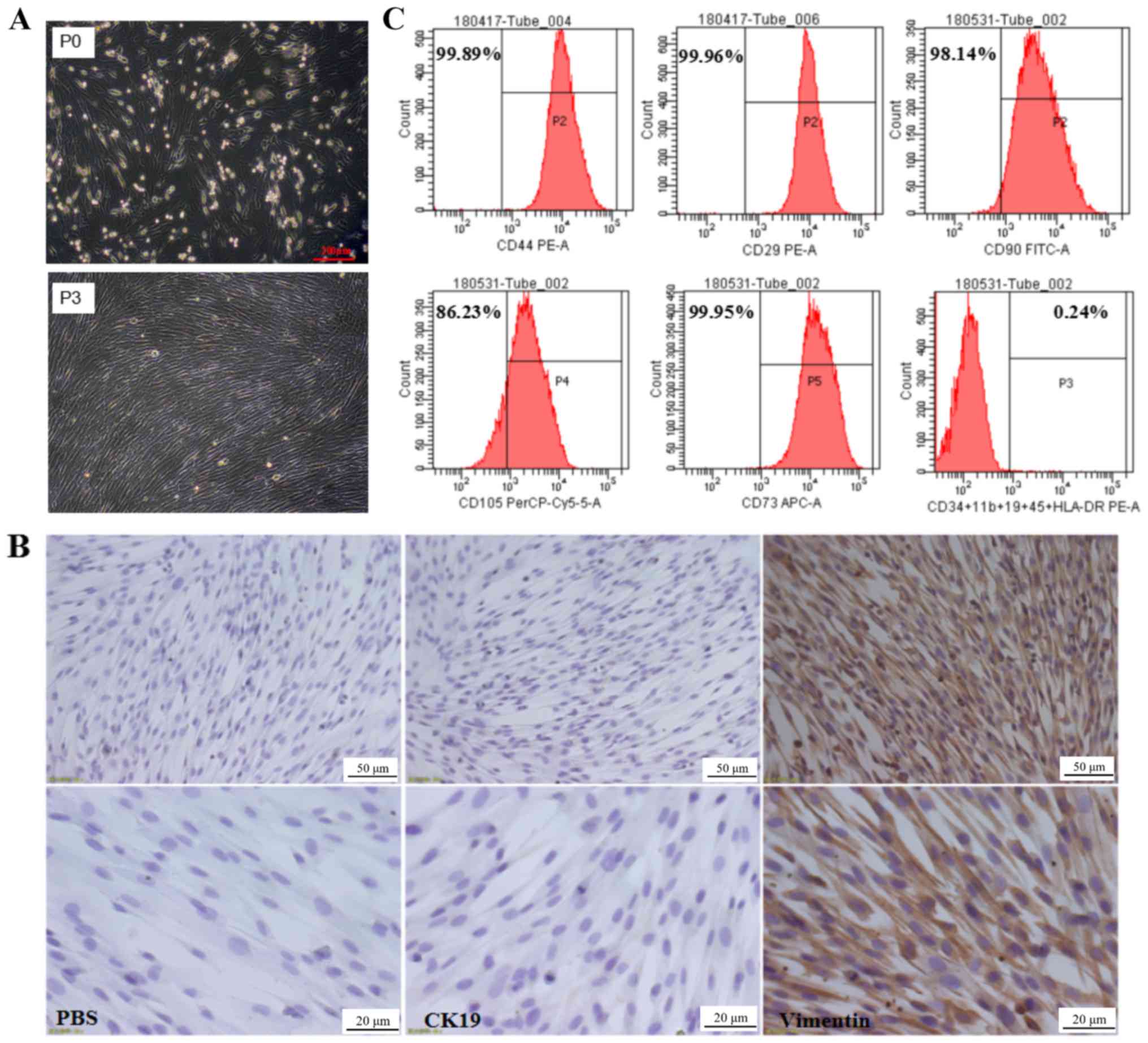
The primary culture of hAMSCs isolated was observed
after 24 h of incubation, revealing that hAMSCs were adherent cells
with irregular cell morphology, as shown in Fig. 1A. Following 3 subcultures, the
third generation of hAMSCs was uniform in morphology with fibrous,
spindle-shaped cells with spiral-like growth (Fig. 1A). Immunocytochemistry results
indicated that hAMSCs highly expressed the MSC marker vimentin, but
not the epithelial cell marker keratin, type 1 cytoskeletal 19
(CK19; Fig. 1B). To further verify
the biological characteristics of hAMSCs, the surface molecular
markers of MSCs were examined by flow cytometry. The results
indicated that hAMSCs highly expressed surface molecular markers of
MSCs, including CD90 (98.14%), CD73 (99.95%), CD105 (86.23%), CD44
(99.89%) and integrin β-1 (99.96%); however, they did not express
cell surface molecules of hematopoietic stem cells, such as
hematopoietic progenitor cell antigen CD34, receptor-type
tyrosine-protein phosphatase C, integrin α-M, B-lymphocyte antigen
CD19 and HLA class II histocompatibility antigen γ chain (Fig. 1C). This was consistent with the MSC
accreditation standards recommended by the International Society
for Cellular Therapy (28) and
thus the isolated hAMSCs exhibited typical characteristics of
MSCs.
Suppression of CD44 inhibits
differentiation of hAMSCs into chondrocytes
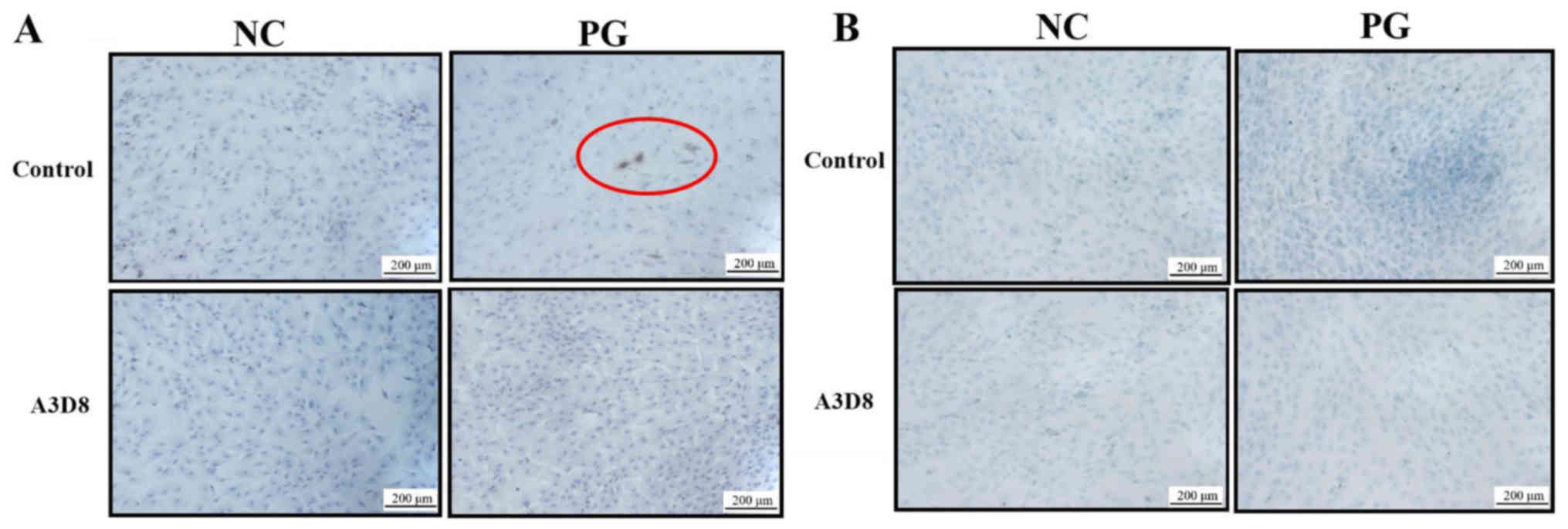
To validate the role of CD44 in the differentiation
of hAMSCs into chondrocytes, the extracellular matrices of
chondrocyte, including type II collagen and aggrecan, were examined
by immunocytochemistry staining in the presence or absence of A3D8,
a CD44 inhibitor. On day 7 of chondrogenic differentiation of
hAMSCs, type II collagen was observed in the PG; however, following
inhibition with A3D8, no such differentiation was observed in the
PG (Fig. 2A). This indicated that
the inhibition of CD44 could inhibit the formation of type II
collagen. Concomitantly, the production of aggrecan showed similar
results. The inhibition of CD44 significantly decreased the
production of aggrecan in the PG (Fig.
2B). These results indicated that suppression of CD44 could
inhibit the differentiation of hAMSCs into chondrocytes.
Inhibition of CD44 decreases the
expression of chondrocyte-associated genes
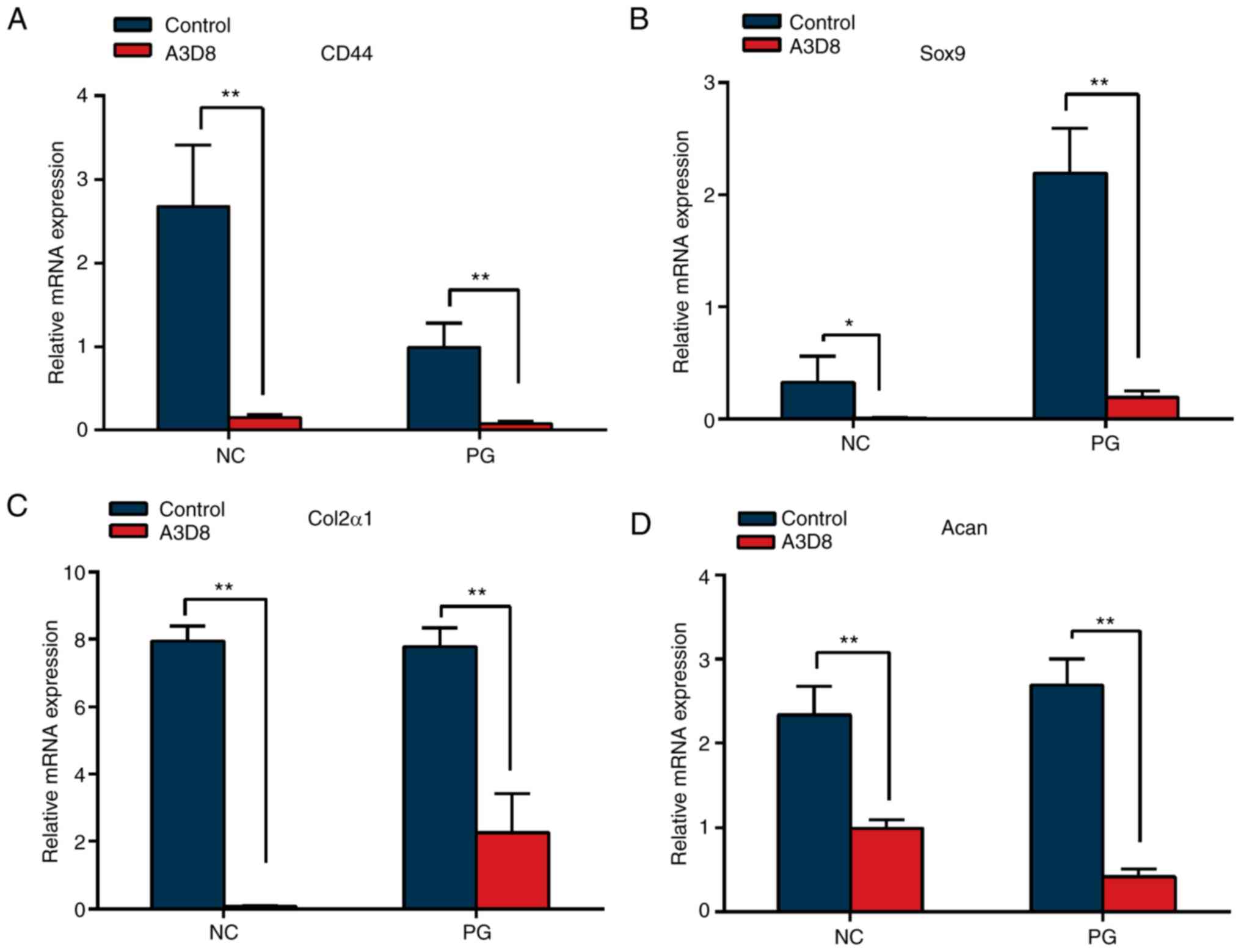
To further verify the effect of CD44 on chondrocyte
differentiation of hAMSCs, the transcriptional levels of
chondrocyte-associated genes, Sox9, Col2α1 and Acan,
were detected by RT-qPCR following suppression of CD44. As shown in
Fig. 3A, 2 µg/ml anti-CD44
antibody, A3D8, could effectively decrease the transcriptional
level of CD44 in hAMSCs. In addition, the transcriptional levels of
Sox9, Col2α1 and Acan were significantly decreased in
the presence of A3D8 (Fig. 3B-D).
These data indicated that suppression of CD44 significantly
attenuated the chondrocyte differentiation potential of hAMSCs.
Suppression of CD44 inhibits ERK1/2
pathway
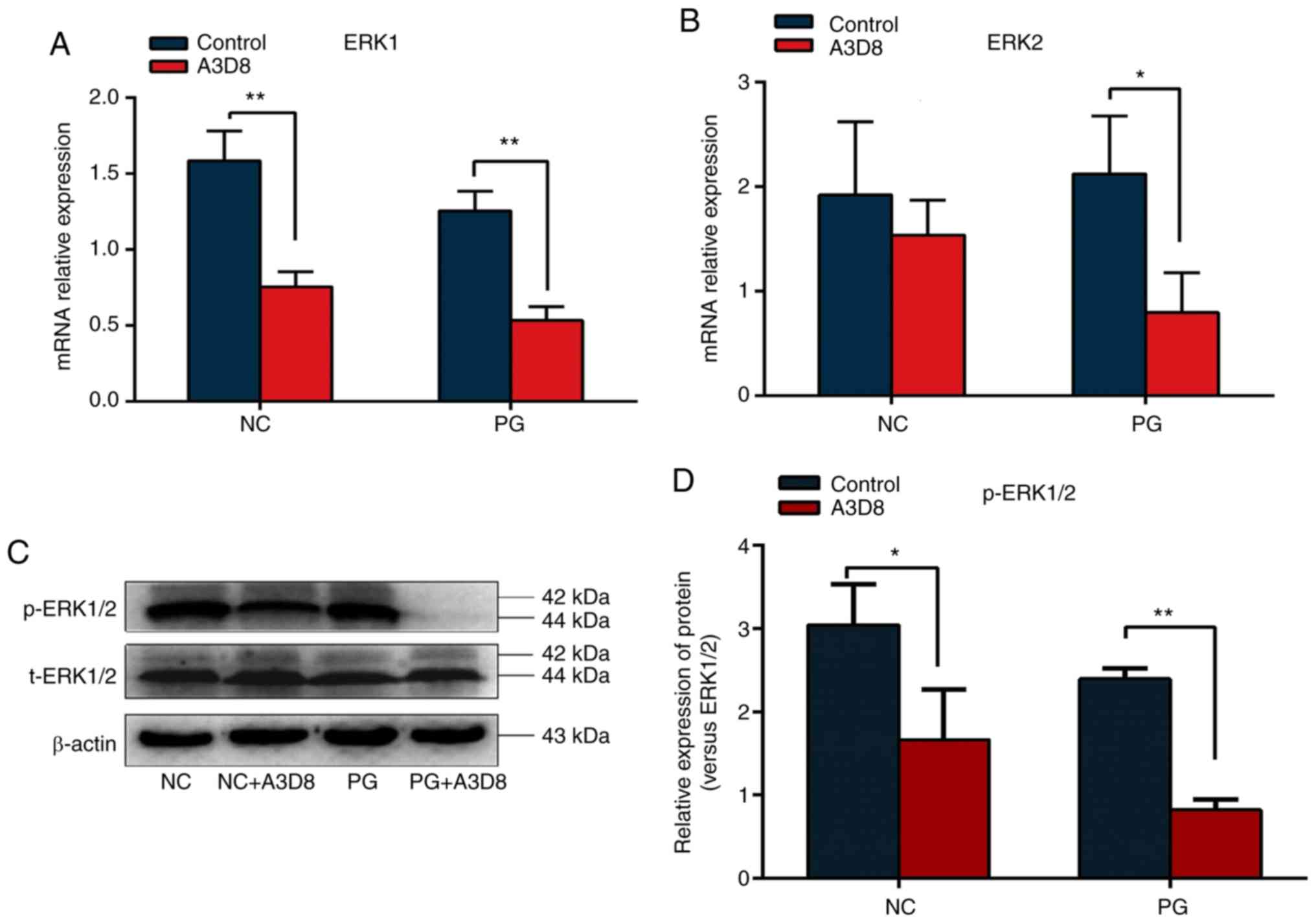
To investigate whether CD44 could regulate
chondrocyte differentiation potential of hAMSCs by ERK signaling
pathway, the relative expression levels of ERK1 and
ERK2 were examined in the presence or absence of A3D8 during
the differentiation of hAMSCs into chondrocytes. Following the
addition of A3D8, the transcriptional levels of ERK1 and
ERK2 were significantly decreased (Fig. 4A and B). To further verify that
CD44 regulated the chondrogenic differentiation of hAMSCs by the
ERK1/2 signaling pathway, the expression of p-ERK1/2 protein
following treatment with A3D8 was examined. As shown in Fig. 4C, no significant change in the
expression of total ERK1/2 protein compared with the normal group
was observed. However, the ratio of p-ERK1/2 to total ERK1/2
protein was significantly decreased following inhibition of CD44
(Fig. 4C and D). These results
indicated CD44 may regulate the chondrocyte differentiation
potential of hAMSCs via the ERK1/2 signaling pathway.
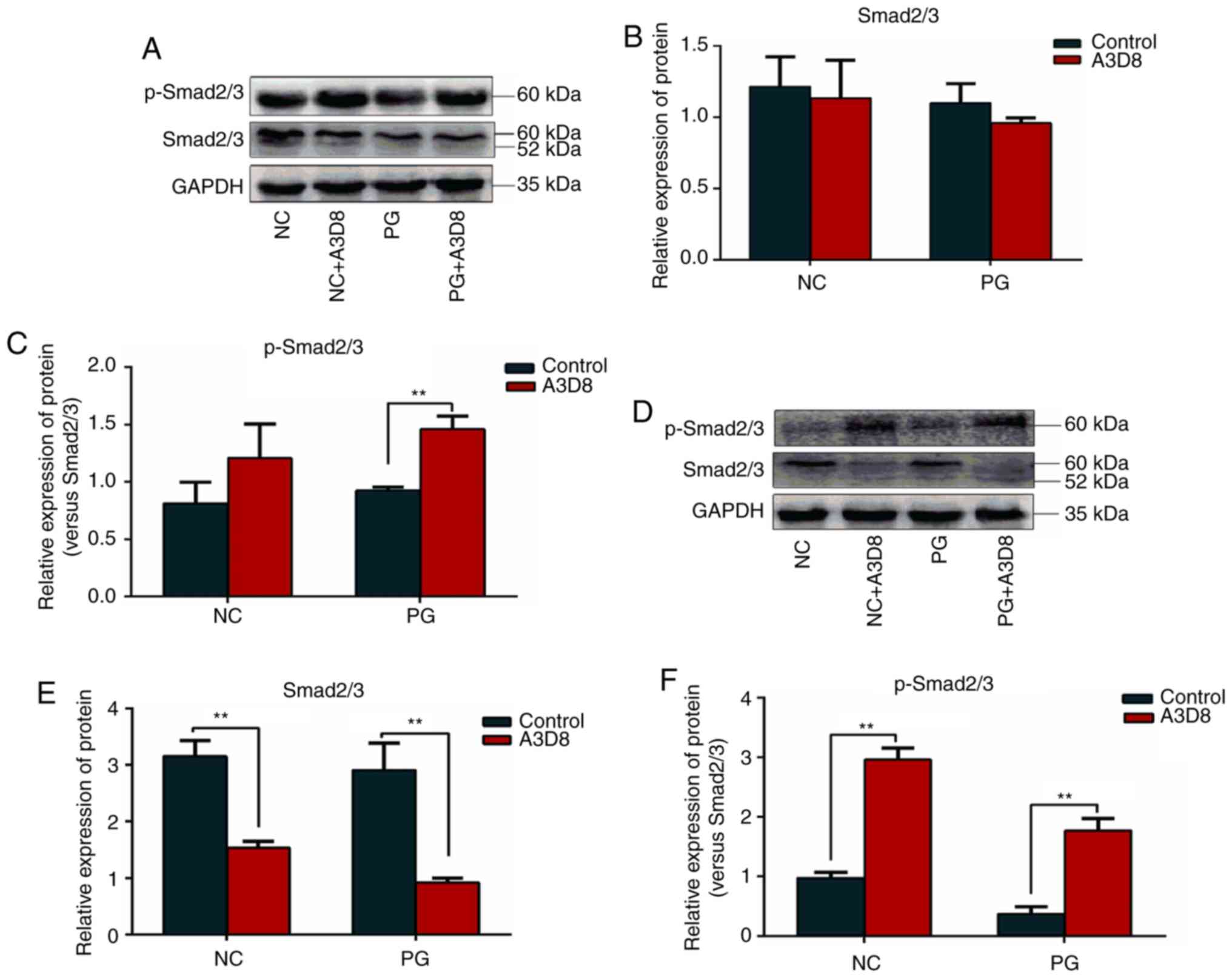
Suppression of CD44-induced Smad2/3
signaling activation
The TGF-β/Smad signaling pathway serves an important
role in chondrogenesis and there was cross-talk between Smad2/3 and
ERK signaling in the regulation of MSC chondrogenesis (29). Therefore, the expression levels of
Smad2/3 and p-Smad2/3 proteins were detected in the presence or
absence of A3D8 by western blot analysis. As shown in Fig. 5, p-Smad2/3 expression was increased
on day 1 and 3 when CD44 expression was inhibited by A3D8 (Fig. 5A and D) and the ratio of p-Smad2/3
to total Smad2/3 was also significantly upregulated following CD44
inhibition (Fig. 5C and F),
particularly on day 3. However, the expression of Smad2/3 was
decreased on day 3 following CD44 blocking (Fig. 5E). This indicated that CD44
regulated the phosphorylation of Smad2/3 directly, not via
regulation of Smad2/3 expression. In addition, the expression of
p-Smad2/3 on day 3 was significantly decreased compared with on day
1 in the PG group (Fig. 5A and D).
Therefore, the effect of CD44 on the differentiation of hAMSCs into
chondrocytes not only regulated the ERK1/2 signal but also the
Smad2/3 signal.
Discussion
CD44 is a principal receptor for hyaluronic acid, a
major extracellular matrix component, and hyaluronic acid exhibits
great potential for regulating the proliferation and
differentiation of stem cells (30). Of note, articular chondrocytes also
express the receptor CD44 and disruption of CD44 has profound
effect on cartilage metabolism (31). Although CD44 serves a critical role
in maintaining cartilage homeostasis, it remains unclear whether
CD44 can regulate the chondrogenic differentiation of stem cells.
The present study first demonstrated that CD44 served an important
role during the differentiation of hAMSCs into chondrocytes.
Suppression of CD44 could decrease the expression of
cartilage-associated genes and inhibit the production of type II
collagen and aggrecan, which are two important markers of
chondrocytes (32). Furthermore,
this process was closely associated with the ERK1/2 signaling
pathway. These results suggested that CD44/ERK may be a key
molecular hub for promoting the differentiation of hAMSCs into
chondrocytes.
MSCs have been widely used as an alternative seed
cell source for cartilage repair due to their chondrogenic
differentiation potential in benign conditions. The chondrogenic
differentiation of MSCs is primarily dependent on chondrocyte
marker genes, such as Sox9, Col2a1 and Acan. In the
present study, hAMSCs (NC group) exhibited high expression of
Col2α1 and Acan and low expression for Sox9.
Other MSCs derived from human adult bone marrow (33) or adipose (34) tissue did not express these
chondrocyte marker genes prior to the induction of chondrogenic
differentiation. In the present study, although hAMSCs in the NC
group did not exhibit formation of type II collagen and aggrecan in
the immunocytochemistry assay, other data were sufficient to
suggest that hAMSCs exhibited stronger chondrogenic potential
compared with other MSCs. In addition, Sox9 was
significantly elevated at transcriptional level in hAMSCs following
the induction of chondrogenic differentiation. However, suppression
of CD44 resulted in a significant decrease in expression level of
Sox9 in PG. As previously described, Sox9 expression
is associated with chondrocyte differentiation (35) and transfection of Sox9
enhances the expression of Col2α1 and Acan (36); Sox9 is an essential
transcription factor for maintaining the cartilage phenotype and
chondrogenesis (37).
CD44 is a transmembrane protein of cell adhesion
molecules and is a well-known surface marker of human MSCs. The
expression of CD44 at the transcriptional level was decreased
significantly during the chondrogenic differentiation in the
present study, and this was consistent with a previous study
(32). However, CD44 is not a
specific negative marker in chondrocytes and has been demonstrated
to be highly expressed in human articular chondrocytes (15). Therefore, it is questionable
whether CD44 could be used as a specific marker for human MSCs or
chondrocytes. As aforementioned, CD44 is a receptor for hyaluronic
acid and also serves an important role in cell differentiation. The
signaling cascade triggered by the interaction between hyaluronic
acid and CD44 significantly contributes to hyaluronic acid-induced
chondrogenesis in human adipose-derived MSCs, while the suppression
of hyaluronic acid interaction with CD44 decreases the chondrogenic
differentiation of human adipose-derived MSCs (33). Another study demonstrated that
dimerization of CD44 is responsive to hyaluronic acid and
contributes to chondrogenic differentiation of human
adipose-derived MSCs (16).
Furthermore, the interaction between hyaluronic acid with CD44 in
chondrocytes is also crucial to maintain cartilage (17). In the present study, suppression of
CD44 significantly attenuated the production of extracellular
matrix of chondrocytes including type II collagen and aggrecan
during the differentiation of hAMSCs into chondrocytes and also led
to a significant decrease in expression of chondrocyte-associated
genes such as Sox9, Col2α1 and Acan. However, these
genes were also inhibited in the NC group following treatment with
A3D8. Thus, it was hypothesized that CD44 may be expressed in both
hAMSCs and chondrocytes and be altered due to the change in
cellular microenvironment, such as the addition of an inducer; the
underlying mechanism requires further investigation.
The differentiation of stem cells into chondrocytes
involves multiple signaling pathways, such as TGF-β/BMP,
Wnt/β-catenin and MAPKs (38–40).
Certain TGF-β superfamily members have been widely used to regulate
cell differentiation by triggering a series of signaling cascades
(41). A previous study showed
that the 3-dimensional alginate culture of human MSCs in defined
induction medium, without TGF-β3, is not sufficient to fully
develop hyaline cartilage-like constructs with lacunae formation
(32), indicating that TGF-β3 is a
key component for the chondro-induction differentiation. Thus,
TGF-β3 was used to induce chondrogenic differentiation in hAMSCs in
the present study.
CD44 is a downstream target gene of the
Wnt/β-catenin signaling pathway, which has been previously reported
to be an important signaling pathway, implicated in the
chondro-induction differentiation (42,43).
MAPK family transduction involves a multistep kinase cascade; ERK
is a major kinase among MAPKs and has been shown to serve a
critical role in mediating chondrogenesis and associated gene
expression (29). Therefore, only
the ERKs of MAPKs signaling pathway were examined in the present
study. For example, during the differentiation of bone marrow
mesenchymal stem cells into chondrocytes, the activation of ERK1/2
negatively regulates the differentiation of chondrocytes (44). In the present study, the
transcription levels of ERK1 and ERK2 genes were downregulated in
hAMSCs following CD44 inhibition and the level of ERK1/2
phosphorylation were also significantly decreased. However, the
total ERK1/2 protein did not change with or without CD44 inhibitor
treatment. Therefore, inhibition of CD44 attenuated the activity of
ERK and ERK served a positive role during the chondro-induction
differentiation of hAMSCs. As described previously, the exact role
of ERK in chondrogenesis remains to be elucidated. For example,
Bobick and Kulyk (44)
reported that ERK signaling serves a negative role in
cartilage-specific gene expression in embryonic limb mesenchyme,
while a recent study indicated that ERK acts as a positive
regulator of hyaluronic acid-induced chondrogenesis in
adipose-derived stem cells (16).
Smad2/3 and ERK1/2 are the downstream molecules of
TGF-β and the major signaling mediator for modulating
chondrogenesis (45). In addition,
they show a cross-talk between Smad2/3 and ERK1/2 signaling during
the regulation of the chondrogenic differentiation of MSC (28). The results of the present study
indicated that p-Smad2/3 expression was increased on days 1 and 3
following the suppression of CD44 and the ratio of p-Smad2/3 to
total Smad2/3 was also significantly upregulated. Notably, it was
identified that the expression of total Smad2/3 was not equal in
each group on day 3 and was different from the change in p-Samd2/3.
It was considered that CD44 inhibition may suppress the expression
of Smad2/3, but it also promoted the phosphorylation of Smad2/3
through other pathways. A previous study reported that suppression
of CD44 inhibited the expression of DUSP10/MKP5, a negative
regulator of p38 MAPK and JNK pathways (46). Thus, CD44 inhibition could directly
activate p38 MAPK and JNK to enhance the phosphorylation of
protein, without being dependent on the regulation of total protein
expression. The results of the present study confirmed our
hypothesis. However, the association between ERK1/2 signaling and
Smad2/3 signaling remains unclear. A previous study has reported
that during the proliferation of smooth muscle cells,
phosphorylation of ERK can be decreased following the use of Smad3
inhibitors (47). Conversely,
Hough et al (48)
identified that phosphorylation of ERK can regulate Smad signaling.
The mechanism through which ERK and Smad interact remains to be
elucidated.
In the present study, the expression of p-Smad2/3 on
day 3 was significantly decreased in the NC group compared with day
1 in PG. Similar results, that the expression of p-Smad2/3 was
gradually decreased in the early stage of chondrogenic
differentiation, have also been reported (49). In the current study, a limitation
was that only one time point was observed for 7 days and the whole
process of cartilage differentiation was not monitored dynamically,
so the role of CD44 during different stages was not fully
understood. In addition, the role of CD44 for cartilage formation
remains unknown in vivo. This is an interesting and complex
question and will be further investigated in the near future.
In summary, the present study suggested that CD44
was a functional regulator of the differentiation of hAMSCs into
chondrocytes by modulating the Smad2/3 and ERK1/2 signaling
pathways. The results provided a potential novel strategy to
enhance the capacity of MSCs to differentiate into
chondrocytes.
Acknowledgements
Not applicable.
Funding
The authors received financial support from National
Natural Science Foundation of China (grant no. 81660363), Science
and Technology Innovation Leading Academics of National High-level
Personnel of Special Support Program (grant no. GKFZ-2018-29),
Guizhou High-Level Innovative Talent Support Program (grant no.
QKH-RC-20154028) and Science and Technology Foundation of Guizhou
(grant no. QKH-2017-1422).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX wrote the manuscript. YX, YQW and ATW performed
the experiments. YL and RML analyzed the data. YJZ performed the
flow cytometry analysis. CYY and JHX designed the experiment,
interpreted the data and modified the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study and use of the human amniotic membrane
were approved [approval no. (2014) 2–085] by the Ethics Committee
of Affiliated Hospital of Zunyi Medical University (Zunyi, China).
The sample was collected after obtaining written informed consent
from the women.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunziker EB: Articular cartilage repair:
Basic science and clinical progress. A review of the current status
and prospects. Osteoarthritis Cartilage. 10:432–463. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caldwell KL and Wang J: Cell-based
articular cartilage repair: The link between development and
regeneration. Osteoarthritis Cartilage. 23:351–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuscik MJ, Hilton MJ, Zhang X, Chen D and
O'Keefe RJ: Regulation of chondrogenesis and chondrocyte
differentiation by stress. J Clin Invest. 118:429–438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barry FP and Murphy JM: Mesenchymal stem
cells: Clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson SM, Kalamegam G, Pushparaj PN,
Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL,
Hoyland JA and Mobasheri A: Mesenchymal stem cells in regenerative
medicine: Focus on articular cartilage and intervertebral disc
regeneration. Methods. 99:69–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fellows CR, Matta C, Zakany R, Khan IM and
Mobasheri A: Adipose, bone marrow and synovial joint-derived
mesenchymal stem cells for cartilage repair. Front Genet.
7:2132016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo M, Yamaoka K and Tanaka Y: Acquiring
chondrocyte phenotype from human mesenchymal stem cells under
inflammatory conditions. Int J Mol Sci. 15:21270–21285. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freitag J, Bates D, Boyd R, Shah K,
Barnard A, Huguenin L and Tenen A: Mesenchymal stem cell therapy in
the treatment of osteoarthritis: Reparative pathways, safety and
efficacy-a review. BMC Musculoskelet Disord. 17:2302016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ
and Blanco FJ: Isolation and characterization of mesenchymal stem
cells from human amniotic membrane. Tissue Eng Part C Methods.
17:49–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nogami M, Tsuno H, Koike C, Okabe M,
Yoshida T, Seki S, Matsui Y, Kimura T and Nikaido T: Isolation and
characterization of human amniotic mesenchymal stem cells and their
chondrogenic differentiation. Transplantation. 93:1221–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ranzoni AM, Corcelli M, Hau KL, Kerns JG,
Vanleene M, Shefelbine S, Jones GN, Moschidou D, Dala-Ali B,
Goodship AE, et al: Counteracting bone fragility with human
amniotic mesenchymal stem cells. Sci Rep. 6:396562016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muiños-López E, Hermida-Gómez T,
Fuentes-Boquete I, de Toro-Santos J, Blanco FJ and Díaz-Prado SM:
Human amniotic mesenchymal stromal cells as favourable source for
cartilage repair. Tissue Eng Part A. 23:901–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morath I, Hartmann TN and Orian-Rousseau
V: CD44: More than a mere stem cell marker. Int J Biochem Cell
Biol. 81:166–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knudson CB: Hyaluronan and CD44: Strategic
players for cell-matrix interactions during chondrogenesis and
matrix assembly. Birth Defects Res C Embryo Today. 69:174–196.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SC, Chen CH, Wang JY, Lin YS, Chang JK
and Ho ML: Hyaluronan size alters chondrogenesis of adipose-derived
stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater.
66:224–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grogan SP, Barbero A, Diaz-Romero J,
Cleton-Jansen AM, Soeder S, Whiteside R, Hogendoorn PC, Farhadi J,
Aigner T, Martin I and Mainil-Varlet P: Identification of markers
to characterize and sort human articular chondrocytes with enhanced
in vitro chondrogenic capacity. Arthritis Rheum. 56:586–595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stanton LA, Underhill TM and Beier F: MAP
kinases in chondrocyte differentiation. Dev Biol. 263:165–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Luo S, Zhang D, Qu X and Tan Y:
Sika pilose antler type I collagen promotes BMSC differentiation
via the ERK1/2 and p38-MAPK signal pathways. Pharm Biol.
55:2196–2204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Wang C, Lin J and Wang X:
Oxidized low-density lipoprotein (ox-LDL) promotes cardiac
differentiation of bone marrow mesenchymal stem cells via
activating ERK1/2 signaling. Cardiovasc Ther. 35:e123052017.
View Article : Google Scholar
|
|
22
|
Wang X, Xue Y, Ye W, Pang J, Liu Z, Cao Y,
Zheng Y and Ding D: The MEK-ERK1/2 signaling pathway regulates
hyaline cartilage formation and the redifferentiation of
dedifferentiated chondrocytes in vitro. Am J Transl Res.
10:3068–3085. 2018.PubMed/NCBI
|
|
23
|
Provot S, Nachtrab G, Paruch J, Chen AP,
Silva A and Kronenberg HM: A-raf and B-raf are dispensable for
normal endochondral bone development, and parathyroid
hormone-related peptide suppresses extracellular signal-regulated
kinase activation in hypertrophic chondrocytes. Mol Cell Biol.
28:344–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Hoss J, Kolind M, Jackson MT, Deo N,
Mikulec K, McDonald MM, Little CB, Little DG and Schindeler A:
Modulation of endochondral ossification by MEK inhibitors PD0325901
and AZD6244 (Selumetinib). Bone. 59:151–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LT, Liu RM, Luo Y, Zhao YJ, Chen DX,
Yu CY and Xiao JH: Hyaluronic acid promotes osteogenic
differentiation of human amniotic mesenchymal stem cells through
the TGF-β/Smad signaling pathway. Life Sci. 232:1166692019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magatti M, Pianta S, Silini A and Parolini
O: Isolation, culture, and phenotypic characterization of
mesenchymal stromal cells from the amniotic membrane of the human
term placenta. Methods Mol Biol. 1416:233–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Schmittgen,
Analysis of relative gene expression data using realtime
quantitative PCR and the 2(-Delta Delta C(T)) method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu RM, Sun RG, Zhang LT, Zhang QF, Chen
DX, Zhong JJ and Xiao JH: Hyaluronic acid enhances proliferation of
human amniotic mesenchymal stem cells through activation of
Wnt/β-catenin signaling pathway. Exp Cell Res. 345:218–229. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knudson W and Loeser RF: CD44 and integrin
matrix receptors participate in cartilage homeostasis. Cell Mol
Life Sci. 59:36–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schnabel M, Marlovits S, Eckhoff G,
Fichtel I, Gotzen L, Vécsei V and Schlegel J:
Dedifferentiation-associated changes in morphology and gene
expression in primary human articular chondrocytes in cell culture.
Osteoarthritis Cartilage. 10:62–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee HJ, Choi BH, Min BH and Park SR:
Changes in surface markers of human mesenchymal stem cells during
the chondrogenic differentiation and dedifferentiation processes in
vitro. Arthritis Rheum. 60:2325–2332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu SC, Chen CH, Chang JK, Fu YC, Wang CK,
Eswaramoorthy R, Lin YS, Wang YH, Lin SY, Wang GJ and Ho ML:
Hyaluronan initiates chondrogenesis mainly via CD44 in human
adipose-derived stem cells. J Appl Physiol (1985). 114:1610–1618.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tew SR, Li Y, Pothacharoen P, Tweats LM,
Hawkins RE and Hardingham TE: Retroviral transduction with SOX9
enhances re-expression of the chondrocyte phenotype in passaged
osteoarthritic human articular chondrocytes. Osteoarthritis
Cartilage. 13:80–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kolettas E, Muir HI, Barrett JC and
Hardingham TE: Chondrocyte phenotype and cell survival are
regulated by culture conditions and by specific cytokines through
the expression of Sox-9 transcription factor. Rheumatology
(Oxford). 40:1146–1156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bi W, Deng JM, Zhang Z, Behringer RR and
de Crombrugghe B: Sox9 is required for cartilage formation. Nat
Genet. 22:85–89. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gómez-Leduc T, Desancé M, Hervieu M,
Legendre F, Ollitrault D, de Vienne C, Herlicoviez M, Galéra P and
Demoor M: Hypoxia is a critical parameter for chondrogenic
differentiation of human umbilical cord blood mesenchymal stem
cells in type I/III collagen sponges. Int J Mol Sci. 18(pii):
E19332017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chowdhury TT, Salter DM, Bader DL and Lee
DA: Signal transduction pathways involving p38 MAPK, JNK, NFkappaB
and AP-1 influences the response of chondrocytes cultured in
agarose constructs to IL-1beta and dynamic compression. Inflamm
Res. 57:306–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu S, Zhang E, Yang M and Lu L:
Overexpression of Wnt11 promotes chondrogenic differentiation of
bone marrow-derived mesenchymal stem cells in synergism with TGF-β.
Mol Cell Biochem. 390:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moustakas A, Pardali K, Gaal A and Heldin
CH: Mechanisms of TGF-beta signaling in regulation of cell growth
and differentiation. Immunol Lett. 82:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deng Y, Lei G, Lin Z, Yang Y, Lin H and
Tuan RS: Engineering hyaline cartilage from mesenchymal stem cells
with low hypertrophy potential via modulation of culture conditions
and Wnt/β-catenin pathway. Biomaterials. 192:569–578. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan X, Liu H, Huang H, Liu H, Li L, Yang
J, Shi W, Liu W and Wu L: The key role of canonical wnt/β-catenin
signaling in cartilage chondrocytes. Curr Drug Targets. 17:475–484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bobick BE and Kulyk WM: The MEK-ERK
signaling pathway is a negative regulator of cartilage-specific
gene expression in embryonic limb mesenchyme. J Biol Chem.
279:4588–4595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY,
Lee CH, Tina Chen SY, Shieh YH, Williams DF and Deng WP:
Synergistic anabolic actions of hyaluronic acid and platelet-rich
plasma on cartilage regeneration in osteoarthritis therapy.
Biomaterials. 35:9599–9607. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Furuta J, Ariyoshi W, Okinaga T, Takeuchi
J, Mitsugi S, Tominaga K and Nishihara T: High molecular weight
hyaluronic acid regulates MMP13 expression in chondrocytes via
DUSP10/MKP5. J Orthop Res. 35:331–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi J, Chen M, Ouyang L, Huang L, Lin X,
Zhang W, Liang R, Lv Z, Liu S and Jiang S: Airway smooth muscle
cells from ovalbumin-sensitized mice show increased proliferative
response to TGFβ1 due to upregulation of Smad3 and TGFβRII. J
Asthma. 54:467–475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hough C, Radu M and Doré JJ: Tgf-beta
induced Erk phosphorylation of smad linker region regulates smad
signaling. PLoS One. 7:e425132012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dexheimer V, Gabler J, Bomans K, Sims T,
Omlor G and Richter W: Differential expression of TGF-β superfamily
members and role of Smad1/5/9-signalling in chondral versus
endochondral chondrocyte differentiation. Sci Rep. 6:366552016.
View Article : Google Scholar : PubMed/NCBI
|