Introduction
As a leading cause of cardiovascular disease,
atherosclerosis is a serious health hazard in humans (1). Endothelial dysfunction, characterized
by changes in sensitivity to apoptosis, coagulation, inflammatory
activity, barrier function and proliferation, is the first step in
atherosclerosis development (2,3).
Recent advances made regarding atherosclerosis have emphasized that
improvement of endothelial function is a potential target for
atherosclerosis treatment (4).
Increasing evidence has demonstrated that the
endothelial dysfunction process is directly influenced by various
factors, including nitric oxide dysfunction, epigenetic factors,
oxidative stress, inflammatory factors and low shear stress (LSS)
(5–11). Among these, LSS, tangential stress
produced by endothelial surface blood flow friction, which is
observed at bifurcations, branching points and inside areas of
curved segments of the coronary artery, induces inflammation and
apoptosis, and subsequently disrupts the endothelial barrier,
eventually leading to atherosclerosis (11–15).
However, to date, no molecular targets have clearly linked LSS with
the endothelial barrier. Thus, it is important to identify
potential molecular targets for the improvement of atherosclerosis
treatment.
As a molecule that causes cell adhesion, the
expression of platelet endothelial cell adhesion molecule-1
(PECAM-1, also termed CD31) is ubiquitous in various types of
cells, including monocytes, neutrophils, T cells and endothelial
cells (ECs), and is crucial for vascular barrier function
regulation as a result of various stimuli, including shear stress
(16). Under certain physiological
conditions, the endothelial barrier function is supported by
PECAM-1 by controlling the junctional and adhesive properties of
ECs (17). During inflammation
observed in vessels affected by atherosclerosis, the function of
PECAM-1 is impaired, leading to an increase in neutrophils and
other leukocytes adhering to ECs, vascular integrity loss and
increased transmigration of leukocytes to the intima media
(18). Of note, increasing
evidence from in vivo experiments has indicated that PECAM-1
removal leads to a decrease in the development of plaque in LSS
environments (19–21). These observations indicate a
proatherogenic role of PECAM-1 under LSS, but its mechanism of
action has not yet been elucidated. The present study hypothesized
that LSS may upregulate PECAM-1 expression, leading to EC apoptosis
and monocyte adhesion that subsequently disrupts the endothelial
barrier, resulting in atherosclerosis.
Materials and methods
Reagents
Antibodies against PECAM-1 (cat. no. 77699S),
PECAM-1 (cat. no. 3528S), phospho-Akt (Ser473; cat. no. 4060S),
phospho-Akt (Thr308; cat. no. 13038S), Akt (cat. no. 9272S),
phospho-forkhead box O (FoxO1; Ser256; cat. no. 84192S), FoxO1
(cat. no. 2880S) and β-actin (cat. no. 8457S) were purchased from
Cell Signaling Technology, Inc. FITC-labeled goat anti-rabbit IgG
(cat. no. GB22303) and horseradish peroxidase (HRP)-labeled goat
anti-rabbit IgG (cat. no. GB23303) were purchased from Wuhan
Servicebio Technology Co., Ltd. FBS, DMEM and RPMI-1640 media were
purchased from Gibco; Thermo Fisher Scientific, Inc. The Annexin
V-FITC/propidium iodide (PI) Apoptosis Detection kit was purchased
from BD Bioscience.
Cell culture
The EA.hy926 cells, obtained from The Type Cell Bank
of Culture Collection of the Chinese Academy of Sciences, are a
human umbilical vein EC (HUVEC) line. The cells were cultured in
10% FBS-supplemented DMEM in a 5% CO2 incubator at 37°C.
The American Type Culture Collection was the source of the human
monocytic leukemia cell line THP-1. RPMI-1640 medium supplemented
with 10% FBS was used to culture the THP-1 cells in a 5%
CO2 incubator at 37°C.
Small interfering RNA (siRNA)
transfection
The siRNA sequences used in the present study were
as follows: PECAM-1 forward, 5′-AUUCUGGUCUCGAGAAUUCUU-3′ and
reverse, 5′-GAAUUCUCGAGACCAGAAUUU-3′; and PECAM-1 negative control
(NC) forward, 5′-ACGUGACACGUUCGGAGAATT-3′ and reverse,
5′-UUCUCCGAACGUGUCACGUTT-3′ (Shanghai GenePharma Co., Ltd.). The
Lipofectamine®-RNAiMAX transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc) was utilized to transfect the HUVECs
with PECAM-1 or NC siRNA as previously described (15). Cells at 70–80% confluency were
co-cultured with 50 nmol/l PECAM-1 or 50 nmol/l NC siRNA and
Lipofectamine®-RNAiMAX at 37°C for 6 h. At 48 h
post-transfection, the cells were either exposed to shear stress or
not. The experiments were performed in triplicate.
Shear stress experiment
Shear stress of 2 dyn/cm2 was applied
in vitro using a parallel-flow chamber as previously
described (15,22,23).
In brief, endothelial monolayers containing cell culture slides
were subjected to LSS for 0, 30, 60 and 120 min after being placed
in a parallel-plate flow chamber. Prior to the flow experiments,
the cells were transferred to serum-free DMEM for 120 min to
maintain their quiescence.
Western blotting analysis
Total protein of the HUVECs was obtained as
previously described (15). The
protein expression of PECAM-1, FoxO1, phospho-FoxO1, Akt, and
phospho-Akt in HUVECs were analyzed using western blotting.
SDS-PAGE (10% gel) was used to separate an equal quantity (40 µg)
of proteins, which were then transferred onto PVDF membranes. After
blocking with 5% BSA (cat. no. SBJ-DB2040; Nanjing SenBeiJia
Biological Technology Co., Ltd.) for 2 h at 25°C, incubation of the
membranes was performed at 4°C with anti-PECAM-1 (1:1,000),
anti-FoxO1 (1:1,000), anti-phospho-FoxO1 (1:1,000), anti-Akt
(1:1,000), anti-phospho-Akt Ser473 (1:1,000), anti-phospho-Akt
Thr308 (1:1,000) and anti-β-actin (1:1,000) antibodies overnight.
The membranes were washed with TBS with 0.1% Tween-20 (cat. no.
T8220; Beijing Solarbio Science & Technology Co., Ltd.) and
incubated with HRP-conjugated secondary antibodies (1:10,000) for 2
h at 25°C. The antibody-bound target antigens were detected using
enhanced chemiluminescence reagents (cat. no. WBKLS0500; EMD
Millipore). A GeneGnome chemiluminescence imaging system (Syngene)
was used to capture images, and ImageJ software (v.1.8.0; National
Institutes of Health) was used to analyze the target protein
expression. The experiments were performed at least in
triplicate.
Adhesion assay
The effects of LSS on THP-1 cell adherence onto
HUVEC monolayers was assessed by an adhesion assay. Following
exposure of the HUVECs to LSS for 60 min, the HUVECs were covered
with THP-1 cells (10,000 cells/ml) and co-incubated for 1 h at
37°C. Attached THP-1 cell quantity was detected using an IX73P2F
light microscope (Olympus Corporation), magnification ×200. The
adherent cell quantity was determined in three high-power
microscopic fields that were randomly chosen.
Flow cytometry for apoptosis
analysis
HUVEC early and late apoptosis determination was
conducted using an Annexin V-FITC/PI Apoptosis Detection kit
according to the manufacturer's instructions. In brief, LSS was
used to stimulate the HUVECs for 90 min, and subsequently Annexin
V-FITC/PI was used to stain the cells at 37°C for 30 min. The
HUVECs were washed twice with PBS, and cold PBS was used to
resuspend the stained cells. The BD FACSCanto II system (BD
Biosciences) was used to perform the flow cytometry, and BD
FACSDiva Software (version 8.0.1; BD Biosciences) was used to
analyze the data.
Immunofluorescence
The HUVECs were fixed in 4% paraformaldehyde for 20
min at 25°C, permeabilized with 0.1% Triton X-100/PBS for 10 min
and blocked with 5% BSA for 30 min at 25°C. Subsequently, the
HUVECs were incubated with a primary rabbit anti-FoxO1 monoclonal
antibody (1:100) overnight at 4°C. After three washes with PBS and
incubation with FITC-labeled goat anti-rabbit IgG (1:200) secondary
antibody for 120 min at 25°C, DAPI (1:1; Beyotime Institute of
Biotechnology) was used to incubate the slides according to the
manufacturer's instructions. Images were captured using an LSM 880
(Carl Zeiss AG) laser scanning confocal microscope (magnification,
×630; 3 fields analyzed per sample).
Animals and surgical procedures
The Institutional Animal Care and Use Committee of
Nanjing Medical University approved the in vivo experimental
procedure (approval no. SYXK2016-0006). According to previous
studies (15,24), 8 week-old male C57BL/6 mice (n=8;
Nanjing Medical University Animal Center, Nanjing, China), weighing
~25 g, fed with a standard chow diet and adequate water, housed in
specific pathogen-free facilities with a 12 h light/dark cycle,
25–26°C controlled temperature, standard atmospheric pressure and
50% humidity, were euthanized with an intraperitoneal injection of
200 mg/kg sodium pentobarbital. Cold PBS was used to rinse the
aortas three times by inserting a cannula into the left ventricle.
The aortic arch and the descending aorta were separated and fixed
in 4% paraformaldehyde for 24 h at 25°C, and then embedded in
paraffin.
Immunohistochemistry of tissues
Fixed and paraffin-embedded aortic tissues were
sectioned at 5 µm. Following previously described protocols
(25), immunochemistry analysis
was conducted. PBS was used to rinse the aortic slices, followed by
30 min of 3% H2O2 treatment at 25°C for
quenching endogenous peroxidase activity and subsequent incubation
for 1 h at 25°C with 5% BSA supplemented with 0.3% Triton X-100.
The sections were incubated overnight at 4°C using a rabbit
anti-PECAM-1 antibody (1:100) for the detection of PECAM-1. PBS was
used for control staining. Incubation with HRP-labeled goat
anti-rabbit IgG (1:200) was conducted for 1 h at 25°C for
visualization. Images were captured using a BX43 light microscope
(Olympus Corporation) at magnification ×400, and CellSens Standard
software (version 1.14; Olympus Corporation). The hormone receptor
score (H-Score) was used to evaluate PECAM-1 expression, as
previously described (26).
Statistical analysis
Data are presented as the mean ± SD of each
experiment performed in triplicate. ANOVA followed by post hoc
Turkey's test or Student's t-test were performed using GraphPad
Prism (version 8.0.1; GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
LSS enhances PECAM-1 expression in
ECs
In order to observe the differences in PECAM-1
expression between the descending aorta and the aortic arch, the
entire mouse aortic arch from the aortic valve to the descending
thoracic aorta was serially sectioned, and the cross-sections were
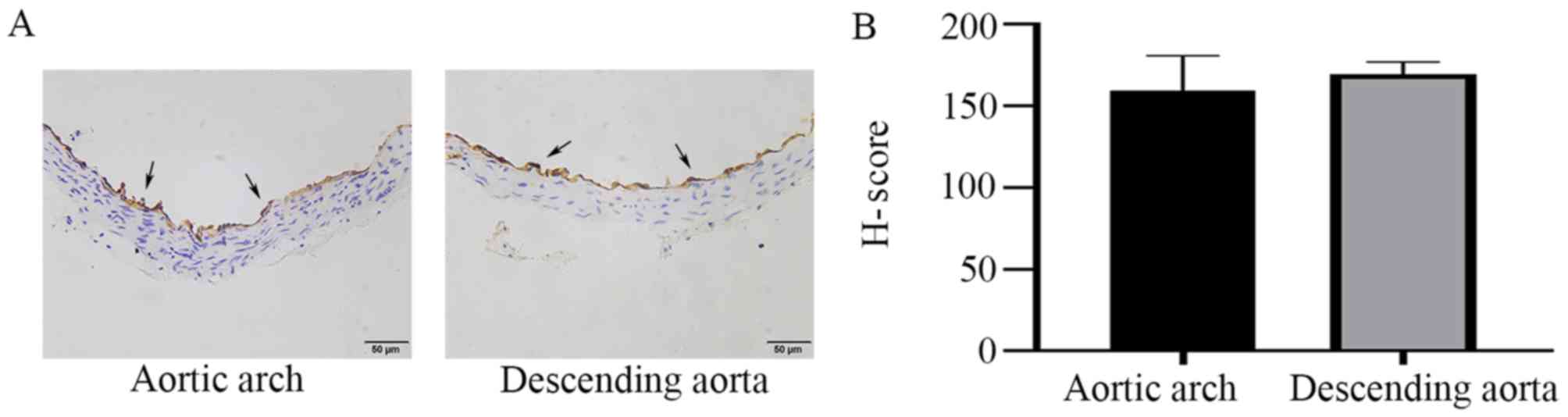
stained by immunohistochemistry. PECAM-1 was expressed in aortic
ECs of mice and was observed at similar levels in the descending
aorta and the aortic arch in the cross-sectional analysis (Fig. 1). To further investigate the
effects of LSS on PECAM-1 expression, in vitro flow
experiments were performed using a parallel-plate flow chamber
system. LSS increased the PECAM-1 protein level in the HUVECs in a
time-dependent manner (Fig. 2A and
B), and this effect was attenuated by transfection with PECAM-1
siRNA (Fig. 2C and D).
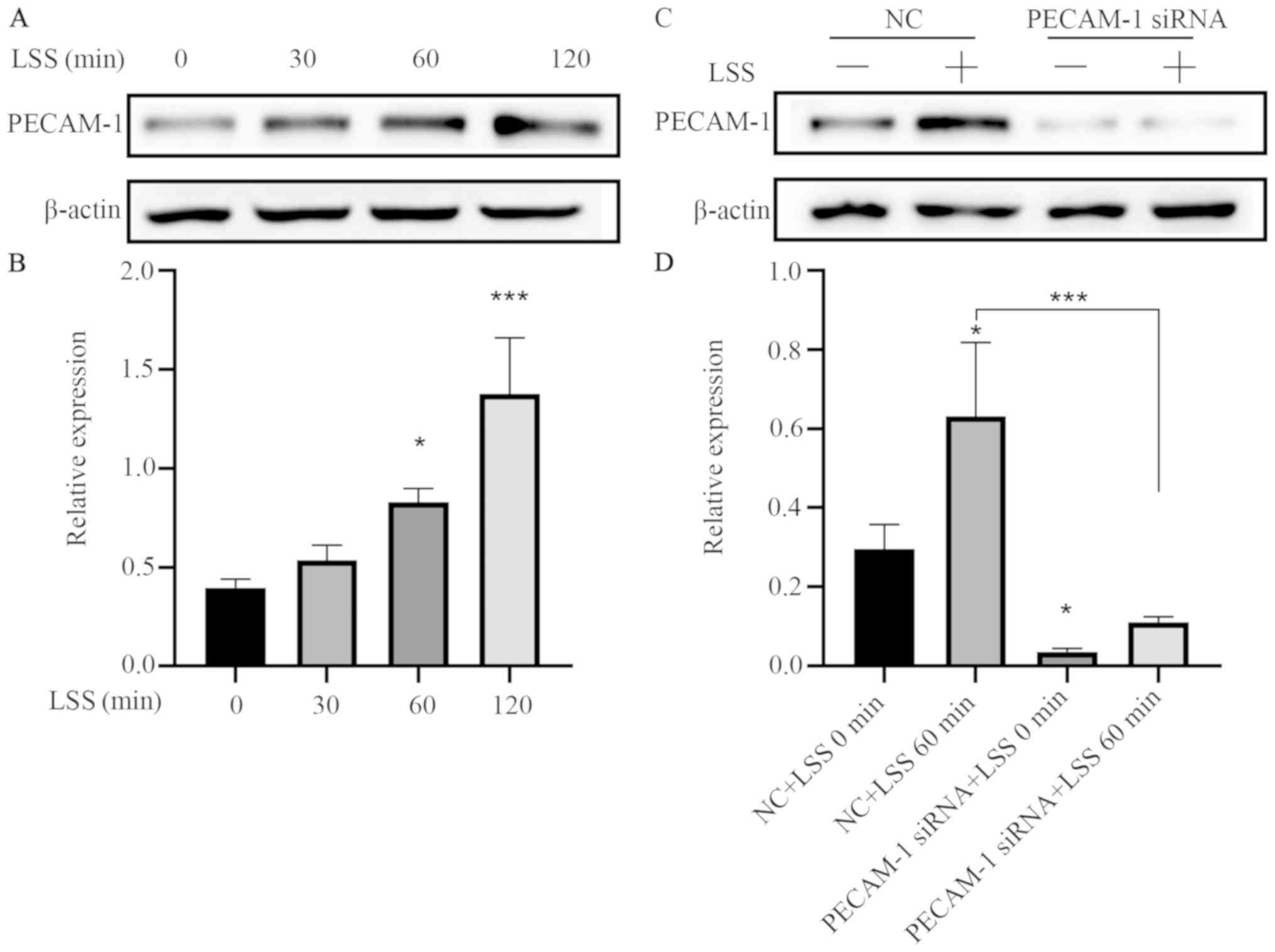 | Figure 2.LSS increases PECAM-1 expression in
HUVECs. (A) Representative immunoblots and (B) quantitative
analysis of PECAM-1 after 0, 30, 60, and 120 min of HUVEC exposure
to LSS. *P<0.05, ***P<0.001 vs. 0 min (C) Representative
immunoblots and (D) quantitative analysis of PECAM-1 in the HUVECs
transfected with NC siRNA or PECAM-1 siRNA after 60-min LSS
exposure. *P<0.05, ***P<0.001 vs. NC+LSS 0 min or as
indicated. HUVECs, human umbilical vein endothelial cells; PECAM-1,
platelet endothelial cell adhesion molecule-1; LSS, low shear
stress; NC, negative control; siRNA, small interfering RNA. |
PECAM-1 knockdown suppresses
LSS-induced adhesion of monocytes to HUVECs
Under LSS conditions, monocytes easily adhere to ECs
(27). Atherosclerosis initiation
involves the adhesion of monocytes to the endothelium, which can be
induced by LSS (27–29). Therefore, the present study
examined whether PECAM-1 regulated monocyte adhesion to HUVECs.
THP-1 cell adhesion to the HUVECs was significantly increased by
LSS compared with the unstimulated HUVECs, and this effect was
inhibited by PECAM-1 siRNA transfection (Fig. 3). These results suggested that
PECAM-1 promoted LSS-induced adhesion of monocytes to HUVECs.
PECAM-1 knockdown inhibits LSS-induced
apoptosis of HUVECs
LSS is an identified risk factor for
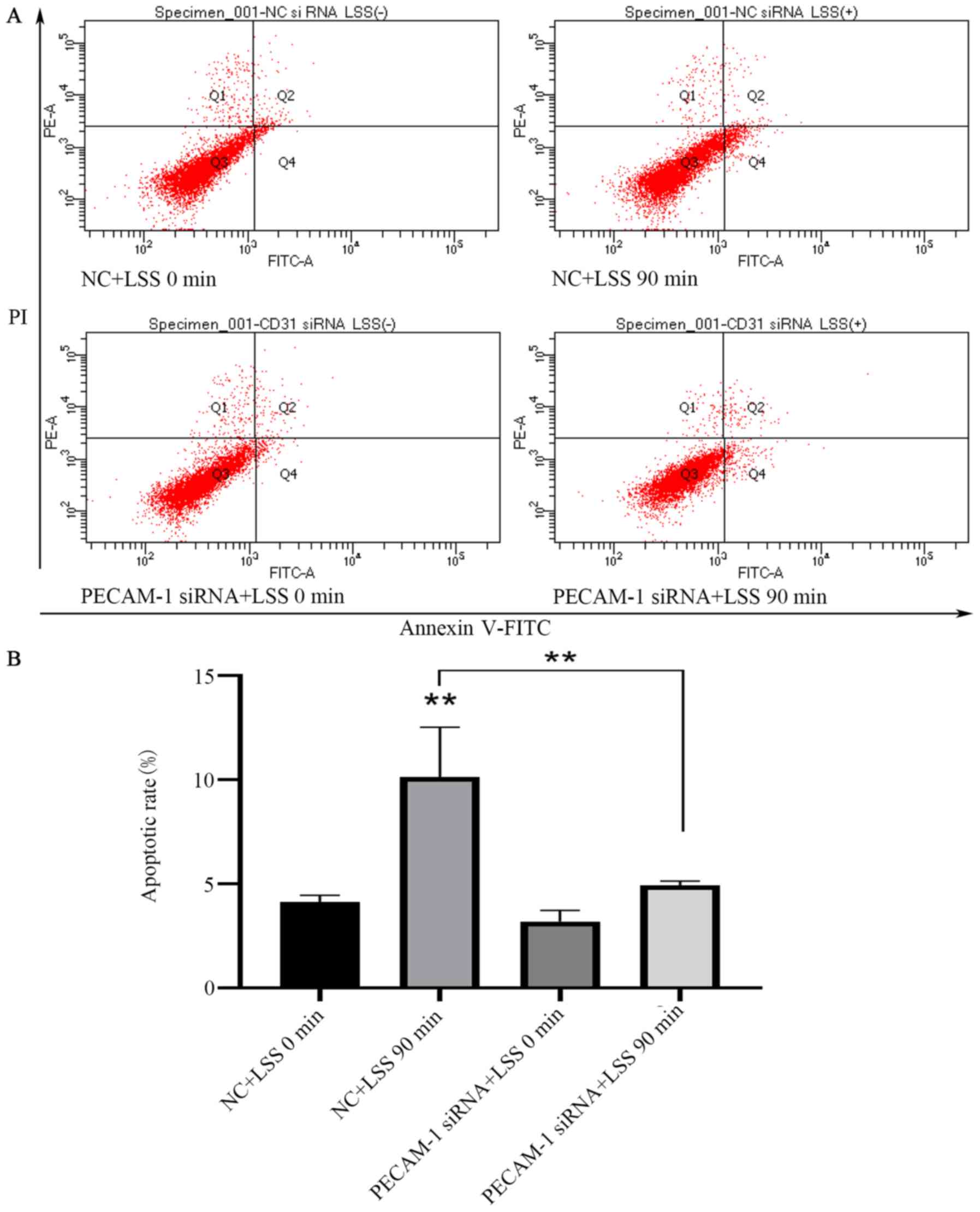
atherosclerosis, which results in the apoptosis of ECs (30). Flow cytometry was used to analyze
the effects of PECAM-1 impact on HUVEC apoptosis induced by LSS.
LSS induced a significant increase in HUVEC apoptosis compared with
unstimulated cells (Fig. 4A and
B). PECAM-1 siRNA transfection decreased the LSS-induced
apoptosis of the HUVECs compared with the NC siRNA-transfected
cells (Fig. 4A and B). These
results suggested that PECAM-1 increased the LSS-induced apoptosis
of HUVECs.
PECAM-1 knockdown increases
LSS-induced Akt and FoxO1 phosphorylation in HUVECs
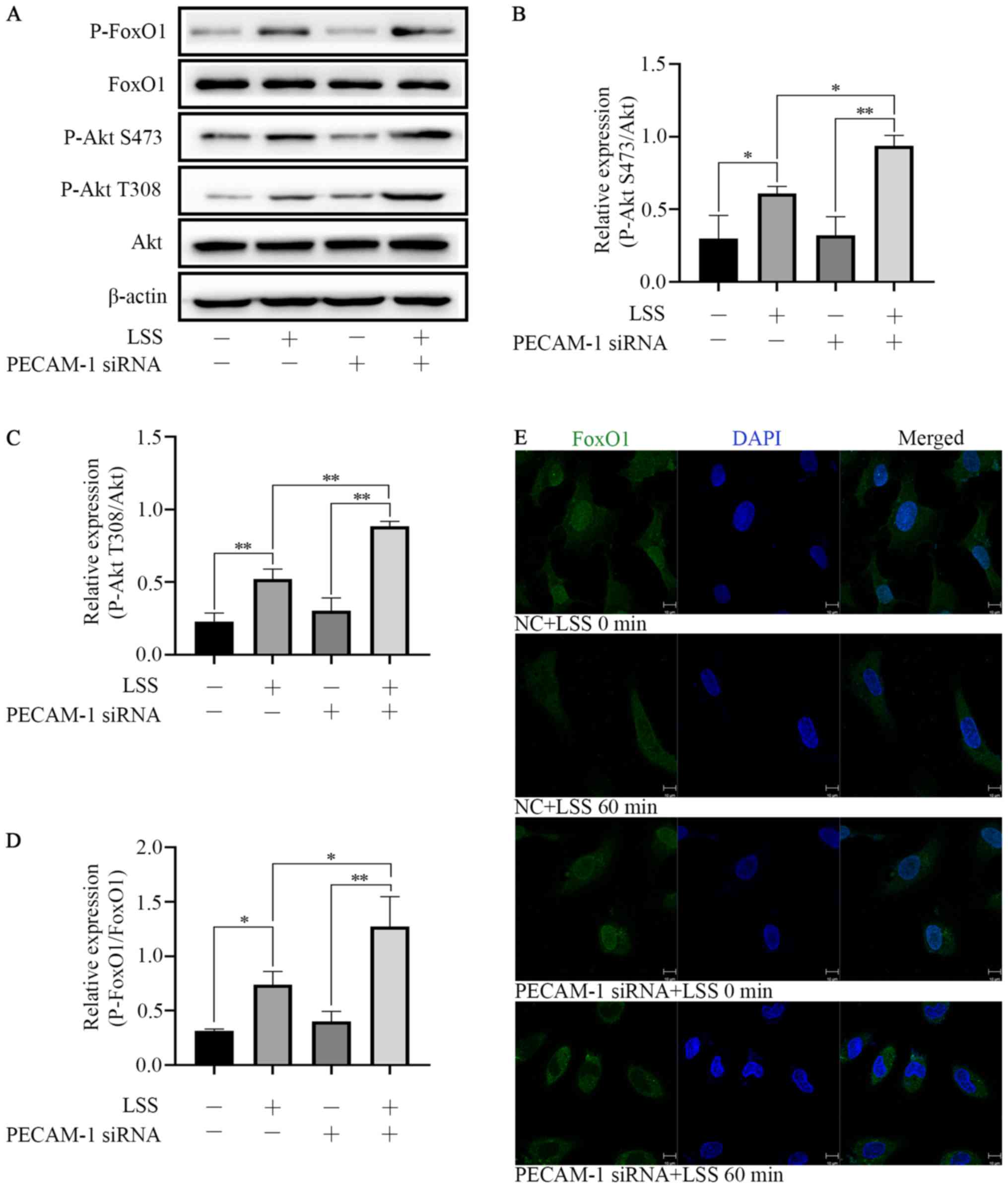
LSS induced Akt phosphorylation at Ser473 and Thr308
and FoxO1 phosphorylation at Ser256. PECAM-1 siRNA transfection
enhanced the effects of LSS-induced phosphorylation of Akt and
FoxO1 in HUVECs compared with NC siRNA-transfected cells (Fig. 5A-D). These results indicated that
both Akt and FoxO1 were regulated by PECAM-1 under LSS conditions.
In order to further define the nuclear or cytoplasmic relocation of
FoxO1 under LSS conditions with or without PECAM-1 siRNA
transfection, immunofluorescence staining was performed. LSS
induced nucleocytoplasmic transportation of FoxO1 compared with
untreated cells, and this effect was enhanced by PECAM-1 siRNA
transfection (Fig. 5E). These
results suggested that PECAM-1 knockdown inhibited LSS-induced
apoptosis of HUVECs by enhancing Akt and FoxO1 phosphorylation.
Discussion
The role of PECAM-1 in LSS-induced EC apoptosis and
monocyte adhesion was the main finding of this study. Maintenance
of vascular integrity is a major role of the endothelial barrier
(17). Macromolecules and cells
leak out of the vascular lumen into the interstitial space,
inducing inflammation if the endothelial barrier is damaged
(13). This induction of
inflammation may lead to chronic vascular diseases, such as
atherosclerosis, in which barrier function is disrupted and vessel
integrity cannot be restored (17). Anti-inflammatory and
anticoagulation states, as well as normal blood flow are maintained
as a result of the vascular integrity provided by dormant ECs under
appropriate physiological conditions. Once a stimuli is received,
ECs are activated and undergo apoptosis, which weakens barrier
function, resulting in increased permeability and extravasation of
inflammatory cells (predominantly leukocytes) and proteins into the
inflamed areas (31). PECAM-1 is
crucial for stimuli, such as shear stress, which regulate vascular
barrier function. Leukocytes, platelets and ECs express PECAM-1 to
regulate cell-cell interactions (32). Emerging evidence has revealed that
PECAM-1 produces either proatherogenic or antiatherogenic effects,
depending on the local hemodynamic environment (19–21).
Specifically, PECAM-1 is proatherogenic in the aortic arch where
LSS is present, but antiatherogenic in the descending aorta where
high shear stress is present (20,21).
Under normal conditions, EC barrier properties are supported by
PECAM-1 and are influenced by the actions of extracellular
messengers, such as cytokines and vascular endothelial growth
factor-A, as well as blood flow (32). Under inflammatory conditions, such
as atherosclerotic vessels, mediators, including proinflammatory
cytokines, which enhance adhesion properties and decrease vascular
integrity, have a large impact on PECAM-1 function. Enhanced
leukocyte transendothelial migration levels contribute to
atherogenesis and atherosclerotic plaque formation (17). High PECAM-1 expression has been
observed in vascular cells, including leukocytes, platelets and
other blood-borne cells, as well as ECs. Junctional and adhesive
properties in ECs are controlled by PECAM-1. Under certain
physiological conditions, PECAM-1 also supports the endothelial
barrier function (33–35). However, atherosclerosis causes
vessel inflammation, which impairs the function of PECAM-1, leading
to an increase in leukocytes adhered to ECs, vascular integrity
loss and increased transmigration of leukocytes to the intima media
(18). The present study
demonstrated that LSS enhanced PECAM-1 expression in a
time-dependent manner in HUVECs. Knockdown of PECAM-1 inhibited
LSS-induced EC apoptosis and monocyte adhesion.
Previous studies have demonstrated that PECAM-1
modulates the phosphorylation of Akt (17,36,37),
which initiates the phosphorylation of FoxO and subsequently rapid
relocation to the cytoplasm from the nucleus, leading to the
inhibition of pro-apoptotic activity (38). Based on these findings, the present
study speculated that the Akt/FoxO1 signaling pathway may be
involved in PECAM-1-mediated LSS-induced HUVEC apoptosis. The
present study further demonstrated that PECAM-1 had an effect on
the phosphorylation of Akt, indicating that PECAM-1 plays a role in
the activation of Akt under LSS. As downstream targets of Akt
(serine/threonine protein kinase B or PKB), FoxO transcription
factors cause apoptosis and quiescence by limiting cell
proliferation (38). The present
study also demonstrated that the phosphorylation of FoxO1 occurred
in the same direction as Akt phosphorylation. Phosphorylation of
FoxOs by Akt leads to nuclear exclusion and inactivation,
inhibiting FoxO transcriptional functions, while enhancing cell
proliferation, growth and survival (38–40).
It was further confirmed that FoxO1 relocalization from the nucleus
to the cytoplasm followed the phosphorylation of FoxO1. FoxOs can
induce the expression of multiple proapoptotic proteins of the
mitochondria-targeting Bcl2 family, whereas the expression of death
receptor ligands are stimulated to enhance apoptosis signaling
and/or cell growth inhibition (38). A method by which cell survival is
promoted by Akt is the sequestration of FoxOs from apoptotic gene
promoters. Therefore, FoxOs can drive the expression of multiple
apoptotic genes by functioning as important Akt signaling effector
arms (41–43). Previous studies have suggested that
FoxO1 is phosphorylated and translocated to the cytoplasm from the
nucleus, causing FoxO1-mediated apoptosis inhibition (42,44).
The results of the present study demonstrated that PECAM-1
knockdown further promoted the phosphorylation of Akt/FoxO1 and
mitigated LSS-induced apoptosis of HUVECs, which suggested that
PECAM-1 knockdown inhibiting LSS-induced HUVEC apoptosis may be
relevant to the Akt/FoxO1 signaling pathway.
In conclusion, the present study revealed that
PECAM-1 promoted LSS-induced EC apoptosis and monocyte adhesion.
PECAM-1 knockdown inhibiting LSS-induced apoptosis of ECs may be
mediated by enhancing the Akt/FoxO1 signaling pathway activation.
The results also indicated that PECAM-1 may be a promising target
for the treatment of atherosclerosis.
Acknowledgements
The authors would like to thank Dr Jian Li
(Department of Immunology) and Dr Yuelin Chao (Department of
Cardiology, Nanjing First Hospital, Nanjing Medical University,
Nanjing, China) for their technical assistance.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81700239 and 81770441).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
XX, FW and SC designed the study. XX, FW, LZ, HY,
DP, YL, XQ, YG and XL performed the experiments. XX performed the
data analysis. XX and SC drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Nanjing Medical University
(approval no. SYXK2016-0006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malekmohammad K, Sewell RDE and
Rafieian-Kopaei M: Antioxidants and atherosclerosis: Mechanistic
aspects. Biomolecules. 9:E3012019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farmakis TM, Soulis JV, Giannoglou GD,
Zioupos GJ and Louridas GE: Wall shear stress gradient topography
in the normal left coronary arterial tree: Possible implications
for atherogenesis. Curr Med Res Opin. 20:587–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandoo A and Kitas GD: A methodological
approach to non-invasive assessments of vascular function and
morphology. J Vis Exp. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gimbrone MA Jr and Garcia-Cardena G:
Endothelial cell dysfunction and the pathobiology of
atherosclerosis. Circ Res. 118:620–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huynh DTN and Heo KS: Therapeutic targets
for endothelial dysfunction in vascular diseases. Arch Pharm Res.
42:848–861. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heo KS, Berk BC and Abe J: Disturbed
flow-induced endothelial proatherogenic signaling via regulating
post-translational modifications and epigenetic events. Antioxid
Redox Signal. 25:435–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penna C, Tullio F, Femmino S, Rocca C,
Angelone T, Cerra MC, Gallo MP, Gesmundo I, Fanciulli A, Brizzi MF,
et al: Obestatin regulates cardiovascular function and promotes
cardioprotection through the nitric oxide pathway. J Cell Mol Med.
21:3670–3678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godo S and Shimokawa H: Divergent roles of
endothelial nitric oxide synthases system in maintaining
cardiovascular homeostasis. Free Radic Biol Med. 109:4–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar A, Hung OY, Piccinelli M, Eshtehardi
P, Corban MT, Sternheim D, Yang B, Lefieux A, Molony DS, Thompson
EW, et al: Low coronary wall shear stress is associated with severe
endothelial dysfunction in patients with nonobstructive coronary
artery disease. JACC Cardiovasc Interv. 11:2072–2080. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siasos G, Sara JD, Zaromytidou M, Park KH,
Coskun AU, Lerman LO, Oikonomou E, Maynard CC, Fotiadis D, Stefanou
K, et al: Local low shearstress and endothelial dysfunction in
patients with nonobstructive coronary atherosclerosis. J Am Coll
Cardiol. 71:2092–2102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plank MJ, Wall DJ and David T:
Atherosclerosis and calcium signalling in endothelial cells. Prog
Biophys Mol Biol. 91:287–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oakley R and Tharakan B: Vascular
hyperpermeability and aging. Aging Dis. 5:114–125. 2014.PubMed/NCBI
|
|
14
|
Green J, Yurdagul A Jr, McInnis MC, Albert
P and Orr AW: Flow patterns regulate hyperglycemia-induced
subendothelial matrix remodeling during earlyatherogenesis.
Atherosclerosis. 232:277–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao Y, Zhu L, Qu X, Zhang J, Zhang J,
Kong X, Gu Y, Pu J, Wu W, Ye P, et al: Inhibition of angiotension
II type 1 receptor reduced human endothelial inflammation induced
by low shear stress. Exp Cell Res. 360:94–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng YM, Chen XH and Zhang X: Roles of
PECAM-1 in cell function and disease progression. Eur Rev Med
Pharmacol Sci. 20:4082–4088. 2016.PubMed/NCBI
|
|
17
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial PECAM-1 and its function in vascular physiology and
atherogenic pathology. Exp Mol Pathol. 100:409–415. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lertkiatmongkol P, Liao D, Mei H, Hu Y and
Newman PJ: Endothelial functions of platelet/endothelial cell
adhesion molecule-1 (CD31). Curr Opin Hematol. 23:253–259. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harry BL, Sanders JM, Feaver RE, Lansey M,
Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, et
al: Endothelial cell PECAM-1 promotes atherosclerotic lesions in
areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb
Vasc Biol. 28:2003–2008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goel R, Schrank BR, Arora S, Boylan B,
Fleming B, Miura H, Newman PJ, Molthen RC and Newman DK:
Site-specific effects of PECAM-1 on atherosclerosis in LDL
receptor-deficient mice. Arterioscler Thromb Vasc Biol.
28:1996–2002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harrison M, Smith E, Ross E, Krams R,
Segers D, Buckley CD, Nash GB and Rainger GE: The role of
platelet-endothelial cell adhesion molecule-1 in atheroma formation
varies depending on the site-specific hemodynamic environment.
Arterioscler Thromb Vasc Biol. 33:694–701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Wang Z, Zhang J, Zuo G, Li B, Mao
W and Chen S: Rapamycin attenuates endothelial apoptosis induced by
low shear stress via mTOR and sestrin1 related redox regulation.
Mediators Inflamm. 2014:7696082014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rezvan A, Ni CW, Alberts-Grill N and Jo H:
Animal, in vitro, and ex vivo models of flow-dependent
atherosclerosis: Role of oxidative stress. Antioxid Redox Signal.
15:1433–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramkhelawon B, Vilar J, Rivas D, Mees B,
de Crom R, Tedgui A and Lehoux S: Shear stress regulates
angiotensin type 1 receptor expression in endothelial cells. Circ
Res. 105:869–875. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Avivi C, Rosen O and Goldstein RS: New
chromogens for alkaline phosphatase histochemistry: Salmon and
magenta phosphate are useful for single- and double-label
immunohistochemistry. J Histochem Cytochem. 42:551–554. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Specht E, Kaemmerer D, Sanger J, Wirtz RM,
Schulz S and Lupp A: Comparison of immunoreactive score,
HER2/neu-Score and H-Score for the immunohistochemical evaluation
of somatostatin receptors in bronchopulmonary neuroendocrine
neoplasms. Histopathology. 67:368–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang M, Sun J, Chen B, Zhao Y, Gong H,
You Y and Qi R: Ginkgolide B inhibits platelet and monocyte
adhesion in TNFα-treated HUVECs under laminar shear stress. BMC
Complement Altern Med. 18:2202018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis FM and Gallagher KA: Epigenetic
mechanisms in monocytes/macrophages regulate inflammation in
cardiometabolic and vascular disease. Arterioscler Thromb Vasc
Biol. 39:623–634. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fong GH: Potential contributions of
intimal and plaque hypoxia to atherosclerosis. Curr Atheroscler
Rep. 17:5102015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong G, Yang S, Cao X, Yu N, Yu J and Qu
X: Low shear stress-induced autophagy alleviates cell apoptosis in
HUVECs. Mol Med Rep. 15:3076–3082. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Danese S, Dejana E and Fiocchi C: Immune
regulation by microvascular endothelial cells: Directing innate and
adaptive immunity, coagulation, and inflammation. J Immunol.
178:6017–6022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarelius IH and Glading AJ: Control of
vascular permeability by adhesion molecules. Tissue Barriers.
3:e9859542015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Muller WA, Weigl SA, Deng X and Phillips
DM: PECAM-1 is required for transendothelial migration of
leukocytes. J Exp Med. 178:449–460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Privratsky JR, Paddock CM, Florey O,
Newman DK, Muller WA and Newman PJ: Relative contribution of
PECAM-1 adhesion and signaling to the maintenance of vascular
integrity. J Cell Sci. 124:1477–1485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Privratsky JR and Newman PJ: PECAM-1:
Regulator of endothelial junctional integrity. Cell Tissue Res.
355:607–619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fleming I, Fisslthaler B, Dixit M and
Busse R: Role of PECAM-1 in the shear-stress-induced activation of
Akt and the endothelial nitric oxide synthase (eNOS) in endothelial
cells. J Cell Sci. 118:4103–4111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones CI, Sage T, Moraes LA, Vaiyapuri S,
Hussain U, Tucker KL, Barrett NE and Gibbins JM: Platelet
endothelial cell adhesion molecule-1 inhibits platelet response to
thrombin and von Willebrand factor by regulating the
internalization of glycoprotein Ib via AKT/glycogen synthase
kinase-3/dynamin and integrin αIIbβ3. Arterioscler Thromb Vasc
Biol. 34:1968–1976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilhelm K, Happel K, Eelen G, Schoors S,
Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger
T, et al: FoxO1 couples metabolic activity and growth state in the
vascular endothelium. Nature. 529:216–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dharaneeswaran H, Abid MR, Yuan L, Dupuis
D, Beeler D, Spokes KC, Janes L, Sciuto T, Kang PM, Jaminet SS, et
al: FoxO1-mediated activation of Akt plays a critical role in
vascular homeostasis. Circ Res. 115:238–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li F, Qu H, Cao HC, Li MH, Chen C, Chen
XF, Yu B, Yu L, Zheng LM and Zhang W: Both FoxO3a and FoxO1 are
involved in the HGF-protective pathway against apoptosis in
endothelial cells. Cell Biol Int. 39:1131–1137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xing YQ, Li A, Yang Y, Li XX, Zhang LN and
Guo HC: The regulation of FoxO1 and its role in disease
progression. Life Sci. 193:124–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu Z and Tindall DJ: FoxOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cifarelli V, Lee S, Kim DH, Zhang T,
Kamagate A, Slusher S, Bertera S, Luppi P, Trucco M and Dong HH:
FoxO1 mediates the autocrine effect of endothelin-1 on endothelial
cell survival. Mol Endocrinol. 26:1213–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|