Introduction
Reports from the World Health Organization have
indicated that hepatocellular carcinoma (HCC) is one of the leading
causes of cancer-related mortality worldwide (1). Although at the beginning treatments
can partially or completely shrink tumors, relapse and metastasis
lead to treatment failure due to the existence of cancer stem cells
(CSCs) (2,3). Moreover, cells isolated by
fluorescence-activated cell sorting techniques based on Hoechst
33342 efflux are known as side population (SP) cells and harbor
CSCs properties, such as self-renewal and multipotential
differentiation (4). The most
significant feature of SP cells is their ability to discharge
Hoechst 33342 from cells due to the high expression of ATP binding
cassette (ABC) transporter proteins (5). Similar to CSCs, SP cells lead to
drug-resistance, tissue invasion and metastasis formation (6–8), but
only a few chemotherapeutic drugs can target SP cells.
It has been shown that 4,4′-bond secalonic acid D
(4,4′-SAD) is an antitumor bioactive substance of secondary
metabolites produced by a marine-derived fungus [Penicillium
(P.) oxalicum] (9).
Furthermore, its analog, SAD, was isolated in 1969 (10). Previous studies have revealed the
antitumor, anti-angiogenic, proapoptotic and antiproliferative
potentials of SAD (11–13). Zhang et al (13) and Hu et al (14) both showed that SAD induces the
degradation of ABC superfamily G member 2 (ABCG2) by activating
calpain-1 in SP cells and decreasing the percentage of SP cells in
lung cancer. Moreover, the authors' previous study identified that
4,4′-SAD significantly inhibits tumor growth via a
caspase-dependent pathway (9).
However, the role of 4,4′-SAD in regulating SP cells remains
unknown.
The present results suggested that the selected SP
cells from human liver cancer cell lines PLC/PRF/5 and HuH-7 were
sensitive to 4,4′-SAD as the ABCG2 expression of SP cells was
suppressed by 4,4′-SAD. Furthermore, it was found that 4,4′-SAD had
significant antimetastatic effects by downregulating matrix
metalloproteinase 9 (MMP-9) and upregulating its antagonist tissue
inhibitor of metalloproteinases 1 (TIMP-1) in vitro and
in vivo. Collectively, the present results may facilitate
the development of novel treatments for liver cancer with drug
resistance, recurrence and metastasis.
Materials and methods
Preparation of 4,4′-SAD
The fungus P. oxalicum was purified from
marine sediments collected from the southeast coastal region of
China and identified through internal transcribed spacer analysis
by Beijing Sunbiotech Co. Ltd. The specimen was preserved in the
China Center for Type Culture Collection (preservation no. CCTCC
M2013714). 4,4′-SAD was prepared after the fermentation and
extraction of P. oxalicum and successive purification by
chromatographic techniques; the detailed procedure was described in
the authors' previous study (9).
The structure of 4,4′-SAD is shown in Fig. 1A.
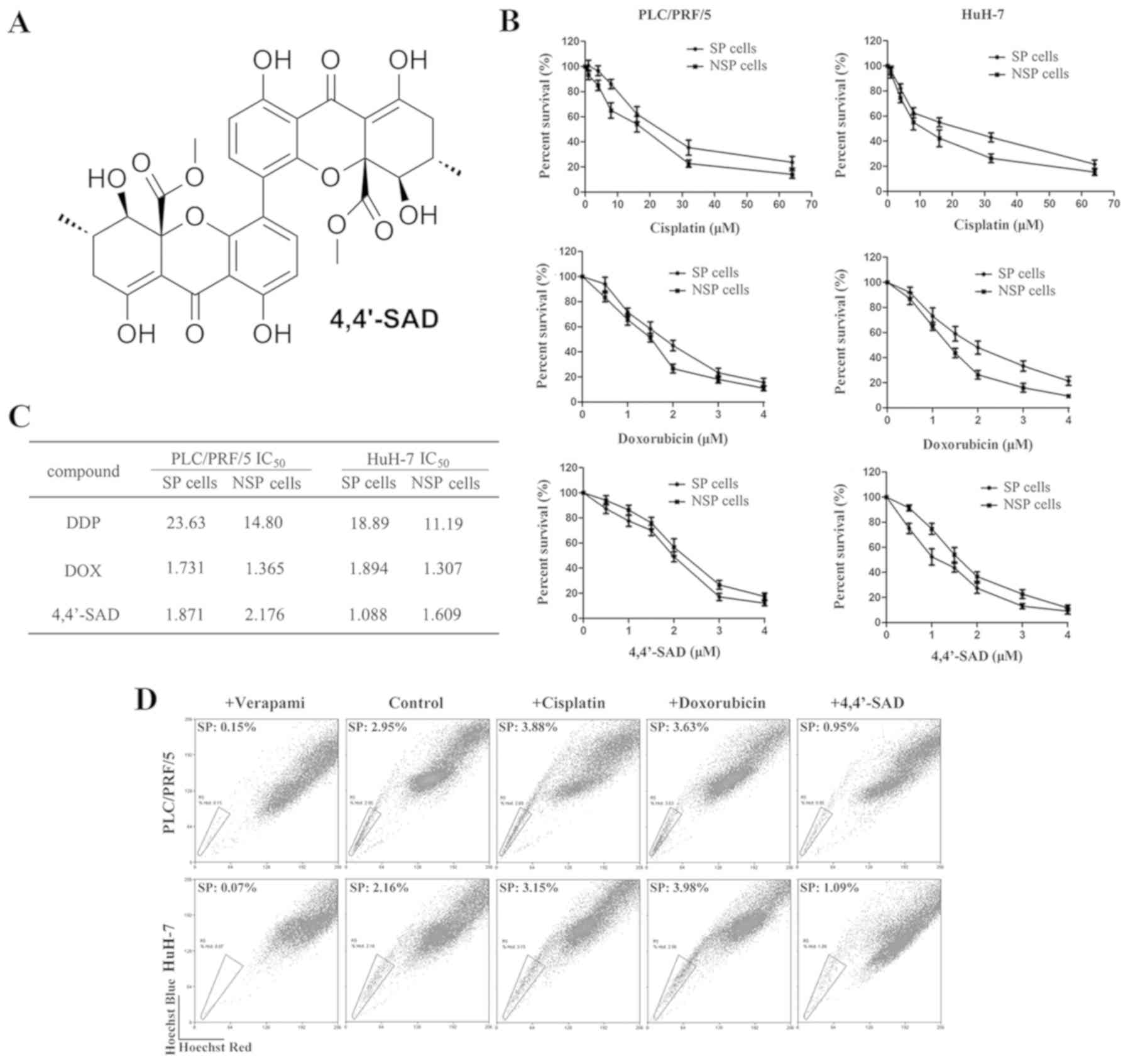 | Figure 1.Treatment with 4,4′-SAD inhibits the
growth of SP cells obtained from PLC/PRF/5 and HuH-7 cells. (A)
Chemical structure of 4,4′-SAD. (B) SP cells were incubated with
gradient concentrations of cisplatin, doxorubicin and 4,4′-SAD for
48 h, then the cellular viability of SP and NSP cells was analyzed
by water-soluble tetrazolium salt assay. SP and NSP cells were
isolated from PLC/PRF/5 and HuH-7 cells by fluorescence activated
cell sorting, based on the intensity of Hoechst 33342 staining.
Data are presented as the mean ± SD of three independent
experiments. (C) IC50 of cisplatin, doxorubicin and
4,4′-SAD in SP and NSP cells. SP and NSP cells were isolated from
PLC/PRF/5 and HuH-7 cells by fluorescence activated cell sorting,
based on the intensity of Hoechst 33342 staining. (D) Percentages
of SP cells in PLC/PRF/5 and HuH-7 cells after treatment of 10 µM
cisplatin, 1 µM doxorubicin and 1 µM 4,4′-SAD for 48 h. Verapamil
was added as a control. 4,4′-SAD, 4,4′-bond secalonic acid D; SP,
side population; NSP, non-side population. |
Culture of cell lines
PLC/PRF/5 and HuH-7 liver cancer cell lines were
purchased from the Shanghai Cell Resource Center. The murine H22
hepatoma cell line was obtained from the Cancer Metastasis Alert
and Prevention Center, Fuzhou University. All cell lines were
cultured in RPMI-1640 (Hyclone; GE Healthcare Life Sciences) or
DMEM (Hyclone; GE Healthcare Life Sciences) supplemented with 10%
FBS (Hyclone; GE Healthcare Life Sciences) at 37°C in a 5% (v/v)
CO2 humidified atmosphere.
Animals
A total of 32 female Kunming (KM) mice (weight,
18–20 g; age, 6 weeks) were purchased from Minhou County Wu
Experimental Animal Trade Co., Ltd. The mice were maintained under
controlled conditions at 19–23°C and 40–60% humidity, with a 12-h
light/dark cycle and free access to drinking water and food. Animal
experiments were approved by the Animal Care and Use Committee of
the Institute of Biomedical and Pharmaceutical Technology (Fuzhou
University), according to the Guide for the Care and Use of
Laboratory Animals (15). All mice
were euthanized with an overdose of sodium pentobarbital (150
mg/kg; intraperitoneally).
SP cell detection and isolation by
flow cytometry
Cells were harvested, washed with PBS and suspended
at 1×106 cells/ml in Hank's balanced salt solution
supplemented with 3% FBS and 10 mM HEPES. Cells were incubated at
37°C for 90 min with 15 µg/ml Hoechst 33342 (Sigma-Aldrich; Merck
KGaA) alone or in the presence of 50 µM verapamil (Sigma-Aldrich;
Merck KGaA). During the incubation, the tubes were shaken every 20
min to mix the cells with the solution. The cells were washed twice
with Hank's solution, 1 µg/ml propidium iodide (PI; Sigma-Aldrich;
Merck KGaA) was added and this was filtered through a 70 µm cell
strainer (BD Biosciences; Becton, Dickinson and Company). SP cell
analysis and sorting were performed using MoFlo XDP (Beckman
Coulter, Inc.). The data were analyzed using Summit software
version 5.0 (Beckman Coulter, Inc.). Hoechst 33342 was excited with
a UV laser at 350 nm and fluorescence emission was measured with
450 nm (Hoechst blue) and 570 nm (Hoechst red) optical filters. PI
labeling was measured for the discrimination of dead cells.
Cell viability analysis
The water-soluble tetrazolium salt (WST-1; Roche
Diagnostics) assay was used to assess cytotoxicity as described
previously (9). Briefly,
4×103 cells per well in a 96-well plate were cultured at
37°C for 24 h and incubated with gradient concentrations of
cisplatin (Sigma-Aldrich; Merck KGaA; 0, 2, 4, 8, 16, 32 and 64
µM), doxorubicin (Sigma-Aldrich; Merck KGaA; 0, 0.5, 1, 1.5, 2, 3
and 4 µM) or 4,4′-SAD (0, 0.5, 1, 1.5, 2, 3 and 4 µM) at 37°C for
48 h. Then, cells were treated with 10% WST-1 reagent for 4 h at
37°C and the optical density at 450 nm was tested with an ELISA
reader (TECAN Infinite M200 Pro; Tecan Group, Ltd.). Each
experiment was carried out in triplicate. The IC50 was
calculated using GraphPad Prism software version 7.0 (GraphPad
Software, Inc.).
Wound healing assay
The wound healing assay was performed to assess cell
motility as described in our previous study (9). PLC/PRF/5 and HuH-7 cells
(8×105 cells/ml) were seeded in 6-well plates and
incubated for 24 h to form confluent cell clusters. The vertical
cell wounds were generated by 10 µl sterile microtips, washed with
PBS three times to remove shedding cells and cultured with 2 ml
FBS-free DMEM containing different concentrations (0, 0.5 and 1 µM)
of 4,4′-SAD. Wound closure was observed at 0, 24 and 48 h with an
inverted fluorescent microscope (magnification, ×100; Nikon
Corporation). Each experiment was performed in triplicate.
Transwell assay
Inhibition capacity against cell invasion was
assessed using the Millipore 24-well Millicell Chamber with an 8-µm
pore size (EMD Millipore). Using a standard procedure, the upper of
Transwell chambers were coated with Matrigel (BD Biosciences;
Becton, Dickinson and Company) at 37°C for 1 h and a 100 µl cell
suspension containing a total of 2×105 cells (previously
treated with 0, 0.5, 1 and 2 µM 4,4′-SAD) in DMEM without FBS were
added to the upper chamber. The lower chamber was filled with 600
µl DMEM with 20% FBS. After incubation at 37°C for 24 h, the
chamber was washed twice with PBS buffer, fixed with precooled 100%
methanol on ice for 15 min and stained with 0.5% crystal violet at
room temperature for 20 min. In total, five random fields were
imaged and the number of invaded cells was counted under a
fluorescent microscope (magnification, ×400; Nikon
Corporation).
Western blot analysis Cells were
harvested and lysed with RIPA buffer (Beyotime Institute of
Biotechnology)
Total protein was quantified using a bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology) and 30 µg
protein/lane was separated with 12% SDS-PAGE by electrophoresis
(Bio-Rad Laboratories, Inc.). The protein sample was transferred to
the nitrocellulose membrane, which was blocked with 5% (w/v)
milk-PBS-0.1%Tween 20 (TBST) at room temperature for 1 h and
incubated with specific primary rabbit antibodies against human
(h)ABCG2 (cat. no. 42078S; Cell Signaling Technology, Inc.), hMMP-9
(cat. no. 3852S; Cell Signaling Technology, Inc.), hTIMP-1 (cat.
no. 8946S; Cell Signaling Technology, USA), mouse (m)MMP-9 (cat.
no. BA2202; Wuhan Boster Biological Technology, Ltd.), mTIMP-1
(cat. no. A00561; Wuhan Boster Biological Technology, Ltd.) and
β-actin (cat. no. 3700; Cell Signaling Technology, Inc.) at 1:1,000
dilutions in 5% (w/v) milk-PBST overnight at 4°C. The membranes
were subsequently washed three times, every 10 min, in PBST and
incubated with a horseradish peroxidase (HRP)-conjugated
anti-rabbit IgG (1:5,000; cat. no. BA1054; Wuhan Boster Biological
Technology, Ltd.) secondary antibody or an HRP-conjugated
anti-mouse IgG (1:5,000; cat. no. BA1050; Wuhan Boster Biological
Technology, Ltd.) secondary antibody at room temperature for 1 h.
Protein bands were detected by the FluorChem E digital darkroom
system (ProteinSimple). Blots were performed in triplicate and
protein expression levels were quantified using ImageJ v.1.8.0
software (National Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from cells with
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
mRNA was synthesized to cDNA with the GoScript Reverse
Transcriptase system (Promega Corporation) at 42°C for 1 h. qPCR
was subsequently performed using a Light Cycler 480 II (Roche
Diagnostics) with the miScript SYBR Green PCR kit (Qiagen GmbH).
The following thermocycling conditions were used: Initial
denaturation at 95°C for 2 min; followed by 40 cycles at 94°C for
15 sec, 55°C for 15 sec and 68°C for 30 sec. The oligonucleotide
primer pairs used in this study were as follows: ABCG2 forward,
5′-CAGGTGGAGGCAAATCTTCGT-3′ and reverse,
5′-ACCCTGTTAATCCGTTCGTTTT-3′; MMP-9 forward,
5′-CCTGGAGACCTGAGAAC-3′ and reverse, 5′-CAGGGACAGTTGCTTCT-3′;
TIMP-1 forward, 5′-ACTCTTGCACATCACTACCT-3′ and reverse,
5′-CATTACTCACAGTCCCTTTT-3′; and GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
The relative mRNA expression levels were quantified using the
2−ΔΔCq method (16).
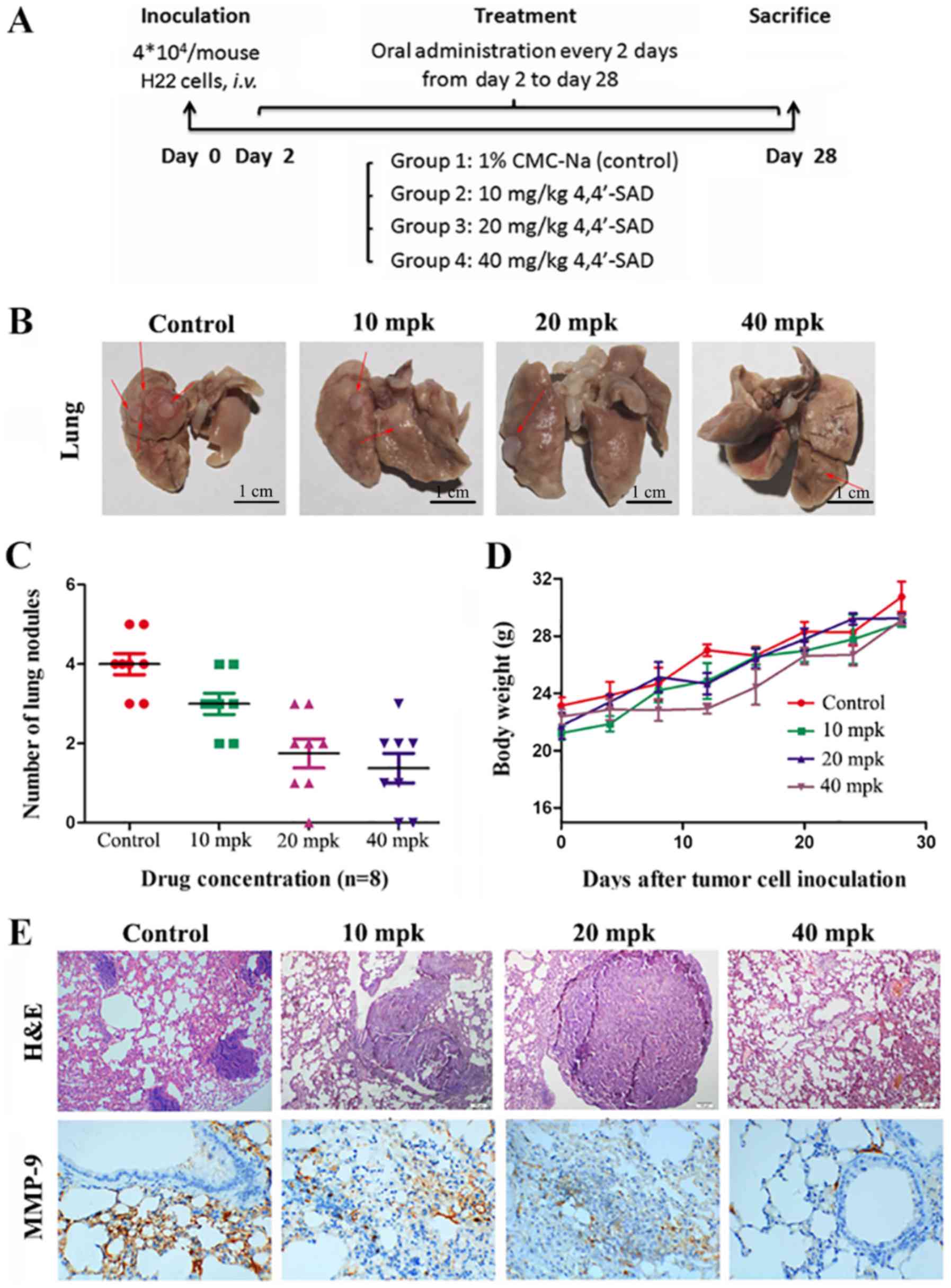
In vivo H22 intravenous (i.v.)
model
To assess the effects of 4,4′-SAD on tumor
metastasis in vivo, the present study established a H22 i.v.
model. In total, 4×104 H22 cells in 200 µl PBS were
injected into the tail vein of KM mice. KM mice were randomly
divided into four groups (n=8 for each group) on day 1. Dry
compounds were suspended in 1% carboxymethylcellulose (CMC)-Na
(Sigma-Aldrich; Merck KGaA) for immediate use. Mice were orally
gavaged with either 200 µl normal 1% CMC-Na solution (control) or
4,4′-SAD (10, 20 or 40 mg/kg) every 2 days from days 2–28. Weight
was measured every 4 days until mice were euthanized on day 28.
Contrastive images were captured after eviscerating the mice lungs.
Lungs were excised, fixed in 10% neutral buffered formalin for 24 h
at room temperature, embedded in paraffin, cut into 3–5 µm sections
and stained with hematoxylin for 5 min and eosin for 1 min, both at
room temperature. The number of tumor nodules on the lung surface
was counted by visual inspection using a magnifying glass and
tissues were observed under a fluorescent microscope
(magnification, ×400; Nikon Corporation).
Immunohistochemistry
Tumor tissues of lungs in KM mice were harvested,
fixed in 10% neutral buffered formalin at room temperature for 24
h, then embedded in paraffin and cut into 3-µm thick sections. The
sections were blocked with 5% (w/v) BSA (EMD Millipore) at room
temperature for 30 min and incubated with anti-MMP-9 antibody
(1:100; cat. no. BA2202; Wuhan Boster Biological Technology, Ltd.)
overnight at 4°C as previously described (17). Following the primary antibody
incubation, the sections were incubated with an HRP-conjugated
anti-rabbit IgG secondary antibody (1:500; cat. no. BA1054; Wuhan
Boster Biological Technology, Ltd.) at room temperature for 30 min.
The expression of MMP-9 was determined with the
3,3-diaminobenzidine assay kit (OriGene Technologies, Inc.),
according to the manufacturer's protocol. All sections were imaged
using a light microscope (magnification, ×400).
Statistical analysis
All data are presented as the mean ± SD of
triplicate repeats. All statistical analyses were conducted using a
Student's t-test or one-way ANOVA followed by Tukey's test, using
SPSS 13.0 software (IBM Corp.). P<0.05 was considered to
indicate a statically significant difference.
Results
4,4′-SAD inhibits the proliferation of
SP cells in PLC/PRF/5 and HuH-7
The present study isolated SP and non-SP (NSP) cells
from human liver cancer cell lines PLC/PRF/5 and HuH-7 based on the
ability of SP cells to actively efflux the Hoechst 33342 dye. To
evaluate the cytotoxic effects of 4,4′-SAD and traditional
chemotherapy drugs on SP and NSP cells, cells were treated with
gradient concentrations of 4,4′-SAD, cisplatin and doxorubicin for
48 h, then cell viability was assessed via the WST-1 assay. It was
found that the IC50 values of cisplatin, doxorubicin and
4,4′-SAD were 23.63, 1.731 and 1.871 µM in PLC/PRF/5 SP cells and
18.89, 1.894 and 1.088 µM in HuH-7 SP cells, respectively (Fig. 1B and C). Therefore, suggesting that
SP cells were more sensitive to 4,4′-SAD compared with traditional
chemotherapeutic drugs. Furthermore, the IC50 values of
cisplatin and doxorubicin were higher in SP cells compared with NSP
cells in both cell lines, whereas the IC50 value of
4,4′-SAD was lower in SP cells compared with NSP cells (Fig. 1B and C), thus indicating that SP
cells were resistant to traditional chemotherapy drugs, but still
sensitive to 4,4′-SAD.
The effect of 4,4′-SAD and the traditional
chemotherapy drugs, cisplatin and doxorubicin, on the percentage
alternation of SP cells was examined in PLC/PRF/5 and HuH-7 cells.
It was found that the fraction of SP cells increased from 2.95 to
3.88 and 3.63%, but decreased to 0.95% in PLC/PRF/5 after the 48 h
treatment of 10 µM cisplatin, 1 µM doxorubicin and 1 µM 4,4′-SAD,
respectively (Fig. 1D). Moreover,
the fraction of SP cells increased from 2.16 to 3.15 and 3.98%, but
decreased to 1.09% in HuH-7 after 48 h treatment of 10 µM
cisplatin, 1 µM doxorubicin and 1 µM 4,4′-SAD, respectively
(Fig. 1D). Collectively, the
results demonstrated that 4,4′-SAD inhibited and had cytotoxic
effects on SP cells, whereas the traditional chemotherapy drugs
cisplatin and doxorubicin had more powerful cytotoxic effects on
NSP cells compared with SP cells.
4,4′-SAD inhibits the invasion and
migration of SP cells
Besides drug resistance, SP cells are also involved
in tumor invasion and metastasis (18). Due to the anti-SP cell efficacy of
4,4′-SAD in vitro, the present study investigated whether
4,4′-SAD could affect the invasion and migration of PLC/PRF/5 and
HuH-7 SP cells using wound healing and Transwell assays. The wound
healing assay results demonstrated a decrease in the migration of
both PLC/PRF/5 and HuH-7 SP cells after treatment with gradient
concentrations of 4,4′-SAD for 24 and 48 h (Fig. 2A). However, 4,4′-SAD was more
effective at suppressing of SP cell migration in PLC/PRF/5 cells
compared with HuH-7 cells.
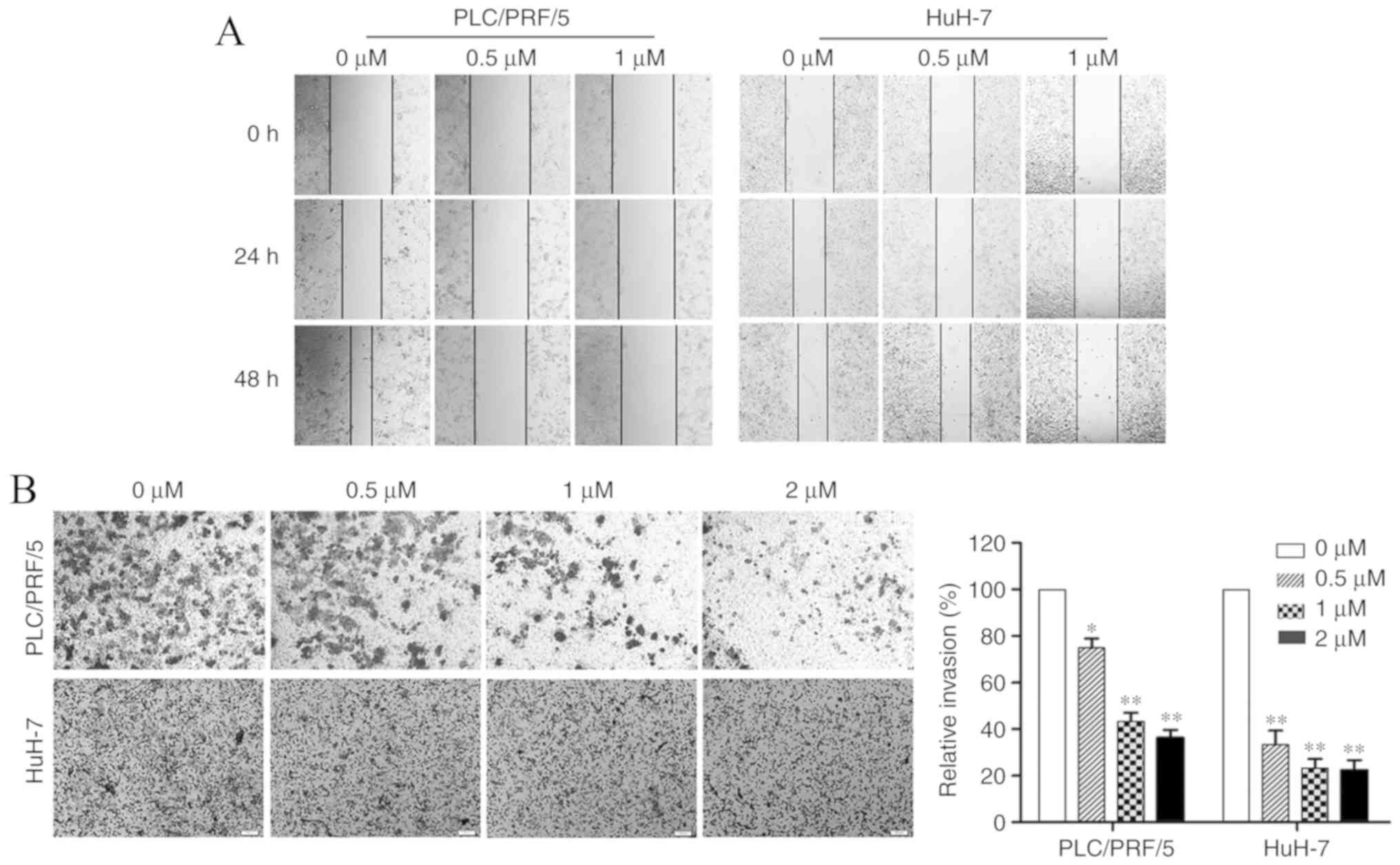 | Figure 2.Treatment with 4,4′-SAD inhibits the
migratory and invasive capacity of PLC/PRF/5 and HuH-7 side
population cells in vitro. (A) Wound healing assay was
performed in PLC/PRF/5 and HuH-7 cells treated with 4,4′-SAD (0,
0.5 and 1 µM) for 0, 24 and 48 h. Magnification, ×100. (B) Cell
invasion was measured by the Transwell assay and quantitatively
analyzed after treating cells with 4,4′-SAD (0, 0.5, 1 and 2 µM)
for 24 h. *P<0.05 and **P<0.01 vs. 0 µM. 4,4′-SAD, 4,4′-bond
secalonic acid D. Magnification, ×400. |
In the Transwell assay, the number of PLC/PRF/5 SP
cells passing through the Matrigel per field under an inverted
microscope (magnification, ×100) was reduced from 203±13 to 141±6,
107±4 and 86±5 after treatment with 0, 0.5, 1 and 2 µM 4,4′-SAD for
24 h, respectively. Moreover, the number of invaded HuH-7 SP cells
per field decreased from 147±7 to 57±4, 28±5 and 23±3 after
treatment with 0, 0.5, 1 and 2 µM 4,4′-SAD for 24 h, respectively
(Fig. 2B). Therefore, it the
results indicated that 4,4′-SAD significantly decreased the
invasion of PLC/PRF/5 and HuH-7 SP cells.
4,4′-SAD decreases the expression levels of ABCG2
and MMP-9 and increases the expression of TIMP-1 in SP cells. To
further examine the role of 4,4′-SAD in inhibiting the
proliferation, migration and invasion of SP cells, related gene
expression levels were measured by western blotting and RT-qPCR. As
ABCG2 is an efflux protein and multidrug resistance marker in SP
cells, ABCG2 expression was assessed and it was found that 4,4′-SAD
reduced the protein and mRNA expression levels of ABCG2 in SP cells
in a concentration-dependent manner (Fig. 3A). The results also indicated that
4,4′-SAD treatment downregulated the expression of MMP-9 at both
the protein and mRNA levels, and upregulated the expression of
TIMP-1, an inhibitor of MMP-9, in a dose-independent manner
(Fig. 3B). Thus, the results
suggested that 4,4′-SAD inhibited metastasis by reducing the
proteolytic degradation of the extracellular matrix (ECM) and
basement membrane near the tumor.
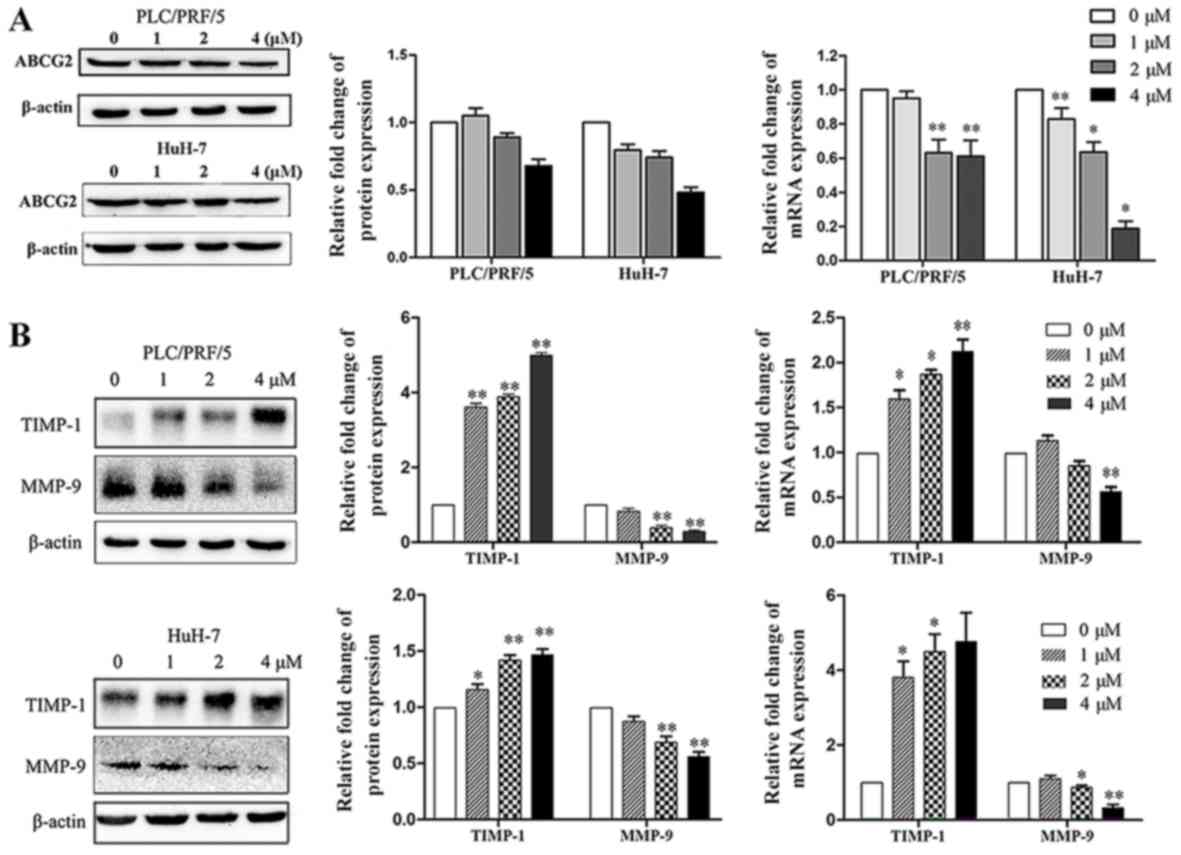 | Figure 3.Treatment with 4,4′-SAD increases the
expression of TIMP-1 and reduced the expression levels of ABCG2 and
MMP-9. (A) Western blotting and RT-qPCR analyses of ABCG2
expression in PLC/PRF/5 and HuH-7 SP cells treated with the
indicated concentrations (0–4 µM) of 4,4′-SAD for 48 h. β-actin was
used as a loading control. (B) Expression levels of MMP-9 and
TIMP-1 in PLC/PRF/5 and HuH-7 SP cells treated with 4,4′-SAD were
detected by western blotting and RT-qPCR. Data are presented as the
mean ± SD. N=3. *P<0.05, **P<0.01 vs. control group (0 µM).
RT-qPCR, reverse transcription-quantitative PCR; 4,4′-SAD,
4,4′-bond secalonic acid D; MMP, matrix metalloproteinase; TIMP-1,
tissue inhibitors of metalloproteinases; ABCG2, ATP-binding
cassette superfamily G member 2. |
Effects of 4,4′-SAD on metastasis in
vivo
The antimetastatic efficacy of 4,4′-SAD was further
assessed using the H22 HCC model in mice. Firstly, the present
study investigated whether the role of 4,4′-SAD on metastasis in
murine H22 cells was the same as in human PLC/PRF/5 and HuH-7 cells
in vitro. It was found that 4,4′-SAD attenuated the
expression of MMP-9 and enhanced the expression of TIMP-1 in H22
cells (Fig. S1), which indicated
that 4,4′-SAD inhibited the metastasis of mouse H22 cells by
regulating MMP-9 and TIMP-1.
The mouse model was constructed by injecting H22
into the tail vein of KM mice at 4×104 cells in 200 µl
PBS. Then, mice were orally gavaged with either normal CMC-Na
(control) or 4,4′-SAD (10, 20 or 40 mg/kg, every other day) from
days 2–28 and were euthanized on day 28 (Fig. 4A). The results suggested that mice
treated with 4,4′-SAD exhibited less lung metastatic loci compared
with mice in the control group (Fig.
4B and C). Moreover, no significant reduction in body weight
change was observed after 4,4′-SAD treatment (Fig. 4D). Histological examinations, which
identified the presence of HCC cells in the lung parenchyma,
demonstrated the presence of metastasis (Fig. 4E). Furthermore, immunohistochemical
staining indicated that MMP-9 expression in lung tissues was
inhibited in a dose-independent manner, which was consistent with
the experimental results at the cellular level (Fig. 4E). Collectively, these results
suggested that 4,4′-SAD efficiently suppressed lung metastasis
without obvious side effects in vivo.
Discussion
HCC is an often fatal malignant tumor type with a
high recurrence rate, poor prognosis and chemoresistance (19). Despite advances in anticancer
therapies such as chemotherapy, radiotherapy and targeted
therapies, the survival rate of HCC remains poor (20). Moreover, CSC-mediated relapse and
metastasis have been identified as key factors in reducing the
efficacy of current anticancer cytotoxic therapies (3). Our previous study showed that
4,4′-SAD, a mycotoxin from the secondary metabolites of P.
oxalicum, promotes apoptosis of tumor cells in the human HCC
cell lines PLC/PRF/5 and HuH-7 by activating apoptosis-related
protein expression, as well as regulating the Bax/Bcl-2 ratio
(9). The present results suggested
that 4,4′-SAD exhibited potent anti-CSC activity by inhibiting the
growth and metastasis of SP cells, which was regulated by ABCG2 and
MMP-9 in vitro and in vivo. Therefore, the results
may facilitate the potential application of 4,4′-SAD in future
liver cancer therapy.
The present results indicated that 4,4′-SAD may have
significant direct cytotoxicity on SP cells, as it was found that
SP cells were resistant to the traditional chemotherapeutic drugs
cisplatin and doxorubicin, but were sensitive to 4,4′-SAD. In
addition, the IC50 values of cisplatin and doxorubicin
in SP cells were higher compared with NSP cells, whereas SP cells
were more sensitive to 4,4′-SAD compared with NSP cells. SP cells,
which are involved in multidrug resistance and tumor initiation,
express high levels of ABC transporters, including ABCG2, to
expulse Hoechst 33342 dye (4,21).
Moreover, previous studies have revealed that SAD reduces the
expression of ABCG2 and percentage of SP cells in lung cancer cells
(13,14). The present results demonstrated
that 4,4′-SAD, as the analog of SAD, targeted SP cells and
regulated ABCG2 expression. Therefore, it was speculated that the
potential of 4,4′-SAD to reverse the drug resistance of SP cells
may be partially due to its inhibition of ABCG2. Furthermore, SAD
induces the degradation of ABCG2 by activating calpain-1 (13,14),
and thus, 4,4′-SAD may regulate ABCG2 via the same pathway.
However, further research is required to elucidate the detailed
mechanism involved.
Self-renewing CSCs are usually associated with the
course of metastatic disease (22). Metastasis is a primary obstacle to
successful cancer therapy and is closely linked to the rates of
morbidity and mortality in liver cancer types (3,23).
As 4,4′-SAD showed high cytotoxic effects on SP cells,
investigating its role in tumor progression and metastasis
inhibition may be valuable (24,25).
The present results indicated that 4,4′-SAD suppressed migration
and invasion by downregulating the expression of MMP-9 and
upregulating the expression of TIMP-1 at both protein and mRNA
levels in vitro. Moreover, 4,4′-SAD reduced lung metastatic
loci in a lung metastatic model of H22 HCC without side effects.
MMP-9, which is an important MMP in the degradation of multiple
elements of the ECM, facilitates the release of numerous
prometastatic factors, such as vascular endothelial growth factor
A, transforming growth factor-β, tumor necrosis factor-α and
interleukin-8, leading to the migration of cancer cells to other
tissues (26–28). However, the activities of MMPs may
be limited by their associated tissue inhibitors of
metalloproteinases, which inhibit tumor growth and invasion
(29). In addition, both CSCs and
the equilibrium between the content of MMP-9 and TIMP-1 play
important roles in the pathogenesis of tumor metastasis (29–32).
In conclusion, the present results suggested
4,4′-SAD had effects on advanced liver cancer. Mechanistically,
4,4′-SAD significantly suppressed the growth of SP cells in
PLC/PRF/5 and HuH-7 cells via the downregulation of ABCG2. In
addition, it was found that 4,4′-SAD reduced the migration and
invasion of tumor cells both in vitro and in vivo,
via MMP-9 and TIMP-1. Combined with previous studies, the present
results indicated that 4,4′-SAD may be a novel therapeutic agent to
treat liver cancer cells that are resistant to current therapies
and are also responsible for relapse and metastasis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Joint Funds for
the Innovations of Science and Technology, Fujian Province (grant
no. 2017Y9075), Key Scientific Project of Ministry of Education in
Fujian Province (grant no. JZ160419), Natural Science Foundation of
Fujian Province (grant nos. 2017J01855 and 2017J01179), Leading
Project Foundation of Fujian Province (grant no. 2018Y0015), ‘Young
Top Creative Talents’ of the Second Batch Special Support ‘Double
Hundred Plan’ of Fujian Province and Science and Technology Program
of Fujian Province, China (grant no. 2018Y2003).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL contributed to the design of this study. LC, MC,
YL and SL performed the experiments. LC and QZ analyzed and
interpreted the experimental data and wrote the manuscript. QL
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments involved in this study were approved
by both the Institutional Review Boards of Fuzhou University and
the Ethics Committee of Fujian Cancer Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
4,4′-SAD
|
4,4′-bond secalonic acid D
|
|
SP
|
side population
|
|
HCC
|
hepatocellular carcinoma
|
|
ABC
|
ATP binding cassette
|
|
SAD
|
secalonic acid D
|
|
NSP
|
non-side population
|
|
MMPs
|
matrix metalloproteinases
|
|
ECM
|
extracellular matrix
|
|
TIMPs
|
tissue inhibitor of
metalloproteinases
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu S, Du R, Gao C, Kang J, Wen J and Sun
T: The role of XBP1s in the metastasis and prognosis of
hepatocellular carcinoma. Biochem Biophys Res Commun. 500:530–537.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Terada T and Maruo H: Unusual extrahepatic
metastatic sites from hepatocellular carcinoma. Int J Clin Exp
Pathol. 6:816–820. 2013.PubMed/NCBI
|
|
4
|
Wolmarans E, Nel S, Durandt C, Mellet J
and Pepper MS: Side population: Its use in the study of cellular
heterogeneity and as a potential enrichment tool for rare cell
populations. Stem Cells Int. 2018:24721372018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H, et al: The ABC transporter Bcrp1/ABCG2 is expressed in
a wide variety of stem cells and is a molecular determinant of the
side-population phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toyoda Y, Takada T and Suzuki H:
Inhibitors of human ABCG2: From technical background to recent
updates with clinical implications. Front Pharmacol. 10:2082019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi YH and Yu AM: ABC transporters in
multidrug resistance and pharmacokinetics, and strategies for drug
development. Curr Pharm Des. 20:793–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Najafi M, Farhood B and Mortezaee K:
Cancer stem cells (CSCs) in cancer progression and therapy. J Cell
Physiol. 234:8381–8395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Li YP, Li XX, Lu ZH, Zheng QH and
Liu QY: Isolation of 4,4′-bond secalonic acid D from the
marine-derived fungus Penicillium oxalicum with inhibitory property
against hepatocellular carcinoma. J Antibiot (Tokyo). 72:34–44.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steyn PS: The separation and detection of
several mycotoxins by thin-layer chromatography. J Chromatogr A.
45:473–475. 1969. View Article : Google Scholar
|
|
11
|
Guru SK, Pathania AS, Kumar S, Ramesh D,
Kumar M, Rana S, Kumar A, Malik F, Sharma PR, Chandan BK, et al:
Secalonic acid-D represses HIF1α/VEGF-mediated angiogenesis by
regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res.
75:2886–2896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang JY, Tao LY, Liang YJ, Yan YY, Dai
CL, Xia XK, She ZG, Lin YC and Fu LW: Secalonic acid D induced
leukemia cell apoptosis and cell cycle arrest of G(1) with
involvement of GSK-3beta/beta-catenin/c-Myc pathway. Cell Cycle.
8:2444–2450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Huang L, Tao L, Zhang J, Wang F,
Zhang X and Fu L: Secalonic acid D induces cell apoptosis in both
sensitive and ABCG2-overexpressing multidrug resistant cancer cells
through upregulating c-Jun expression. Acta Pharm Sin B. 9:516–525.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu YP, Tao LY, Wang F, Zhang JY, Liang YJ
and Fu LW: Secalonic acid D reduced the percentage of side
populations by down-regulating the expression of ABCG2. Biochem
Pharmacol. 85:1619–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals: 8th edition. The National
Academies Press. (Washington, DC). 2011.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wellner UF, Hopt UT and Brabletz T: Tumour
stem cells and metastasis. Zentralbl Chir. 135:318–322. 2010.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gravitz L: Liver cancer. Nature.
516:S12014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parisod L, Duran R, Denys A and Digklia A:
Treatment of advanced hepatocellular carcinoma: Novel agents and
role of local therapy. Rev Med Suisse. 13:1032–1034. 2017.(In
French). PubMed/NCBI
|
|
21
|
Challen GA and Little MH: A side order of
stem cells: The SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McClements L, Annett S, Yakkundi A,
O'Rourke M, Valentine A, Moustafa N, Alqudah A, Simões BM, Furlong
F, Short A, et al: FKBPL and its peptide derivatives inhibit
endocrine therapy resistant cancer stem cells and breast cancer
metastasis by downregulating DLL4 and Notch4. BMC Cancer.
19:3512019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altekruse SF, Henley SJ, Cucinelli JE and
McGlynn KA: Changing hepatocellular carcinoma incidence and liver
cancer mortality rates in the United States. Am J Gastroenterol.
109:542–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Zhang E, Long J, Hu Z, Peng J,
Liu L, Tang F, Li L, Ouyang Y and Zeng Z: Immune infiltration in
renal cell carcinoma. Cancer Sci. 110:1564–1572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nandy SB and Lakshmanaswamy R: Cancer stem
cells and metastasis. Prog Mol Biol Transl Sci. 151:137–176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ward MP and Spiers JP: Protein phosphatase
2A regulation of markers of extracellular matrix remodelling in
hepatocellular carcinoma cells: Functional consequences for tumour
invasion. Br J Pharmacol. 174:1116–1130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giusca SE, Caruntu ID, Amalinei C and
Avadanei ER: Prognostic significance of MMP-9 and TIMP-1 in liver
metastases. Romanian journal of morphology and embryology =. Rev
Roum Morphol Embryol. 56:357–364. 2015.
|
|
30
|
Ogata Y, Ookita A and Kakegawa T:
Significance of MMP-9 and TIMP-1 production during liver metastasis
in colorectal cancer. Nihon Rinsho. 53:1811–1815. 1995.(In
Japanese). PubMed/NCBI
|
|
31
|
Wang D, Plukker JTM and Coppes RP: Cancer
stem cells with increased metastatic potential as a therapeutic
target for esophageal cancer. Semin Cancer Biol. 44:60–66. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agliano A, Calvo A and Box C: The
challenge of targeting cancer stem cells to halt metastasis. Semin
Cancer Biol. 44:25–42. 2017. View Article : Google Scholar : PubMed/NCBI
|