Introduction
The small intestine of mammals is one of the most
sensitive organs to ionizing radiation (1). Leucine-rich repeat-containing G
protein-coupled receptor 5 (Lgr5)-positive stem cells present in
the crypts of the small intestine are crucial for radiation-induced
intestinal regeneration (2). The
death of Lgr5-positive stem cells due to high-dose radiation causes
the disruption of intestinal homeostasis. The loss of the
proliferative capacity of cells in the crypts of the small
intestinal results in the depletion of many cells in the intestinal
mucosa, leading to intestinal tract destruction. Exposure to
radiation doses of ≥10 Gy results in the development of classic
gastrointestinal acute radiation syndrome (GI-ARS) in humans,
usually within 5–10 days, if not appropriately treated (3). Other symptoms include the loss of
appetite, diarrhea, infections, the loss of fluid and electrolytes,
weight loss, malabsorption, and significant leukopenia (4,5). In
1999, three individuals involved in the Tokai-mura JCO accident
experienced severe acute radiation syndrome during; of these, two
died owing to severe GI-ARS (6–8). The
development of radioprotective agents that can mitigate the effects
of high-dose radiation exposure is necessary and has been
highlighted by many researchers. Verginadis et al, have
demonstrated the radioprotective action of curcumin on the
intestinal tract (9). Yamamoto
et al, have reported that treatment with ascorbic acid prior
to radiation exposure prevents fatal GI-ARS in mice (10). To develop effective radioprotective
agents, thoroughly investigating the functional changes in the
small intestine due to radiation exposure is essential.
MicroRNAs (miRNAs) are short single-stranded RNAs
comprising approximately 20 bases. They are involved in regulating
gene expression via the post-transcriptional regulation of
messenger RNA (mRNA) and the inhibition of protein translation
(11). Previously, we reported
altered gene expression in cells exposed to radiation (12,13).
Other studies have also reported changes in mRNA expression in the
small intestine following high-dose radiation exposure (14,15).
However, information on gene expression changes in miRNAs following
such exposure is limited.
In this study, we analyzed the effects of changes in
miRNA gene expression in the small intestine of mice following
whole body irradiation with 10 Gy of X-rays.
Materials and methods
Mice and X-ray irradiation
All experiments were performed in accordance with
The Guidelines for Animal Experimentation of the Hirosaki
University. The procedures were approved and monitored by The
Animal Research Committee of Hirosaki University (approval nos.
G12003 and G19005). Male C57BL/6NJcl mice were purchased from CLEA,
Japan. All mice were housed in a conventional animal room with 12-h
light/dark cycles; all mice were provided food and water ad
libitum. Eight-week-old mice were exposed to X-rays
(MBR-1520R-3 X-ray machine, Hitachi Medical Corporation) at a rate
of 1.0 Gy/min (150 kVp, 20 mA, 0.5 mm aluminum, and 0.3 mm copper
filters). Mice were observed for up to 30 days after irradiation.
Before dissection, mice were anesthetized with 2% isoflurane
(Pfizer). To minimize distress as a humane end point, we performed
cervical dislocation before death of the mice. Mice with severe
diarrhea were sacrificed.
Fluorescent TdT-mediated dUTP nick end
labeling (TUNEL) assay
The small intestine from mice at 72 h after
non-irradiation and 10 Gy irradiation of X-ray was directly excised
under anesthesia. For tissue analysis, the small intestine was
fixed with 4% paraformaldehyde solution in Dulbecco's
phosphate-buffered saline (−) [D-PBS (−), pH 7.2]. The fixed small
intestine was embedded in paraffin. Sections were cut at a
thickness of 4 µm and placed on glass slides. Paraffin-embedded
sections of small intestine isolated from mice exposed to 10 Gy of
X-rays on glass slides were deparaffinized with xylene and ethanol,
followed by washing with D-PBS (−). Cell death analysis was
performed using the DeadEnd™ Fluorometric TUNEL System
(Promega) according to the manufacturer's instructions. Nuclei were
stained using the ProLong Gold Antifade Reagent with
4′,6-diamidino-2-phenylindole (Thermo Fisher Scientific, Inc.). The
stained tissues were examined using a confocal laser scanning
microscope LSM710 (Carl Zeiss).
Total RNA extraction
The non-irradiated and irradiated mice were
sacrificed after 72 h for collection of small intestine. Total RNA
was extracted from the small intestine using the Isogen II reagent
(Nippon Gene) according to the manufacturer's instructions. RNA
content in the small intestine was assessed using a NanoDrop
spectrophotometer (NanoDrop Technologies). RNA samples had
260/280-nm absorbance ratios of 1.8–2.0. Total RNA quality was
confirmed using the Agilent 2100 Bioanalyzer and Agilent RNA 6000
Pico kit (both Agilent Technologies, Inc.) as per the
manufacturer's instructions.
Microarray analysis
Cyanine 3 (Cy3)-labeled miRNA was synthesized from
total RNA obtained from the small intestines of irradiated (10 Gy,
n=4) and non-irradiated (0 Gy, n=4) mice using the miRNA Complete
Labeling Reagent and Hyb kit (Agilent Technologies). SurePrint G3
mouse miRNA microarray slides (8×60 K, v.21.0) were hybridized with
the Cy3-labeled miRNA in a hybridization solution, which was
prepared using a Gene Expression Hybridization kit (Agilent
Technologies) as per the manufacturer's instructions. The images of
Cy3 fluorescence signals on slides were obtained using the SureScan
microarray scanner (Agilent Technologies) and were processed using
the Feature Extraction software (v.10.7) based on the
manufacturer's instructions. Expression data obtained were
processed using the GeneSpring GX14.5 software (Agilent
Technologies) to normalize all values to 90% percentile shift on
the respective microarrays, followed by the normalization of the
median expression level of all samples. miRNAs That were
upregulated and downregulated by more than 2.000-fold in irradiated
small intestine samples (10 Gy) compared with that in
non-irradiated small intestine samples (0 Gy) were analyzed
(P<0.05, unpaired t-test). The Benjamini Hochberg FDR was
used for multiple testing correction. Moreover, OmicsNet
(https://www.omicsnet.ca/faces/home.xhtml) was used to
predict target miRNA genes. To predict target genes of miRNAs and
their pathways, TargetScan Mouse (http://www.targetscan.org/mmu_72/) analysis and
WikiPathways (https://www.wikipathways.org/index.php/WikiPathways)
analysis were performed using the GeneSpring 14.5 software (Agilent
Technologies). Pathway data of Mus musculus were downloaded
from WikiPathways.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was used for synthesizing complementary
DNAs (cDNAs) for miRNA expressions using the TaqMan™ miRNA RT kit
and the prescribed 5×RT primer (both from Thermo Fisher Scientific,
Inc.). Real-time PCR for miRNA expression was performed using a
FastStart TaqMan probe master (Roche Diagnostics), 20× probe and
the StepOne Plus Real-Time PCR system (Thermo Fisher Scientific,
Inc.) under the following conditions: 10 min at 95°C, followed by
45 cycles at 95°C for 15 sec and 60°C for 60 sec. The comparative
Cq method was used to assess miRNA expression levels. U6 small
nuclear RNA was used as an internal control.
Western blotting
Western blotting was performed in the same protocol
as before (16). In this study,
the primary antibodies used were as follows: Anti-Forkhead box P1
(FoxP1) (D35D10) rabbit monocle antibody (no. 4402; Cell Signaling
Technology, Inc.) and anti-Gapdh (D16H11) rabbit monocle antibody
(no. 5174; Cell Signaling Technology, Inc.).
Statistical analysis
A Kaplan-Meier plot was used for assessing the
survival of irradiated and non-irradiated mice. Statistical
differences between irradiated and non-irradiated mice were
determined using the log-rank test. P<0.05 was considered to
indicate a statistically significant difference. The Student's
t-test was used to compare the results of the two groups.
Results
Exposure to 10 Gy X-rays in mice is
fatal
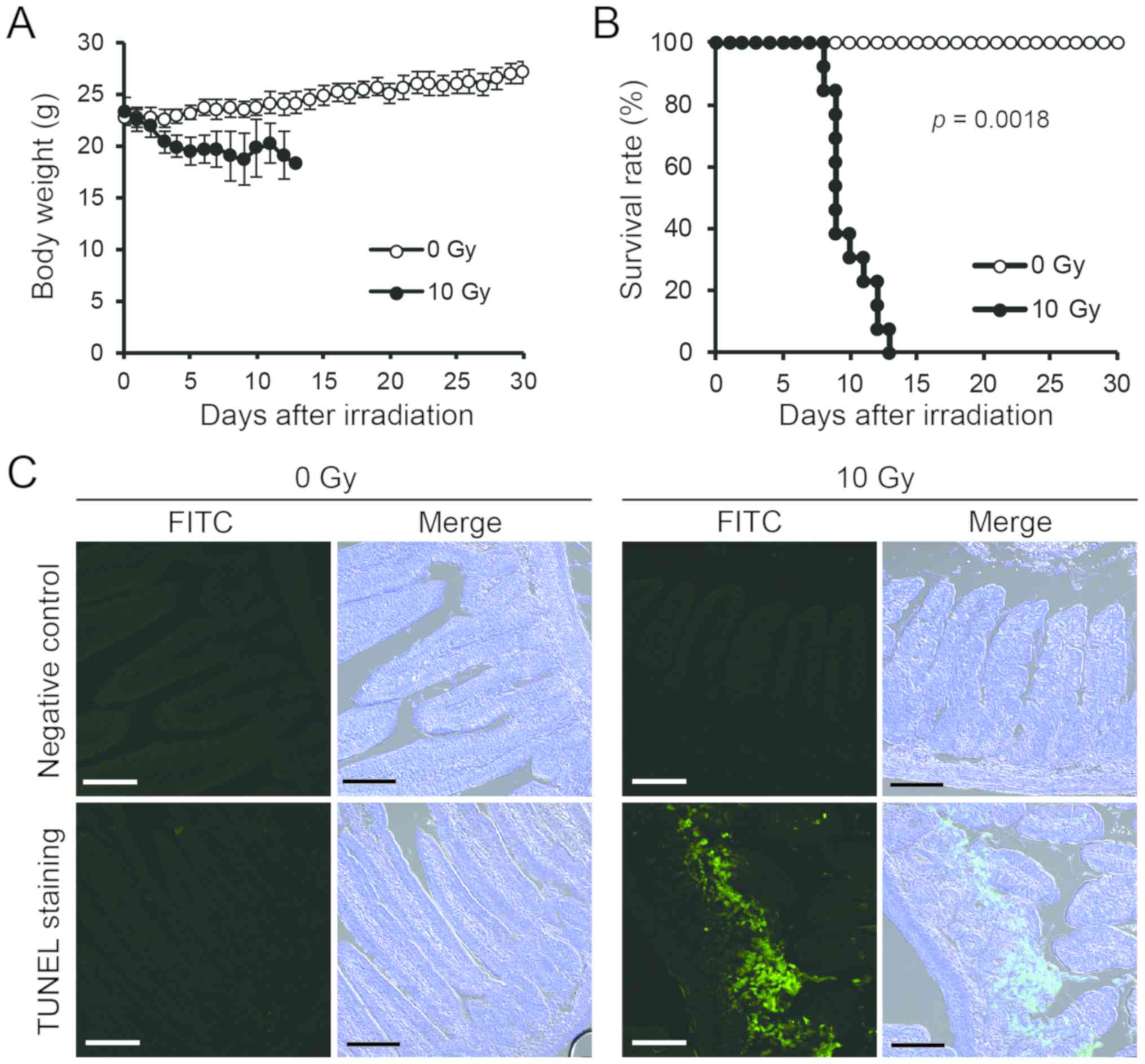
We examined changes in body weight and survival time
after exposure to 10 Gy X-rays. Weight loss was observed several
days after irradiation (Fig. 1A).
The 50% survival time was approximately 9 days following radiation
exposure (Fig. 1B), indicating
that exposure to 10 Gy X-rays in mice is fatal. The TUNEL assay
confirmed DNA damage in the intestinal epithelial cells following
radiation exposure. At 72 h after radiation exposure, the green
fluorescent TUNEL labeling increased, showing many positive images
of the small intestinal pit site, particularly rich in small
intestinal epithelial stem cells (Fig.
1C). These results demonstrate that cell death is induced in
the small intestine of mice exposed to 10-Gy X-rays.
Identification of upregulated and
downregulated miRNAs in the small intestine of mice following
radiation exposure (10 Gy X-rays)
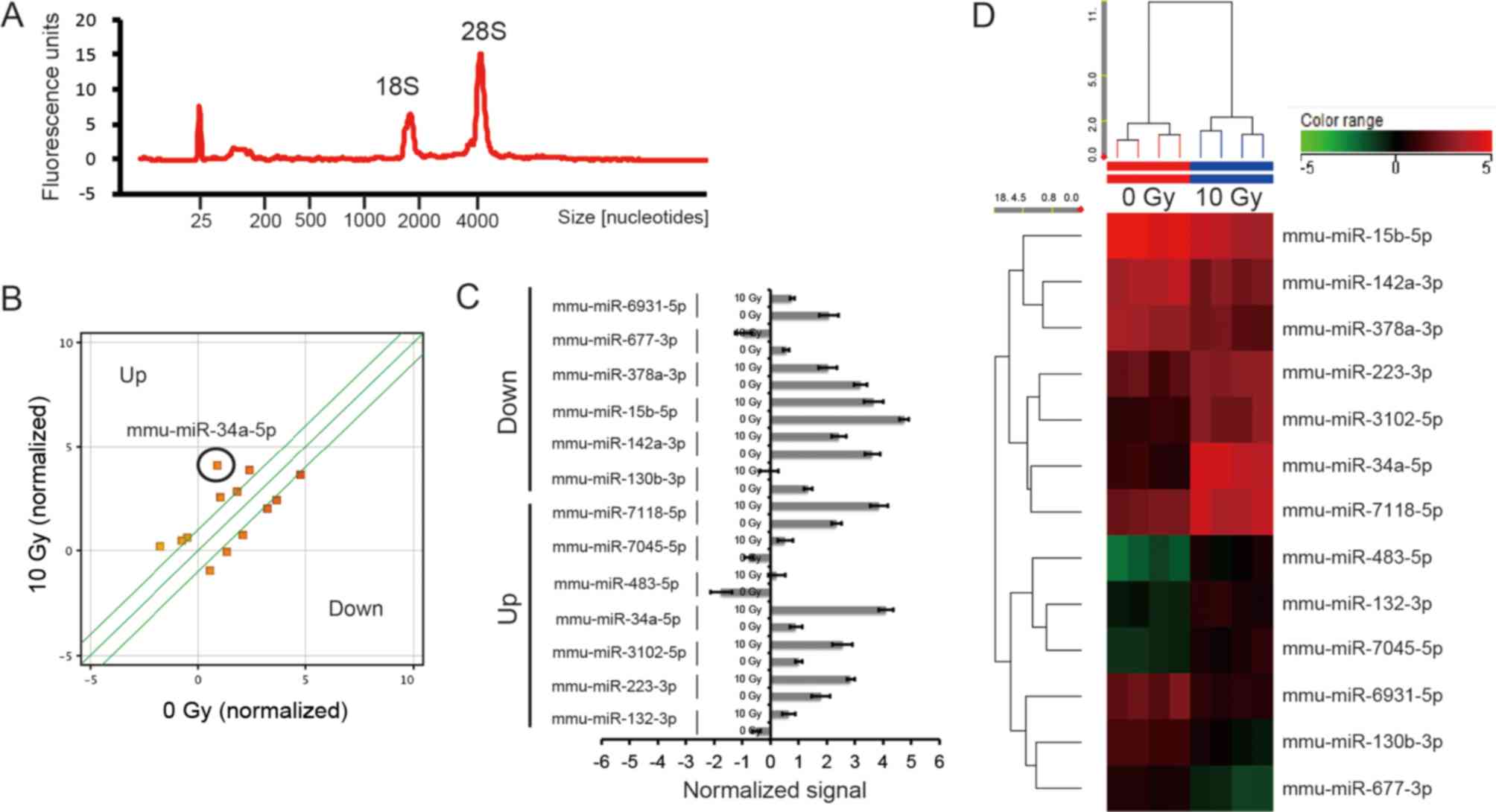
The small intestines of 8-week-old mice exposed to
10 Gy X-rays were extracted at 72 h after irradiation. Total RNA
appeared to have an RNA integrity number of >9.0 (Fig. 2A). Change in miRNA expressions in
the small intestine were investigated using microarray analysis. We
selected changes in miRNA expressions of over 2.000-fold
(P<0.05) and identified seven upregulated and six downregulated
miRNA, respectively, (Fig. 2 and
Table I).
 | Table I.Upregulated and downregulated miRNAs
(>2.000-fold) in mouse small intestine at 72 h after exposure to
10 Gy of X-ray irradiation. |
Table I.
Upregulated and downregulated miRNAs
(>2.000-fold) in mouse small intestine at 72 h after exposure to
10 Gy of X-ray irradiation.
|
Systematic_name | Mirbase accession
no. | Fold change (10 Gy
vs. 0 Gy) | P-value |
|---|
| mmu-miR-132-3p | MIMAT0000144 | 2.209±0.131 | 0.000332 |
| mmu-miR-223-3p | MIMAT0000665 | 2.081±0.536 | 0.001907 |
|
mmu-miR-3102-5p | MIMAT0014933 | 2.952±0.836 | 0.000360 |
| mmu-miR-34a-5p | MIMAT0000542 | 9.137±0.624 | 0.000004 |
| mmu-miR-483-5p | MIMAT0004782 | 3.911±1.901 | 0.000393 |
|
mmu-miR-7045-5p | MIMAT0027994 | 2.447±0.836 | 0.000407 |
|
mmu-miR-7118-5p | MIMAT0028133 | 2.826±0.642 | 0.000346 |
|
mmu-miR-130b-3p | MIMAT0000387 | −2.626±0.314 | 0.000525 |
|
mmu-miR-142a-3p | MIMAT0000155 | −2.261±0.771 | 0.001618 |
| mmu-miR-15b-5p | MIMAT0000124 | −2.117±0.331 | 0.002784 |
|
mmu-miR-378a-3p | MIMAT0003151 | −2.241±0.283 | 0.002295 |
| mmu-miR-677-3p | MIMAT0017246 | −2.866±0.234 | 0.000189 |
|
mmu-miR-6931-5p | MIMAT0027762 | −2.473±0.828 | 0.000583 |
Prediction of target genes in
upregulated and downregulated miRNAs
To predict the target mRNA of the upregulated and
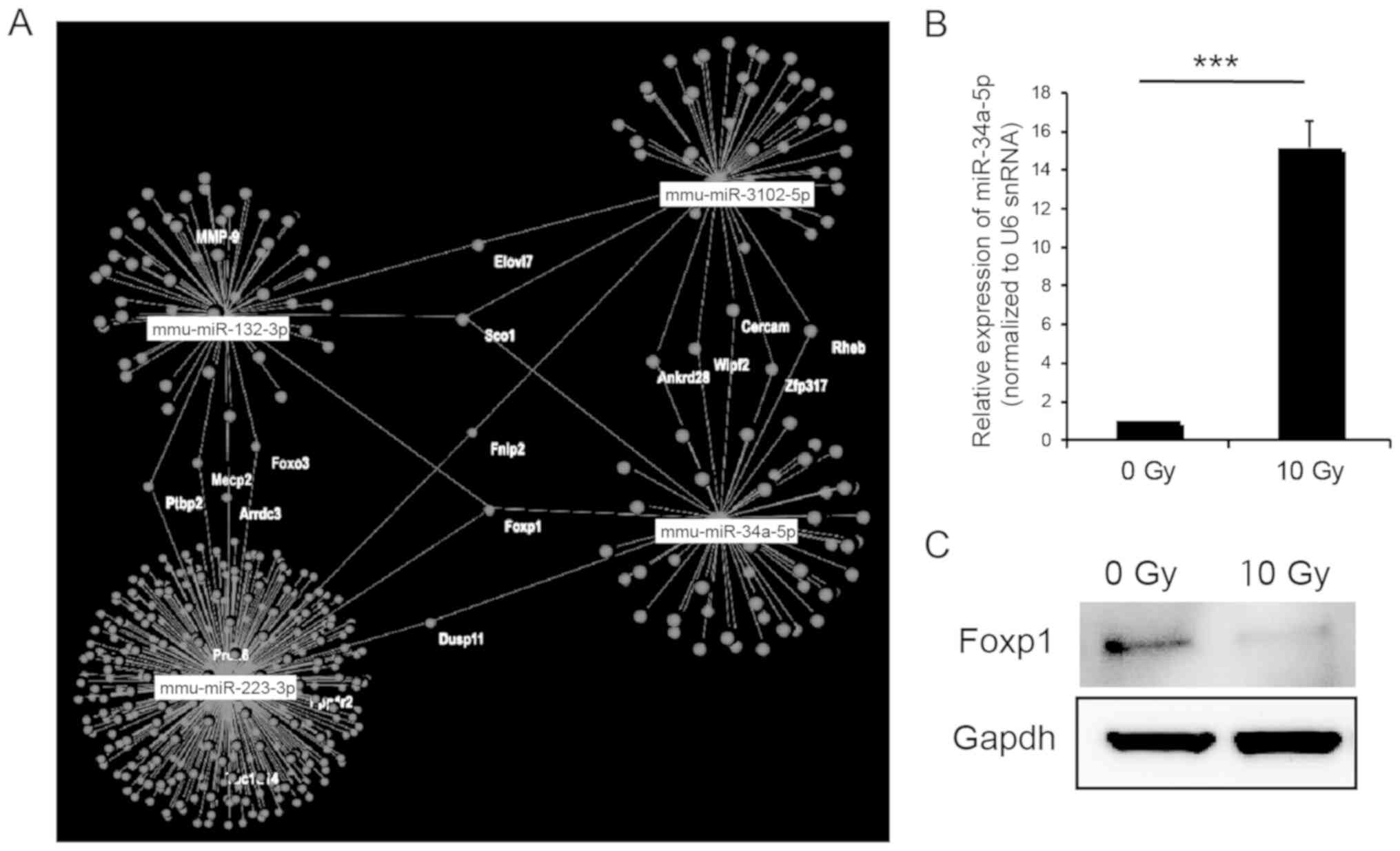
downregulated miRNAs, we used OmicsNet. Foxp1 was predicted
to be a target of the mRNAs of upregulated miR-34a-5p, miR-132-3p
and miR-223-3p via analysis using OmicsNet (Fig. 3A). In particular, miR-34-5p was
highly expressed, which was also validated using real-time PCR
(Fig. 3B). Whereas, decreased
Foxp1 expression in the small intestine following radiation
exposure was confirmed using western blotting (Fig. 3C). These results suggest that
Foxp1 may be the target gene of miR-34a-5p and be involved
in response to high-dose radiation exposure in the small
intestine.
Prediction of pathways involved in
target genes of miRNAs
To predict target genes of miRNAs and their
pathways, WikiPathways analysis were performed. Using TargetScan,
201 genes were predicted as targets of the upregulated miRNAs
(Table SI) and 857 of the
downregulated miRNAs (Table SII).
Under a p-value of 0.05, 65 and 122 pathways were predicted as
target genes and pathways of the upregulated and downregulated
miRNAs, respectively (Tables SIII
and SIV). The top 10 pathways
involved in up- and downregulated miRNAs are presented in Table II. Among these, the ‘TNF-α NF-κB
Signaling Pathway’ and ‘Regulation of Actin Cytoskeleton’ are
presented in Fig. S1.
 | Table II.Top 10 pathways predicted to be
regulated by miRNAs up- and downregulated >2.000-folds in the
small intestine of mice exposed to 10 Gy of X-ray irradiation. |
Table II.
Top 10 pathways predicted to be
regulated by miRNAs up- and downregulated >2.000-folds in the
small intestine of mice exposed to 10 Gy of X-ray irradiation.
| A, Upregulated
miRNAs |
|---|
|
|---|
| Pathway name | P-value |
|---|
|
Mm_PluriNetWork_WP1763_89515 |
1.63×10−18 |
|
Mm_mRNA_processing_WP310_78419 |
7.06×10−11 |
|
Mm_Non-odorant_GPCRs_WP1396_69993 |
2.45×10−8 |
|
Mm_Metapathway_biotransformation_WP1251_94721 |
2.45×10−8 |
|
Mm_Myometrial_Relaxation_and_Contraction_Pathways_WP385_95806 |
2.45×10−8 |
|
Mm_IL-6_signaling_Pathway_WP387_72091 |
2.45×10−8 |
|
Mm_Focal_Adhesion-PI3K-Akt-mTOR-signaling_pathway_WP2841_94308 |
2.45×10−8 |
|
Mm_Focal_Adhesion_WP85_94410 |
2.45×10−8 |
|
Mm_Chemokine_signaling_pathway_WP2292_97515 |
2.45×10−8 |
|
Mm_Cytoplasmic_Ribosomal_Proteins_WP163_78425 |
8.49×10−6 |
|
| B, Downregulated
miRNAs |
|
| Pathway
name | P-value |
|
|
Mm_mRNA_processing_WP310_78419 |
9.54×10−43 |
|
Mm_PluriNetWork_WP1763_89515 |
3.56×10−33 |
|
Mm_Chemokine_signaling_pathway_WP2292_97515 |
2.91×10−31 |
|
Mm_MAPK_signaling_pathway_WP493_78412 |
2.38×10−29 |
|
Mm_TNF-alpha_NF-kB_Signaling_Pathway_WP246_69201 |
1.94×10−27 |
|
Mm_Insulin_Signaling_WP65_88446 |
1.94×10−27 |
|
Mm_Non-odorant_GPCRs_WP1396_69993 |
1.58×10−25 |
|
Mm_EGFR1_Signaling_Pathway_WP572_82883 |
1.58×10−25 |
|
Mm_Regulation_of_Actin_Cytoskeleton_WP523_71326 |
1.05×10−21 |
|
Mm_G_Protein_Signaling_Pathways_WP232_89955 |
8.51×10−20 |
Discussion
This study revealed changes in the expression of
various miRNA in the small intestine of mice following radiation
exposure (10 Gy X-rays). In particular, miR-34a-5p was highly
expressed at 72 h after radiation exposure, suggesting that this
miRNA was the high-dose radio-responsive miRNA in the small
intestine.
The body weight of irradiated mice decreased after
several days of radiation exposure; in these mice, the 50% survival
period was approximately 9 days (Fig.
1A and B). Fluorescent TUNEL assay confirmed that the small
intestine was damaged by radiation exposure and TUNEL-positive
cells were detected in the irradiation group and were not detected
in the non-irradiated group. DNA damage was observed in the cells
of the small intestine, particularly in stem cells, as the
fluorescence of the small intestinal pit region strongly increased
(Fig. 1C). Liu et al
reported that intestinal Lgr5-positive stem cells underwent
apoptosis following exposure to high-dose X-rays (17). Lgr5 is a marker of intestinal
epithelial stem cells (18), and
the death of Lgr5-positive cells indicates intestinal injury that
is potentially lethal owing to poor regeneration ability of these
cells. It will be interesting to examine if Lgr5-positive cells
co-localize with TUNEL-positive cells in a future study.
We identified seven and six upregulated and
downregulated miRNAs, respectively, in the small intestine of mice
exposed to 10 Gy X-rays using miRNA microarray analysis.
Particularly, miR-34a-5p overexpression by more than nine fold was
observed in irradiated mice compared with that in non-irradiated
mice. This suggests that miR-34a-5p is a radiation-responsive miRNA
in the small intestine. Reportedly, miR-34a-5p regulates various
target mRNAs involved in the cell cycle, cell proliferation,
senescence, migration, and invasion, including cyclin-dependent
kinase 4/6, E2F transcription factor 3, cyclin E2, hepatocyte
growth factor receptor, B-cell lymphoma 2 (Bcl-2), NAD-dependent
deacetylase sirtuin-1, Myc, Notch, and CD44 (19,20).
Furthermore, miR-34a-5p primarily induces p53-mediated apoptosis
and cell cycle arrest in the G1 phase and during senescence
(21,22). Radiation exposure has been reported
to increase miR-34a-5p expression in different types of cells such
as breast cancer cells (23), lung
cancer cells (24), prostate
cancer cells (25), lymphocytes
(26), spleen cells (21), and colorectal cancer cells
(27). However, the present study
is the first to report on the increased miR-34a-5p expression in
the small intestine following high-dose radiation exposure.
Reportedly, elevated miR-34a-5p expression in the
small intestine suppresses Bcl-2 and Notch expressions, thus
promoting apoptosis and resulting in the deterioration of the small
intestine. Conversely, it is expected that increased miR-34a-5p
expression suppresses RAD51, reduces double-strand break repair,
and enhances radiosensitivity. Interestingly, miR-34a-5p has been
reported to an indicator of radiation exposure (28); this report revealed that the serum
miR-34a-5p level increases following radiotherapy in patients with
breast cancer. In the future, it will necessary to examine the
possibility of using serum miR-34a-5p as a biomarker for
determining intestinal high-dose radiation exposure.
Foxp1 belongs to subfamily P of the forkhead
box transcription factor family and plays important roles in organ
development (29). Moreover, it is
a valuable prognostic biomarker in lymphoma (30). However, the role of Foxp1 in
the intestinal tissue is slightly unclear. De Smedt et al,
suggested that the loss of FOXP1 was associated with decreased
survival of patients with colorectal cancer (CRC) and affected CRC
proliferation and inflammatory responses (31). Presumably, Foxp1 is a target
of miR-34a-5p; however, the relationship between high-dose
radiation and Foxp1 expression has not been reported till
date. The maintenance of Foxp1 expression with the use of an
miR-34a-5p inhibitor may suppress radiation-induced enteritis;
thus, an miR-34a-5p inhibitor may be used as a novel
radioprotective agent.
Gene expression is predicted to be upregulated and
downregulated by radiation exposure, and pathway analysis showed
the enhancement of ‘TNF-α NF-κB Signaling Pathway’ (Table II). TNF-α stimulation and ROS
generation following radiation cause NF-κB activation via IκB
kinase signaling and induce enteritis (32,33).
The predicted enhancement of the ‘TNF-α NF-κB Signaling Pathway’
suggests radiation-induced enteritis (Table II). Recently, radioprotective
agents, such as α-lipoic acid and L-carnitine, have been studied,
which alleviate radiation-induced enteritis as a side effect during
radiotherapy (34,35). Understanding the underlying
molecular mechanisms may lead to the development of novel effective
radioprotective agents.
In this study, we examined miRNA expression in the
small intestine after 72 h of radiation exposure using 8 week-old
mice. In a previous study, we examined miRNA expression at various
time-course with a high-dose (7 Gy) of irradiation and observed a
significant difference at 72 h compared with that at baseline
(36). Based on these findings,
changes after 72 h were examined in this study; however, there may
be a possibility that the results of these two studies differ owing
to the use of two radiation doses. In the future, analyzing
miR-34a-5p expression at various time-course with 10 Gy irradiation
will be necessary. In addition, the effects of radiation on the
small intestine may vary depending on the age of the mouse.
However, since it is difficult to use mice of all ages, 8 weeks of
age was used as a representative of mice that reached adulthood. We
plan to investigate the effects on the intestinal tract of children
and older mice in the future.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by The JSPS
KAKENHI (grant nos. JP25670264, JP17H04761 and JP17K19779) and a
grant from The Takeda Science Foundation in 2016.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MC was a major contributor in performing the
experiments and writing the manuscript. HU, IN, HK and SM helped
conduct the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
The Guidelines for Animal Experimentation of the Hirosaki
University. The procedures were approved and monitored by The
Animal Research Committee of Hirosaki University (approval nos.
G12003 and G19005).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Atomic Energy Agency, .
Diagnosis and treatment of radiation injures. IAEA Safety Report
Series. 2:IAEA. (Vienna). 1998.
|
|
2
|
Metcalfe C, Kljavin NM, Ybarra R and de
Sauvage FJ: Lgr5+ stem cells are indispensable for
radiation-induced intestinal regeneration. Cell Stem Cell.
14:149–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leibowitz BJ, Wei L, Zhang L, Ping X,
Epperly M, Greenberger J, Cheng T and Yu J: Ionizing irradiation
induces acute haematopoietic syndrome and gastrointestinal syndrome
independently in mice. Nat Commun. 5:34942014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berger ME, Christensen DM, Lowry PC, Jones
OW and Wiley AL: Medical management of radiation injuries: Current
approaches. Occup Med (Lond). 56:162–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macià I Garau M, Lucas Calduch A and López
EC: Radiobiology of the acute radiation syndrome. Rep Pract Oncol
Radiother. 16:123–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka SI: Summary of the JCO criticality
accident in Tokai-mura and a dose assessment. J Radiat Res. 42
(Suppl):S1–S9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki MS, Hayata I, Kamada N, Kodama Y
and Kodama S: Chromosome aberration analysis in persons exposed to
low-level radiation from the JCO criticality accident in
Tokai-mura. J Radiat Res. 42:S107–S116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asano S: Current status of hematopoietic
stem cell transplantation for acute radiation syndromes. Int J
Hematol. 95:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verginadis II, Kanade R, Bell B, Koduri S,
Ben-Josef E and Koumenis C: A novel mouse model to study
image-guided, radiation-induced intestinal injury and preclinical
screening of radioprotectors. Cancer Res. 77:908–917. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto T, Kinoshita M, Shinomiya N,
Hiroi S, Sugasawa H, Matsushita Y, Majima T, Saitoh D and Seki S:
Pretreatment with ascorbic acid prevents lethal gastrointestinal
syndrome in mice receiving a massive amount of radiation. J Radiat
Res. 51:145–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiba M, Miura T, Kasai K, Monzen S,
Kashiwakura I, Yasue H and Nakamura T: Identification of
up-regulated and down-regulated cis-natural antisense transcripts
in the human B lymphoblastic cell line IM-9 after X-ray
irradiation. Mol Med Rep. 5:1151–1157. 2012.PubMed/NCBI
|
|
13
|
Chiba M: Radiation-responsive
transcriptome analysis in human lymphoid cells. Radiat Prot
Dosimetry. 152:164–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strup-Perrot C, Mathé D, Linard C, Violot
D, Milliat F, François A, Bourhis J and Vozenin-Brotons MC: Global
gene expression profiles reveal an increase in mRNA levels of
collagens, MMPs, and TIMPs in late radiation enteritis. Am J
Physiol Gastrointest Liver Physiol. 287:G875–G885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng J, Wang J, Pouliot M, Authier S,
Zhou D, Loose DS and Hauer-Jensen M: Gene expression profiling in
non-human primate jejunum, ileum and colon after total-body
irradiation: A comparative study of segment-specific molecular and
cellular responses. BMC Genomics. 16:9842015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiba M, Kubota S, Sakai A and Monzen S:
Cell-to-cell communication via extracellular vesicles among human
pancreatic cancer cells derived from the same patient. Mol Med Rep.
18:3989–3996. 2018.PubMed/NCBI
|
|
17
|
Liu Z, Liu H, Jiang J, Tan S, Yang Y, Zhan
Y and Wu B: PDGF-BB and bFGF ameliorate radiation-induced
intestinal progenitor/stem cell apoptosis via Akt/p53 signaling in
mice. Am J Physiol Gastrointest Liver Physiol. 307:G1033–G1043.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P and Caraglia M: Mir-34: A new weapon against cancer? Mol
Ther Nucleic Acids. 3:e1942014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tarasov V, Jung P, Verdoodt B, Lodygin D,
Epanchintsev A, Menssen A, Meister G and Hermeking H: Differential
regulation of microRNAs by p53 revealed by massively parallel
sequencing: miR-34a is a p53 target that induces apoptosis and
G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stankevicins L, Almeida da Silva AP,
Ventura Dos Passos F, Dos Santos Ferreira E, Menks Ribeiro MC, G
David M, J Pires E, Ferreira-Machado SC, Vassetzky Y, de Almeida CE
and de Moura Gallo CV: MiR-34a is up-regulated in response to low
dose, low energy X-ray induced DNA damage in breast cells. Radiat
Oncol. 8:2312013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salzman DW, Nakamura K, Nallur S, Dookwah
MT, Metheetrairut C, Slack FJ and Weidhaas JB: miR-34 activity is
modulated through 5′-end phosphorylation in response to DNA damage.
Nat Commun. 7:109542016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
John-Aryankalayil M, Palayoor ST, Makinde
AY, Cerna D, Simone CB II, Falduto MT, Magnuson SR and Coleman CN:
Fractionated radiation alters oncomir and tumor suppressor miRNAs
in human prostate cancer cells. Radiat Res. 178:105–117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girardi C, De Pittà C, Casara S, Sales G,
Lanfranchi G, Celotti L and Mognato M: Analysis of miRNA and mRNA
expression profiles highlights alterations in ionizing radiation
response of human lymphocytes under modeled microgravity. PLoS One.
7:e312932012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Zhou C, Gao F, Cai S, Zhang C, Zhao
L, Zhao F, Cao F, Lin J, Yang Y, et al: MiR-34a in age and tissue
related radio-sensitivity and serum miR-34a as a novel indicator of
radiation injury. Int J Biol Sci. 7:221–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Halimi M, Shahabi A, Moslemi D, Parsian H,
Shari SM, Satire R, Yeganeh F and Zabihi E: Human serum miR-34a as
an indicator of exposure to ionizing radiation. Radiat Environ
Biophys. 55:423–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Usui N, Araujo DJ, Kulkarni A, Co M,
Ellegood J, Harper M, Toriumi K, Lerch JP and Konopka G: Foxp1
regulation of neonatal vocalizations via cortical development.
Genes Dev. 31:2039–2055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gascoyne DM and Banham AH: The
significance of FOXP1 in diffuse large B-cell lymphoma. Leuk
Lymphoma. 58:1037–1051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Smedt L, Palmans S, Govaere O, Moisse
M, Boeckx B, De Hertogh G, Prenen H, Van Cutsem E, Tejpar S,
Tousseyn T and Sagaert X: Expression of FOXP1 and colorectal cancer
prognosis. Lab Med. 46:299–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He LX, Wang JB, Sun B, Zhao J, Li L, Xu T,
Li H, Sun JQ, Ren J, Liu R, et al: Suppression of TNF-α and free
radicals reduces systematic inflammatory and metabolic disorders:
Radioprotective effects of ginseng oligopeptides on intestinal
barrier function and antioxidant defense. J Nutr Biochem. 40:53–61.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalita B, Ranjan R, Singh A, Yashavarddhan
MH, Bajaj S and Gupta ML: A combination of podophyllotoxin and
rutin attenuates radiation induced gastrointestinal injury by
negatively regulating NF-κB/p53 signaling in lethally irradiated
mice. PLoS One. 11:e01685252016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tas S, Ozkan OF, Cikman O, Kiraz A, Akgun
Y and Karaayvaz M: L-carnitine has a protective effect on the
colonic mucosa during abdominopelvic radiotherapy in rats. Acta Cir
Bras. 31:615–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong BK, Song JH, Jeong H, Choi HS, Jung
JH, Hahm JR, Woo SH, Jung MH, Choi BH, Kim JH and Kang KM: Effect
of alpha-lipoic acid on radiation-induced small intestine injury in
mice. Oncotarget. 7:15105–15117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiba M, Monzen S, Iwaya C, Kashiwagi Y,
Yamada S, Hosokawa Y, Mariya Y, Nakamura T and Wojcik A: Serum
miR-375-3p increase in mice exposed to a high dose of ionizing
radiation. Sci Rep. 8:13022018. View Article : Google Scholar : PubMed/NCBI
|