Introduction
Bacillus Calmette-Guérin (BCG) has been used to
treat non-muscle-invasive bladder cancer (BCa) for nearly 40 years
(1). It is one of the most
successful biotherapies for BCa currently in use (2,3).
Despite extensive clinical experience with BCG, the mechanism by
which it achieves its therapeutic effect remains a matter for
investigation. Abundant evidence indicates that BCG therapy for BCa
results in extensive activation of the immune system (4). The requirements for effective live
BCG therapy include an intact immune system and close contact of
BCG with BCa cells (5). Important
constituents of the cellular inflammatory response to BCG include
CD4+ and CD8+ lymphocytes, natural killer
cells and granulocytes (6–8). Important elements of the humoral
immune response to BCG include tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL), interleukin (IL)-2, IL-8, IL-18,
IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α
(9). In addition, the macrophage
response to BCG therapy is evident in the bladder wall of patients
as well as in their urine in vitro (6,10).
Another indirect piece of evidence of the response of macrophages
to BCG is the variety of cytokines found in the urine of patients
treated with BCG, including IL-6, IL-12, and TNF-α, which are known
to be secreted by macrophages exposed to BCG (9,11,12).
However, there are currently no published reports that confirm the
role of macrophages in BCG therapy for BCa in vivo, to the
best of our knowledge.
In the present study, with the aim of examining the
potential functions of BCG in activating macrophages in
vivo, functional macrophages were observed in a T24 carcinoma
tumor-bearing NOD/scid IL2Rg−/− (NSI) BCa mouse model
after injection via the tail vein of live BCG. The results showed
macrophage recruitment in the blood and maturation of macrophages
in the bone marrow. Moreover, macrophages were expressed at high
levels in the tumor environment. Due to the relationship between
macrophages and tumor cells, the present study attempted to clarify
the mechanism of BCG treatment in BCa. Independent in vitro
experimental results demonstrated BCG ‘targeting’ of macrophages.
The results demonstrated that BCG was the basis for successful
cancer treatment and that one of the mechanisms was the targeting
of macrophages could be relevant to the future treatment of
BCa.
Materials and methods
BCa in the NSI mouse model
The human BCa cell line T24 was obtained from the
American Type Culture Collection. Cells were incubated in culture
media consisting of RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and a 1% penicillin-streptomycin solution (Gibco;
Thermo Fisher Scientific, Inc.). Cells were cultured in 5% CO2 and
a normal level of oxygen at 37°C.
NSI mice (N=24) were obtained from Guangzhou
Institutes of Biomedicine and Health (GIBH); this is a novel strain
of immunodeficient mice with the absence of T, B and NK cells.
Animal experiments were performed in the Laboratory Animal Center
of GIBH, and all animal procedures were approved by the Animal
Welfare and Ethical Committee of GIBH (approval no. N2014050). Mice
were maintained in a barrier facility by sister-brother mating and
were specific pathogen-free, according to the current Federation
for Laboratory Animal Associations guidelines, which include
pathogens such as parvovirus, Sendai virus, Hantan virus,
coronavirus, reovirus, cytomegalovirus, Pasteurellaceae and
Mycoplasma pulmonis. All of the mice were kept in a
climate-controlled environment (temperature: 22–26°C; humidity:
40–50%) with a 14 h light/10 h dark cycle, and were provided with
autoclaved food and water ad libitum. The establishment of a
mouse T24 carcinoma tumor-bearing model for study has been reported
in other studies (13,14). In brief, 24 male NSI mice (18–20 g)
were randomized into 4 groups (n=6) and inoculated with
5×106 T24 cells/mouse. After 3 weeks, each mouse
received a single tail-vein injection of BCG at 0.25, 1.25 or 6.25
µg/mouse; one dose was administered each week. The control group
was treated with PBS. Analysis was performed during the following
week after the administration of the last dose of BCG. Animals were
anesthetized with Avertin (2,2,2-tribromoethanol) at a dose of 240
mg/kg body weight, and sacrificed by cervical dislocation. Samples
including blood, spleen and tumor tissue were collected for further
analysis. The formula for calculating the tumor volume was V=1/2 ×
a × b2. The weight of each group's spleen was weighed by
electronic balance (WANT Balance Instrument Co., Ltd).
Flow cytometry analysis
For the analysis of macrophage surface antigen
expression, allophycocyanin-, phycoerythrin-, Precp- and
FITC-conjugated anti-mouse CD11b (cat. no. 17-0112-81), F4/80 (cat.
no. 17-4801-82), CD206 (cat. no. 85-12-2061-82) and Ly-6G (Gr-1;
cat. no. 11-5931-82) antibodies were used, diluted 1:50. Mouse
isotype controls were also used. The cells were washed and stained
for 30 min at 4°C. All of the antibodies were obtained from
eBioscience unless otherwise stated. Flow cytometric analysis was
performed using ACEA NovoCyte™ II (ACEA Biosciences, Inc.). Data
analysis was performed using FlowJo 7.6.1 software (FlowJo
LLC).
Immunohistochemistry analyses
Standard protocols were used for
immunohistochemistry. Tumor tissue was pre-treated with 4%
paraformaldehyde for 1 h at room temperature, and the tissue was
collected and sequentially cut into 4-µm thick sections. The
sections were heated in a tissue-drying oven for 120 min at 65°C
and deparaffinized, followed by rehydration through a gradient
ethanol series (100, 95 and 70%). Antigen retrieval procedures were
necessary for the exposure of all epitopes, which was achieved by
microwaving the sections in 0.01 M citrate buffer (pH 6.0) on full
power for 3×5 min. The sections were incubated for 30 min at 37°C
with 5% BSA (cat. no. A8806-1G; Sigma-Aldrich; Merck KGaA). The
sections were covered with the F4/80 antibody, which was diluted in
diluent buffer to 1:100, overnight at 4°C. The sections were then
washed three times with PBS for 5 min each. The sections were
covered with the peroxidase (HRP)-conjugated goat anti-rabbit
secondary antibody (cat. no. ab6728; 1:2,000; Abcam) for 30 min at
37°C, then washed three times with PBS for 5 min each. The sections
were stained with DAB for 10 min, and counterstained with
hematoxylin for 1 min at room temperature after being rinsed twice
in PBS. Photomicrographs were captured using a confocal system
(Olympus FV500-IX81; Olympus Corporation) and analyzed with
Image-Pro Plus 6 (Media Cybernetics, Inc.).
Measurement of cytokine and growth
factor production
Parallel 96-well plates were prepared for multiplex
assay analysis to measure TNF-α, IL-1β, IL-6, IL-12P70, vascular
endothelial growth factor (VEGF), tumor necrosis factor ligand
superfamily member 11 (RANKL), macrophage colony stimulating factor
(M-CSF) and monocyte chemotactic protein 1 (MCP-1). The multiplex
kits were custom designed in-house and manufactured by R&D
Systems (cat. no. LXSAMSM-09; lot no. L121798) according to the
manufacturer's instructions. Briefly, the medium was collected from
triplicate wells and stored at −80°C until analysis. Each 96-well
filter plate (EMD Millipore) was blocked with 100 µl blocking
buffer for 30 min and vacuum-filtered at 2 psi. The 25× multiplex
bead mix was mixed using a vortex mixer for 1 min and then mixed
for 30 sec before diluting. The bead mix was diluted in wash
buffer, and 50 µl was immediately added to each well. Different
samples were then added to the assay well and mixed at room
temperature for 2 h. After washing, 50 µl of detection antibody was
added to each well, and the plates were incubated on a shaker at
room temperature for 1 h in the dark. After a final washing step,
the beads were then resuspended in 150 µl of wash buffer for
analysis. The fluorescence intensity of the detection antibody was
determined using the Luminex 200 System (Luminex Corporation).
Fluorescence intensity readings of 50 beads/cytokine were collected
for each standard and sample dilution.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was determined using RT-qPCR and extracted
with TRIzol® (GenStar Technologies). The EasyScript
First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech Co.,
Ltd.) was used to prepare cDNA from 1,000 ng of RNA under the
following conditions: 25°C for 10 min, 37°C for 60 min and 70°C for
10 min. cDNA was then amplified with a TransStart Green qPCR
SuperMix kit (TransGen Biotech Co., Ltd.) in a Bio-Rad CFX96
Real-Time PCR System (Bio-Rad Laboratories Inc.). The primers were
from Shanghai Jierui Biotechnology Co., Ltd., with the following
sequences: 5′-GCTGTCTTGGGTGCATTGGA-3′ (forward) and
5′-AAGGGACTTCCTGTAACAATGCA-3′ (reverse) for β-actin;
5′-CGGGGCTGATCTGGGAAAAT-3′ (forward) and 5′-CACAGCGTAACCTCGTCTTC-3′
(reverse) for interferon regulatory factor 8 (IRF-8);
5′-CAAGCGCATGACGTATCAGAA-3′ (forward) and
5′-GCTGTCAAACTGGTAGGTGAG-3′ (reverse) for Spi-1 proto-oncogene
(PU.1); 5′-GGACGGTGTTGCAGCAGAT-3′ (forward) and
5′-GCAGTCTGAGTTCCAGTGGTA-3′ (reverse) for RANK;
5′-TCCCCACAGTTGCCTTCAC-3′ (forward) and 5′-GAGCGGCGTCTTGCCTTTA-3′
(reverse) for NF-κB; 5′-ACTTTGCGCCTACAATTCAGG-3′ (forward) and
5′-AACTTGCCAGGGAATGGAACT-3′ (reverse) for EGR-1;
5′-TGTCATCGAGCCTAGTGGC-3′ (forward) and 5′-CGGGAGATTCAGGGTCCAAG-3′
(reverse) for colony stimulating factor 1 receptor (C-FMS);
5′-TTGAGCGATCATCCCGGTC-3′ (forward) and 5′-GCGTGAGTCCATACTGGCAAG-3′
(reverse) for proto-oncogene c-Fos (c-Fos). The following thermal
profile was used for all qPCR experiments: 50°C for 2 min; 95°C for
10 min; and 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
fit point algorithm of the Light Cycler software was used to
calculate Cq (15) values. For the
normalization of RNA expression, the levels of β-actin mRNA were
used as the internal control, and the gene-specific mRNA expression
was normalized against β-actin expression. All samples were
prepared and tested in triplicate.
Statistical analysis. Data are
expressed as the mean ± SD
Significant differences between the control and
treatment group were analyzed by one-way ANOVA followed by Tukey's
post-hoc test, or by two-tailed unpaired Student's t-test.
Statistical analysis was performed using GraphPad Prism Software
version 5.0 (GraphPad Software, Inc.). FlowJo 7.6.1 software was
used to analyze the flow cytometry data. P<0.05 was considered
to indicate a statistically significant difference.
Results
BCG enhances the macrophage-mediated
inhibition of BCa growth in vivo
To assess the effects on macrophage of live BCG
during BCa growth in vivo, NSI mice were inoculated with
human BCa T24 cells. Although every mouse in the model-control
group and BCG-treatment groups developed tumors (100% tumor
incidence) after a 3-week latency, the treatment effect of live BCG
(delivered intravenously to the tail) was assessed for 4 weeks. In
the present study, no mouse presented with multiple. The largest
subcutaneous tumor had a diameter of 1.18 cm, and the largest tumor
as a percentage of body weight was 5.1%. It was also observed that
no mice were found to have succumbed during this study, and no
significant abnormalities were found between the treatment group
and the control group. Interestingly, during the treatment period,
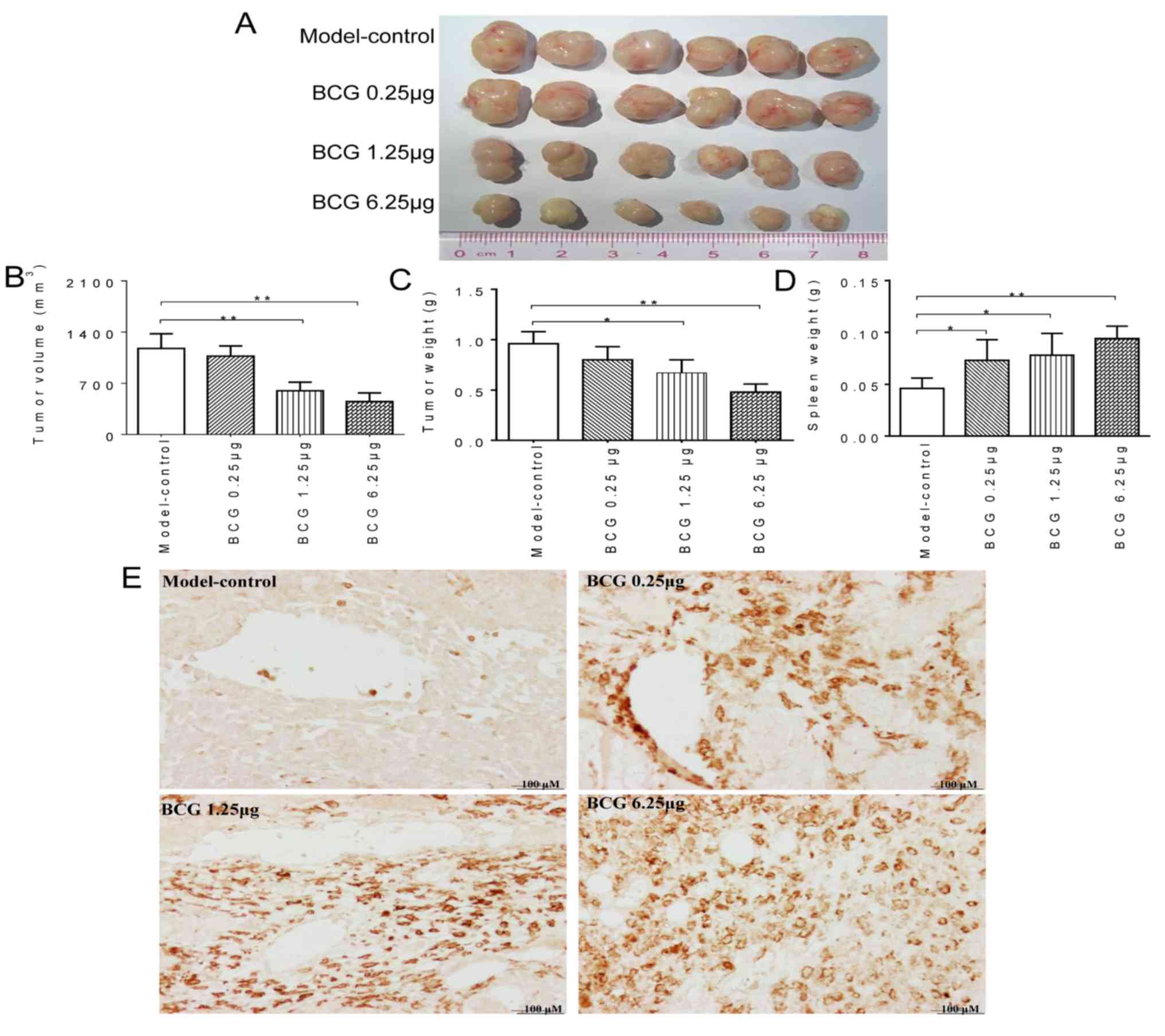
a significant reduction in tumor growth was observed. Tumor burden,
as assessed by tumor size, weight and volume (Fig. 1A-C), in the BCG treated groups
(1.25 and 6.25 µg/mouse) were significantly lower (P<0.05) than
in the control group. In addition, the weight of the spleen in the
BCG-treated groups increased compared to that in the control group
(Fig. 1D). Similarly, the
infiltration of macrophages (detected with anti-F4/80) was markedly
increased in NSI mice compared to mice in the control group
(Fig. 1E). Based on these results,
macrophages were selected for further experimentation and
analyses.
BCG enhances macrophage recruitment
and differentiation/transformation in the immune system
After observing increased numbers of macrophages in
BCa tumors using F4/80 staining, the blood, spleen and bone marrow
isolated from the studied NSI mouse model were examined to
determine whether immune system parameters were consistent with the
results generated using tumor samples. After the mice had been
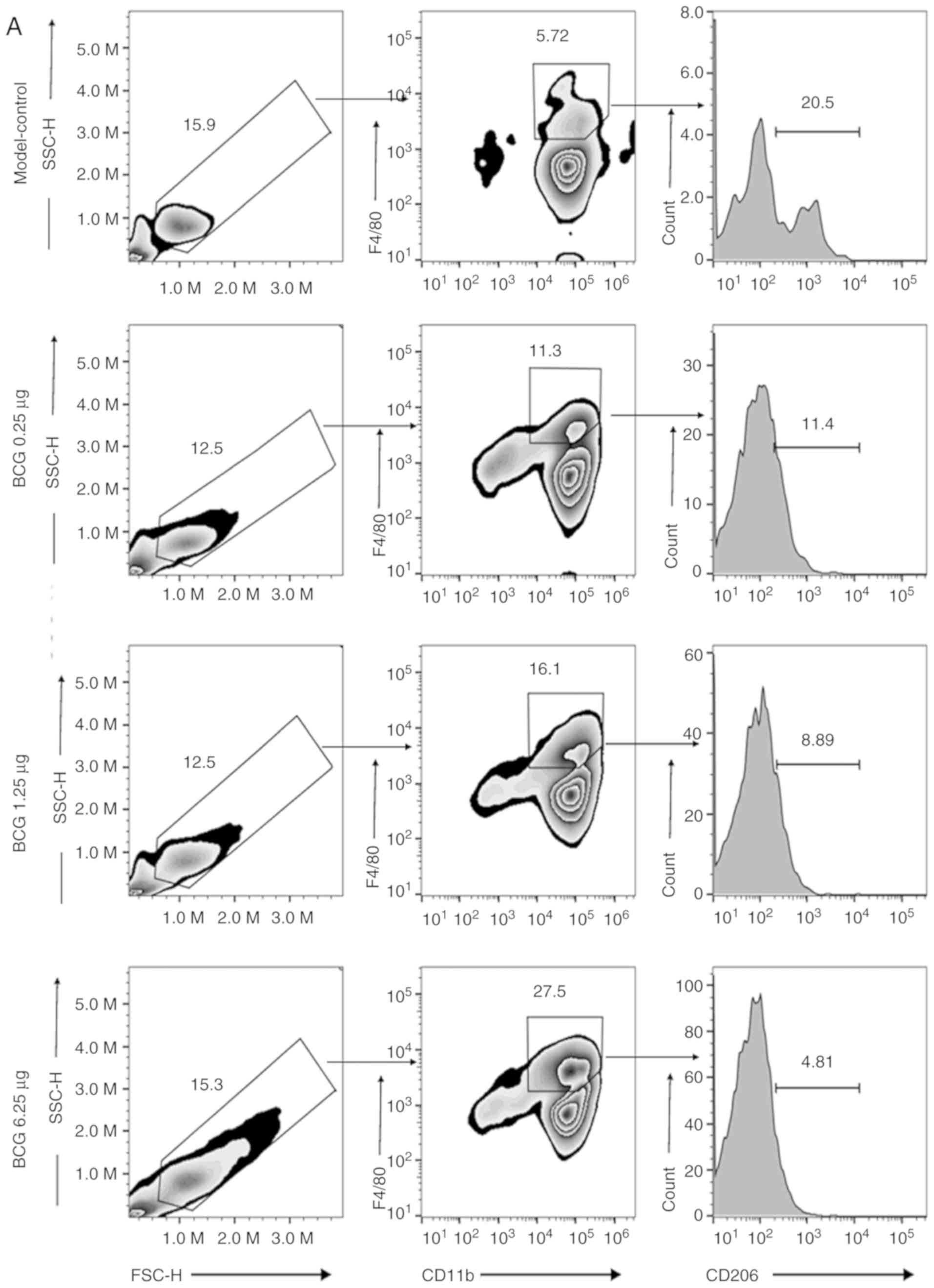
sacrificed, blood-derived macrophages were analyzed using flow
cytometry staining with anti-CD11b and F4/80. The M2 phenotype was
also extensively analyzed using the marker CD206 (Fig. 2A, E and F). It was observed that
BCG caused a marked increase in the number of macrophages and that
the proportion of M2 decreased; however,
CD11b+/Gr-1+ myeloid-derived suppressor cells
(MDSCs) did not significantly change in the blood (Fig. 2D and I). To determine a more direct
mechanism, further analysis of dissociated spleen cells using flow
cytometry confirmed that the expression mainly consisted of
CD11b+/F4/80+ macrophages, with a six-fold
increase in active macrophages compared to the model control
(Fig. 2B and G). To determine
whether macrophage precursor cells were detected by flow cytometry
with anti-CD11b+/F4/80+, macrophage
differentiation/transformation was evaluated using the
anti-CD11b+/F4/80+ macrophage marker in bone
marrow (Fig. 2C and H). Notably,
it was found that this difference was consistent with the results
from the spleen and blood. These data indicated that BCG-induced
differentiation/transformation of macrophages began in the bone
marrow and affected the entire immune system in a sweeping
manner.
BCG increases the expression of TNF-α,
IL-1β, IL-6, IL-12P70, VEGF, RANKL, M-CSF and MCP-1, associated
with macrophages
The present study suggested that BCG may function by
recruiting macrophages to suppress BCa via an increased quantity of
macrophage-released soluble cytotoxic factors. The mouse magnetic
Luminex assay was performed to evaluate the changes in the
expression levels of cytokines; serum and tumor supernatants were
collected for this test (Fig. 3).
Treatment with BCG markedly increased the recruitment of
macrophages, resulting in the upregulation of pro-inflammatory
cytokines such as TNF-α, IL-1β, IL-6 and IL-12P70, in a
dose-dependent manner (0.25–3.25 µg BCG/mice). Chemokines enhance
chemotaxis and the activating factors of macrophages, thereby
accelerating the generation of macrophages. In our study, the
expression level of MCP-1 was also upregulated after treatment with
BCG. In addition, the recruitment of macrophages led to the
secretion of RANKL, which strengthened the immune response to BCG.
Otherwise, the levels of vascular VEGF and M-CSF in the serum and
tumor supernatants were decreased compared to those in the normal
group.
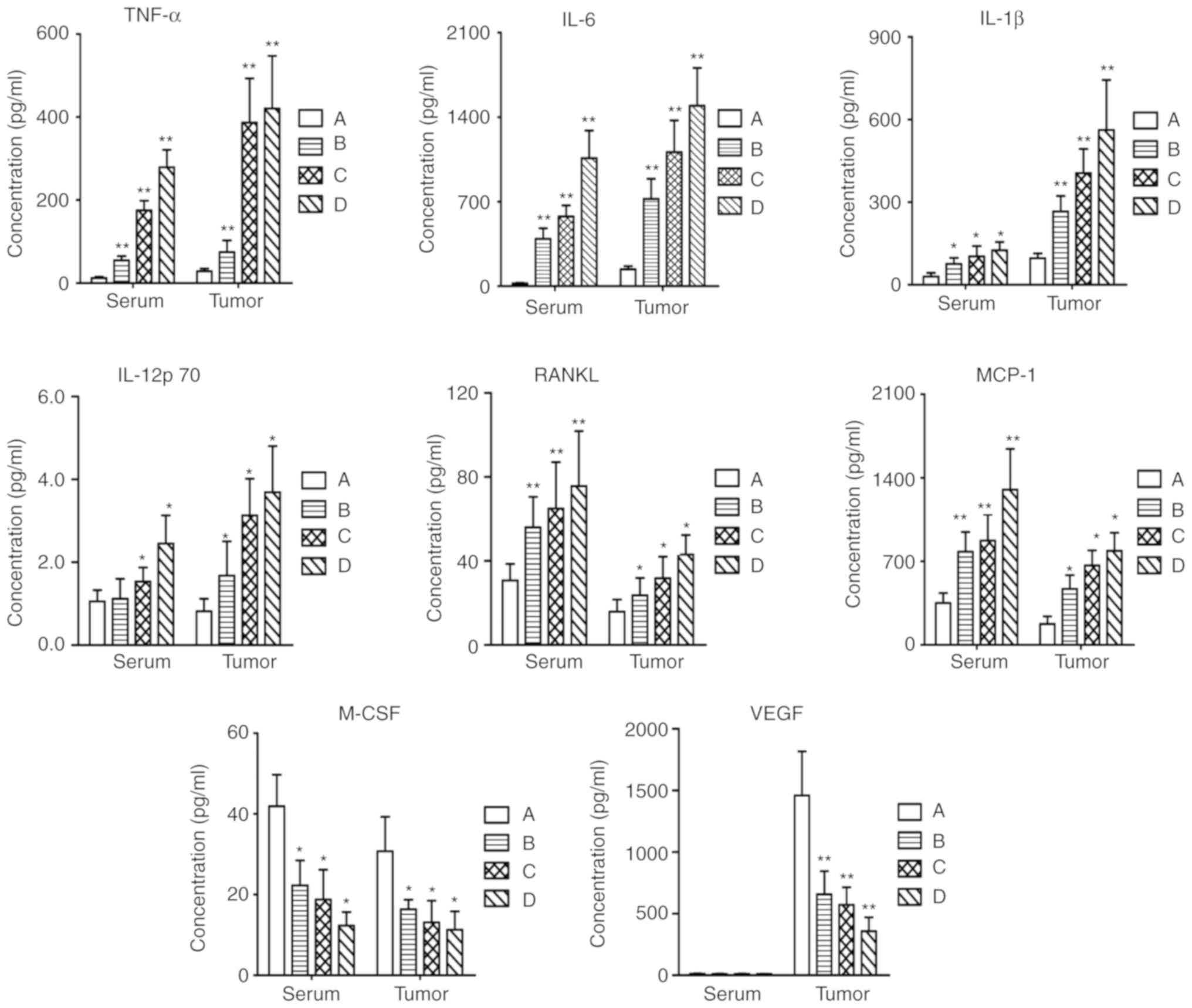 | Figure 3.Serum and tumor supernatants were
analyzed using a multiplex assay for the production of TNF-α, IL-6,
IL-1β, IL-12P70, RANKL, MCP-1, M-CSF and VEGF after treatment of
NOD/scid IL2Rg−/− mice with BCG. Each value is expressed
as the mean ± SD of three separate experiments. *P<0.05 and
**P<0.01 vs. respective control. A, control group; B, BCG 0.25
µg group; C, BCG 1.25 µg group; D, BCG 6.25 µg group; BCG, Bacillus
Calmette-Guérin; IL, interleukin; TNF-α, tumor necrosis factor-α;
VEGF, vascular endothelial growth factor; RANKL, tumor necrosis
factor ligand superfamily member 11; M-CSF, macrophage colony
stimulating factor; MCP-1, monocyte chemotactic protein 1. |
Effect of BCG on IRF-8, PU.1, RANK,
EGR-1, NF-κB, C-FMS and c-Fos mRNA expression in bone marrow
Growing evidence demonstrates that IRF-8, PU.1,
RANK, EGR-1, NF-κB, C-FMS and c-Fos may be linked to mature
macrophage differentiation from monocyte-macrophage precursor cells
(16–18). Thus, the present study further
investigated the effects of BCG on the gene expression levels in
bone marrow tissue from a BCa model. RT-qPCR analysis showed that
the expression levels of four genes in bone marrow treated with BCG
were significantly overexpressed compared to the control group. As
shown in Fig. 4, different doses
of BCG in treated mice exhibited 1.4–3.6-fold, 2.5–3.1-fold,
1.7–3.6-fold, and 1.3–3.8-fold up-regulation of the PU-1, EGR-1,
NF-κB and c-Fos mRNA levels (P<0.05), respectively.
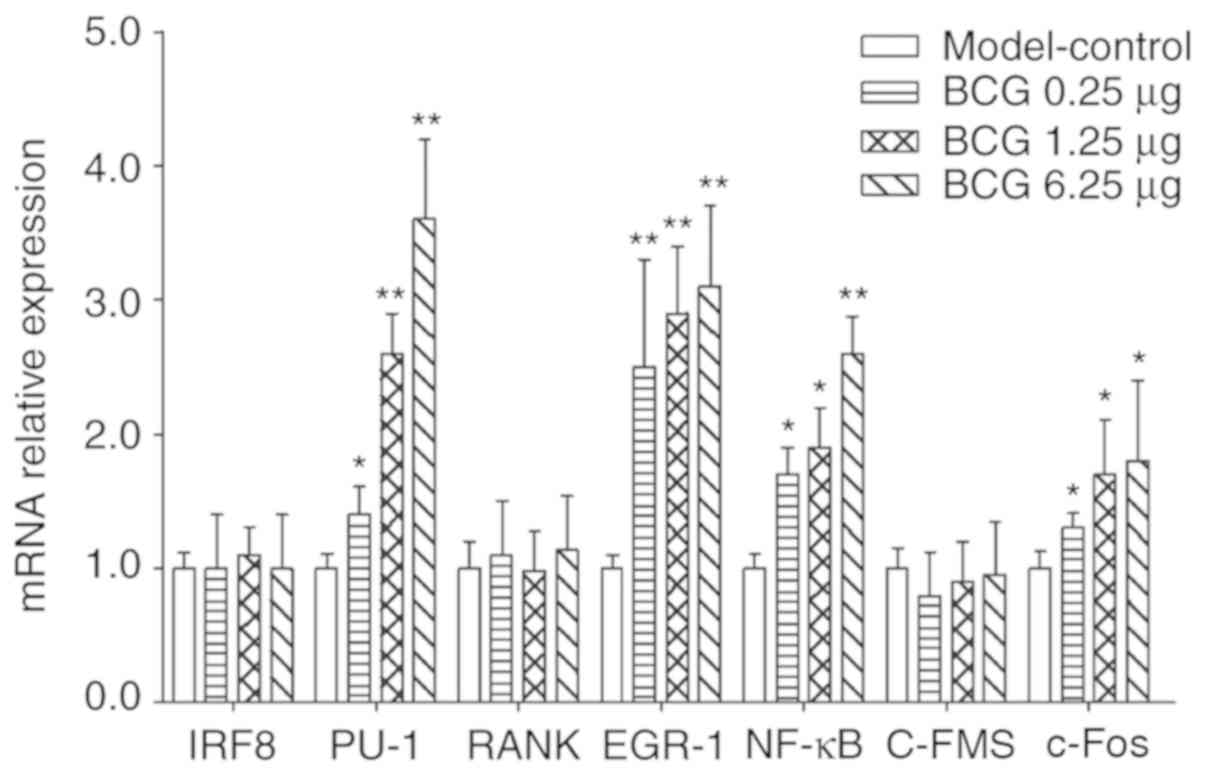 | Figure 4.mRNA levels of IRF8, PU-1, RANK,
EGR-1, NF-κB, C-FMS and c-Fos in BCG-treated mice were detected
using reverse transcription-quantitative PCR in bone marrow.
*P<0.05 and **P<0.01 vs. respective model-control group. The
results represent the mean ± SD of three separate experiments. BCG,
Bacillus Calmette-Guérin; IRF-8, interferon regulatory factor-8;
PU.1, Spi-1 proto-oncogene; NF-κB, nuclear factor-κB; C-FMS, colony
stimulating factor 1 receptor; c-Fos, c-FOS proto-oncogene. |
Discussion
In 1891, the use of microbial products for the
treatment of cancer was pioneered by Dr. William B. Coley, who
treated cancer patients with intratumoural injections of live
Streptococcus pyogenes (19). The biologist Pearl (1928) (1), who performed an autopsy study and
observed that cancer was less common in patients who had lesions of
active tuberculosis, first suggested the idea that mycobacteria
might be useful as a therapy for cancer. A study conducted at the
Sloan-Kettering Institute showed that mice infected intravenously
with BCG were more resistant to the transplantation of tumors
(20), leading to the discovery of
TNF-α in the serum of BCG-infected mice. Since BCG was first used
by Morales et al (21) in
1976, the efficacy of BCG therapy for BCa has been confirmed.
Intravesical BCG has been an effective immunotherapy that
represents the current standard treatment for patients with high
risk non-muscle-invasive BCa (22). However, several gaps in the
knowledge still exist, which should be addressed in future efforts
to understand this biotherapy for cancer. No published reports have
confirmed the role of macrophages in BCG therapy for BCa in
vivo, to the best of our knowledge. This study comprised
innovative exploration of BCG injection in NSI mice; the treatment
was administered twice a week for 1 month, relatively close to the
duration of clinical treatment BCG in patients with BCa, typically
once a week for 6 weeks (23). The
upper limit of tumor size was set at 2 cm3, which was
defined as the experimental ethical endpoint. Animals exhibiting
signs of this humane endpoint would be sacrificed immediately,
although this did not occur in the present study.
The NSI strain does not harbor T, B or NK cells.
However, the mice exhibit normal macrophages and other monocytes,
and the strain can be adapted to study the physiological roles and
mechanisms of macrophages in tumor models (24). The present study showed that BCG
directs monocyte precursor cells to differentiate into functional
mature macrophages. Upon BCG treatment, monocyte progenitor cells
induce the macrophage-specific marker F4/80 and enhance CD11b
expression in vivo. These cells have overexpressed gene
levels of PU-1, EGR-1, NF-κB and c-Fos, which may be linked to the
differentiation of macrophages to a mature form. The secretion of
cytokines, such as TNF-α, IL-1β, IL-6 and IL-12P70, is also a
hallmark of mature macrophage function. Moreover, it was identified
that BCG induced a high level of MCP-1, which macrophages require
to stimulate differentiation responses. Another observation that
supports the role of BCG in stimulating authentic macrophage
differentiation is that the proportion of macrophages increased in
the blood in the present study.
In the present study, the immunotherapy effect of
BCG depended on macrophage activation and tissue infiltration, the
increase in the number of macrophages in the bladder tumor, and the
secretion of tumor-killing cytokines. Notably, the therapeutic
effect of live BCG treatment in the NSI BCa mouse model was
significant inhibition of tumor growth, evidence of the important
role of macrophages in tumor immunotherapy. The proportion of
CD11b+/Gr-1+ MDSCs in the blood did not
significantly change, meaning that the macrophages were
immune-activated. In the present investigation, based on knowledge
of macrophage maturation and immune activation, administration of
BCG led to a high state of macrophage immunotherapy, which allowed
tumoricidal activity to continue for 1 month.
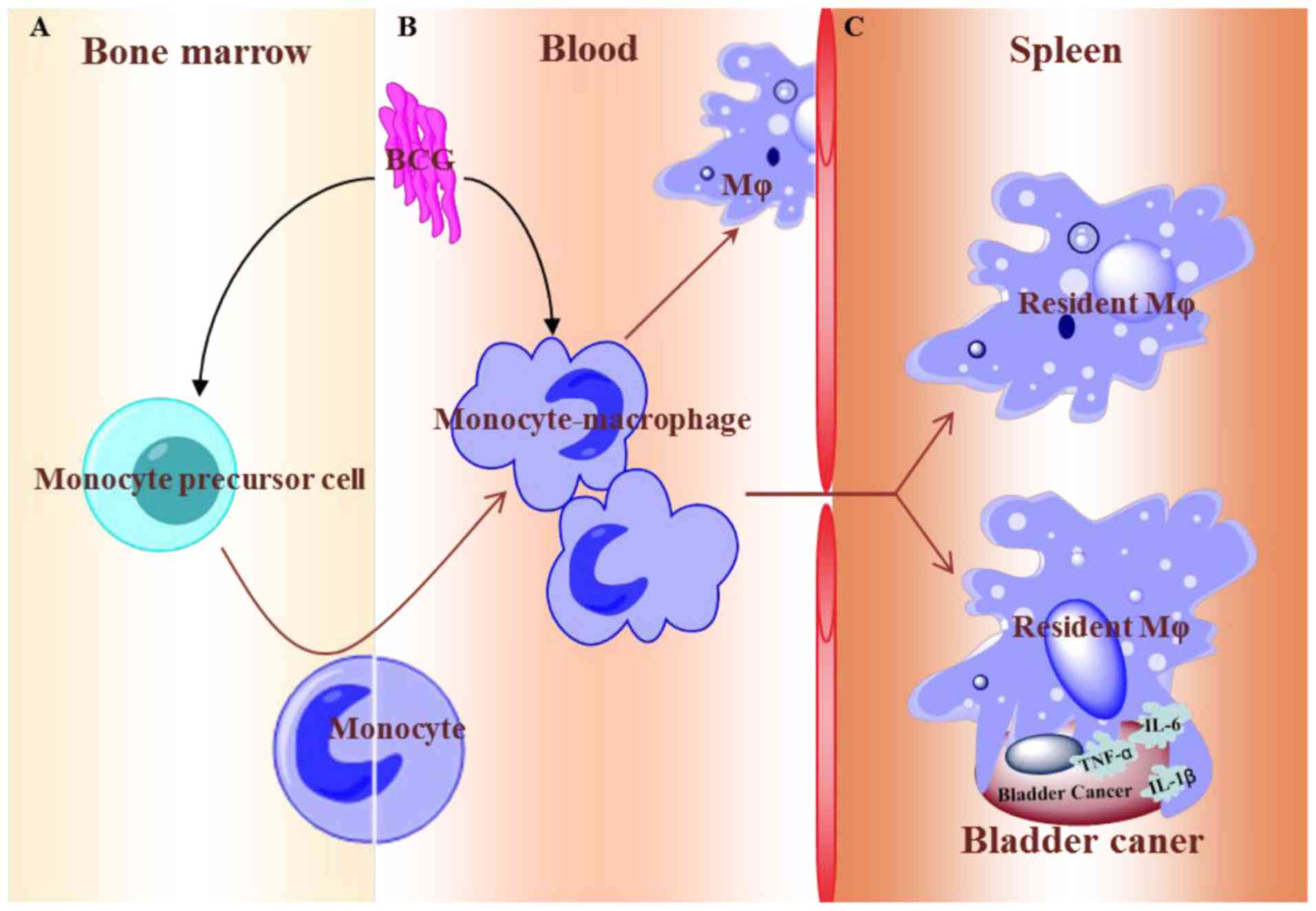
In summary, the present study may provide an
additional theoretical and experimental basis for the use of BCG
for tumor immunotherapy (Fig. 5).
BCG stimulated the differentiation and maturation of macrophages,
thereby increasing the proportion of macrophages in the blood.
Moreover, macrophages were activated and the tumoricidal effects
were maintained. Relieving the immunosuppressive effects of
macrophages, such as tumor progression, vascularization, invasion
and metastasis in the tumor microenvironment, is also beneficial to
the enhancement of other immunotherapies. The present study is the
first, to the best of our knowledge, conducted to examine the
mechanism of BCG in targeting macrophages against BCa in an NSI
mouse model. Since a mouse model with a healthy immune system was
not used, there can be no clear conclusions about the in
vivo effects of BCG on lymphocytes. Further study is necessary
to determine the effects of BCG as an immunotherapeutic agent for
cancer in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573769), the
Natural Science Foundation of Guangdong province (grant no.
2014A030313415), the Research Funds for High-level University
Construction from Guangzhou University of Chinese Medicine [grant
nos. (2016)64 and (2017)10], and the Project for Excellent Doctor
Training supported by Guangzhou University of Chinese Medicine in
2015.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZe, QLT and CYZ designed the research study and
wrote the paper. QLT, CYZ, LC, CPL, ML, WXX and XZh performed the
research study. XZe, XZh and ML revised the manuscript. XZh helped
with revising the language of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Welfare
and Ethical Committee of Guangzhou Institutes of Biomedicine and
Health (approval no. 2014050).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mathé G, Amiel JL, Schwarzenberg L,
Schneider M, Cattan A, Schlumberger JR, Hayat M and De Vassal F:
Active immunotherapy for acute lymphoblastic leukaemia. Lancet.
1:697–699. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Old LJ, Clarke DA and Benacerraf B: Effect
of bacillus Calmette-Guérin infection on transplanted tumours in
the mouse. Nature. 184:291–292. 1959. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redelmansidi G, Glickman MS and Bochner
BH: The mechanism of action of BCG therapy for bladder cancer-a
current perspective. Nat Rev Urol. 11:153–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suriano F, Santini D, Perrone G, Amato M,
Vincenzi B, Tonini G, Muda A, Boggia S, Buscarini M and Pantano F:
Tumor associated macrophages polarization dictates the efficacy of
BCG instillation in non-muscle invasive urothelial bladder cancer.
J Exp Clin Canc Res. 32:872013. View Article : Google Scholar
|
|
6
|
De Boer EC, De Jong WH, Van Der Meijden
AP, Steerenberg PA, Witjes JA, Vegt PD, Debruyne FM and Ruitenberg
EJ: Presence of activated lymphocytes in the urine of patients with
superficial bladder cancer after intravesical immunotherapy with
bacillus Calmette-Guérin. Cancer Immunol Immun. 33:411–416. 1991.
View Article : Google Scholar
|
|
7
|
Beatty JD, Islam S, North ME, Knight SC
and Ogden CW: Urine dendritic cells: A noninvasive probe for immune
activity in bladder cancer? Bju Int. 94:1377–1383. 2014. View Article : Google Scholar
|
|
8
|
Siracusano S, Vita F, Abbate R, Ciciliato
S, Borelli V, Bernabei M and Zabucchi G: The role of granulocytes
following intravesical BCG prophylaxis. Eur Urol. 51:1589–1599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Boer EC, De Jong WH, Steerenberg PA,
Aarden LA, Tetteroo E, De Groot ER, Van der Meijden AP, Vegt PD,
Debruyne FM and Ruitenberg EJ: Induction of urinary interleukin-1
(IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical
immunotherapy with bacillus Calmette-Guérin in superficial bladder
cancer. Cancer Immunol Immun. 34:306–312. 1992. View Article : Google Scholar
|
|
10
|
Böhle A, Gerdes J, Ulmer AJ, Hofstetter AG
and Flad HD: Effects of local bacillus Calmette-Guerin therapy in
patients with bladder carcinoma on immunocompetent cells of the
bladder wall. J Urol. 144:53–58. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackson AM, Alexandroff AB, Kelly RW,
Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD and James K:
Changes in urinary cytokines and soluble intercellular adhesion
molecule-1 (ICAM-1) in bladder cancer patients after bacillus
Calmette-Guérin (BCG) immunotherapy. Clin Exp Immunol. 99:369–375.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shintani Y, Sawada Y, Inagaki T, Kohjimoto
Y, Uekado Y and Shinka T: Intravesical instillation therapy with
bacillus Calmette-Guérin for superficial bladder cancer: Study of
the mechanism of bacillus Calmette-Guérin immunotherapy. Int J
Urol. 14:140–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jitao W, Jinchen H, Qingzuo L, Li C, Lei
S, Jianming W and Zhenli G: Androgen receptor inducing bladder
cancer progression by promoting an epithelial-mesenchymal
transition. Andrologia. 46:1128–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noguera-Ortega E, Rabanal RM,
Secanella-Fandos S, Torrents E, Luquin M and Julián E: γ Irradiated
mycobacteria enhance survival in bladder tumor bearing mice
although they are less efficacious than live mycobacteria. J Urol.
195:198–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anderson KL, Smith KA, Perkin H, Hermanson
G, Anderson CG, Jolly DJ, Maki RA and Torbett BE: PU.1 and the
granulocyte- and macrophage colony-stimulating factor receptors
play distinct roles in late-stage myeloid cell differentiation.
Blood. 94:2310–2318. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamura T, Nagamura-Inoue T, Shmeltzer Z,
Kuwata T and Ozato K: ICSBP directs bipotential myeloid progenitor
cells to differentiate into mature macrophages. Immunity.
13:155–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagamura-Inoue T, Tamura T and Ozato K:
Transcription factors that regulate growth and differentiation of
myeloid cells. Int Rev Imm. 20:83–105. 2001. View Article : Google Scholar
|
|
19
|
Sharma P, Old LJ and Allison JP:
Immunotherapeutic strategies for high-risk bladder cancer. Semin
Oncol. 34:165–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pearl R: On the Pathological relations
between cancer and tuberculosis. Exp Biol Med. 26:73–75. 1928.
View Article : Google Scholar
|
|
21
|
Morales A, Eidinger D and Bruce AW:
Intracavitary Bacillus Calmette-Guerin in the treatment of
superficial bladder tumors. J Urol. 116:180–183. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alexandroff A, Jackson A, Skibinska A and
James K: Production of IL-5, a classical T(H)2 cytokine, following
bacillus calmette guerin immunotherapy of bladder cancer. Int J
Oncol. 9:179–182. 1996.PubMed/NCBI
|
|
23
|
Mugiya S, Ozono S, Nagata M, Takayama T,
Ito T, Maruyama S, Hadano S and Nagae H: Long-term outcome of a
low-dose intravesical Bacillus Calmette-Guerin therapy for
carcinoma in situ of the bladder: Results after six successive
instillations of 40 mg BCG. Jpn J Clin Oncol. 35:395–399. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye W, Jiang Z, Li GX, Xiao Y, Lin S, Lai
Y, Wang S, Li B, Jia B, Li Y, et al: Quantitative evaluation of the
immunodeficiency of a mouse strain by tumor engraftments. J Hematol
Oncol. 8:592015. View Article : Google Scholar : PubMed/NCBI
|