Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-associated death worldwide (1,2).
Approximately 85% of HCC cases occur in developing countries, with
the majority of cases occurring in Africa and Asia (3). Hepatitis B virus (HBV) is a small DNA
virus with a full length of 3,200 bp. HBV affects the malignant
phenotype of HCC by increasing chromosome instability and promoting
cell proliferation (4). The
carcinogenic mechanism of HBV is extremely complex and is not
completely understood (5). Further
investigation into the mechanism underlying HCC development
associated with HBV could aid with the identification of novel
therapeutic strategies for HCC (6).
HBV infection is currently considered to be a high
risk factor for primary HCC (7,8).
Persistent HBV infection can lead to chronic liver disease and
accounts for ~50% of all HCC cases (7). In China, >80% of patients with HCC
have chronic HBV infection, and patients with HBV have a ≥300-fold
increased risk of developing primary HCC (9). HBV contains four overlapping open
reading frames: S region (surface protein HBS encoding the virus),
P region (polymerase protein encoding the virus), X region (X
protein encoding the virus, HBX) and pre C region (encoding the
viral e and c antigens) (5,10,11).
HBV DNA can integrate into host genes, altering chromosomal
instability and the function of endogenous genes, thereby inducing
HCC (8,12,13).
Since the initial discovery of HBV DNA integration by Edman et
al (14) in 1980, the
association between HBV DNA integration and the pathogenesis of HCC
has become a key research topic. The HBV integration mechanism has
been explored by a number of research teams, including teams based
at the University of Hong Kong, the National University of
Singapore, the Shenzhen University of China Genetics Research
Institute, and the Asian Cancer Research Organization, with the aim
of producing a highly precise, unbiased HBV integration map
(15–17). Genome wide sequencing of cancer and
paracancerous tissue samples from 81 HBV-positive patients with HCC
and 7 HBV-negative patients with HCC suggested that HBV integration
was a common phenomenon in HCC. Furthermore, the study reported
that the frequency of HBV integration was significantly higher in
tumor tissues (86.4%) compared with adjacent normal tissues (30.7%)
(18).
The zinc finger protein ZBTB20 is a novel
transcriptional repressor (19,20).
ZBTB20 contains a conserved BTB domain at the N-terminus, which
typically mediates protein interactions, and five conserved C2H2
zinc finger domains at the C-terminus, which result in postnatal
transcriptional loss of the AFP gene in the liver (21). Previous studies have reported that
liver-specific ZBTB20 knockout mice display high liver AFP
expression; however, the liver tissue structure is normal and
hepatocytes remain in a normal resting state. These findings
suggested that there was not an intrinsic relationship between
hepatocyte AFP expression and cell proliferation (21,22).
Therefore, at present, it is unclear whether HBV DNA integrates
into the zinc finger protein ZBTB20.
The aim of the present study was to identify the HBV
DNA integration site and investigate the expression of ZBTB20 in
HCC tissues of patients with chronic HBV and HCC. The results of
the present study may provide a basis for the diagnosis and
treatment of HCC.
Materials and methods
Specimens
A total of 30 patients with HCC (age, 45–75 years;
median age, 57.07±7.83 years; 23 male and 7 female; 22 cases of
stage II and 8 cases of stage III) were recruited from Taizhou Enze
Medical Center Enze Hospital between September 2017 and July 2019.
The patients were diagnosed by clinical and pathological
examination in accordance with the Guidelines for Prevention and
Treatment of Chronic HBV (2015 edition) (23). None of the participants had
received any anticancer or antiviral treatment prior to the study,
and were serum HBsAg-positive. Patients with other viral
infections, who had a drug addiction or who were alcoholics, or who
had autoimmune hepatitis were excluded. HCC, paracancerous (≤2 cm
from the edge of the tumor) and normal control (≥5 cm from the edge
of the tumor) tissues were obtained from patients with HCC by
surgical resection at the Taizhou Enze Medical Center Enze
Hospital. All tissue samples were cut into 0.5 cm3
slices, frozen in liquid nitrogen and stored at −80°C until further
analysis. The present study was approved by the Ethics Committee of
Taizhou Central Hospital (approval no. T13010123Y), and written
informed consent was obtained from each patient.
DNA extraction
HCC, paracancerous and normal control tissues
(0.027–3.125 cm3) were cut. Following the addition of
400 µl OmniPur DNA extraction buffer (EMD Millipore), the tissues
were homogenized in a tissue homogenizer and rinsed with 100 µl DNA
extraction buffer. The resulting solution was mixed with 100–200
µg/ml proteinase K in a 37°C water bath for 5–12 h, mixed with 500
µl equilibrated phenol, and centrifuged at 11,180 × g for 1 min at
4°C. The supernatant was collected, mixed with 500 µl chloroform
and centrifuged at 11,180 × g for 1 min at 4°C. The supernatant was
collected, mixed with 60 µl NaOAc (3 M; pH 4.8) and 1,000 µl
ice-cold ethanol at −20°C for 2 h, and centrifuged at 21,912.8 × g
for 15 min at 4°C. Subsequently, the supernatant was discarded and
the pellet was washed with pre-cooled 70% ethanol, dried, dissolved
in double-distilled water, and stored at −20°C until further
analysis. DNA concentration was quantified by spectrophotometry at
an absorbance of 260 nm.
HBV-Alu-PCR amplification
reaction
The primers used for PCR amplification are presented
in Table I. The amplification
primers used for the first round of HBV-Alu-PCR amplification were
pUTP and UP5. The total reaction volume (300 µg DNA in 25 µl)
consisted of 2.5 µl EX-Taq DNA polymerase (Thermo Fisher
Scientific, Inc.), 2 µl 2.5 mmol/l NTP, 1 µl 10 µmol/l primer, 1 µl
1 U EX Taq DNA polymerase and 18.5 µl ddH2O. The
following thermocycling conditions were used for the first round of
PCR amplification: Pre-denaturation at 94°C for 1 min, followed by
10 cycles of denaturation at 94°C for 30 sec, annealing at 59°C for
30 sec, and 70°C extension for 1 min, set 30 cycles and at the end
of procedure 70°C extension for 5 min. Subsequently, 0.5 U uracil
DNA glycosidase was added to the system at 37°C for 30 min to
disrupt the dUTP-containing DNA. The reaction was stopped by an
incubation at 94°C for 10 min. Subsequently, the MM37 and UP6 (10
µmol/l; 1 µl each) primers were added to the PCR system for the
second round of PCR amplification, using the following
thermocycling conditions: Pre-denaturation at 94°C for 1 min;
followed by 40 cycles of denaturation at 94°C for 30 sec and
annealing from 65–55°C for 30 sec (the temperature decreased by 1°C
for the first 10 cycles and was maintained at 55°C for the last 20
cycles); and a final extension at 72°C for 3 min. Subsequently, for
the third round of PCR amplification, 1 µl PCR product was used as
a template, and MM60 and UP6 primers were used in a 50 µl
amplification system (as with the first round, above) following the
thermocycling conditions used for the second round of PCR
amplification.
 | Table I.Primer sequences used for HBV-Alu-PCR
amplification. |
Table I.
Primer sequences used for HBV-Alu-PCR
amplification.
| Name | Primer sequence | HBV location
(bp) | Annotation |
|---|
| MM37 |
5′-TGCCAAGTGTTTGCTGACGC-3′ | 1,174-1,193 | HBVX |
| UP5 |
5′-CAGUGCCAAGUGUUUGCUGACGCCAAAGUGCUGGGAUUA-3′ |
| Alu-forward |
| UP6 |
5′-CAAGTGGCTGACGCCAAAG-3′ |
| Alu-forward
(Tag) |
| pUTP |
5′-ACAUGAACCUUUACCCCGUUGC-3′ | 1,131-1,152 | HB1 (HBVX) |
| MM60 |
5′-CTGCCGATCCATACTGCGGAAC-3′ | 1,258-1,279 | HB3 (HBVX) |
DNA sequencing
The PCR products obtained following the third round
of PCR amplification were confirmed by 1% agarose gel
electrophoresis and visualized by ethidium bromide using a 2500 Gel
Imaging system (Tanon Science & Technology Co., Ltd.).
Subsequently, DNA purification by QIAquick PCR Purification kit
(Qiagen GmbH) and sequencing were performed. Sequencing was
performed using an ABI-3730XL fully automated sequencer (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Sequence analysis and
information regarding the sequencing of HBV DNA localized on human
chromosomes was retrieved using the National Center for
Biotechnology Information (NCBI) Basic Local Alignment Search Tool
(BLAST 2.6.0+; www.ncbi.nlm.nlh.gov/BLAST), NCBI Map Viewer v2018
(www.ncbinlm.nih.gov/mapview) and
University of California Santa Cruz BLAST-like Alignment Tool
(samtools 1.2–3-ge2bb18f, www.genome.ucsc.edu/cgi bin/hgBlat).
HBV DNA trapping and analysis of the
HBV integration site
Based on the 8 HBV genotypes (A-H), their reported
subtypes and the mutant information available in the Genome
Information Broker for Viruses database release 1.0 (24), the HBV capture probe was designed
using the MyGenostics algorithm (BED tools 1.119, Beijing
MyGenostics Co., Ltd.). Genomic DNA fragments from HCC,
paracancerous and normal control tissues (cut to 150–200 bp) were
purified, mixed with a polyA tail, ligated, and linked to connector
sequences to identify sequences for the construction of the basic
sequencing library. After the hybridization library was captured
and enriched by magnetic beads with an HBV DNA probe at 47°C for 24
h, the unbound fragments were removed. The magnetic bead enriched
fragments were eluted and amplified o generate a sequencing
library. The KAPA-DNA polymerase (KAPA Biosystems) with primers (F:
AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATC; R:
CAAGCAGAAGACGGCATACGAGATNNNNNNNNGTGACTGGAGTTCAGACGTGTGCTCTTCCGAT)
was used with the following thermocycling conditions:
Pre-denaturation at 98°C for 45 sec; followed by 8 cycles of
denaturation at 98°C for 15 sec and annealing at 65°C for 30 sec
and 72°C for 30 sec; and a final extension at 72°C for 1 min. The
raw read data were obtained on a Hiseq 2000 Sequencing Platform
(Illumina, Inc.), and clean data were generated by quality control
and de redundancy processing. Positive HBV integration was defined
as DNA fragments containing both a human genome sequence and an HBV
sequence. According to the HBV integration sites obtained, the
corresponding specimens were selected for PCR amplification and
verified by Sanger sequencing. The KAPA-DNA polymerase (KAPA
Biosystems) with primers (F: AATGAT ACGGCGACCACCGA; R:
CAAGCAGAAGACGGCAT ACG) was used with the following thermocycling
conditions: Pre-denaturation at 95°C for 4 min; followed by 10–12
cycles of denaturation at 98°C for 20 sec and annealing at 58°C for
30 sec and 72°C for 30 sec; and a final extension at 72°C for 5
min. Reads that completely overlapped with the HBV reference
sequence were removed, and other fusion reads were retained; the
sequences were then assembled as paired end (PE) reads.
Subsequently, the Burrows-Wheeler Aligner (BWA) software (v.
0.7.12-r1044; http://bio-bwa.sourceforge.net/) was used to align the
PE reads with the human reference sequence and HBV reference
sequence to complete the annotation. Reads with both a human genome
sequence and an HBV sequence indicated a HBV integration site.
Western blot analysis
HCC, paracancerous and normal control tissues were
lysed on ice in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1
mM Na3VO4, 1 mM NaF, 1% Nonidet P-40, 0.25%
sodium deoxycholate, 1 mM PMSF, 1 mg/ml aprotinin and 1 mg/ml
pepsin inhibitor. Proteins (20 µg) were quantified using a BCA kit
(Beyotime Institute of Biotechnology), separated via SDS PAGE (10%
gels) and transferred to PVDF membranes. The membranes were blocked
in 5% non-fat milk at 25°C for 1 h. Subsequently, the membranes
were incubated with a primary antibody targeted against ZBTB20
(cat. no. ab48889; 1:500; Abcam) at 4°C overnight. Following
primary incubation, the membranes were incubated with a HRP
conjugated secondary antibody (ZB-2305; 1:10,000; OriGene
Technologies, Inc.) at 25°C for 1 h. Protein bands were visualized
using an ECL immunoreactive western blot detection reagent (Pierce;
Thermo Fisher Scientific, Inc.). β-actin (CW0096; 1:1,000, CoWin
Biosciences) was used as the loading control. ImageJ (1.4.3.67,
National Institutes of Health) was used for densitometry.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 19.0; IBM Corp.). Data were presented as mean ±
SD and analysed using one way ANOVA followed by the least
significant difference post hoc-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
HBV X DNA integration sequences
Among the HCC tissues assayed by HBV-Alu-PCR, HBV
integration sites were identified in ~70% of them (21/30; data not
shown). Detection of HBV integrated with subgene X suggested that
14 of the integrated specimens (67%) were inserted into the host X
gene in the forward direction, 12 (57%) in the reverse direction, 5
(24%) in both the forward and reverse directions, and 8 (38%) had
two HBV integration sites (data not shown). NCBI BLAST was used to
identify HCC specimens displaying high homology to the nucleotide
sequence of the HBV X gene, and the results suggested that
95.0–3.7% of specimens displayed high homology (data not
shown).
HBV integrates into ZBTB20
High throughput sequencing methods were used to
analyse four normal liver tissues, five HCC tissues and six
paracancerous tissues (not all 30 specimens were used for
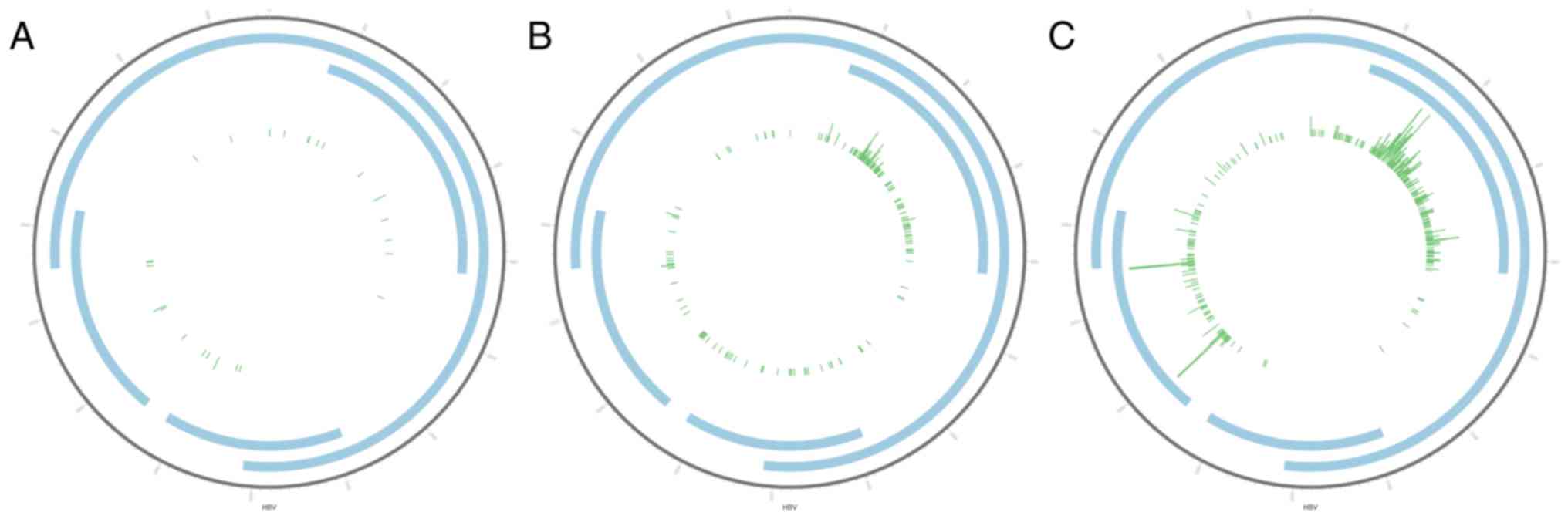
high-throughput sequencing). The distribution of viral and host DNA
integration sites are presented in Circos plots (Fig. 1). In total, 3,320 HBV integration
sites were identified, including 718 in the normal liver tissues,
1,397 in the HCC tissues and 1,205 in the paracancerous tissues
(Fig. 1A-C). The number of
integration sites between the three tissue types was significantly
different (P<0.05).
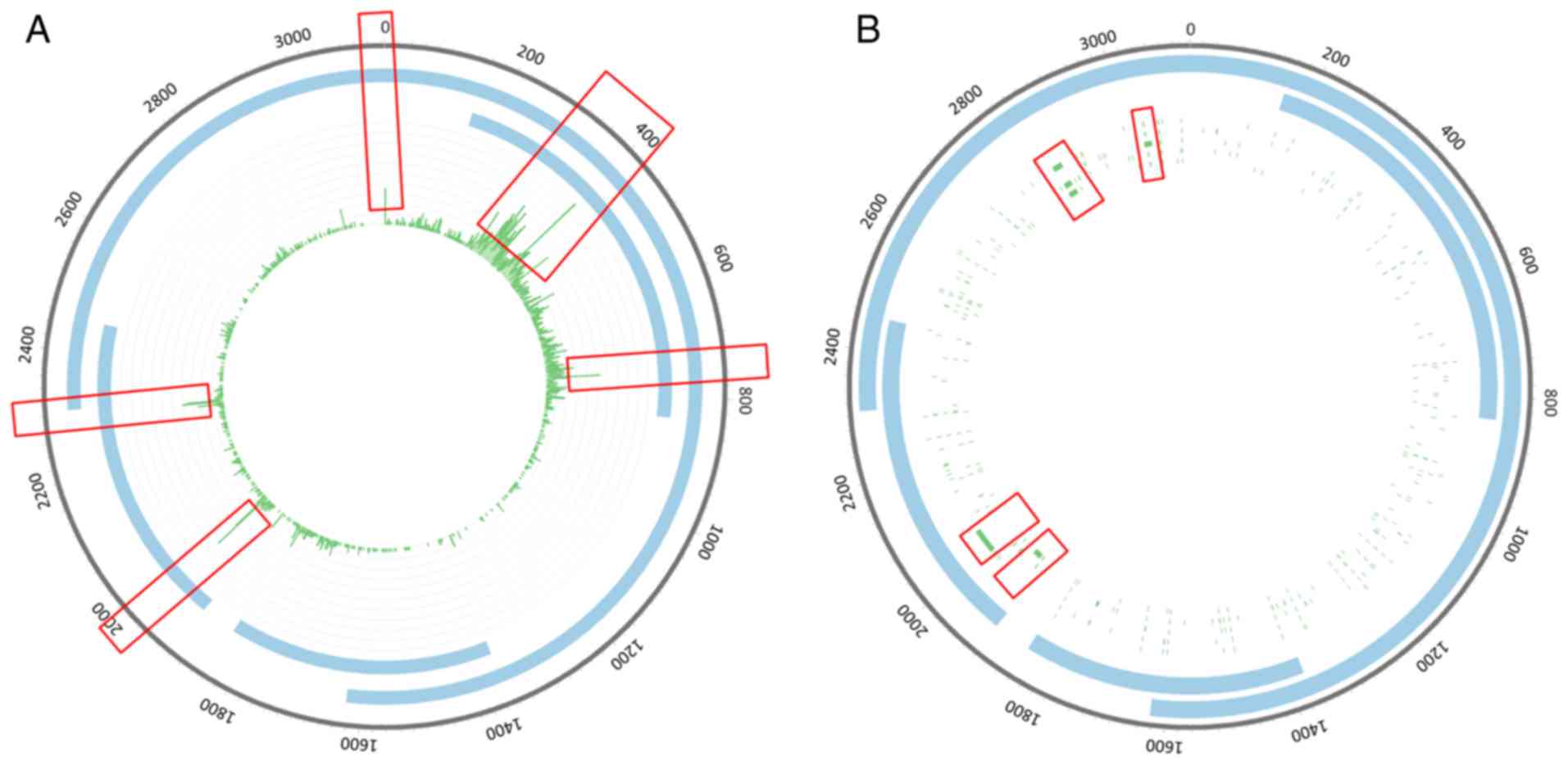
The integration sites in the HBV genome were
scattered throughout the genome, and were notably enriched
(Fig. 2A). Integration sites with
high integration frequencies were concentrated in the 200–800 bp
region (Fig. 2A). However,
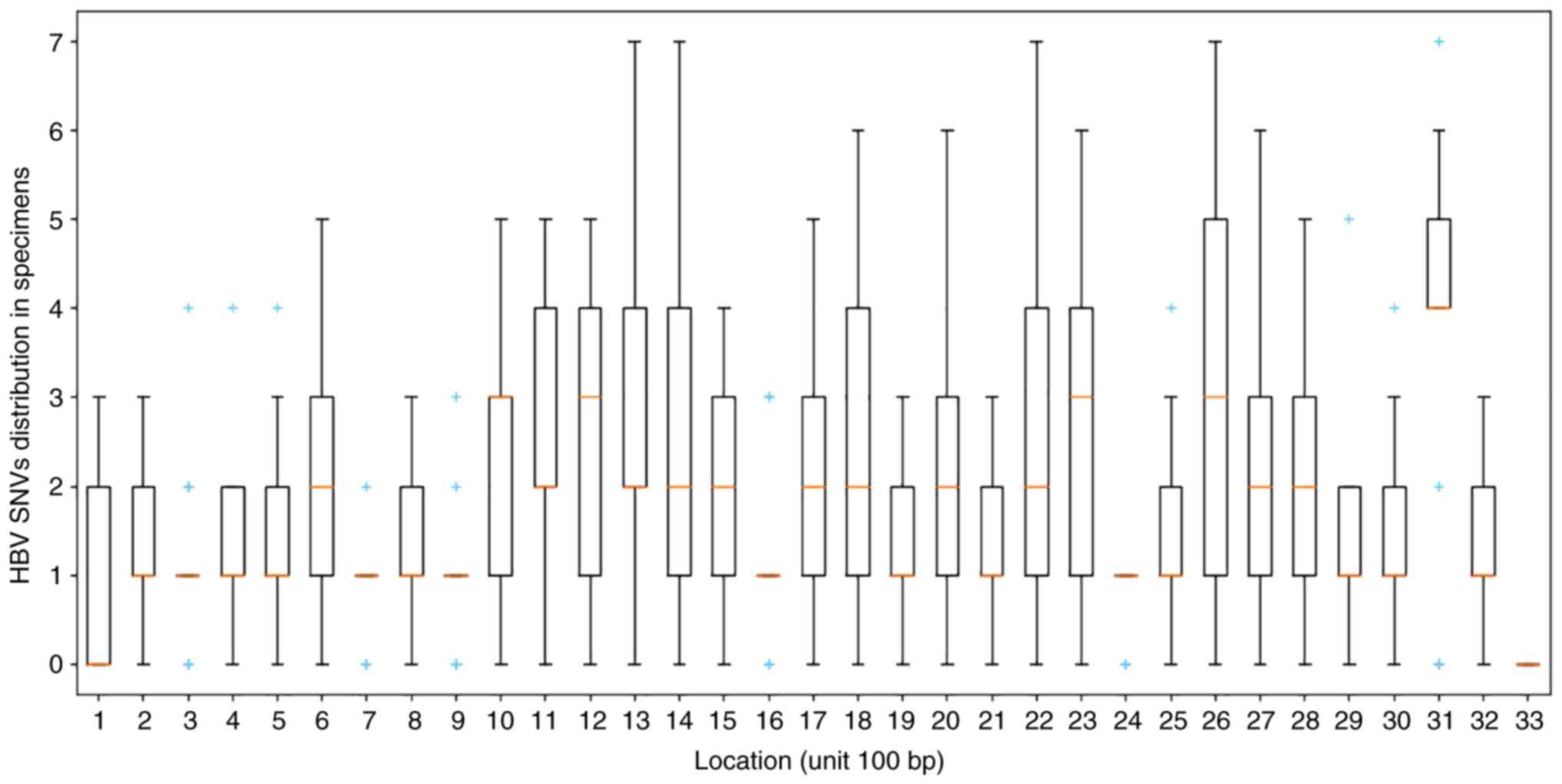
mutation sites with high frequencies were enriched in the
2,840-2,860 bp region (Figs. 2B
and 3).
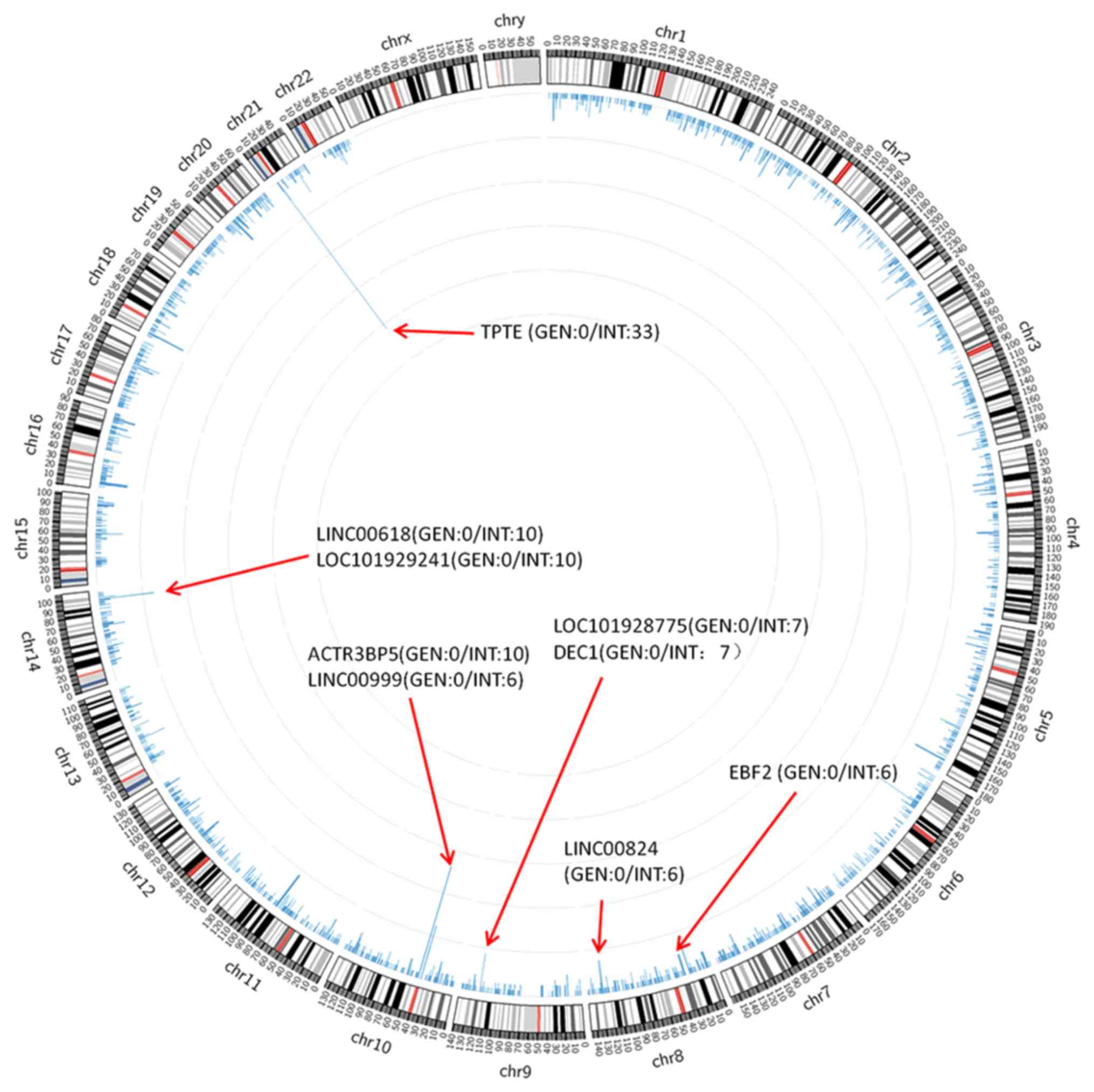
The integration of HBV into the human genome
integration sites was analyzed, and the annotations with the
highest integration site frequencies were transmembrane phosphatase
with tensin homology (TPTE), long intergenic non-protein coding RNA
(LINC)00618, LOC101929241, ACTR3 pseudogene 5 (ACTR3BP5),
LINC00999, LOC101928775, deleted in oesophageal cancer 1 (DEC1),
LINC00824, EBF transcription factor 2 (EBF2) and ZBTB20 (Fig. 4).
HBV integration sites were identified in the
vicinity of ZBTB20: one in the downstream region; 38 in the
intergenic region; 18 in the intronic region; one in the upstream
region; one in the 3′-untranslated region (UTR); and one in the
5′-UTR (Table II).
 | Table II.Zinc finger protein integration site
statistics. |
Table II.
Zinc finger protein integration site
statistics.
| Gene | Location | Frequency |
|---|
| ZBTB20 | Downstream | 1 |
|
| Intergenic | 38 |
|
| Intronic | 18 |
|
| Upstream | 1 |
|
| 3′-UTR | 1 |
|
| 5′-UTR | 1 |
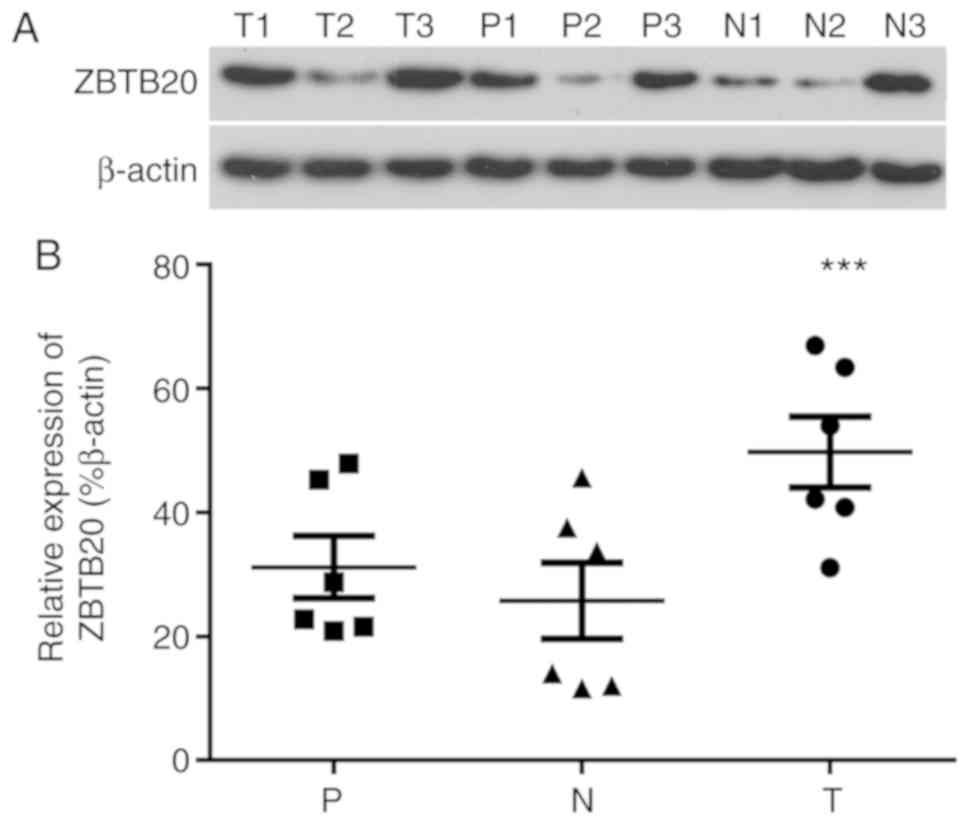
ZBTB20 is upregulated in HCC
tissues
The expression of ZBTB20 in HCC, paracancerous and
normal control liver tissues was analyzed by western blotting
(Fig. 5). The expression of ZBTB20
in tissues varied among the individuals, however; the overall trend
was significantly increased expression in HCC tissues compared with
normal control liver tissues, which displayed the lowest expression
levels among the three tissue types (Fig. 5). Among the HCC specimens, higher
ZBTB20 expression levels were observed in the HCC tissues with
higher integration frequencies (T3, 53 HBV DNA integrations vs. T1,
28 HBV DNA integrations, as descripted in Fig. 5), suggesting that HBV integration
frequency was related to the level of ZBTB20 expression. The
results indicated that the expression of ZBTB20 was affected by the
frequency of HBV integration.
Discussion
HBV DNA host genome integration is an important
cancer-inducing factor in HBV-associated HCC. Although the level of
HBV DNA integration into host genes is random, a number of genes
including TP53, PIK3CA, AXIN1 and DENG are associated with high
frequency HBV integration (25).
The integrated HBV fragment can act as an enhancer by upregulating
the transcription level of genes adjacent to the integration site
(26). HBV-Alu PCR allows HBV DNA
integration sites to be separated; however, it can only detect
human gene Alu fragments and integrated fragments near specific
viral gene sequences (27).
High-throughput sequencing technology can detect an increased
number of integration sites and identify more integration regions
compared with HBV-Alu-PCR. In the present study, HCC samples
containing integration sites were selected by HBV-Alu-PCR, and HBV
integration was further analyzed by high-throughput sequencing. The
results suggested that 67% of the specimens displayed integration
into the host X gene in the forward direction, 57% in the reverse
direction, 24% in both the forward and reverse insertions, and 38%
had two HBV integration sites. A total of 3,320 HBV virus
integration breakpoints were identified, including 1,397 in HCC
tissues, 1,205 in paracancerous tissues and 718 in normal liver
tissues. Furthermore, the results suggested that HBV was highly
integrated into TPTE, LINC00618, LOC101929241, ACTR3BP5, LINC00999,
LOC101928775, DEC1, LINC00824, EBF2 and ZBTB20 in HCC tissues.
Additionally, the HBV integration fragment displayed significant
enrichment in the 200–800 bp region, and the mutation sites were
specifically enriched in the 2,840-2,860 bp region.
The zinc finger protein ZBTB20 contains an ~100
amino-acids long conserved BTB domain at the N-terminus, which
mediates interactions between proteins, as well as a C-terminal
C2H2 zinc finger domain containing five conservative amino acids
(28). The C-terminus of ZBTB20
can interact with DNA to regulate chromatin modifications and
transcriptional activation or inhibition, and thus participate in a
variety of cellular functions, including transcription regulation,
cell proliferation, apoptosis, cell morphology, ion channels,
collection and ubiquitin protein degradation (29,30).
In addition, ZBTB20 plays an important role in DNA damage and tumor
development (31), and is widely
expressed in malignant tumor cells of the hematopoietic system
(32). ZBTB20 is also a key
transcription inhibitor of the hepatic AFP gene inactivation after
birth (33). Previous studies have
reported that liver AFP expression in hepatic specific ZBTB20
knockout mice is high, but there is no obvious internal
relationship between the expression of AFP in liver cells and cell
proliferation (21). It has also
been reported that the expression of ZBTB20 is upregulated in
clinical HCC cells and displays a positive regulatory effect on
hepatocyte proliferation (29).
HCC with high ZBTB20 expression has been associated with increased
malignancy, which manifests as metastasis and post-operative
recurrence with a low 5-year survival rate (29). It has also been reported that
ZBTB20 expression is closely related to the prognosis of HCC
(29). In the present study,
ZBTB20 was upregulated in HCC tissues and HBV integrated into
ZBTB20. Moreover, the expression of ZBTB20 in tumor tissues was
associated with HBV integration frequency. However, a relationship
between clinical characteristics and the frequency of HBV
integration or ZBTB2 expression was not identified, therefore,
further investigation is required.
In summary, the present study suggested that HBV DNA
integration and ZBTB20 expression are associated, and may promote
the occurrence and development of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nature
Science Fund of the Zhejiang Provincial of China (grant no.
LY16H030001) and the Medical Science Fund of Taizhou of China
(grant no. 2015A33259).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and QC contributed to the conception or design of
the study; ZH, JZ, JM, HZ and QC the acquisition, analysis or
interpretation of data for the study; ZH, JZ, JM, HZ and CQ drafted
the manuscript, revised it and checked the final version. All
authors reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Taizhou Central Hospital (approval no. T13010132Y).
All participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singal AG, Li X, Tiro J, Kandunoori P,
Adams-Huet B, Nehra MS and Yopp A: Racial, social, and clinical
determinants of hepatocellular carcinoma surveillance. Am J Med.
128:90.e91–90.e97. 2015. View Article : Google Scholar
|
|
2
|
Zeng Y: Advances in mechanism and
treatment strategy of cancer. Cell Mol Biol. 64:1–3. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Younossi ZM, Stepanova M, Saab S, Ahmed A,
Lam B, Srishord M, Venkatesan C, Wai H and Henry L: The impact of
viral hepatitis related hepatocellular carcinoma to post transplant
outcomes. J Viral Hepat. 23:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chisari FV and Ferrari C: Hepatitis B
virus immunopathogenesis. Annu Rev Immunol. 13:29–60. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang M, Xi D and Ning Q: Virus-induced
hepatocellular carcinoma with special emphasis on HBV. Hepatol Int.
11:171–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng Y, Yao X, Liu X, He X, Li L, Liu X,
Yan Z, Wu J and Fu BM: Anti-angiogenesis triggers exosomes release
from endothelial cells to promote tumor vasculogenesis. J Extracell
Vesicles. 8:16298652019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lupberger J and Hildt E: Hepatitis B
virus-induced oncogenesis. World J Gastroenterol. 13:74 812007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hino O, Ohtake K and Rogler CE: Features
of two hepatitis B virus (HBV) DNA integrations suggest mechanisms
of HBV integration. J Virol. 63:2638–2643. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maddrey WC: Hepatitis B: an important
public health issue. J Med Virol. 61:362–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shlomai A, de Jong YP and Rice CM: Virus
associated malignancies: The role of viral hepatitis in
hepatocellular carcinoma. Semin Cancer Biol. 26:78–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dandri M and Locarnini S: New insight in
the pathobiology of hepatitis B virus infection. Gut. 61 (Suppl
1):i6–i17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minami M: Integration of hepatitis B virus
genome into the host gene: Its significance to
hepatocarcinogenesis. Nihon Rinsho. 73 (Suppl 9):409–413. 2015.(In
Japanese). PubMed/NCBI
|
|
13
|
Jiang S, Yang Z, Li W, Li X, Wang Y, Zhang
J, Xu C, Chen PJ, Hou J, McCrae MA, et al: Re-evaluation of the
carcinogenic significance of hepatitis B virus integration in
hepatocarcinogenesis. PLoS One. 7:e403632012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edman JC, Gray P, Valenzuela P, Rall LB
and Rutter WJ: Integration of hepatitis B virus sequences and their
expression in a human hepatoma cell. Nature. 286:535–538. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu T, Budzinska MA, Shackel NA and Urban
S: HBV DNA Integration: Molecular Mechanisms and Clinical
Implications. Viruses. 9:92017. View
Article : Google Scholar
|
|
16
|
Yang L, Ye S, Zhao X, Ji L, Zhang Y, Zhou
P, Sun J, Guan Y, Han Y, Ni C, et al: Molecular Characterization of
HBV DNA Integration in Patients with Hepatitis and Hepatocellular
Carcinoma. J Cancer. 9:3225–3235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu S, Koh SS and Lee CG: Hepatitis B
Virus X Protein and Hepatocarcinogenesis. Int J Mol Sci.
17:9402016. View Article : Google Scholar
|
|
18
|
Sung WKZH, Zheng H, Li S, Chen R, Liu X,
Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, et al:
Genome-wide survey of recurrent HBV integration in hepatocellular
carcinoma. Nat Genet. 44:765–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kan H, Huang Y, Li X, Liu D, Chen J and
Shu M: Zinc finger protein ZBTB20 is an independent prognostic
marker and promotes tumor growth of human hepatocellular carcinoma
by repressing FoxO1. Oncotarget. 7:14336–14349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao M, Yao DF, Bian YZ, Wu W, Yan XD, Yu
DD, Qiu LW, Yang JL, Zhang HJ, Sai WL, et al: Values of circulating
GPC-3 mRNA and alpha-fetoprotein in detecting patients with
hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int.
12:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Shi JH, Jiang H, Wang K, Lu JY,
Jiang X, Ma X, Chen YX, Ren AJ, Zheng J, et al: ZBTB20 regulates
EGFR expression and hepatocyte proliferation in mouse liver
regeneration. Cell Death Dis. 9:4622018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Z, Zhang H, Tsai W, Zhang Y, Du Y,
Zhong J, Szpirer C, Zhu M, Cao X, Barton MC, et al: Zinc finger
protein ZBTB20 is a key repressor of alpha fetoprotein gene
transcription in liver. Proc Natl Acad Sci U S A. 105:10859–10864.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Health Organization (WHO), .
Guidelines for the prevention, care and treatment of persons with
chronic hepatitis B infection. WHO. (Geneva). 2015.
|
|
24
|
Masaki H, Takashi A, Naoto T, Kuwana Y,
Shigemoto Y, Miyazaki S, Suzuki Y and Sugawara H: Genome
Information Broker for Viruses (GIB-V): database for comparative
analysis of virus genomes. Nucleic Acids Res. 35((Database issue)):
D339–D342. 2007.PubMed/NCBI
|
|
25
|
Zucman-Rossi J and Laurent-Puig P: Genetic
diversity of hepatocellular carcinomas and its potential impact on
targeted therapies. Pharmacogenomics. 8:997–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guerrero RB and Roberts LR: The role of
hepatitis B virus integrations in the pathogenesis of human
hepatocellular carcinoma. J Hepatol. 42:760–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murakami Y, Saigo K, Takashima H, Minami
M, Okanoue T, Bréchot C and Paterlini-Bréchot P: Large scaled
analysis of hepatitis B virus (HBV) DNA integration in HBV related
hepatocellular carcinomas. Gut. 54:1162–1168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Mi J, Li N, Sui L, Wan T, Zhang
J, Chen T and Cao X: Identification and characterization of DPZF, a
novel human BTB/POZ zinc finger protein sharing homology to BCL-6.
Biochem Biophys Res Commun. 282:1067–1073. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Tan YX, Ren YB, Dong LW, Xie ZF,
Tang L, Cao D, Zhang WP, Hu HP and Wang HY: Zinc finger protein
ZBTB20 expression is increased in hepatocellular carcinoma and
associated with poor prognosis. BMC Cancer. 11:2712011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagao M, Ogata T, Sawada Y and Gotoh Y:
Zbtb20 promotes astrocytogenesis during neocortical development.
Nat Commun. 7:111022016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chevrier S and Corcoran LM: BTB-ZF
transcription factors, a growing family of regulators of early and
late B-cell development. mmunol Cell Biol. 92:481–488. 2014.
View Article : Google Scholar
|
|
32
|
Maeda T: Regulation of hematopoietic
development by ZBTB transcription factors. Int J Hematol.
104:310–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao D, Ma X, Cai J, Luan J, Liu AJ, Yang
R, Cao Y, Zhu X, Zhang H, Chen YX, et al: ZBTB20 is required for
anterior pituitary development and lactotrope specification. Nat
Commun. 7:111212016. View Article : Google Scholar : PubMed/NCBI
|