Introduction
Idiopathic scoliosis is the most common form of
scoliosis; it is characterized by a complicated three-dimensional
spinal deformity that is accompanied by a rotation of the vertebrae
body (1–6). Several studies have reported on the
influence of the pineal body on experimentally induced spinal
scoliosis, and numerous researchers hypothesize that defects in the
synthesis and metabolism of melatonin secreted from the pineal body
is a key factor underlying spinal scoliosis (7–9). The
pineal body secretes the neuroendocrine hormone melatonin, which
serves an important role in regulating physiological and
pathological effects in the human body, including bone growth and
vertebrae body molding (10,11).
A prospective study demonstrated that melatonin deficiency may play
a key role in the prognosis of idiopathic scoliosis, the progress
of which may be prevented by melatonin supplements (12).
Our previous studies demonstrated that melatonin
exerts a dual effect on osteoblast proliferation that is dependent
on concentration; 1 nM-100 µM melatonin can stimulate cell
proliferation, whereas 1 mM melatonin can significantly delay
osteoblast proliferation (13–14).
It was also found that melatonin could upregulate the concentration
of calcium ions, activate the calcium pathway, disrupt homeostasis
of intracellular calcium, and lead to calcium overload (15–20).
Calcium overload induces endoplasmic reticulum stress (ERS), which
is associated with autophagy and apoptosis. Septins belong to a
class of cytoskeletal proteins with GTPase activity, which can form
intracellular filamentous scaffolds. Previous studies have
demonstrated that septins are involved in numerous biological
processes, such as cell mitosis, polarity determination, vesicle
trafficking and apoptosis (21–23).
In addition, our previous study revealed that septin-7, a member of
septin family, has an inhibitory effect in melatonin-induced
apoptosis (15). However, the
mechanisms underlying the inhibition of apoptosis by septin-7
hasn't been described comprehensively and the effect of autophagy
in this process is still unclear. The present study was designed to
further clarify the mechanisms by which melatonin induces
osteoblast apoptosis, thus offering an improved understanding of
the roles of ERS, autophagy and apoptosis in melatonin-induced
osteoblasts. The insights from the present study may be useful in
the investigation of melatonin as a potential treatment of
idiopathic scoliosis.
Materials and methods
Reagents
Melatonin, ERS inhibitor 4PBA, autophagy inhibitor
3MA and Hoechst 33342 stain were obtained from Sigma-Aldrich (Merck
KGaA). An Annexin V-FITC/PI Apoptosis Detection kit was obtained
from Nanjing KeyGen Biotech Co., Ltd. Primary antibodies against
microtubule-associated protein 1 light chain 3β (LC3; 1:1,000; cat.
no. ab128025), glucose-regulated protein, 78 kDa (GRP78; 1:1,000;
cat. no. ab21685), septin7 (1:1,000; cat. no. ab186021) were
obtained from Abcam. Primary antibodies against poly (ADP-ribose)
polymerase 1 (PARP-1; 1:1,000; cat. no. 9532), C/EBP homologous
protein (CHOP; 1:1,000; cat. no. 5554), β-actin (1:1,000; cat. no.
4970) were obtained from Cell Signaling Technology, Inc., as were
fluorescent anti-rabbit secondary antibodies (1:500; cat. no.
4414).
Cell culture
The human fetal osteoblastic cell line hFOB 1.19
kindly provided by Dr Mamayannan Subramaniam (Department of
Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN,
USA), was maintained in a 1:1 mixture of Ham's F-12
medium/Dulbecco's modified Eagle's medium without phenol red
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10%
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences) at
37°C in a humidified 5% CO2 atmosphere. The medium was
changed every other day. The cells were utilized at passages 8–12
and plated at 104 cells/cm2 for 4, 24 and 48
h before treatment. Cells were treated with melatonin, which was
dissolved in 0.2% dimethyl sulfoxide (DMSO), or vehicle (0.2% DMSO
in culture medium only) media containing 10% FBS.
Apoptosis assay
Apoptosis was detected using the Annexin V-FITC/PI
Apoptosis Detection kit according to the manufacturer's protocols.
Briefly, hFOB 1.19 cells were seeded onto 6-well plates
(5×105 cells/well). After treatment with 1, 2, 4 and 8
mM melatonin for 24 h in 5% CO2 at 37°C, cells were
harvested by trypsin digestion without EDTA, resuspended in 500 µl
final volume of binding buffer, and incubated with 5 µl of Annexin
V-FITC and 5 µl of propidium iodide (PI) solution for 15 min at
room temperature in the dark. Finally, cells were analyzed using a
BD FACScan flow cytometer equipped with ModFit LT v3.0 (Becton,
Dickinson and Company) within 1 h. Viable cells (Annexin
V-FITC−/PI−), early apoptotic cells (Annexin
V-FITC+/PI−), late apoptotic cells (Annexin
V-FITC+/PI+) and necrotic cells (Annexin
V-FITC−/PI+).
Chromatin condensation assay
Chromatin condensation in the nucleus was observed
using the fluorescent Hoechst 33342 stain. hFOB 1.19 were seeded
onto 24-well plates (2×103 cells/well). After treatment
with 2 mM melatonin for 24 h, cells were fixed in 4%
paraformaldehyde fix solution for 15 min at room temperature, and
incubated with a concentration of 2 µg/ml of Hoechst 33342 for 15
min at room temperature in the dark. After washing three times with
PBS, cells were observed and images captured under a fluorescent
microscope.
Septin7 small interfering (si)RNA
transfection
Cells were cultured as aforementioned. Using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols, cells
were transfected with 60 nm septin7 siRNA (forward,
5′-CGACUACAUUGAUAGUAAAUU-3′ and reverse,
5′-UUUACUAUCAAUGUAGUCGAU-3′) purchased from Shanghai GeneChem Co.,
Ltd., at 37°C for 48 h. There were three control groups: i) An
untreated blank control; ii) transfection reagent control (to
control for potentially toxic influence of Lipofectamine 2000 and
the influence of Lipofectamine 2000 on the expression of the target
gene); and scramble siRNA control (5′-GAAATTTATAACGATCAGTCT-3′)
purchased from Shanghai GeneChem Co., Ltd. The time interval
between transfection and subsequent experimentation was 24 h.
Overexpression plasmid construction
and rescue experiment
Septin7 was cloned into GV230 plasmids (200 ng;
Shanghai GeneChem Co., Ltd.) between XhoI and KpnI
restriction sites to overexpress septin7 in hFOB 1.19 cells. The
full-length septin7 gene (4,377 bp; reference sequence
NM_001011553) was amplified by PCR using the following primers:
Forward, 5′-CTGCTCACAATAGTTGATACCCC-3′ and reverse,
5′-TGTTCACTCGTGATTCTGCATT-3′. PrimeSTAR HS DNA polymerase was
obtained from GeneChem, Inc., and cycled for 30 cycles following
initial denaturation (98°C for 5 min) with the following
parameters: 72°C for 8 min. Following enzyme digestion
(Exnase™ II; Vazyme Biotech Co., Ltd.; 1 µl) using
ClonExpress II One Step Cloning kit (Vazyme Biotech Co., Ltd.) and
sequencing, the PCR product was cloned into the
XhoI/KpnI sites of the GV230 expression vector. The
recombinant GV230-septin7 plasmid was confirmed by endonuclease
digestion and DNA sequencing (GeneChem, Inc.) prior to transfection
into osteoblasts using Lipofectamine 2000 (37°C for 48 h; 90% cell
density) according to the manufacturer's protocols (Invitrogen;
Thermo Fisher Scientific, Inc.). The rescue experiment using 45 nM
septin7 overexpression plasmid was performed following transfection
of the septin7 siRNA. The time interval between transfection and
subsequent experimentation was 24 h.
Immunoblotting
Cells were lysed in RIPA buffer on ice for 30 min.
Protein fractions were centrifuged for 15 min at 4°C at 12,000 × g,
and supernatants containing total protein were harvested. Then,
aliquots containing 50 µg protein were separated by 12% SDS-PAGE
and transferred onto PVDF membranes. The membranes were blocked
with 5% BSA (Beyotime Institute of Biotechnology) at 4°C for 2 h,
and incubated with primary antibodies against PARP-1, LC3-II,
GRP78, CHOP, septin7 or β-actin overnight at 4°C. Following
incubation, a fluorescent anti-rabbit secondary antibody was
applied for 2 h at 4°C, and blots were visualized using an ECL
system (Analytik Jena US, LLC). Protein levels were normalized to
the corresponding β-actin band, and the optical density was
semi-quantified using ImageJ Software (National Institutes of
Health).
Statistical analysis
Statistical analysis among groups was performed
using one-way ANOVA with Tukey's test to evaluate the differences
between melatonin, Septin 7 siRNA or overexpression plasmid groups
(RNA and protein level), and two-way ANOVA with Tukey's test was
used to evaluate the differences between melatonin, 4PBA, 3MA or
combined groups (protein level). Data were expressed as the mean ±
SEM. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of melatonin on osteoblast
apoptosis
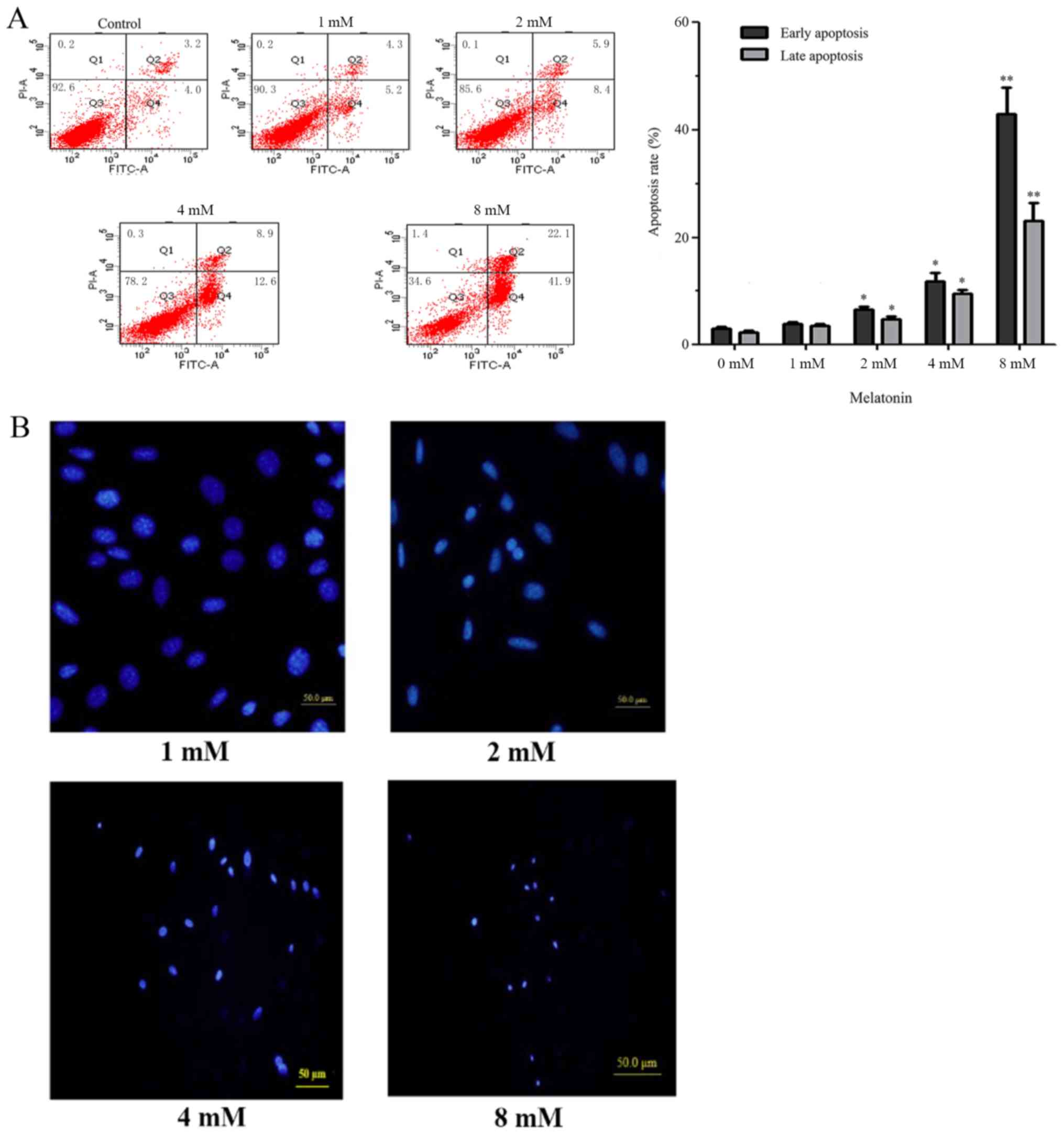
Osteoblast apoptosis was detected by flow cytometry
with the Annexin V-FITC/PI Apoptosis Detection kit. The results
indicated that when hFOB 1.19 cells were treated with melatonin (1,
2, 4 and 8 mM) for 24 h, the early and late apoptotic rates of
osteoblast cells increased significantly compared with the control
group in a concentration-dependent manner (Fig. 1A). The inhibitory effect at 2 mM
concentration was most suitable; at 4 and 8 mM, early apoptotic
rates increased significantly, resulting in cell death.
Subsequently, fluorescence microscopy with the fluorescent Hoechst
33342 stain was used to observe chromatin condensation in the
nucleus and apoptotic body formation. The results demonstrated that
the nuclei became dense in a melatonin concentration-dependent
manner (Fig. 1B).
Effects of melatonin on PARP-1
expression
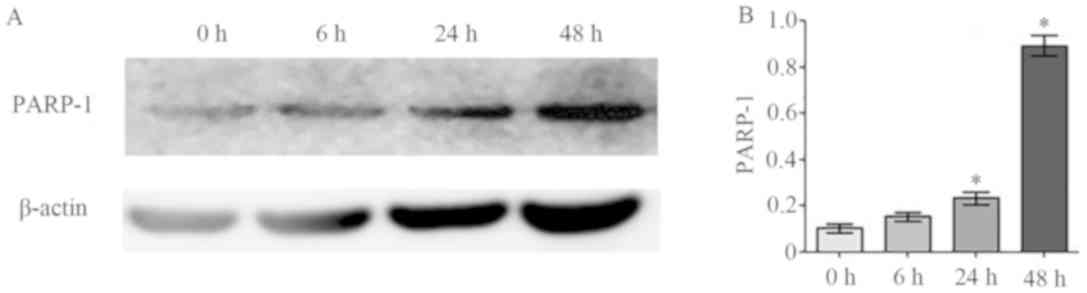
The aforementioned results indicated that apoptosis
rates increased in osteoblasts induced by 2 mM melatonin. This was
further examined by measuring protein expression levels of the
apoptosis marker protein PARP-1 in cells treated with 2 mM
melatonin at 0, 6, 24 and 48 h (Fig.
2). The results demonstrated that PARP-1 expression was
significantly increased in a time-dependent manner.
Effects of melatonin on CHOP and GRP78
expression
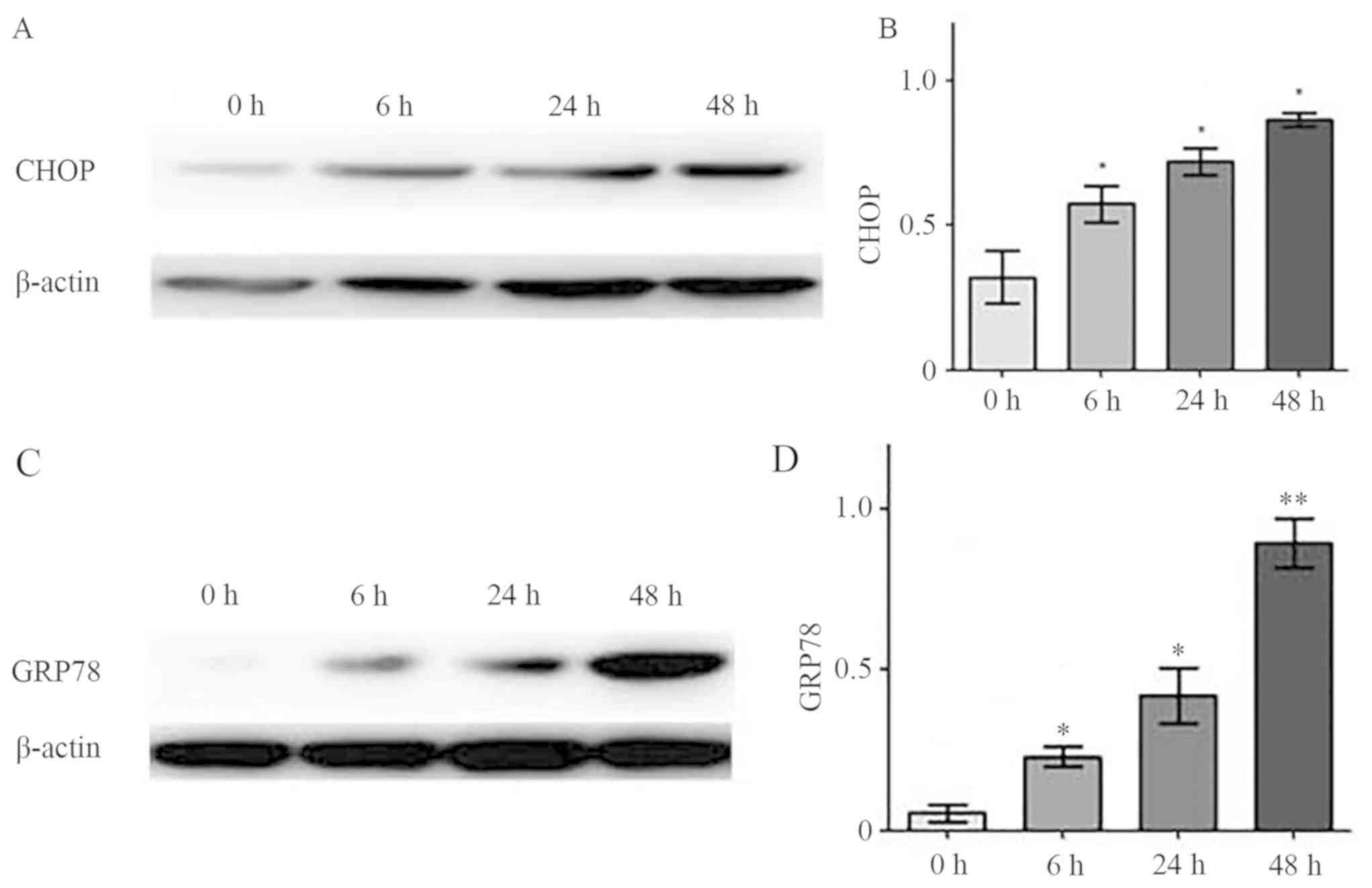
CHOP and GRP78 are marker proteins of ERS-induced
apoptosis; as such, the expression levels of these proteins were
examined in hFOB 1.19 cells treated with 2 mM melatonin for 0, 6,
24 or 48 h. Western blotting results indicated that CHOP and GRP78
expressions increased significantly over time compared with
treatment for 0 h, and this was in a time-dependent manner
(Fig. 3A and B, respectively).
Effects of melatonin on osteoblast
autophagy
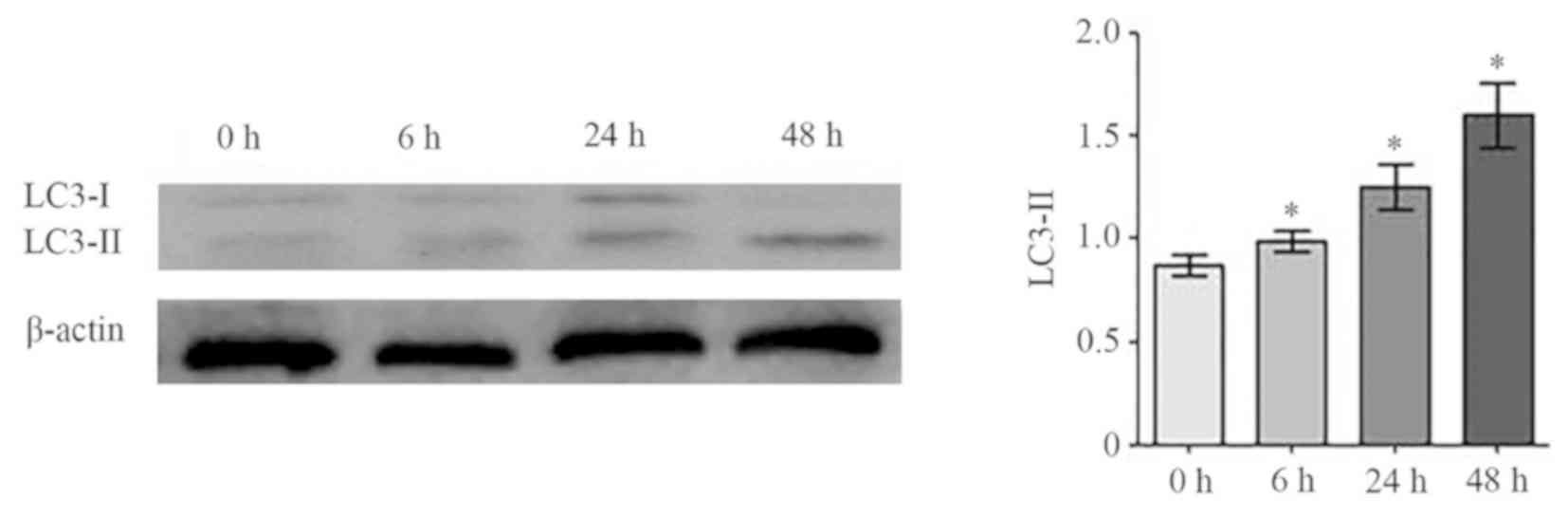
To examine the role of autophagy in
melatonin-induced osteoblast apoptosis, expression levels of the
autophagy marker protein LC3-I/II was examined in cells treated
with 2 mM melatonin at 6, 24 and 48 h. As presented in Fig. 4, LC3-II protein expression
increased significantly over time compared with the control
(P<0.05), with the highest levels observed at 48 h.
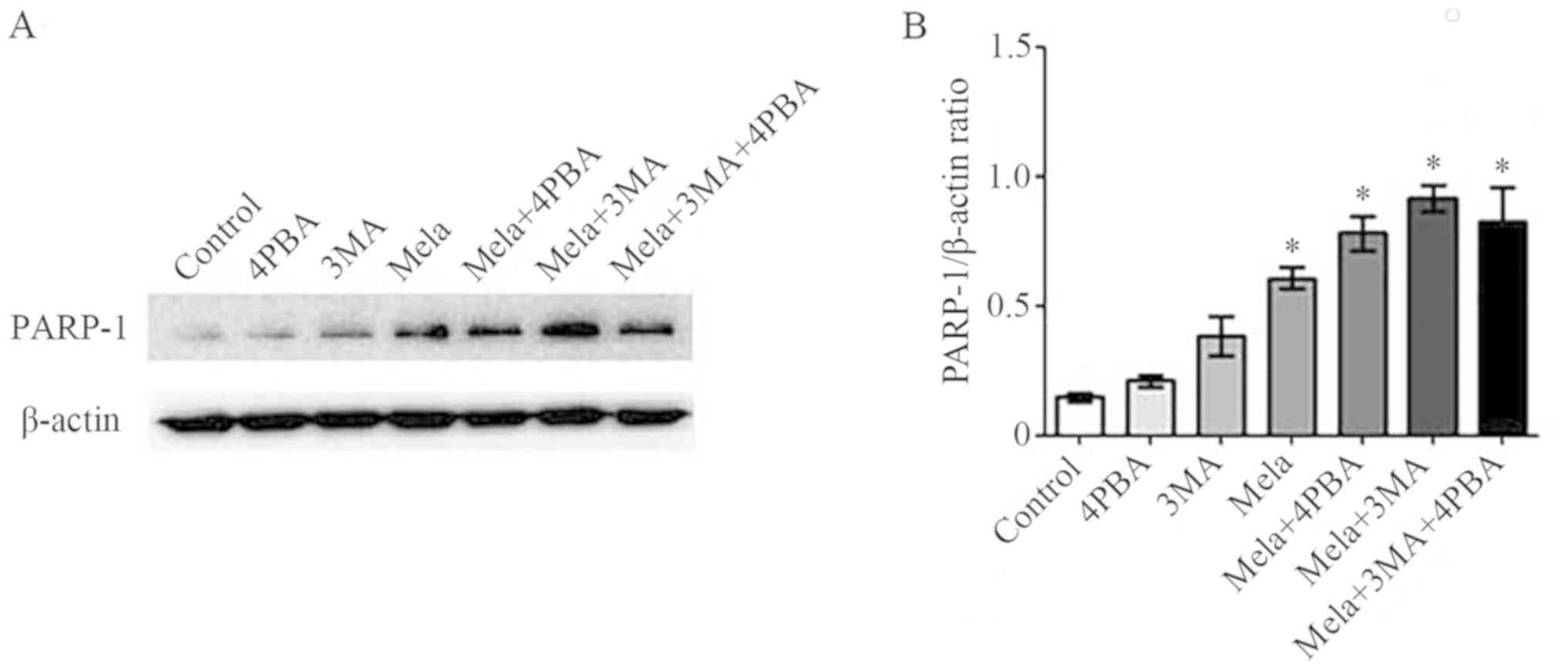
Relationship between apoptosis,
autophagy and ERS
To further investigate the association between
apoptosis, autophagy and ERS in melatonin-treated osteoblasts,
cells were incubated with either 2 mM melatonin, 4PBA (ERS
inhibitor), 3MA (autophagy inhibitor), or two or three of these in
combination for 24 h, and the expression levels of PARP-1 protein
was determined using western blotting. The results showed that
expression of PARP-1 was not significantly different when cells
were treated with 4PBA or 3MA alone compared with the control;
however, when osteoblast cells were treated with a combination of
4PBA or 3MA and 2 mM melatonin (or all three) for 24 h, the
expression of PARP-1 increased significantly compared with the
control (Fig. 5).
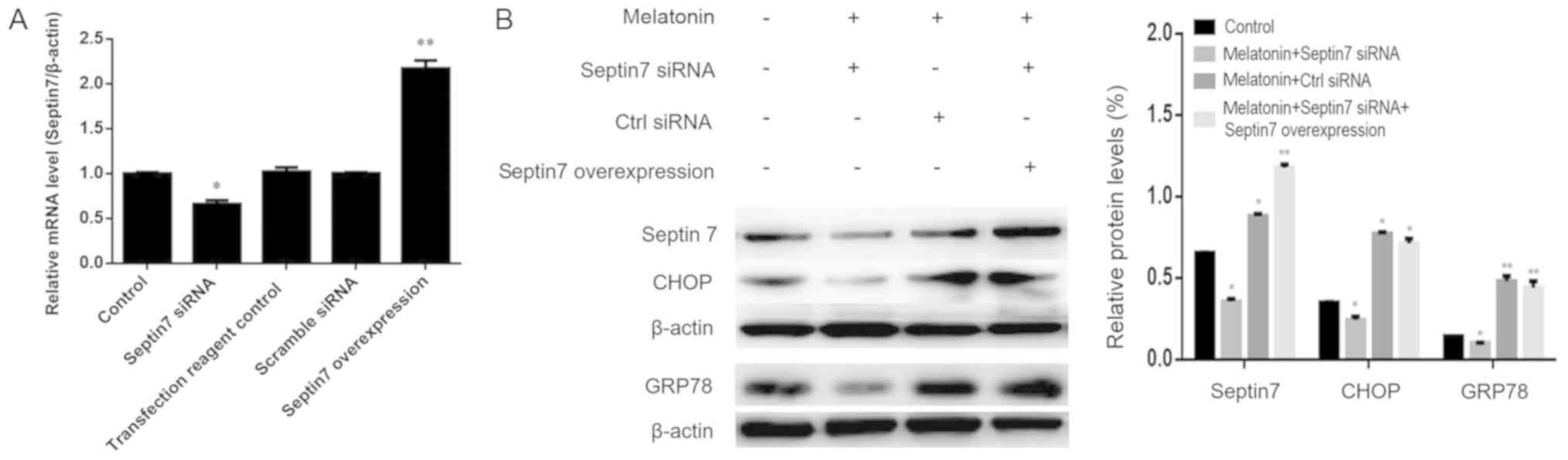
Relationship between septin7 and
melatonin-induced ERS
To improve the understanding of the relationship
between septin7 and ERS in melatonin-induced osteoblasts, septin7
expression was knocked down using siRNA. Osteoblasts transfected
with septin7 siRNA exhibited a significant decrease in septin7 mRNA
expression compared with the scramble siRNA, confirming successful
transfection (Fig. 6A). septin7
siRNA transfection led to a decrease in GRP78 and CHOP protein
expression levels (Fig. 6B),
although the changes were not significant, even when in combination
with melatonin. These data suggested that melatonin-mediated ERS
may induce osteoblast proliferation by regulating septin7
expression.
It was also determined whether exogenous expression
of septin7 could rescue the inhibition of GRP78 and CHOP expression
caused by septin7 knockdown in osteoblasts by co-transfections with
a septin7 overexpression plasmid. The expression levels of septin7,
GRP78 and CHOP proteins were evaluated by western blotting. As
shown in Fig. 6B, exogenous
expression of septin7 restored cellular septin7 levels in septin7
knockdown cells induced by melatonin. It was also found that when
transfected with the septin7 overexpression plasmid, osteoblasts
showed significantly higher GRP78 and CHOP expression compared with
the control.
Discussion
Melatonin is a neuroendocrine hormone mainly
secreted by the pineal body that plays an important part in
regulating physiological and pathological processes in humans, such
as bone growth and vertebral body molding (24–28).
Melatonin has been shown to trigger apoptosis in human alveolar
rhabdomyosarcoma cells and could be considered a promising drug for
future multitargeted therapies (28), but the role of melatonin in
osteoblast apoptosis remains unclear. Our previous study
demonstrated that a high concentration of melatonin could increase
the concentration of calcium ions, causing an imbalance in the
homeostasis of intracellular calcium, thus leading to calcium
overload (15–20). Calcium overload induces ERS, which
is associated with autophagy and apoptosis. But in our previous
studies, the role of autophagy in melatonin-induced osteoblast
apoptosis and the relationship between autophagy and ERS were not
investigated, thus leading us to perform the present study to
explore this. Based on preliminary experiments, four melatonin
concentrations were chosen as they have been shown to have negative
effects in vitro: 1, 2, 4 and 8 mM. In this work, it was
confirmed that 2 mM melatonin readily induced apoptosis. In
addition, a high concentration of melatonin caused intracellular
calcium overload, which resulted in ERS, induced osteoblast
autophagy and promoted osteoblast apoptosis.
Apoptosis is a type of programmed cell death
controlled by genes, including Bax and Bcl-2, that occurs as a
homeostatic mechanism to maintain cell populations in the internal
environment (11,29). Tumor cell studies have shown that
melatonin can induce apoptosis (30); the present study demonstrated that
early and late apoptotic rates of osteoblast cells treated with
melatonin increased significantly as the concentration increased.
The data also indicated that chromatin condensation in the nucleus
and high expression of PARP-1 could be observed in osteoblast cells
treated with 2 mM melatonin. PARP-1 is an apoptosis marker protein
and is closely related to the repair of DNA under stress (31); it is activated by recognizing the
DNA fragment of structural damage and is considered to be the
receptor of DNA damage (32). The
findings of the present study indicate that melatonin may induce
osteoblast apoptosis, although the mechanism is unclear.
ERS is an essential biological phenomenon that can
be activated by a number of conditions, including fatigue, calcium
imbalance and oxidative stress (33). The endoplasmic reticulum activates
a series of unfolded protein responses to protect cells from damage
and promote cell survival. However, when the ERS is too strong or
has endured too much damage to repair, the endoplasmic reticulum
can initiate a GRP78 or CHOP pathway to induce apoptosis (19). Previous experiments by the authors
demonstrated that a high concentration of melatonin can cause
calcium overload and inhibit osteoblast cell proliferation
(13–15). Other research has demonstrated that
melatonin modulates ERS and the AKT/glycogen synthase kinase-3β
signaling pathway in a rat model of renal warm ischemia reperfusion
(34); thus, we hypothesized that
melatonin-induced osteoblast apoptosis is related to ERS. To
examine this possibility, the effect of melatonin on GRP78 and CHOP
expression was investigated in the present study. The results
showed that melatonin increased GRP78 and CHOP expression as
treatment time increased, which suggested that melatonin activated
the ERS to initiate a specific apoptotic pathway to induce
apoptosis (19).
Conversely, activated unfolded protein responses can
initiate the autophagy pathway to eliminate the misfolded protein
and restore homeostasis of the endoplasmic reticulum to promote
cell survival; however, an excessive autophagy pathway can also
cause programmed cell death (35).
A previous study also examined the involvement of autophagy in
melatonin-induced osteoblasts (36). Autophagy is an important cellular
mechanism that degrades damaged macromolecules and cytoplasmic
organelles; it plays a key role in cell survival, renewal,
substance reuse and maintenance of internal homeostasis (37–39).
The role of autophagy in bone metabolism has been previously
reported. For example, autophagy attenuates oxidative
stress-induced apoptosis of MC3T3-E1 osteoblasts (40). Qi et al (41) indicated that autophagy maintains
the function of bone marrow mesenchymal stem cells to prevent
estrogen deficiency-induced osteoporosis. The present findings
showed that autophagy was triggered when osteoblasts were treated
with melatonin, and the degree of autophagy increased gradually
over time, as shown by the autophagy marker LC3-II. Apoptosis and
autophagy are two programmed cell death processes that operate
independently in numerous biological activities, this includes
roles in morphology, as biochemical indicators and as regulators of
cell death (42–46). To further understand the
relationship between ERS, autophagy and apoptosis in
melatonin-induced osteoblasts, osteoblasts were treated with either
the ERS inhibitor 4PBA, autophagy inhibitor 3MA and 2 mM melatonin,
or all three, after which PARP-1 expression increased
significantly, indicating an increase in apoptosis. However in the
present study, 4PBA and 3MA could expand the effect of melatonin,
suggesting that both ERS and autophagy can play a protective role
in promoting survival, which attenuated melatonin-induced apoptosis
to some extent.
The endoplasmic reticulum is sensitive to stress. It
maintains its structure and function by regulating membrane
proteins and selectively transports these proteins to transmit
biological effects (47). It is
currently unclear how the endoplasmic reticulum is involved in the
melatonin-induced changes in protein expression and how it
transports the related membrane protein to transmit the biological
information to the cell membrane. A previous study by the authors
found that melatonin treatment induced osteoblast proliferation,
and this was associated with septin7 protein expression (15). Another previous study has
demonstrated that septin4 is highly expressed on the endoplasmic
reticulum and Golgi membrane of HeLa cells, as detected using the
siRNA screen technique (48). When
stimulated, septin4 redistributes rapidly on the plasma membrane,
resulting in an influx of calcium ions in the cell membrane,
suggesting that the septin superprotein family have roles in
apoptosis via the induction of ERS. The septin7 superprotein family
may also be the target protein of melatonin acting on osteoblasts,
which can initiate the upregulation of calcium ions and cause
calcium overload. The findings of the present study indicated that
septin7 may be a target protein of melatonin-induced ERS. Melatonin
acting on septin7 at the endoplasmic reticulum membrane may
initiate ERS and induce osteoblast autophagy followed by osteoblast
apoptosis as autophagy increases. However, when septin7 was knocked
down and overexpressed the results were not significant, which
suggests that there may be another target protein involved.
In conclusion, results from the present study
suggested that ERS and autophagy may occur in an early state of
treatment with a high concentration of melatonin (≥2 mM), both of
which can play a protective role in promoting survival. In later
stages of treatment (>48 h), they might interact and contribute
to the induction of apoptosis. Moreover, septin7 may be a target
protein of melatonin-induced ERS. These data provide a theoretical
basis and new ideas for therapies using melatonin to treat patients
with idiopathic scoliosis.
Acknowledgements
The authors would like to acknowledge Dr M.
Subramaniam (The Department of Biochemistry and Molecular Biology
Research, Mayo Clinic, Rochester, MN, USA) for providing the hFOB
1.19 cell line.
Funding
This study was supported by two grants from The
National Natural Foundation Science of China (grant nos. 81472044
and 81271939) and a grant from Shenyang Science and Technology
Foundation of Population and Health Project (grant no.
17230904).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC contributed to the study design, data collection
and analysis, as well as the writing of the manuscript. YZ
conceived, designed and coordinated the study. TL and ZG
contributed to the experimental methods. All authors read and
approved the final manuscript.
Ethics approval and consent to
patriciate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burwell RG, Dangerfield PH, Moulton A and
Anderson SI: Etiologic theories of idiopathic scoliosis: Autonomic
nervous system and the leptin-sympathetic nervous system concept
for the pathogenesis of adolescent idiopathic scoliosis. Stud
Health Technol Inform. 140:197–207. 2008.PubMed/NCBI
|
|
2
|
Letellier K, Azeddine B, Blain S, Turgeon
I, Wang da S, Boiro MS, Moldovan F, Labelle H, Poitras B, Rivard
CH, et al: Etiopathogenesis of adolescent idiopathic scoliosis and
new molecular concepts. Med Sci (Paris). 23:910–916. 2007.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carpintero P, Mesa M, Garcia J and
Carpintero A: Scoliosis induced by asymmetric lordosis and
rotation: An experimental study. Spine (Phila Pa 1976).
22:2202–2206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azeddine B, Letellier K, Wang da S,
Moldovan F and Moreau A: Molecular determinants of melatonin
signaling dysfunction in adolescent idiopathic scoliosis. Clin
Orthop Relat Res. 462:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grivas TB, Vasiliadis E, Mouzakis V, Mihas
C and Koufopoulos G: Association between adolescent idiopathic
scoliosis prevalence and age at menarche in different geographic
latitudes. Scoliosis. 23:1–9. 2006.
|
|
6
|
Weinstein SL and Dolan LA: The evidence
base for the prognosis and treatment of adolescent idiopathic
scoliosis: The 2015 orthopaedic research and education foundation
clinical research award. J Bone Joint Surg Am. 97:1899–1903. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poon AM, Cheung KM, Lu DS and Leong JC:
Changes in melatonin receptors in relation to the development of
scoliosis in pinealectomized chickens. Spine (Phila Pa 1976).
31:2043–2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machida M, Dubousset J, Yamada T, Kimura
J, Saito M, Shiraishi T and Yamagishi M: Experimental scoliosis in
melatonin-deficient C57BL/6J mice without pinealectomy. J Pineal
Res. 41:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung KM, Wang T, Poon AM, Carl A,
Tranmer B, Hu Y, Luk KD and Leong JC: The effect of pinealectomy on
scoliosis development in young nonhuman primates. Spine (Phila Pa
1976). 30:2009–2013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maria S and Witt-Enderby PA: Melatonin
effects on bone: Potential use for the prevention and treatment for
osteopenia, osteoporosis, and periodontal disease and for use in
bone-grafting procedures. J Pineal Res. 56:115–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Proksch S, Strobel SL, Vach K, Abouassi T,
Tomakidi P, Ratka-Krüger P and Hellwig E: Melatonin as a candidate
therapeutic drug for protecting bone cells from
chlorhexidine-induced damage. J Periodontol. 85:e379–e389. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goultidis TT, Papavasiliou KA, Petropoulos
AS, Philippopoulos A and Kapetanos GA: Higher levels of melatonin
in early stages of adolescent idiopathic scoliosis: Toward a new
scenario. J Pediatr Orthop. 34:768–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Prevention of ERK activation involves melatonin-induced G(1) and
G(2)/M phase arrest in the human osteoblastic cell line hFOB 1.19.
J Pineal Res. 53:60–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Melatonin delays cell proliferation by inducing G1 and G2/M phase
arrest in a human osteoblastic cell line hFOB 1.19. J Pineal Res.
50:222–231. 2011.PubMed/NCBI
|
|
15
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
Overexpression of septin-7 inhibits melatonin-induced cell
apoptosis in human fetal osteoblastic cells via suppression of
endoplasmic reticulum stress. Mol Med Rep. 17:4817–4822.
2018.PubMed/NCBI
|
|
16
|
Tao L and Zhu Y: Melatonin regulates
CRE-dependent gene transcription underlying osteoblast
proliferation by activating Src and PKA in parallel. Am J Transl
Res. 10:86–100. 2018.PubMed/NCBI
|
|
17
|
Liu L, Zhu Y, Han X and Wu Y: The creation
of scoliosis by scapula-to-contralateral ilium tethering procedure
in bipedal rats a kyphoscoliosis model. Spine (Phila Pa 1976).
6:1340–1349. 2011. View Article : Google Scholar
|
|
18
|
Xiong XC, Zhu Y, Ge R, Liu LF and Yuan W:
Effect of melatonin on the extracellular-regulated kinase signal
pathway activation and human osteoblastic cell line hFOB 1.19
proliferation. Int J Mol Sci. 16:10337–10353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
Periostin has a protective role in melatonin-induced cell apoptosis
by inhibiting the eIF2α-ATF4 pathway in human osteoblasts. Int J
Mol Med. 41:1003–1012. 2018.PubMed/NCBI
|
|
20
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
MicroRNA-125b-1-3p mediates intervertebral disc degeneration in
rats by targeting teashirt zinc finger homeobox 3. Exp Ther Med.
15:2627–2633. 2018.PubMed/NCBI
|
|
21
|
He X, Bao J, Chen J, Sun X, Wang J, Zhu D,
Song K, Peng W, Xu T and Duan Y: Adenovirus-mediated
over-expression of Septin4 ameliorates hepatic fibrosis in mouse
livers infected with Schistosoma japonicum. Parasitol Int.
64:487–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen S, Liu M, Wu Y, Saiyin H, Liu G and
Yu L: Involvement of SEPT4_i1 in hepatocellular carcinoma: SEPT4_i1
regulates susceptibility to apoptosis in hepatocellular carcinoma
cells. Mol Biol Rep. 39:4519–4526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinoshita M: Diversity of septin
scaffolds. Curr Opin Cell Biol. 18:54–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Y, Kim YM, Kim HS and Lee KY:
Melatonin promotes osteoblast differentiation by regulating Osterix
protein stability and expression. Sci Rep. 7:57162017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dimitri P and Rosen C: The central nervous
system and bone metabolism: An evolving story. Calcif Tissue Int.
100:476–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vriend J and Reiter RJ: Melatonin, bone
regulation and the ubiquitin-proteasome connection: A review. Life
Sci. 145:152–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roforth MM, Farr JN, Fujita K, McCready
LK, Atkinson EJ, Therneau TM, Cunningham JM, Drake MT, Monroe DG
and Khosla S: Global transcriptional profiling using RNA sequencing
and DNA methylation patterns in highly enriched mesenchymal cells
from young versus elderly women. Bone. 76:49–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burattini S, Battistelli M, Codenotti S,
Falcieri E, Fanzani A and Salucci S: Melatonin action in tumor
skeletal muscle cells: An ultrastructural study. Acta Histochem.
118:278–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pourhanifeh MH, Sharifi M, Reiter RJ,
Davoodabadi A and Asemi Z: Melatonin and non-small cell lung
cancer: New insights into signaling pathways. Cancer Cell Int.
19:1312019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh MP, Chauhan AK and Kang SC: Morin
hydrate ameliorates cisplatin-induced ER stress, inflammation and
autophagy in HEK-293 cells and mice kidney via PARP-1 regulation.
Int Immunopharmacol. 56:156–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Luo W and Wang Y: PARP-1 and its
associated nucleases in DNA damage response. DNA Repair (Amst).
81:1026512019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hadj Ayed Tka K, Mahfoudh Boussaid A,
Zaouali MA, Kammoun R, Bejaoui M, Ghoul Mazgar S, Rosello Catafau J
and Ben Abdennebi H: Melatonin modulates endoplasmic reticulum
stress and Akt/GSK3-beta signaling pathway in a rat model of renal
warm ischemia reperfusion. Anal Cell Pathol (Amst).
2015:6351722015.PubMed/NCBI
|
|
35
|
Gump JM and Thorburn A: Autophagy and
apoptosis: What is the connection? Trends Cell Biol. 21:387–392.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WL, Meng HZ, Yang RF, Yang MW, Sun
GH, Liu JH, Shi PX, Liu F and Yang B: Melatonin suppresses
autophagy in type 2 diabetic osteoporosis. Oncotarget.
7:52179–52194. 2016.PubMed/NCBI
|
|
37
|
Sun X, Yang X, Zhao Y, Li Y and Guo L:
Effects of 17β-estradiol on mitophagy in the murine MC3T3-E1
osteoblast cell line is mediated via G protein-coupled estrogen
receptor and the ERK1/2 signaling pathway. Med Sci Monit.
24:903–911. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Zhao Z, Na Y, Meng C, Wang J and
Bai R: Dexamethasone-induced production of reactive oxygen species
promotes apoptosis via endoplasmic reticulum stress and autophagy
in MC3T3-E1 cells. Int J Mol Med. 41:2028–2036. 2018.PubMed/NCBI
|
|
39
|
Li X, Lu Q, Xie W, Wang Y and Wang G:
Anti-tumor effects of triptolide on angiogenesis and cell apoptosis
in osteosarcoma cells by inducing autophagy via repressing
Wnt/β-Catenin signaling. Biochem Biophys Res Commun. 496:443–449.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoo YM, Han TY and Kim HS: Melatonin
suppresses autophagy induced by clinostat in preosteoblast MC3T3-E1
cells. Int J Mol Sci. 17:5262016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qi M, Zhang L, Ma Y, Shuai Y, Li L, Luo K,
Liu W and Jin Y: Autophagy maintains the function of bone marrow
mesenchymal stem cells to prevent estrogen deficiency-induced
osteoporosis. Theranostics. 7:4498–4516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song HS, Jang S and Kang SC: Bavachalcone
from Cullen corylifolium induces apoptosis and autophagy in HepG2
cells. Phytomedicine. 40:37–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
San-Miguel B, Crespo I, Sánchez DI,
González-Fernández B, Ortiz de Urbina JJ, Tuñón MJ and
González-Gallego J: Melatonin inhibits autophagy and endoplasmic
reticulum stress in mice with carbon tetrachloride-induced
fibrosis. J Pineal Res. 59:151–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Luxán-Delgado B, Potes Y,
Rubio-González A, Caballero B, Solano JJ, Fernández-Fernández M,
Bermúdez M, Rodrigues Moreira Guimarães M, Vega-Naredo I, Boga JA
and Coto-Montes A: Melatonin reduces endoplasmic reticulum stress
and autophagy in liver of leptin-deficient mice. J Pineal Res.
61:108–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moreira AJ, Ordoñez R, Cerski CT, Picada
JN, García-Palomo A, Marroni NP, Mauriz JL and González-Gallego J:
Melatonin activates endoplasmic reticulum stress and apoptosis in
rats with diethylnitrosamine-induced hepatocarcinogenesis. PLoS
One. 10:e01445172015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wongprayoon P and Govitrapong P: Melatonin
protects SH-SY5Y neuronal cells against methamphetamine-induced
endoplasmic reticulum stress and apoptotic cell death. Neurotox
Res. 31:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma S, Quintana A, Findlay GM, Mettlen
M, Baust B, Jain M, Nilsson R, Rao A and Hogan PG: An siRNA screen
for NFAT activation identifies septins as coordinators of
store-operated Ca2+ entry. Nature. 499:238–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|