Introduction
Aortic aneurysm, dissection and dilation share a
common pathological feature of cystic medial necrosis and the
histological characteristics of cystic medial necrosis include the
loss of contractile vascular smooth muscle cells (VSMCs), leading
to apoptosis and phenotypic switching, elastic fiber degradation
and inflammatory cell infiltration (1). To date, the mechanism contributing to
aortic remodeling remains unclear (2).
Previous studies have demonstrated that sympathetic
activation and over-innervation promotes aortic dissection
(3,4) and that norepinephrine (NE) released by
sympathetic nerve endings can upregulate the expression of matrix
metallopeptidase-2 (MMP2) and promote aortic remodeling (5). However, the signaling pathway involved
in NE regulation of aortic remodeling is still unknown.
Transforming growth factor (TGF) β signaling serves
a central role in aortic remodeling. Mutations of TGFβ family
members such as TGFR-1/TGFR-2 result in a hereditary aortic
aneurysm such as Loeys-Dietz syndrome (6). Marfan syndrome is a result of
fibrillin-1 modification due to a defect or a mutation of the gene
that encodes it, which is considered to regulate TGFβ
bioavailability and activity by controlling access to, or the
efficiency of, TGFβ activators (7).
The dysregulation of the downstream TGFβ pathway signaling is also
associated with aortic aneurysm (7). Since the homeostasis of TGFβ signaling
is important in maintaining a normal structure of the aortic wall,
the present study aimed to explore whether the sympathetic system
may also regulate aortic remodeling via the TGFβ pathway.
Little is known regarding the effect of the
sympathetic system on TGFβ signaling. Yang et al (8) investigated the interaction between the
α1 adrenergic receptor and TGFβ type I receptor kinase
(ALK5) pathways; however, the study was insufficient to clarify the
relationship between the sympathetic system and TGFβ signaling.
Therefore, the present study was designed to test a new hypothesis
that the sympathetic system may regulate ALK5-mediated TGFβ
signaling, thus serving a role in aortic remodeling. Previous
studies have provided evidence on the use of ALK5 as a therapeutic
target; for example, galunisertib, an ALK5 inhibitor, has antitumor
activity in tumor-bearing animal models of breast, colon and lung
cancers, and hepatocellular carcinoma (9); a phase II study has revealed that
galunisertib treatment exerts hematologic improvements in low- and
intermediate-risk myelodysplastic syndromes (10). Thus, the possibility of using ALK5
as a therapeutic target in aortic aneurysm was also explored in the
present study.
Materials and methods
Animal experiments
As previously described (5), 50 male Sprague-Dawley rats (8 weeks,
weight 267–299 g) were brought from ABLIII experimental animal
laboratory of Wuhan university and housed in an animal room under
controlled conditions of 20–26°C and 40–70% humidity on a 12/12-h
light/dark cycle. Normal chow was supplied to the control group,
where as 0.25% β-aminopropionitrile (BAPN) chow was supplied to the
angiotensin II (AngII) and BAPN group to loosen the cross-link
among elastic fibers (11–13). Chemical sympathetic denervation
(CSD) was performed under pentobarbital anesthesia (1%; 30 mg/kg)
through a left paraspinal chest incision. The descending aorta
between the left subclavian artery and the diaphragm was dissected
and covered by a gauze pre-soaked in 20 µg/µl guanethidine for 30
min. An osmotic minipump (Alzet, Durect Corp.) was implanted into
the peritoneal cavity to infuse 1,000 ng/kg/min AngII continuously
for 4 weeks. The same operation and osmotic minipump was used in
the control group where saline was used instead of guanethidine or
AngII. At the end of 4 weeks, all surviving mice were sacrificed by
CO2 (100% CO2, 2.5 liters per min, 5 min) and
survival rate was calculated as survived/total. The experiments
were approved by The Ethics Committee of Renmin Hospital (Wuhan,
China).
Cell culture and treatment
Mouse VSMC cell line (MOVAS) was obtained from ATCC
and cultured in DMEM (Procell Life Science & Technology Co.,
Ltd.) containing 10% FBS (Procell Life Science & Technology
Co., Ltd.) at 37°C with 5% CO2 and 95% air. The cells
were sub-cultured to 70% confluence and subsequently cultured in
DMEM without serum for 12 h before treatment; 1% FBS was added to
the medium during any treatment.
ALK5 overexpression
Mouse ALK5 coding sequence was cloned into a pcw107
(V5) vector (Hanbio Biotechnology Co., Ltd.). A lentivirus was
obtained using the PPMD2.G (Hanbio Biotechnology Co., Ltd.) and
psPAX2 vectors (Hanbio Biotechnology Co., Ltd.) in 293T cells
(China Center for Type Culture Collection). The lentivirus was
aliquoted and transfected to the mouse VSMCs at the unified
concentration using polybrene (8 µg/ml, Sigma-Aldrich; Merck KGaA)
for 72 h.
Histology and immunostaining
Histology and immunostaining were performed as
previously described (14).
Briefly, sections were cut at 4 µm from the paraffin-embedded
aortic specimens of the rat model or control. The sections were
stained with hematoxylin and eosin or elastica Van Gieson staining
and immunostained with antibodies against each target protein (TH;
1:100, CST Biological Reagents Co., Ltd.; cat. no. 58844S; ALK5;
1:200, Abcam cat. no. ab31013). For the cell staining, having been
seeded on the slides for 24 h, the cells (~105
cells/cm2) were fixed in 4% paraformaldehyde (MACKLIN,
China, Cat No. 30525-89-4) at 4°C for 20 min and stained using the
same antibodies (incubated overnight at 4°C) as above. An Olympus
BX53 fluorescent microscope (Olympus Corporation) was used to
investigate and capture images. Sympathetic nerve densities were
determined by Image-Pro Plus 6.0 (Media Cybernetics, Inc.,) in
tyrosine hydroxylase (TH) staining slides as previously described
(3). Nerve density was calculated
as the nerve area divided by the total area examined
(µm2/mm2).
Realtime quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells (MOVAS) using
RNAiso plus (Takara Bio, Inc.) according to the manufacturer's
instructions followed by reverse transcription (SMART MMLV cDNA
synthesis kit, Takara Biotechnology Co., Ltd.). A total of 20 µl
was used (2 µl cDNA, 10 µl SYBR® Green (Thermo Fisher
Scientific, Inc.), 2 µl primer (Servicebio), 6 µl water) on an ABI
9700 qPCR machine (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The settings were: 93°C for 40 sec, 58°C for 30 sec and 72°C
for 60 sec (35 cycles). The primers are listed in Table I. The result was calculated by the
2−ΔΔCq method (15).
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Target genes | Forward 5′-3′ | Reverse 5′-3′ |
|---|
| Mouse
ALK5 |
GAAAAGCAGTCAGCTGGCCTT |
CTTCATTTGGCACACGGTGG |
| Mouse
TGFβ1 |
CTGCTGACCCCCACTGATAC |
AGCCCTGTATTCCGTCTCCT |
| Mouse
INHBA |
AAATCAGAACGCCTCCGCTA |
TCCCGAGTGTAGAGTTCGGT |
| Mouse
BMP4 |
TCCGTCCCTGATGGGATTCT |
TGGTGTCTCATTGGTTCCTGC |
Western blotting
RIPA lysis buffer (Beyotime Biotechnology, Inc. cat
no. P0013B) was used to extract total proteins from cells and
bicinchoninic acid assay was used to measure the protein
concentration. Protein (20–30 µg) was loaded onto a 15% SDS-PAGE
gel and ran at 100 V. Then the protein was transferred onto PVDF
membranes and blocked by 5% milk at room temperature for 1 h.
Primary antibodies were incubated with the membrane overnight at
4°C: ALK5, Abcam, cat. no. ab31013, 1:1,000; SMAD2/3, CST
Biological Reagents Co., Ltd., cat. no. 5678, 1:2,000; p-SAMD2/3,
Santa Cruz Biotechnology, Inc., cat. no. sc-11769,1:200; ERK1/2,
Biorbyt Technology, Inc., cat. no. orb216186, 1:500; p-ERK1/2, CST
Biological Reagents Co., Ltd., cat. no. 8544, 1:1,000; JNK1/2,
Santa Cruz Biotechnology, Inc., cat. no. sc-7345, 1:200; p-JNK,
Invitrogen (Thermo Fisher Scientific, Inc.), cat. no. 700031,
1:1,000; GAPDH, Santa Cruz Biotechnology, Inc., cat. no. sc47724,
1:1,000. The membrane was then incubated with the secondary
antibodies and chemiluminescence method was used to acquire images.
Image-Pro Plus 6.0 (Media Cybernetics, Inc.) was used to analysis
the bands.
Cell migration assay
Cell migration assay was performed using mouse
VSMCs. Cells were cultured in DMEM supplemented with 1% FBS as
VSMCs stopped proliferating in 1% FBS (Fig. S1). The scratch was created with a
200 µl pipette tip and the scratch closure was monitored at 24 and
48 h. Scratch closure was quantified using ImageJ 1.52t (National
Institutes of Health).
NE concentration assay
Small segments of the aorta were immediately placed
in 0.1 M Perchloric acid (HClO4) solution after harvest and kept
overnight and then stored under −80°C. The samples was ground and
dissolved in the same volume of saline (50 mg/ml). The
concentration of AngII and NE in the aorta were detected using
ELISA kits (AngII, Cloud-Clone Corp. (CEA005Ra); NE, Eagle
Biosciences, Inc. (SKU: NOR31-K01) according to the manufacturers'
instructions.
Cell proliferation assay
Cell Counting Kit-8 (CCK8) proliferation assay kit
was obtained from Biosharp Life Sciences (cat. no: BS350B) and used
according to the manufacturer's protocol. Mouse VSMCs were seeded
in 96-well plates at a density of 3×103 cells/well.
After 24 h, DMEM containing 1% FBS and 10 µl CCK8 was added into
each well and incubated for 4 h at 37°C. The optical density was
read at 450 nm using a Multiskan MK3 microplate reader (Thermo
Fisher Scientific, Inc.). The NE and FBS concentration gradient
experiment was performed with different dosages of NE or FBS in
DMEM by cell counting. Briefly, MOVAS cells were passaged and
synchronized in 1% FBS for 12 h and 1, 10, 50, 100 and 200 nm NE
and 0, 1, 2, 5 and 10% FBS DMEM was used to culture cells. Cell
numbers were counted at 24, 48 and 72 h.
Apoptosis assay
DMEM containing 1% FBS was applied to cells treated
with NE, NE+ALK5 or PBS in each group. According to the
manufacturer's instructions of the apoptosis assay kit (Nanjing
KeyGen Biotech Co., Ltd.), after trypsinization, cells were washed
twice, centrifuged (800 × g, 4°C for 5 min) and resuspended in 500
µl Binding Buffer from the kit). Next, the suspension was mixed
with 5 µl AnnexinV-FITC, and 5 µl propidium iodide (PI) was added.
After incubation in the dark for 5–15 min, flow cytometry assay was
performed using a CytoFLEX flow cytometer with Cytexpert software
(Beckman Coulter, Inc.; version 2.3). The negative control was
without AnnexinV-FITC and PI. Late apoptosis was assessed and the
most significant apoptotic group was used as a positive control, as
suggested by the kit manufacturer.
Statistical analysis
All quantitative data are presented as mean ±
standard deviation. Statistical analysis was performed using
GraphPad Prism software (version 6; GraphPad Software Inc.).
Unpaired Student's t-test was performed to calculate the
differences between two groups, and one-way ANOVA was performed
when three groups were compared. Tukey's post hoc test was used for
multiple comparisons. P<0.01 was considered to indicate a
statistically significant difference.
Results
Aortic CSD protects AngII-induced
aortic remodeling
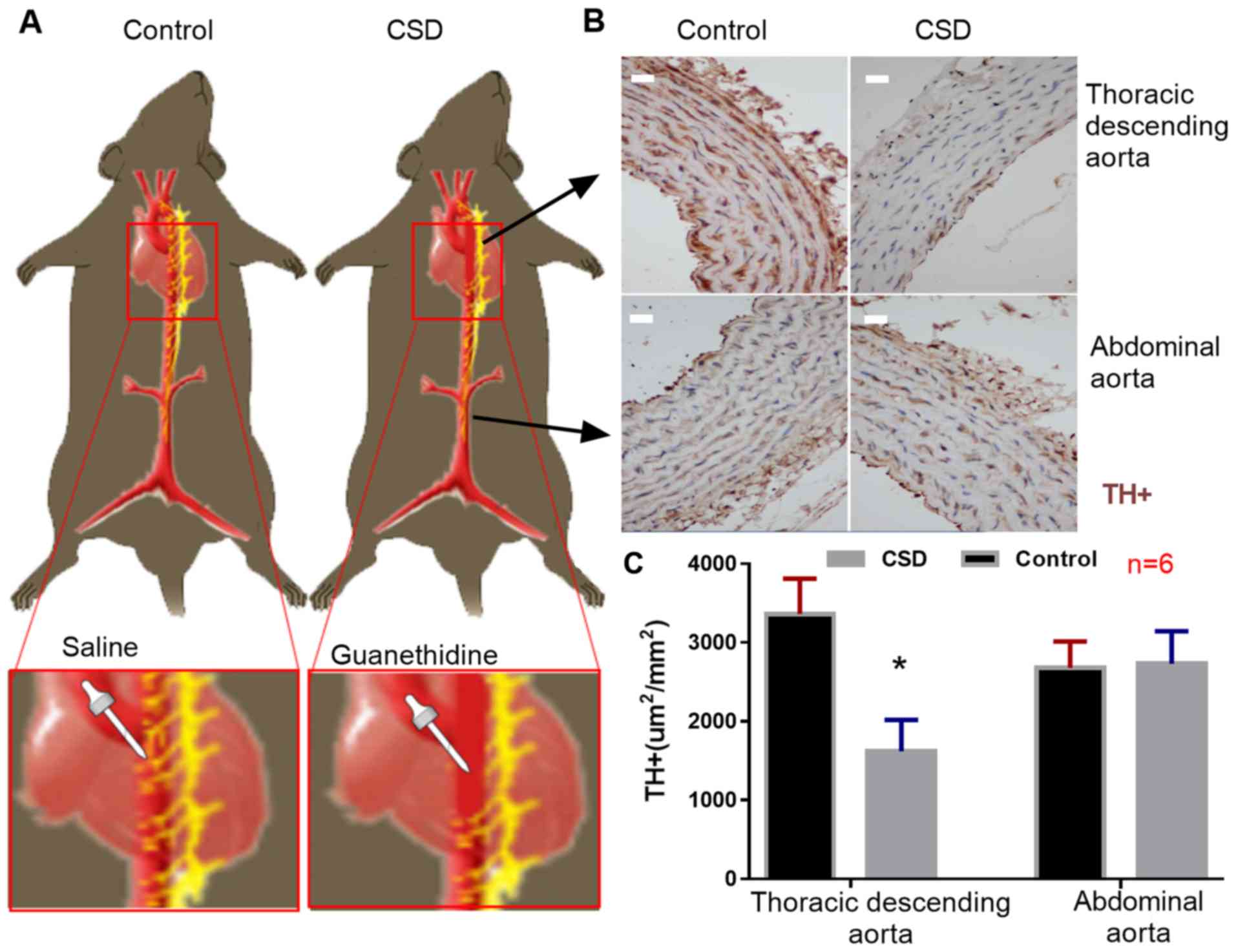
At the end of the animal experiment, TH-positive
(TH+) cells (sympathetic nerve endings) were
immunostained. The number of TH+ cells were
significantly decreased in the descending aortas, but not in the
abdominal aortas of the CSD group compared with those in the
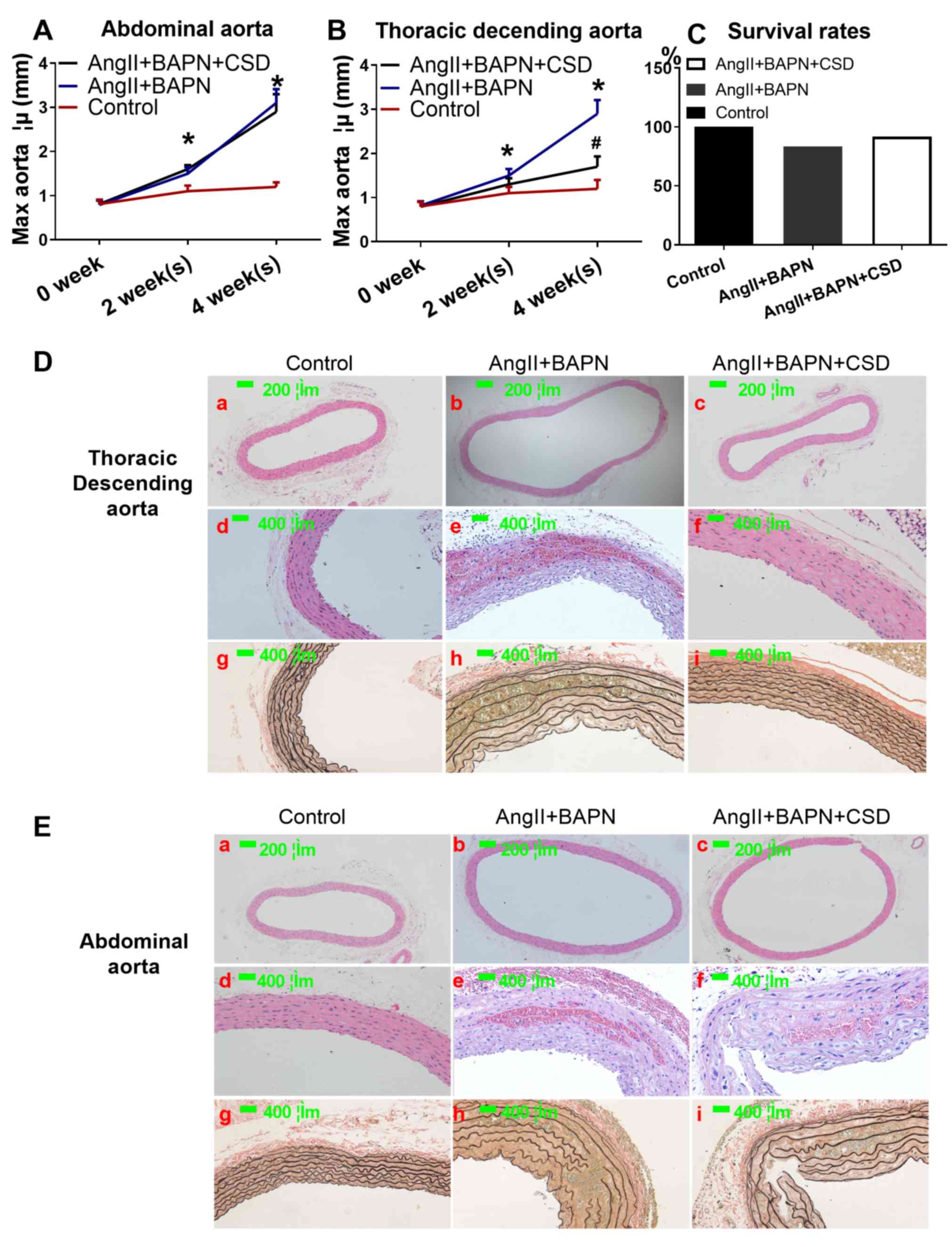
control groups (Fig. 1). No aortic
rupture was observed in the control group, but two (16.7%) ruptures
in the AngII+BAPN group and one in the AngII+BAPN+CSD group (8.3%)
were present (Fig. 2C). The
diameter of both the descending and abdominal aorta increased with
time in the AngII- and BAPN-treated groups (Fig. 2A, B, D-a, D-b, E-a and E-b). CSD
rescued the aortic dilation in the descending, but not in the
abdominal aorta (Fig. 2A, B, D-c and
E-c). AngII and BAPN induced intramural aortic hematoma and
elastic fiber destruction, which was not observed in the control
group. In addition, CSD rescued intramural aortic hematoma and
elastic fiber destruction in the descending, but not in the
abdominal aorta (Fig. 2D-d-i and
E-d-i).
CSD alleviates AngII-induced NE
release and ALK5 downregulation in the aorta
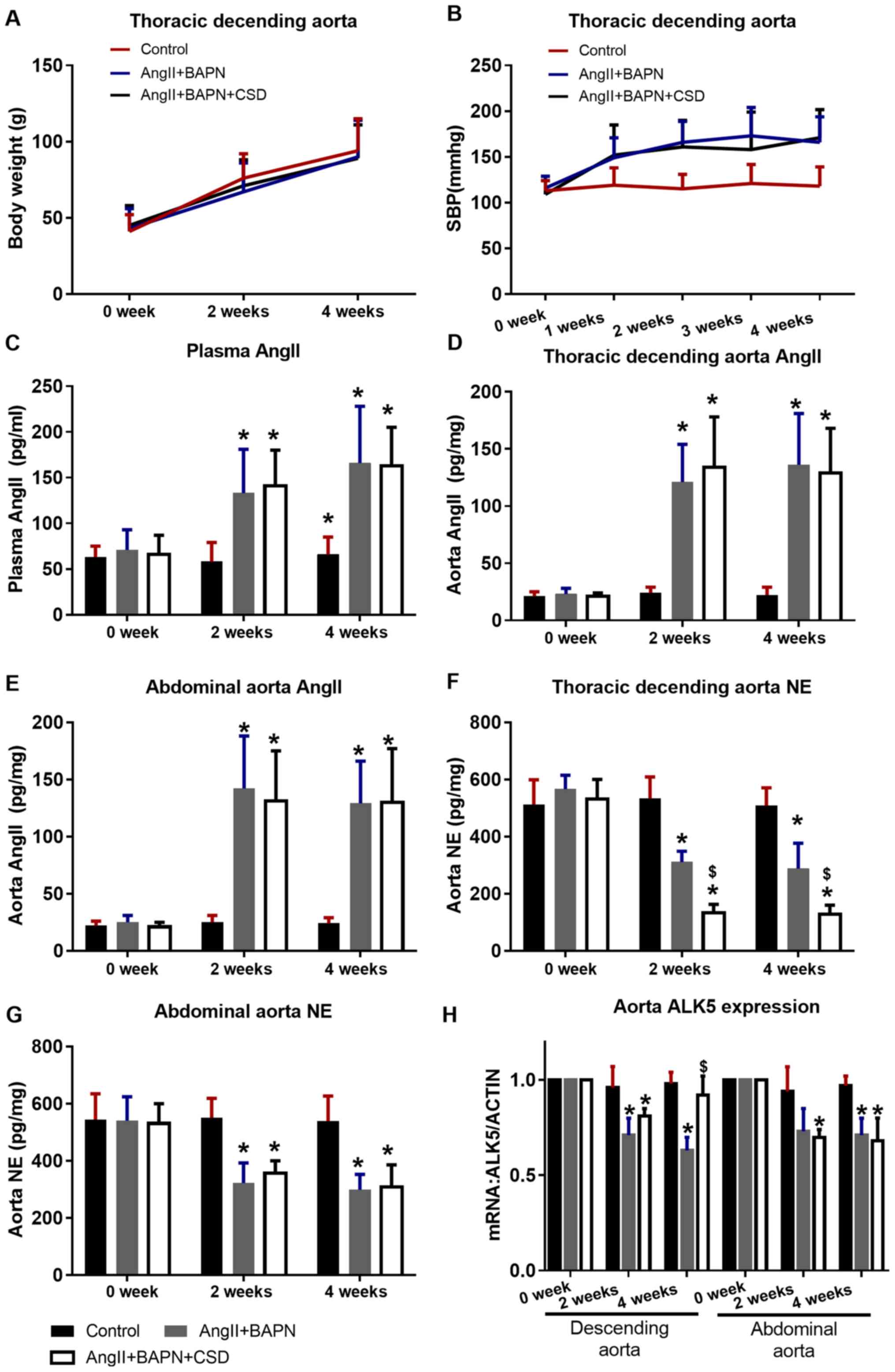
No significant difference in body weight was
observed among the treatment groups (Fig. 3A). AngII increased the systemic
blood pressure compared with the control group, and regional CSD
did not alleviate the AngII-induced hypertension (Fig. 3B). In the AngII-pumped groups, the
concentrations of AngII in the plasma, the thoracic descending and
the abdominal aorta significantly increased compared with those in
the control group, and regional CSD did not affect AngII
concentration in the plasma and aortic tissue (Fig. 3C-E). AngII also reduced the NE
concentration in the thoracic descending and abdominal aortas of
the AngII-pumped groups compared with the control group. In the CSD
segment, the NE concentration in the thoracic descending aorta was
lower compared with that in the aorta of the AngII+BAPN group, and
this difference was not observed in the abdominal aorta (Fig. 3F and G). ALK5 expression was
downregulated in the aortas of the rats in the AngII+BAPN group
compared with those in the control group, and CSD rescued this
effect in the thoracic descending aorta (significant at the end of
4 weeks), but not in the abdominal aorta (Fig. 3H).
NE modulates TGFβ signaling by
suppressing ALK5 expression in VSMCs
Based on the concentration gradient experiment, 100
nM NE was used to treat mouse VSMCs. Compared with the control
group the expression of ALK5 was significantly downregulated at 24
h after NE treatment was further downregulated at 48 h according to
the results obtained by western blotting (Fig. 4B and C), qPCR (Fig. 4D), and immunofluorescence (Fig. 4E-I); however the expression of the
TGFβ receptor ligands; TGFβ1, Inhibin Subunit β A (INHBA) and Bone
Morphogenetic Protein 4 (BMP4) as determined by qPCR did not change
(Fig. 4D). NE treatment also
altered the dominance of TGFβ signaling, as it suppressed the
phosphorylation of SMAD2/3 and promoted the phosphorylation of
ERK1/2 and JNK1/2, which was partially reversed by ALK5
overexpression (Fig. 4K and L).
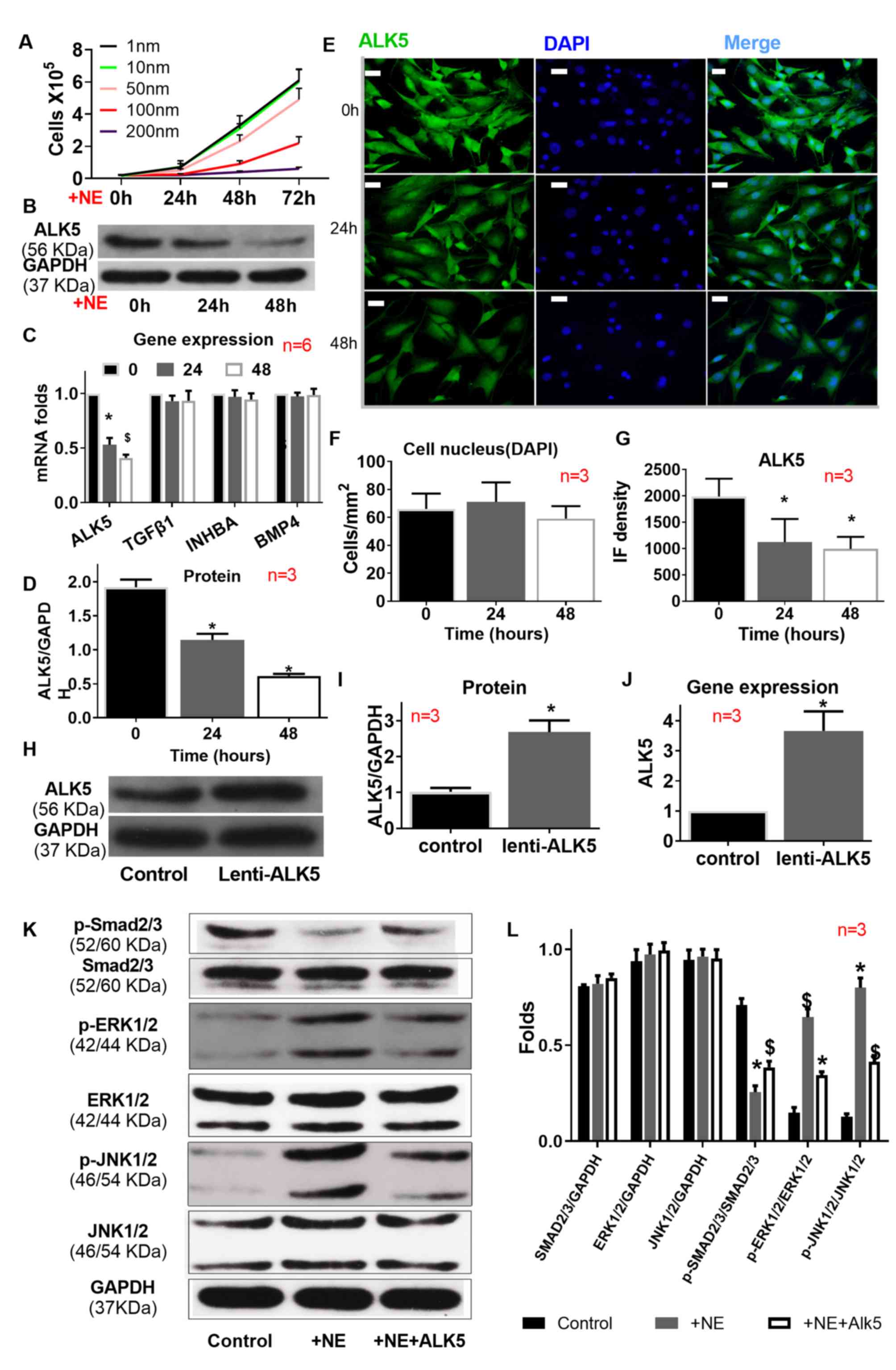 | Figure 4.Impact of ALK5 overexpression on ALK5
expression and signaling transduction. (A) NE (100 nm)
significantly decreased VSMC proliferation and suppressed ALK5
expression, as indicated by (B-D) western blotting (C) reverse
transcription-quantitative PCR and (E-G) immunofluorescence, but
(C) did not affect TGFβ1, INHBA or BMP4 expression. (H-J) ALK5 was
successfully overexpressed.(K and L) NE suppressed SMAD2/3
signaling, but activated ERK1/2 and JNK1/2 signaling. ALK5
overexpression partially rescued the effect of NE. Scale bar=400
µm. *P<0.01 vs. control; $P<0.01 vs. AngII+BAPN.
NE, norepinephrine; ALK5, transforming growth factor β type I
receptor kinase; TGF, transforming growth factor; AngII,
angiotensin II; BAPN, β-aminopropionitrile; p, phosphorylated; IF,
immunofluorescence; INHBA, inhibin subunit β A; BMP4, bone
morphogenetic protein 4. |
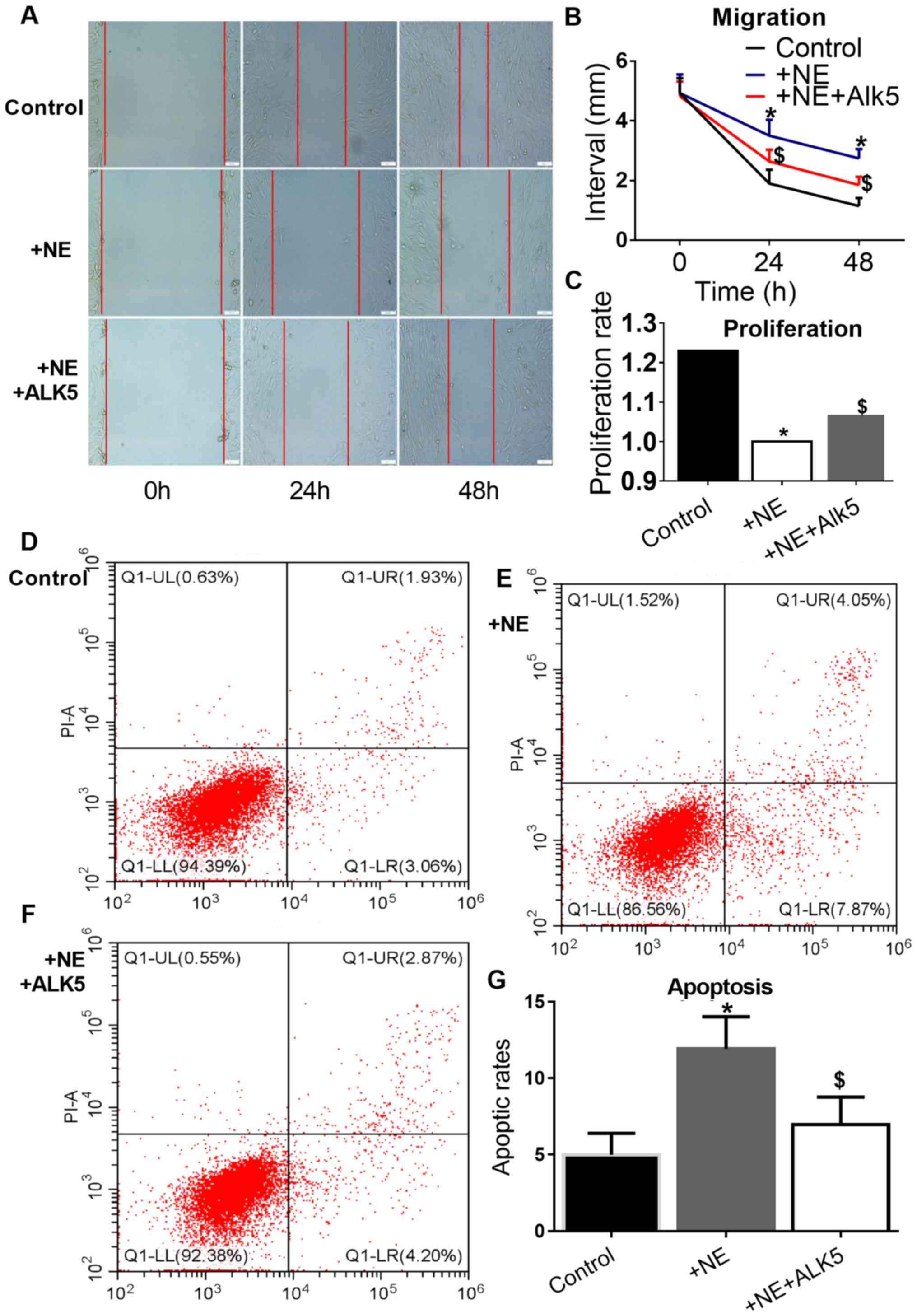
ALK5 overexpression reverses the
effects of NE on VSMC proliferation, migration and apoptosis
CCK8 assay was used to investigate the effects of
100 nM NE on VSMC proliferation. A significant inhibition of mouse
VSMC proliferation and migration in the wound healing assay by NE
was observed. In addition, a promoting effect of NE on VSMC
apoptosis was observed. However, the effect of NE on VSMC
proliferation, migration and apoptosis was reversed by ALK5
overexpression (Fig. 5).
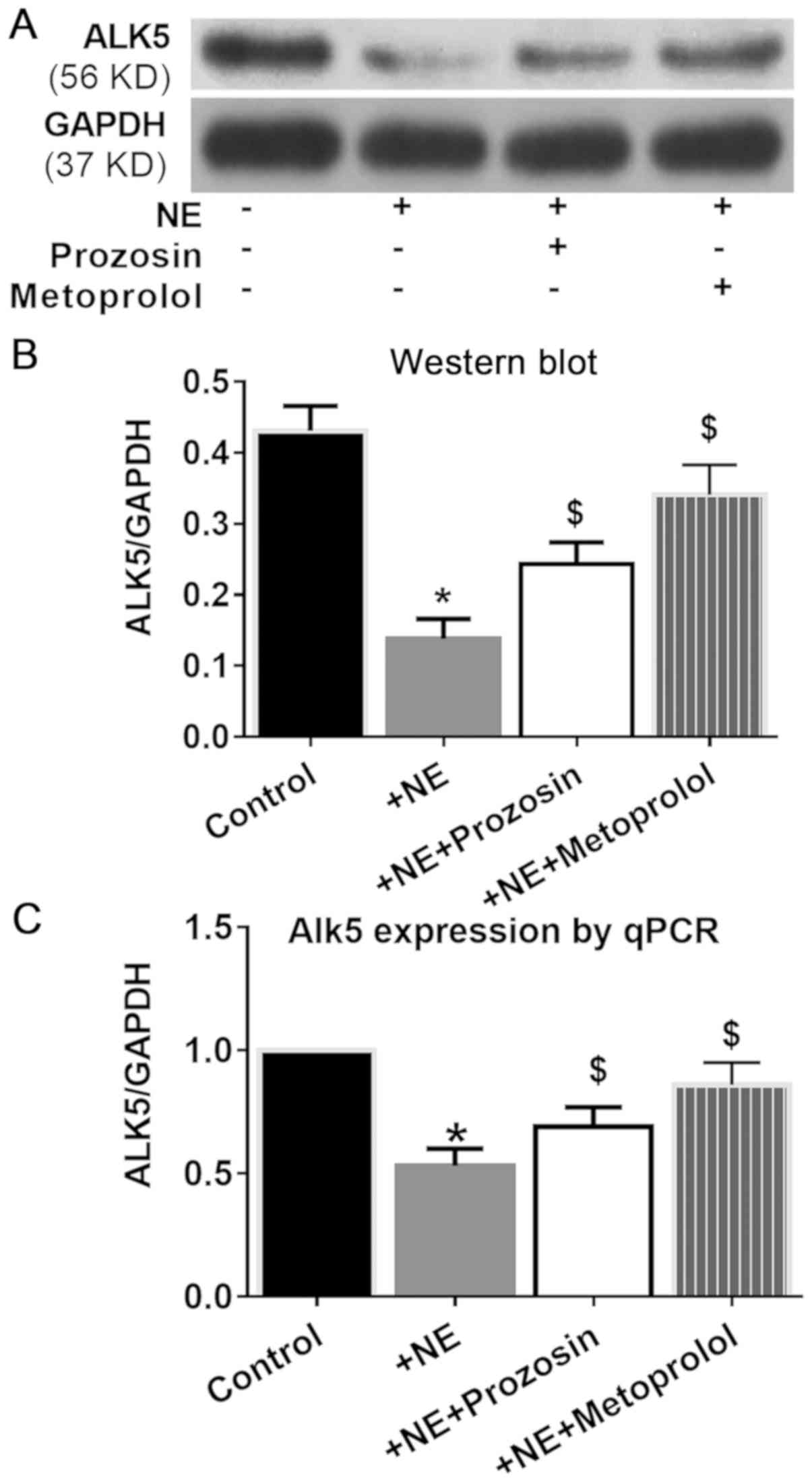
α- and β-adrenergic receptors are
involved in NE-ALK5 signaling
Among the adrenoceptors, α1 and
β1 receptors are very important in maintaining artery
structure homeostasis (16,17). To clarify which adrenergic receptors
were involved in NE-ALK5 signaling, selective receptor antagonists
10 µM prazosin and metoprolol were used to block α1- or
β1-adrenergic receptors, respectively. Both prazosin and
metoprolol partially reversed the inhibition of NE on ALK5
expression, with metoprolol exerting a slightly stronger effect
(Fig. 6).
Discussion
A number of studies have focused on the regulation
of the vessel tone and blood pressure by the sympathetic system,
but have neglected its impact on aortic wall structure (18,19).
Evidence indicates that the sympathetic system not only regulates
the arteries from a functional aspect, but also from a structural
aspect. First, there is a clear difference in the sympathetic
innervation of arteries and veins; sympathetic innervation is rich
in arteries but poor in veins (20). Furthermore, sympathetic innervation
increases during development (21).
Chronic hypoxia is a risk factor of aortic (22–24)
and small artery (25,26) diseases, as well as an inducer of
arterial sympathetic innervation (27). Thus, a regional CSD method was used
in the present study to investigate the role of aortic sympathetic
innervation. Compared with global sympathetic denervation or
surgical aortic sympathetic denervation (thoracic sympathectomy),
this method is less likely to affect systemic blood pressure
(28). Blood pressure is a strong
factor promoting aortic remodeling (29). The histological and pathological
features of the descending (CSD segment) and abdominal aorta
(non-CSD segment) were compared in order to exclude the possibility
that CSD may also affect the non-CSD area. In the descending aorta,
the number of TH+ cells (sympathetic nerve endings) was
significantly decreased, and the intramural aortic hematoma and
elastic fiber destruction were rescued compared with the control
group. By contrast, in the abdominal aorta (non-CSD segment), the
number of TH+ cells was not decreased, and the
intramural aortic hematoma and elastic fiber were not rescued.
These results indicated that regional CSD was only effective in the
treated region.
The present study identified ALK5 as a target of the
sympathetic nervous system. NE suppressed TGFβ receptor1 expression
without any impact on the TGFβ receptor ligands (although the
expression of TGFβ receptor ligands was only tested by qPCR, not
western blotting). To date, only a limited number of studies have
explored whether and how the autonomic nervous system regulates
ALK5 expression and signaling. In a rat cerebral
ischemia/reperfusion model, Ma et al (30) have demonstrated that stimulation of
the vagus nerve regulates Growth Differentiation Factor 11 (GDF11)
and ALK5 expression, and hypothesized that GDF11/ALK5 may represent
a potential target for stroke therapy. Neuropilins1, an axon
elongation inducer, inhibits the expression of both ALK1 and ALK5
(31). Yang et al (8) demonstrated that
α1-adrenergic receptor/ALK5 interaction contributes to
doxazosin-induced apoptosis, which is further enhanced by TGFβ1 in
association with attenuating SMAD3 phosphorylation in H9C2 cells.
By contrast, the results of the present study demonstrated that
both α- and β-adrenergic receptors were involved in the regulation
of NE on ALK5 expression. The present study and the aforementioned
previous studies further confirmed the interaction between TGFβ
signaling and the autonomic nervous system.
ALK5 is a membrane-bound receptor protein of the
TGFβ superfamily of signaling ligands (32). When bound to TGFβ, ALK5 transduces
the TGFβ signal from the cell surface to the cytoplasm. Abnormal
expression and/or activation of ALK5 induce changes in the
downstream signaling transduction and diseases (30). Mutations in the ALK5 gene are
associated with the Loeys-Dietz aortic aneurysm syndrome (6). The present study revealed that
postnatal modulation of ALK5 by a sympathetic transmitter also
contributes to aortic remodeling.
TGFβ signaling consists of SMAD2/3-dependent and
non-SMAD2/3-dependent cascades; the former is termed canonical TGFβ
signaling, and the latter is termed non-canonical TGFβ signaling
(33,34). Inhibition of the non-canonical TGFβ
signaling molecules such as ERK1/2 and/or JNK1 may rescue aortic
aneurysms (33,35,36).
The results of the present study suggested that ALK5 repression by
NE also induced TGFβ signaling dominance switch and aortic
remodeling. This indicated a strong impact of NE and the
sympathetic nervous system on ALK5 expression and downstream
signaling. TGFβ signaling serves broad biological functions
(34). Its normal status is
essential in the development and maintenance of the physiological
balance, but when disturbed, may result in a number of diseases
(34). Thus, it is possible that NE
and the sympathetic nervous system may also exert important roles
in other diseases, such as arterial diseases, via modulating ALK5
expression and TGFβ signaling.
A possible limitation of the present study was the
NE tissue concentration. Our previous study has demonstrated that
AngII promotes NE release from the sympathetic nerve endings
(5). Subsequently, cells in the
tissue (such as VSMCs) interact with more NE, and the NE in the
extracellular fluid is washed away by the blood flow. Thus, more NE
release results in lower tissue NE concentration (33). CSD also reduces tissue NE by
reducing the number of sympathetic nerve endings and NE release,
resulting in less NE acting on cells (VSMCs and others). This is
consistent with another study (37). The mortality rate in the present
study was lower compared with some studies (12,38) as
older rats were used in the present study instead of 3-week-old
rats. Tatsuo Kawai et al (11) used a similar protocol in modeling
abdominal aortic aneurysm and observed similar mortality.
The biological function of the sympathetic nervous
system is broad. NE and ALK5 may not be the only molecules in the
sympathetic nervous system to regulate TGFβ signaling, and TGFβ
signaling modulation may not be the only mechanism of the
sympathetic nervous system to serve a role in aortic disease
pathogenesis. However, based on previous and the present research,
the sympathetic nervous system, or even other autonomic nervous
systems, such as the vagal nerve system, may serve as a therapeutic
target to combat aortic diseases. Whether directly inhibiting ALK5
in control cells without NE treatment also produces the same
results requires further research. Although this was a limitation
of the present study, it did not affect the conclusions as previous
studies have demonstrated similar findings on the role of ALK5 in
VSMC proliferation and migration (32,35).
The present study confirmed the important role of
sympathetic system dysregulation in aortic remodeling. In addition,
the present study also revealed a new signaling pathway regulating
the sympathetic-adrenergic system. This pathway and its in
vivo function have not been previously investigated.
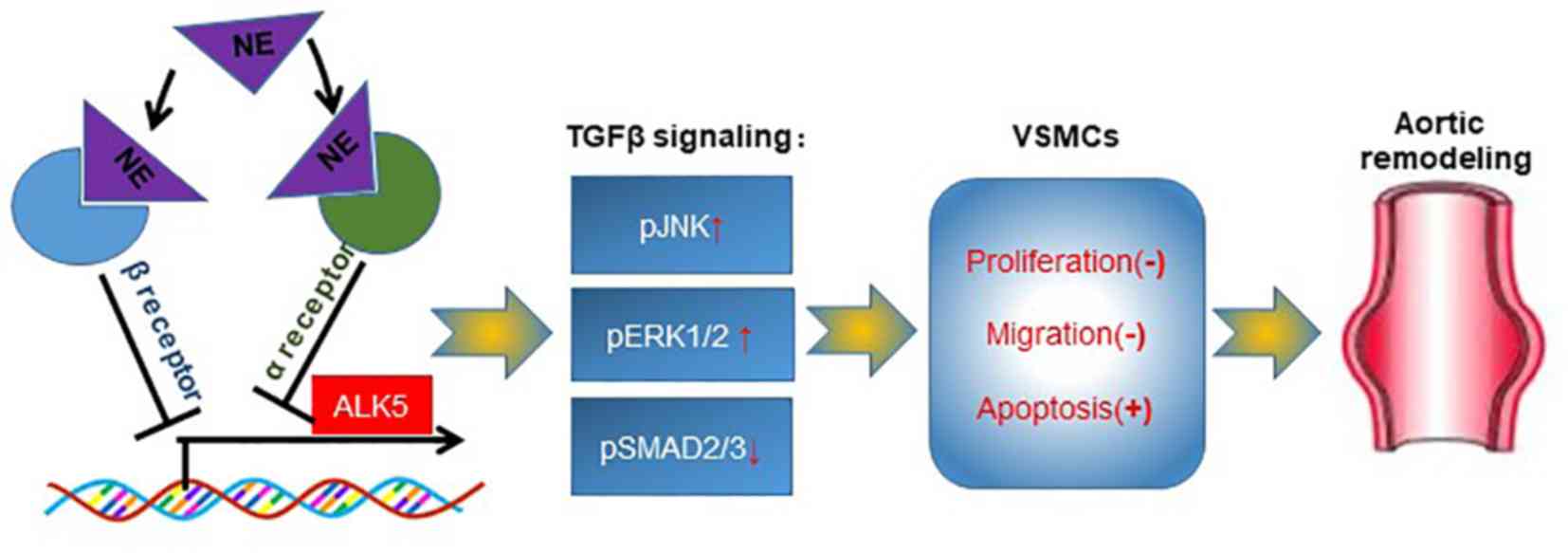
In conclusion, the results of the present study
demonstrated that regional CSD protected the rats from aortic
aneurysm. The sympathetic transmitter NE modulated TGFβ signaling
by suppressing ALK5 expression, serving an important role in the
biological functions of VSMCs. Both α- and β-adrenergic receptors
may be involved in the regulation of NE on ALK5 expression. Thus,
abnormal sympathetic innervation of the aorta may serve as a
therapeutic target in aortic diseases (Fig. 7).
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Dr Junmou Hong from the Zhongshan
Hospital at Xiamen University for his valuable advice to the
present study.
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81600367 to Zhipeng Hu).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW and ZH conceived the hypothesis and designed the
study protocol. ZH wrote the manuscript. BL, RC, QW and ZH
performed most of the experiments. XH, MZ, and FJ participated in
some of the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the ethics
committee of Renmin hospital, Wuhan University (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akutsu K, Kawamoto M, Sato N, Yamamoto T,
Tamura K, Mizuno K and Tanaka K: Acute aortic dissection associated
with cystic medial necrosis of unknown etiology. J Nippon Med Sch.
79:159–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fraga-Silva RA and Trachet B: Editorial:
Novel insights on aortic aneurysm. Curr Pharm Des. 21:3993–3995.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhipeng H, Zhiwei W, Lilei Y, Hao Z,
Hongbing W, Zongli R, Hao C and Xiaoping H: Sympathetic
hyperactivity and aortic sympathetic nerve sprouting in patients
with thoracic aortic dissection. Ann Vasc Surg. 28:1243–1248. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu R, Wang Z, Ren Z and Liu M: Autonomic
remodeling may be responsible for decreased incidence of aortic
dissection in STZ-induced diabetic rats via down-regulation of
matrix metalloprotease 2. BMC Cardiovasc Disord. 16:2002016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Z, Wang Z, Wu H, Yang Z, Jiang W, Li L
and Hu X: Ang II enhances noradrenaline release from sympathetic
nerve endings thus contributing to the up-regulation of
metalloprotease-2 in aortic dissection patients' aorta wall. PLoS
One. 8:e769222013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loeys BL, Schwarze U, Holm T, Callewaert
BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S,
Manouvrier S, et al: Aneurysm syndromes caused by mutations in the
TGF-beta receptor. N Engl J Med. 355:788–798. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lindsay ME and Dietz HC: Lessons on the
pathogenesis of aneurysm from heritable conditions. Nature.
473:308–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang YF, Wu CC, Chen WP and Su MJ:
Transforming growth factor-beta type I receptor/ALK5 contributes to
doxazosin-induced apoptosis in H9C2 cells. Naunyn Schmiedebergs
Arch Pharmacol. 380:561–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbertz S, Sawyer JS, Stauber AJ,
Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba
SC, Benhadji KA, et al: Clinical development of galunisertib
(LY2157299 monohydrate), a small molecule inhibitor of transforming
growth factor-beta signaling pathway. Drug Des Devel Ther.
9:4479–4499. 2015.PubMed/NCBI
|
|
10
|
Santini V, Valcárcel D, Platzbecker U,
Komrokji RS, Cleverly AL, Lahn MM, Janssen J, Zhao Y, Chiang A,
Giagounidis A, et al: Phase II study of the ALK5 inhibitor
galunisertib in very low-, low-, and intermediate-risk
myelodysplastic syndromes. Clin Cancer Res. 25:6976–6985. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai T, Takayanagi T, Forrester SJ,
Preston KJ, Obama T, Tsuji T, Kobayashi T, Boyer MJ, Cooper HA,
Kwok HF, et al: Vascular ADAM17 (a Disintegrin and
Metalloproteinase Domain 17) is required for angiotensin
II/β-aminopropionitrile-induced abdominal aortic aneurysm.
Hypertension. 70:959–963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurihara T, Shimizu-Hirota R, Shimoda M,
Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N and Okada
Y: Neutrophil-derived matrix metalloproteinase 9 triggers acute
aortic dissection. Circulation. 126:3070–3080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagashima H, Uto K, Sakomura Y, Aoka Y,
Sakuta A, Aomi S, Hagiwara N, Kawana M and Kasanuki H: An
angiotensin-converting enzyme inhibitor, not an angiotensin II
type-1 receptor blocker, prevents beta-aminopropionitrile
monofumarate-induced aortic dissection in rats. J Vasc Surg.
36:818–823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong J, Hu Z, Wu Q, Tang C, Hu J, Chen R,
Li B and Wang Z: The deregulation of STIM1 and store operative
calcium entry impaired aortic smooth muscle cells contractility in
aortic medial degeneration. Biosci Rep. 39:BSR201815042019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mallem Y, Holopherne D, Reculeau O, Le Coz
O, Desfontis JC and Gogny M: Beta-adrenoceptor-mediated vascular
relaxation in spontaneously hypertensive rats. Auton Neurosci.
118:61–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirai K, Song M, Suzuki J, Kurosu T,
Oyama T, Nagayama D, Miyashita Y, Yamamura S and Takahashi M:
Contradictory effects of β1- and α1-aderenergic receptor blockers
on cardio-ankle vascular stiffness index (CAVI)-CAVI independent of
blood pressure. J Atheroscler Thromb. 18:49–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas P and Dasgupta I: The role of the
kidney and the sympathetic nervous system in hypertension. Pediatr
Nephrol. 30:549–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parati G and Esler M: The human
sympathetic nervous system: Its relevance in hypertension and heart
failure. Eur Heart J. 33:1058–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eichmann A and Brunet I: Arterial
innervation in development and disease. Sci Transl Med.
6:252ps2592014. View Article : Google Scholar
|
|
21
|
Woolgar JR and Scott TM: The relationship
between innervation and arterial structure in late prenatal and
early postnatal development of the rat jejunal artery. J Anat.
167:57–70. 1989.PubMed/NCBI
|
|
22
|
Hernigou J, Dakhil B, Belmont L,
Couffinhal JC and Bagan P: Sleep apnea syndrome and abdominal
aortic aneurysm: Study of the prevalence of sleep apnea syndrome in
patients with aneurysm and research of association. Clinical study
on 52 patients. J Med Vasc. 42:162–169. 2017.(In French).
PubMed/NCBI
|
|
23
|
Sampol G, Romero O, Salas A, Tovar JL,
Lloberes P, Sagalés T and Evangelista A: Obstructive sleep apnea
and thoracic aorta dissection. Am J Respir Crit Care Med.
168:1528–1531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yanagi H, Imoto K, Suzuki S, Uchida K,
Masuda M and Miyashita A: Acute aortic dissection associated with
sleep apnea syndrome. Ann Thorac Cardiovasc Surg. 19:456–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bradley TD and Floras JS: Obstructive
sleep apnoea and its cardiovascular consequences. Lancet.
373:82–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schiza SE, Mermigkis C and Bouloukaki I:
The effect of obstructive sleep apnea syndrome and snoring severity
to intima-media thickening of carotid artery. Sleep Breath.
19:25–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruijtenbeek K, le Noble FA, Janssen GM,
Kessels CG, Fazzi GE, Blanco CE and De Mey JG: Chronic hypoxia
stimulates periarterial sympathetic nerve development in chicken
embryo. Circulation. 102:2892–2897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angouras DC, Dosios TJ, Dimitriou CA,
Chamogeorgakis TP, Rokkas CK, Manos TA and Sokolis DP: Surgical
thoracic sympathectomy induces structural and biomechanical
remodeling of the thoracic aorta in a porcine model. J Surg Res.
172:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brady AR, Thompson SG, Fowkes FG,
Greenhalgh RM and Powell JT; UK Small Aneurysm Trial Participants,
: Abdominal aortic aneurysm expansion: Risk factors and time
intervals for surveillance. Circulation. 110:16–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Zhang L, He G, Tan X, Jin X and Li
C: Transcutaneous auricular vagus nerve stimulation regulates
expression of growth differentiation factor 11 and activin-like
kinase 5 in cerebral ischemia/reperfusion rats. J Neurol Sci.
369:27–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aspalter IM, Gordon E, Dubrac A, Ragab A,
Narloch J, Vizán P, Geudens I, Collins RT, Franco CA, Abrahams CL,
et al: Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting
downstream of Notch. Nat Commun. 6:72642015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomas M, Docx C, Holmes AM, Beach S,
Duggan N, England K, Leblanc C, Lebret C, Schindler F, Raza F, et
al: Activin-like kinase 5 (ALK5) mediates abnormal proliferation of
vascular smooth muscle cells from patients with familial pulmonary
arterial hypertension and is involved in the progression of
experimental pulmonary arterial hypertension induced by
monocrotaline. Am J Pathol. 174:380–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holm TM, Habashi JP, Doyle JJ, Bedja D,
Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, et al:
Noncanonical TGFβ signaling contributes to aortic aneurysm
progression in Marfan syndrome mice. Science. 332:358–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmit BM, Yang P, Fu C, DeSart K, Berceli
SA and Jiang Z: Hypertension overrides the protective effect of
female hormones on the development of aortic aneurysm secondary to
Alk5 deficiency via ERK activation. Am J Physiol Heart Circ
Physiol. 308:H115–H125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carta L, Smaldone S, Zilberberg L, Loch D,
Dietz HC, Rifkin DB and Ramirez F: p38 MAPK is an early determinant
of promiscuous Smad2/3 signaling in the aortas of fibrillin-1
(Fbn1)-null mice. J Biol Chem. 284:5630–5636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fabiani ME, Sourial M, Thomas WG, Johnston
CI, Johnston CI and Frauman AG: Angiotensin II enhances
noradrenaline release from sympathetic nerves of the rat prostate
via a novel angiotensin receptor: Implications for the
pathophysiology of benign prostatic hyperplasia. J Endocrinol.
171:97–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li JS, Li HY, Wang L, Zhang L and Jing ZP:
Comparison of β-aminopropionitrile-induced aortic dissection model
in rats by different administration and dosage. Vascular.
21:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|