Introduction
In addition to being minimally invasive, safe,
effective and amenable to repeated treatments, thermal ablation has
been demonstrated to produce satisfactory long-term follow-up
results in the treatment of hepatocellular carcinoma (HCC) and is
considered an important radical curative therapy for this disease
(1). Due to the effect of the
ablation mechanism, the pathological characteristics of liver
cancer and the anatomical characteristics of the liver, a certain
proportion of the residual tumor remains even following ablation
therapy, which is the main factor affecting the survival rate of
patients with HCC following ablation (2,3).
New strategies based on an improved understanding of
residual tumor biology may help to maximize the efficacy of current
treatments. There has been steadily increasing interest in changes
in heat shock protein (HSP) expression and its antagonistic effect
on apoptosis (4–8). The application of HSP90 inhibitors to
improve the therapeutic effect on tumors has also been explored in
several studies (9–17). However, due to the regulatory
effects and complex downstream molecular changes HSPs exert on
numerous companion proteins (14),
current research on the treatment of advanced tumors remains
limited.
The autophagy system serves multiple roles in the
degradation of dysfunctional proteins. In the case of incomplete
ablation, we first reported the decreased expression of
autophagy-associated proteins and a concomitant increase in the
expression of HSPs (18). HSP90,
as a molecular chaperone, is essential for the proper function of
Akt as it forms a chaperone-substrate protein complex, and a
decrease in HSP90-Akt binding results in Akt inactivation (19–21).
The activation of AKT is characterized by high levels of Akt
phosphorylation (p-Akt), resulting in activation of the mTOR
complex. mTOR acts as a major checkpoint regulating autophagy
(22). Further, it has been
suggested that the HSP90/Akt/mTOR pathway is involved in signal
transmission between autophagy and HSPs. Autophagy can either
protect against or promote apoptosis in a stimulus-dependent manner
(23–25). The relationship between the two in
the incomplete ablation model is unclear. Specifically, it is
unclear whether the use of autophagy inhibitors promotes apoptosis
or if there a synergistic effect when autophagy inhibitors and
HSP90 inhibitors are used in combination. Autophagy was assessed by
analyzing the conversion of microtubule-associated protein 1A (LC3)
protein from cytosolic LC3-I to the autophagosome-associated
lipid-modified form LC3-II, indicative of the onset of autophagy
(24). The present study
hypothesized that autophagy serves an important role in apoptosis
during the treatment of residual tumors with HSP90 inhibitors
following incomplete ablation, and it analyzed the coordinating
mechanisms mediating the interplay between these processes. The
HSP90 inhibitor 17-N-allylamino-17-demethoxygeldanamycin (17-AAG),
which is a derivative of the antibiotic geldanamycin, has already
been used in stage III clinical trials (NCT00546780) (26) and 3-methyladenine (3-MA) has been
well established as an autophagy inhibitor. This present study
builds upon previous studies that explore the possibility of
improving the therapeutic effect of thermal ablation by combining
inhibitors of autophagy and HSP90.
Materials and methods
Animal model
A total of 28 nude mice transplanted with the Huh7
tumor cell line were randomly divided into 4 groups of 7 mice each.
All model animals underwent laser ablation with different drug
combination therapies. The protocol of this study was approved by
the Research Ethics Committee of the First Affiliated Hospital,
College of Medicine, Zhejiang University. In brief, healthy female
nude mice (body weight 12–16 g; 4 weeks of age; n=28) were
purchased from the Shanghai Cancer Institute. The nude mice were
housed in a specific pathogen-free (SPF) animal room at a
temperature of 20–25°C, humidity of 50–60%, and were subjected to a
12-h light/dark cycle with free access to food and tap water. All
experimental procedures followed the operational specifications for
experimental animals at Zhejiang University (http://www.lac.zju.edu.cn/cms/12997). Huh7 tumor
tissue exhibiting sufficient growth up to 10 mm in length was cut
into 2-mm3 pieces and subcutaneously implanted into the
right flanks of nude mice using a 20-gauge trocar. A total of 28
mice were used for the experiments after 3 weeks, when tumors had
grown to an average diameter of 0.8–3.0 cm. The grouping was
performed using the random number method. On the day of ablation,
the average diameter of the tumors was 1.0–3.2 cm and average
volume of tumors was 617.9±138.7 mm3. The largest tumor
diameter was 1.2–3.4 cm and multiple tumors were not present at the
end of the experiment.
Palliative ablation method
Intraperitoneal anesthesia was administered to the
nude mice in the experimental and control groups with approximately
200 µl 4% chloral hydrate at a dose of 400 mg/kg mouse weight. The
conditions established in a previous study were applied to build a
model of incomplete ablation (18). Mice were fixed on the operating
table with local disinfection and the tumor size was observed and
measured by high-frequency ultrasound. An ultrasound-guided 21G PTC
needle was used to puncture the tumor and the inner core was
withdrawn. Subsequently, a laser fiber was inserted into the needle
(Mylab™ Twice, Esaote SpA). After the fiber was ultrasonically
confirmed to be positioned at 1/3 the length of the tumor, the
power was set at 1 W and the foot switch was activated for
continuous laser ablation; the treatment time was 30 sec (total
dose of 30 J). Following treatment, the fiber was removed and local
hemostasis was conducted by applying gentle pressure.
Drug preparation and intravenous
injection
The 17-AAG inhibitor (Selleck Chemicals LLC) was
prepared by dissolving 50 mg in 800 µl DMSO solution and then
diluting this to a concentration of 5 mg/ml with normal saline
solution. The 3-MA inhibitor (Selleck Chemicals LLC) was prepared
by dissolving 50 mg directly in 10 ml normal saline to produce a 5
mg/ml stock concentration. The method of drug administration was as
follows: In group 1, each mouse received an intraperitoneal
injection of 300 µl DMSO (concentration 0.8%); in group 2, each
mouse received an intraperitoneal injection of 200 µl 17-AAG
(concentration 5 mg/ml); in group 3, each mouse received an
intraperitoneal injection of 300 µl 3-MA (concentration 5 mg/ml);
in group 4, each mouse received an intraperitoneal injection of 200
µl 17-AAG (concentration 5 mg/ml) and, 30 min later, an
intraperitoneal injection of 300 µl 3-MA (concentration 5 mg/ml)
was administered. The drugs were administered twice, at 25 h and 1
h prior to surgery.
Sample collection
Nude mice were euthanatized via intraperitoneal
injection of 2% sodium pentobarbital at a dose of 150 mg/kg body
weight. Cervical dislocation was used to confirm the death of mice.
The nude mice were sacrificed 24 h post-surgery; the tumors were
then extracted and their volumes were measured. The tumors were cut
along their diameter and three-quarters of each tumor was kept in a
liquid nitrogen jar, whereas the remaining one-quarter was fixed in
4% paraformaldehyde for 48 h at room temperature.
TUNEL
TUNEL (G3250; Promega Corporation) was used to
determine apoptosis in HCC model mice that underwent incomplete
ablation. Previously fixed tumor tissue was dehydrated in graded
ethyl alcohol (70, 80, 90 and 100%), embedded in paraffin, sliced
into 4 µm sections, and rehydrated (100, 95, 85 and 75%). The
slides were immersed in 4% formaldehyde in PBS for 15 min and then
in PBS for 5 min. Equilibration buffer (100 µl) was added and the
slides equilibrated at room temperature for 5–10 min.50 µl TdT
reaction mix was added to the pretreatment slide on an area ≤5
cm2 for 60 min at 37°C in a humidified chamber protected
from light. Reactions were stopped by immersing slides in 2X
saline-sodium citrate buffer (15 min at room temperature). Slides
were washed 3 times for 5 min each in PBS to remove unincorporated
fluorescein-12-dUTP. Then, mounting medium was added to the slides.
To visualize the nuclei, Vectashield with DAPI (Vector
Laboratories, Inc.; Maravai LifeSciences) was used. Samples were
analyzed immediately by fluorescence microscopy (Olympus
Corporation). For each slide, 5 high-power fields (magnification,
×200) with >500 cells were observed. ImagePro Plus 6.0 (Media
Cybernetics, Inc.) was used to calculate the number of apoptotic
cells. The apoptosis index (AI) was calculated by counting the
number of cells that emitted green fluorescence using the following
formula:
AI=(number of positive cells/total cell count) ×
100%.
Western blot analysis
The small tumor samples were lysed on ice using RIPA
buffer containing a protease inhibitor cocktail with constant
shaking for 30 min; cellular debris was pelleted by centrifugation
at 10,000 × g for 5 min at 4°C and supernatants were harvested.
Protein concentrations were measured by BCA protein assay kit
(ab207007; Abcam). Target proteins (30 µg per lane) were separated
via SDS-PAGE. Following separation with different concentrations of
acrylamide gel (6% for mTOR; 10% for AKT and P62; 12% for caspase-3
and cleaved caspase-3; 15% for LC3II/I), the proteins were
transferred onto PVDF membranes. The membrane containing the
proteins was successively incubated in blocking buffer (overnight
at 4°C), with a primary antibody (37°C for 1 h) and with a
secondary antibody (37°C for 1 h). The following primary antibodies
were used: Anti-LC3II/I (1:200, 12741), anti-p-Akt (1:400, 4060s;
both from Cell Signaling Technology, Inc.); anti-Akt (1:400,
ET1609-47), anti-mTOR (1:400, ET1608-5), anti-Caspase-3 (1:200,
ER30804) and anti-p62 (1:200, R1309-8) (all from Hangzhou HuaAn
Biotechnology Co., Ltd.); and anti-p-mTOR (1:400, ab52757; Abcam);
anti-actin (1:400, ab179467; Abcam). HRP-conjugated goat anti-mouse
IgG H&L (1:5,000, ab205719; Abcam) was used as the secondary
antibody. Chemiluminescence detection was achieved by exposure to
film in a darkroom. Following development and fixation with washing
buffer at 20–25°C for 10 min (P0019, Beyotime Institute of
Biotechnology), the film was photographed by FluorChem HD2
(ProteinSimple). The densities of the protein bands were determined
using ImageJ software (v1.46; National Institutes of Health),
normalized to actin expression and quantified using Microsoft Excel
software (version 2016, Microsoft Corporation).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS Inc.). Data are expressed as the mean ± standard deviation.
The differences among different groups were examined by one-way
analysis of variance followed by Tukeys post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Combined use of 17-AAG and 3-MA
significantly promotes apoptosis
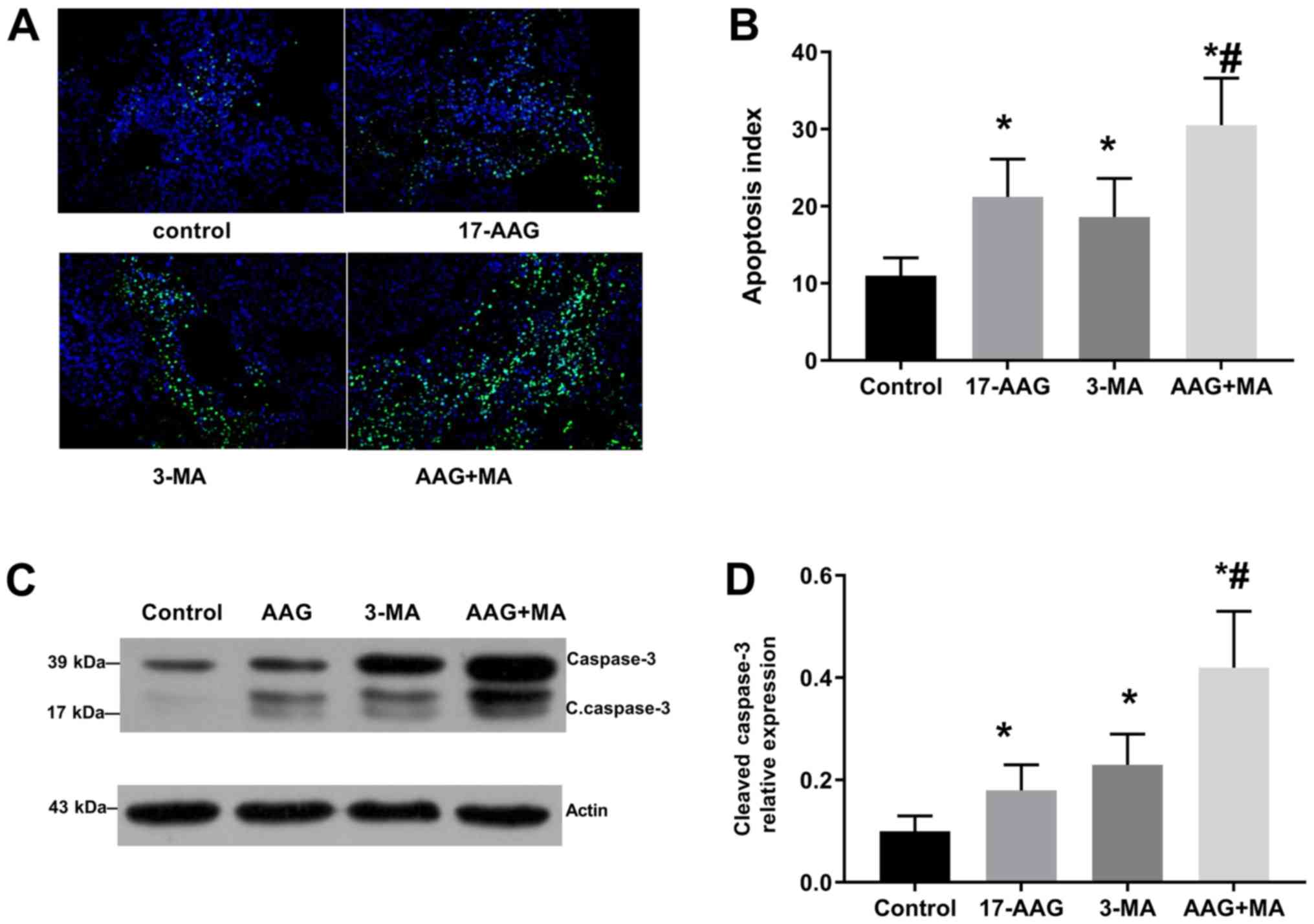
In Fig. 1A,
compared with group 1, groups 2–4 showed green fluorescent
protein-LC3 accumulation. The apoptosis indices in groups 1 to 4
were 11.0±2.3, 21.2±4.9, 18.6±5.0 and 30.5±6.1%, respectively
(Fig. 1B). Differences for all
groups were statistically significant compared with the results of
Group 1 (P<0.05) and there was a significant difference between
groups 2 and 4 (P<0.05). The relative quantities of
cleaved-capase-3 protein detected by western blot analysis in
groups 1–4 were 0.10±0.03, 0.18±0.05, 0.23±0.06 and 0.42±0.11
(Fig. 1C), respectively, with a
statistically significant difference between group 1 and the other
three groups (Fig. 1D). The
expression of apoptosis proteins was highest in Group 4 and there
was a significant difference between groups 2 and 4 (Fig. 1).
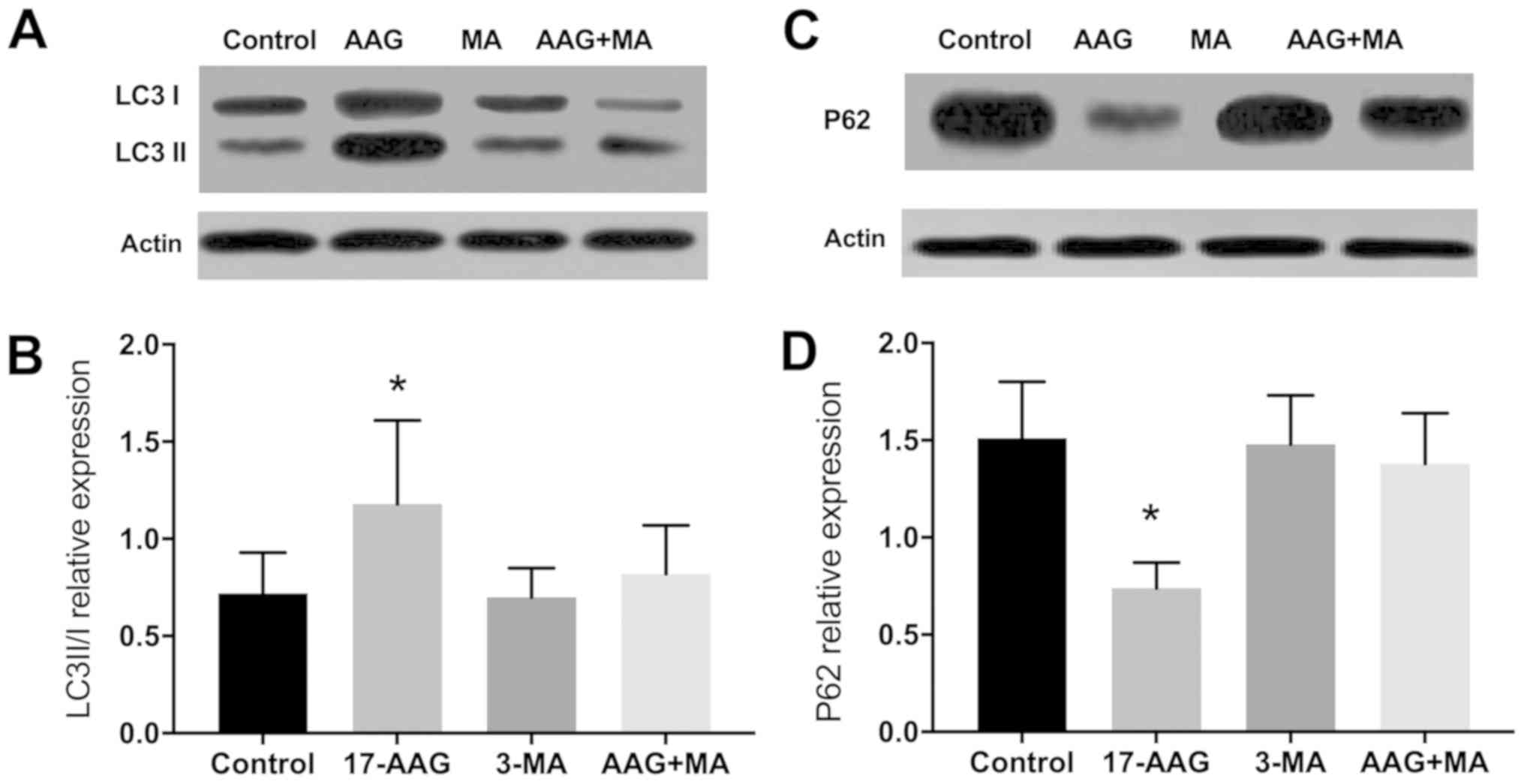
17-AAG-treatment significantly
enhances autophagy
Western blot analysis of LC3 II/I expression
revealed that the protein levels in groups 1–4 were 0.72±0.21,
1.18±0.43, 0.70±0.15 and 0.82±0.25, respectively, and were
significantly different between groups 1 and 2 (P<0.05). The
relative protein expression levels of p62 in groups 1–4 were
1.51±0.29, 0.74±0.13, 1.48±0.25 and 1.38±0.26, respectively, and
were significantly different between groups 1 and 2 (P<0.05;
Fig. 2).
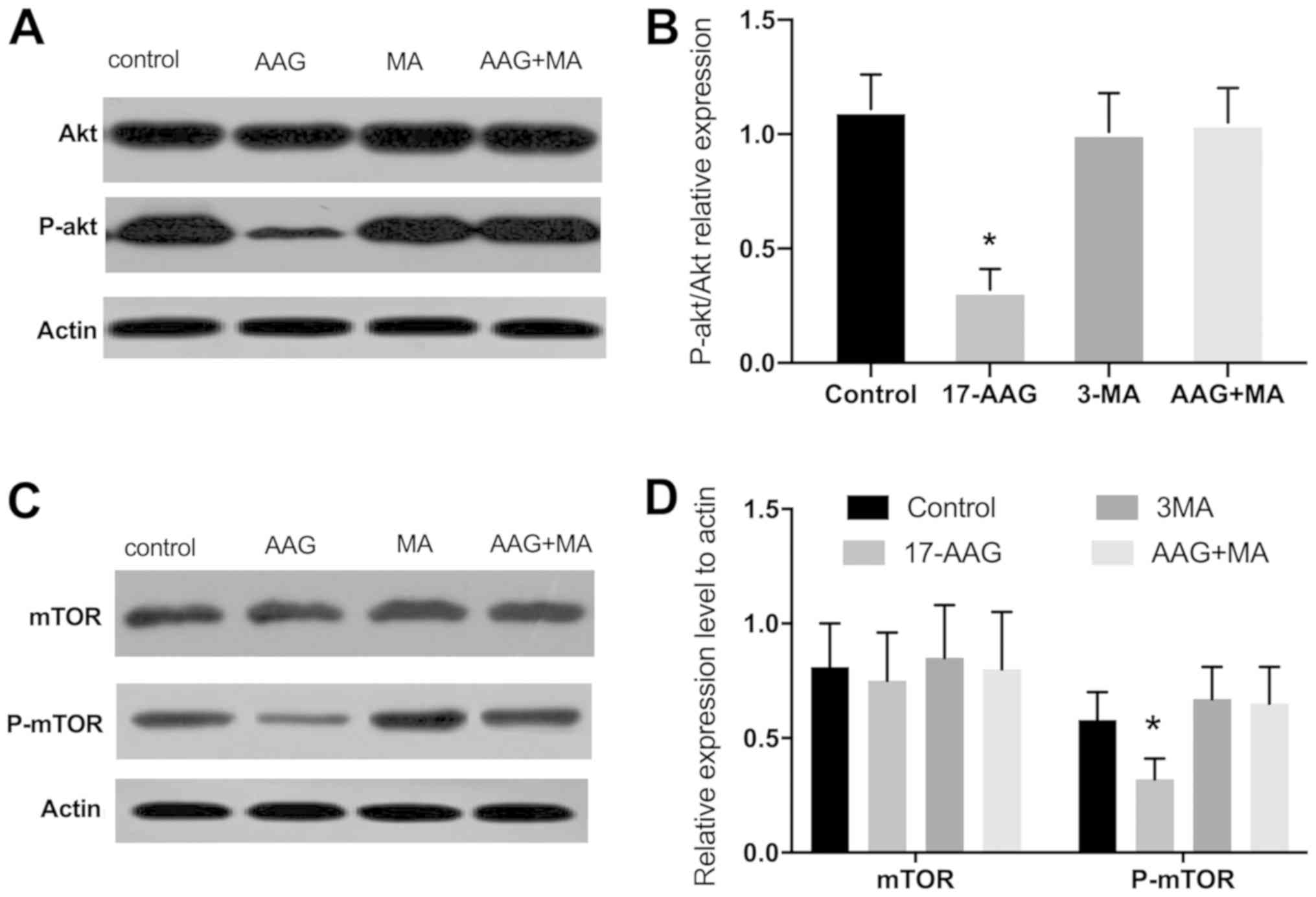
17-AAG treatment significantly
decreases the expression levels of p-Akt and p-mTOR
The quantities of p-Akt relative to Akt levels in
groups 1–4, as measured by western blot analysis, were 1.11±0.15,
0.32±0.09, 1.01±0.17 and 1.05±0.15, respectively. A significant
difference was observed between groups 2 and 1 (P<0.05). The
relative expression of p-mTOR in groups 1–4 was 0.58±0.12,
0.32±0.09, 0.67±0.14 and 0.65±0.16, respectively. A significant
difference between groups 2 and 1 was revealed (P<0.05). The
relative expression of mTOR was 0.81±0.19, 0.75±0.21, 0.85±0.23 and
0.80±0.25, respectively (17-AAG vs. control, P>0.05; Fig. 3).
Discussion
The present study demonstrated that suppressing
autophagy enhanced HSP90 inhibitor-induced apoptosis in residual
tumors. These results clearly indicated that autophagy provided a
pro-survival function following incomplete ablation. Furthermore,
the combination of inhibitors targeting these 2 pathways could
potentially kill residual tumor cells more effectively, thereby
providing a rationale for combining these drugs as a treatment
strategy for incomplete ablation. Finally, the results clearly
suggested that there was an association between changes in
autophagy and regulation of the Akt/mTOR signaling pathway.
The HSP90 inhibitor 17-AAG has already been used in
stage III clinical trials (NCT00546780) (26). The results from stage I/II/III
clinical trials of 17-AAG reveal limitations on its efficacy for
the treatment of advanced tumors and particularly in allowing tumor
cells to enter a quiescent phase (NCT00103272, NCT00098423,
NCT00118092 and NCT001048897) (27–30).
To achieve the optimum therapeutic effect for this oncotherapy,
17-AAG needs to be administered long-term or be considered for
combined treatment with other anti-cancer regimens (NCT00103272)
(27).
Previous studies have investigated on the
combination of various drugs with HSP90 inhibitors. Lang et
al (14) reported that an
HSP90 inhibitor increases the efficacy of rapamycin against HepG2
and Huh7 cells by inhibiting rapamycin-induced Akt and NF-kB
activation, decreasing the expression of platelet-derived growth
factor receptor β in vascular smooth muscle cells and vascular
endothelial growth factor 2 expression in the vascular endothelium.
Another study on non-small cell lung cancer cell lines by Webber
et al (15) indicated that
combining an HSP90 inhibitor (17-AAG) and a focal adhesion kinase
inhibitor (PF-573228) suppresses the Akt-mTOR pathway, consequently
inhibiting colony formation and promoting the activation of
apoptosis-inducing proteins. Furthermore, Yang et al
(16) describes the inhibition of
HSP90 expression and enhancement of apoptosis using Thy-1 membrane
glycoprotein (Thy-1)-targeted thermosensitive
magnetoliposome-encapsulated 17-AAG for Thy-1 + liver cancer stem
cells (LSCSs) selected from the BEL-7404 cell line and in a nude
mouse model transplanted with Thy-1 + LCSCs tumors.
To generate the incomplete ablation model, the
present study used a laser fiber with a diameter of 300 µm and a
transplanted Huh7 tumor mouse to provide a model that can more
easily measure molecular changes for subsequent studies (18). Our previous study (18) indicated that HSP90 inhibitors may
promote apoptosis in the area of incomplete ablation, although an
increase in efficiency was not observed. Another notable result is
that 17-AAG not only induces apoptosis, but also activates
autophagy in the residual tumor. Upon treatment with 17-AAG, a
decreased level of LC3-I to LC3-II conversion was observed and a
decrease in p62 protein levels, all of which are markers of
autophagy activation.
The Akt/mTOR signaling pathway has emerged as the
central conduit in the regulation of autophagy. Accumulating
evidence has emphasized that the inhibition of Akt and its
downstream target mTOR contributes to the initiation of autophagy
(23–25). The present study assessed the
Akt/mTOR pathway proteins using western blot analysis, which
indicated that the 17-AAG group exhibited significantly decreased
levels of p-Akt and p-mTOR expression with increased autophagy
activity. In the group treated with a combination of 17-AAG and
3-MA, p-Akt and p-mTOR levels were not decreased and the
corresponding increase in levels of autophagy was diminished. It
could be hypothesized that this is due to a 3-MA-based inhibition
of PI3K, which is important for a number of signaling pathways that
control mTOR activation. 3-MA blocks class I PI3K persistently,
whereas its suppressive effect on class III PI3K is transient.
Class I PI3K is a heterodimer composed of p85-regulated and p110
catalytic subunits, resulting in AKT activation. Fully activated
AKT leads to mTOR activation and the subsequent inhibition of
autophagy. Although the possibility that other 17-AAG-mediated
mechanisms may be responsible for the observed activation of
autophagy cannot be completely excluded, accumulating evidence
suggests that Akt/mTOR inhibition is probably the mechanism of
autophagy induction (22,31).
An increasing body of evidence supports the
existence of crosstalk between apoptosis and autophagy, including
both positive and negative interactions (23–25).
Recent evidence suggests that autophagy may attenuate drug-induced
apoptotic responses (31,32). In the present study, an increase in
the activation of caspase-3 was observed following treatment with
3-MA, which is a mark of apoptosis. Compared with treatment with
17-AAG alone, a combination of 17-AAG and 3-MA inhibited the
increase of autophagy in a complimentary manner, resulting in a
markedly enhanced level of apoptosis. To the best of our knowledge,
this is the first study to highlight the interaction between
apoptosis and autophagy in an animal model of residual tumors. This
antagonism between autophagy and apoptosis can also be observed in
an HCC incomplete ablation model, which suggests that the
activation of autophagy has a protective effect on HCC cells and
decreases the occurrence of apoptosis during incomplete
ablation.
In summary, the results of the present study
demonstrated that incomplete ablation and HSP90 inhibitor-induced
autophagy involved enhanced autophagosomal synthesis and may
negatively regulate apoptosis in Huh7 transplantation tumors.
Therefore, autophagy has a pro-survival function in
incompletely-ablated tumors treated with HSP90 inhibitors.
Consistent with these results, the inhibition of autophagy may
enhance the anti-cancer effects of HSP90 and therefore could be
therapeutically targeted to improve treatment efficacy of
combination therapy. In conclusion, the combined application of
both drugs described in the present study has promise in clinical
settings.
Acknowledgements
Not applicable.
Funding
This work was supported by Zhejiang Provincial Basic
Public Welfare Research Program (grant no. LGD19C04007 to Fen
Chen). The funders had no role in study design, data collection and
analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
SZ and FC conceived and designed the study. FC, HB
and HX performed experiments. FC, HX and LV analyzed and
interpreted the data. FC and LV drafted the manuscript. FC
critically revised the manuscript for important intellectual
content. FC performed statistical analysis. FC obtained funding. FC
and HX provided technical or material support. SZ supervised the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol of this study was approved by the
Research Ethics Committee of the First Affiliated Hospital, College
of Medicine, Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yun KC, Rhim H and Noh S: Radiofrequency
ablation versus surgical resection as primary treatment of
hepatocellular carcinoma meeting the Milan criteria: A systematic
review. J Gastroenterol Hepatol. 26:1354–1360. 2011.PubMed/NCBI
|
|
2
|
Hermida M, Cassinotto C, Piron L, Assenat
E, Pageaux GP, Escal L, Pierredon-Foulongne MA, Verzilli D, Jaber S
and Guiu B: Percutaneous thermal ablation of hepatocellular
carcinomas located in the hepatic dome using artificial carbon
dioxide pneumothorax: Retrospective evaluation of safety and
efficacy. Int J Hyperthemia. 35:90–96. 2018. View Article : Google Scholar
|
|
3
|
Medhat E, Abdel Aziz A, Nabeel M, Elbaz T,
Zakaria Z, Shousha H, Amer A, Fouad Fathalah W, Maher R and Musa S:
Value of microwave ablation in treatment of large lesions of
hepatocellular carcinoma. J Dig Dis. 16:456–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schueller G, Kettenbach J, Sedivy R, Stift
A, Friedl J, Gnant M and Lammer J: Heat shock protein expression
induced by percutaneous radiofrequency ablation of hepatocellular
carcinoma in vivo. Int J Oncol. 24:609–613. 2004.PubMed/NCBI
|
|
5
|
Hinz S, Tepel J, Röder C, Kalthoff H and
Becker T: Profile of serum factors and disseminated tumor cells
before and after radiofrequency ablation compared to resection of
colorectal liver metastases-a pilot study. Anticancer Res.
35:2961–2967. 2015.PubMed/NCBI
|
|
6
|
Rai R, Richardson C, Flecknell P,
Robertson H, Burt A and Manas DM: Study of apoptosis and heat shock
protein (HSP) expression in hepatocytes following radiofrequency
ablation (RFA). J Surg Res. 129:147–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang W, Ahmed M, Tasawwar B, Levchenko T,
Sawant RR, Collins M, Signoretti S, Torchilin V and Goldberg SN:
Radiofrequency ablation combined with liposomal quercetin to
increase tumour destruction by modulation of heat shock protein
production in a small animal model. Int J Hyperthermia. 27:527–538.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schueller G, Kettenbach J, Sedivy R,
Bergmeister H, Stift A, Fried J, Gnant M and Lammer J: Expression
of heat shock proteins in human hepatocellular carcinoma after
radiofrequency ablation in an animal model. Oncol Rep. 12:495–499.
2004.PubMed/NCBI
|
|
9
|
He W, Ye X, Huang X, Lel W, You L, Wang L,
Chen X and Qian W: Hsp90 inhibitor, BIIB021, induces apoptosis and
autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive
and-resistant chronic myeloid leukemia cells. Int J Oncol.
48:1710–1720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leng AM, Liu T, Yang J, Cui JF, Li XH, Zhu
YN, Xiong T, Zhang G and Chen Y: The apoptotic effect and
associated signalling of HSP90 inhibitor 17-DMAG in hepatocellular
carcinoma cells. Cell Biol Int. 36:893–899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Chen S, Tu J, Cai W and Xu Q: HSP90
inhibits apoptosis and promotes growth by regulating HIF-1α
abundance in hepatocellular carcinoma. Int J Mol Med. 37:825–835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Ban LL, Luo G, Li ZY, Li YF, Zhou
YC, Wang XC, Jin CG, Ye JG, Ma DD, et al: Acquired resistance to
HSP90 inhibitor 17-AAG and increased metastatic potential are
associated with MUC1 expression in colon carcinoma cells.
Anticancer Drugs. 27:417–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Youn P, Pysher TJ, Scaife CL and
Furgeson DY: Tumour eradication using synchronous thermal ablation
and Hsp90 chemotherapy with protein engineered triblock
biopolymer-geldanamycin conjugates. Int J Hyperthermia. 30:550–564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lang SA, Moser C, Fichnter-Feigl S,
Schachtschneider P, Hellerbrand C, Schmitz V, Schlitt HJ, Geissler
EK and Stoeltzing O: Targeting heat-shock protein 90 improves
efficacy of rapamycin in a model of hepatocellular carcinoma in
mice. Hepatology. 49:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Webber PJ, Park C, Qui M, Ramalingam SS,
Khuri FR, Fu H and Du Y: Combination of heat shock protein 90 and
focal adhesion kinase inhibitors synergistically inhibits the
growth of non-small cell lung cancer cells. Oncoscience. 2:765–776.
2015.PubMed/NCBI
|
|
16
|
Yang R, Tang Q, Miao F, An Y, Li M, Han Y,
Wang X, Wang J, Liu P and Chen R: Inhibition of heat-shock protein
90 sensitizes liver cancer stem-like cells to magnetic hyperthermia
and enhances anti-tumor effect on hepatocellular carcinoma-burdened
nude mice. Int J Nanomedicine. 10:7345–7358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moussa M, Goldberg SN, Kumar G, Sawant RR,
Levchenko T, Torchilin V and Ahmed M: Radiofrequency
ablation-induced upregulation of hypoxia-inducible factor-1α can be
suppressed with adjuvant bortezomib or liposomal chemotherapy. J
Vasc Interv Radiol. 25:1972–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen F, Bao H, Xie H, Tian G and Jiang T:
Heat shock protein expression and autophagy after incomplete
thermal ablation and their correlation. Int J Hyperthermia.
36:95–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa
WC and Min W: Hsp90-Akt phosphorylates ASK1 and inhibits
ASK1-mediated apoptosis. Oncogene. 24:3954–3963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato S, Fujita N and Tsuruo T: Modulation
of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA.
97:10832–10837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oral O, Akkoc Y, Bayraktar O and Gozuacik
D: Physiological and pathological significance of the molecular
cross-talk between autophagy and apoptosis. Histol Histopathol.
31:479–498. 2016.PubMed/NCBI
|
|
22
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nazim UM and Park SY: Attenuation of
autophagy flux by 6-shogaol sensitizes human liver cancer cells to
TRAIL-induced apoptosis via p53 and ROS. Int J Mol Med. 43:701–708.
2019.PubMed/NCBI
|
|
24
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Talaei S, Mellatyar H, Asadi A, Akbarzadeh
A, Sheervalilou R and Zarghami N: Spotlight on 17-AAG as an Hsp90
inhibitor for molecular targeted cancer treatment. Chem Biol Drug
Des. 93:760–786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walker AR, Klisovic R, Johnston JS, Jiang
Y, Geyer S, Kefauver C, Binkley P, Byrd JC, Grever MR, Garzon R, et
al: Pharmacokinetics and dose escalation of the heat shock protein
inhibitor 17-allyamino-17-demethoxygeldanamycin in combination with
bortezomib in relapsed or refractory acute myeloid leukemia. Leuk
Lymphoma. 54:1996–2002. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaufmann SH, Karp JE, Litzow MR, Mesa RA,
Hogan W, Steensma DP, Flatten KS, Loegering DA, Schneider PA,
Peterson KL, et al: Phase I and pharmacological study of cytarabine
and tanespimycin in relapsed and refractory acute leukemia.
Haematologica. 96:1619–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heath EI, Hillman DW, Vaishampayan U,
Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, et
al: A phase II trial of 17-allylamino-17-demethoxygeldanamycin in
patients with hormone-refractory metastatic prostate cancer. Clin
Cancer Res. 14:7940–7946. 2018. View Article : Google Scholar
|
|
30
|
Pacey S, Gore M, Chao D, Banerji U, Larkin
J, Sarker S, Owen K, Asad Y, Raynaud F, Walton M, et al: A Phase II
trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG,
tanespimycin) in patients with metastatic melanoma. Invest New
Drugs. 30:341–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strozyk E and Kulms D: The role of
AKT/mTOR pathway in stress response to UV-irradiation: Implication
in skin carcinogenesis by regulation of apoptosis, autophagy and
senescence. Int J Mol Sci. 14:15260–15285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janku F, Mcconkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|