Introduction
Bladder outlet obstruction (BOO), which is primarily
caused by benign prostatic hyperplasia (BPH), is a common disease
in aging male individuals (1). BOO
leads to urothelial inflammasome activity, bladder hypertrophy and
fibrosis, and bladder smooth muscle cell (BSMC) proliferation
(2,3). Stress stimulation, hypoxia and other
conditions induce bladder remodeling during BOO, which can also
result in progressive tissue remodeling of the bladder (4,5).
Pathological alterations in BOO-induced bladder remodeling occur in
three stages: hypertrophy, compensation and decompensation
(6). Serum/glucocorticoid
regulated kinase 1 (SGK1), a kinase under powerful genomic
regulation and activated by phosphorylation via the
phosphoinositol-3-phosphate signaling pathway, has been reported to
regulate several enzymes and transcription factors. SGK1
contributes to the regulation of transport, hormone release,
neuroexcitability, inflammation, cell proliferation and apoptosis
(7,8). Our previous study revealed that
different cyclic hydrodynamic pressures display different effects
on promoting the proliferation of human BSMCs (HBSMCs) cultured in
scaffolds via the PI3K/SGK1 signaling pathway (9). The nuclear factor of activated T-cell
(NFAT) family of transcription factors is composed of four
calcium-responsive proteins (NFAT1-4). NFAT is important for
regulating the survival, proliferation and function of multiple
cell types, including mast cells, coronary endothelial cell and
ventricular myocytes. NFAT has been reported to regulate heart
valve development, skeletal and smooth muscle cell differentiation,
and vascular development (10). In
addition, numerous studies have demonstrated that NFAT2 plays a
critical role in promoting cell proliferation (11–13).
Therefore, it was hypothesized that SGK1 and NFAT2 may be
associated with promoting mice BMSC (MBSMC) proliferation. During
the decompensation phase of bladder remodeling, the wall
contractility and emptying functions of the bladder deteriorate.
During BOO, intravesical pressure increases, and if the stress on
the cells is increased beyond the capacity of the compensatory
responses, cells undergo pyroptosis (14). Therefore, we propose that acute
obstruction could exacerbate cell pyroptosis, leading to rapid
decompensation of bladder function.
Pressure stimulation of BMSCs during BOO is
different compared with normal conditions. The majority of BOOs
involve chronic and progressive pathological processes; however,
previous findings have commonly used acute obstruction models that
do not accurately mimic the natural course of BOO (15). A number of studies have reported
that the mortality rate of BOO is usually ≥15% (16,17),
even when a highly standardized method of obstruction, such as
surgery, is applied to induce BOO (18). Cellular molecular mechanisms
identified via traditional direct obstruction models may be
inconsistent with the mechanisms underlying the progression of the
clinical disease; therefore, developing an accurate model for
investigating the pathogenesis of BOO is required.
In a previous study, a BOO model that successfully
avoided trauma to the bladder was established (19). However, compared with human BPH,
other models are potentially more acute and strict. In the present
study, a method of gradually narrowing the outer urethra of mice to
mimic the natural course of the BOO, based on previous research
(9), was employed. This method
involved inducing directly aggravated BOO (DBOO) and gradually
aggravated BOO (GBOO) that displayed the 1/2 urethral meatus
stricture (UMS) at the same time, thus establishing the same degree
of BOO. GBOO is a gradually developing model of BOO, but it
typically models acute BOO. Accordingly, the present study aimed to
investigate whether there was a difference in pathology between
DBOO and GBOO.
Materials and methods
Animals
A total of 27 female BALB/c mice (age, 6–8 weeks;
weight, 20–30 g) were purchased from the Dashuo Laboratory Animal
Technology Co. Mice were housed at 24°C with 12-h light/dark
cycles, 35–40% humidity, and free access to food and water.
Mice were randomly divided into three groups (n=9
per group): control, GBOO and DBOO. Animals in the BOO groups were
subjected to isoflurane inhalation anesthesia prior to surgery. The
method of BOO induction was performed as previously described
(19). The GBOO group was
pre-treated with this method before constructing the 1/2 UMS so the
GBOO group displayed successfully established 1/3 UMS at 1 week and
1/4 UMS at 2 weeks prior to the establishment of 1/2 UMS. Whereas,
the DBOO group was not pre-treated before constructing the 1/2 UMS.
The DBOO and GBOO groups displayed 1/2 UMS at the same time. The
control group did not undergo any treatment. At 1, 2 and 4 weeks
post-1/2 UMS construction, three mice from each group were
euthanized. Bladder tissues were harvested and the bladder was cut
horizontally into two pieces down the midline. Half of the bladder
tissue was used for subsequent biochemical analyses, and the other
half was fixed in 4% paraformaldehyde for one day at room
temperature for subsequent histological analysis.
Western blotting
Bladder tissues were homogenized in RIPA lysis
buffer (cat. no. CW2333S; CoWin Biosciences) containing a protease
inhibitor. The protein concentration was detected by bicinchoninic
acid protein assay kit (cat. no. CW0014; CoWin Biosciences). The
homogenates were centrifuged at 15,000 × g for 10 min at 4°C and
the supernatants were obtained. Proteins (50 µg per lane) were
separated by SDS-PAGE on a 12% gel and transferred onto PVDF
membranes. Non-specific binding was blocked with 5% skimmed milk
powder for 1 h at room temperature. Subsequently, the membranes
were incubated overnight at 4°C with primary antibodies targeted
against: NFAT2 (cat. no. ab175134; 1:1,000; Abcam), SGK1 (cat. no.
ab59337; 1:1,000; Abcam), proliferating cell nuclear antigen (PCNA;
cat. no. D3H8PXP; 1:1,000; Cell Signaling Technology, Inc.),
interleukin (IL)-1β (cat. no. 3A6; 1:1,000; Cell Signaling
Technology, Inc.) and β-actin (cat. no. ab8227; 1:1,000; Abcam).
Following primary incubation, the membranes were washed three times
for 10 min in PBS with 0.1% Tween-20 and subsequently incubated
with horseradish peroxidase conjugated-secondary antibodies (cat.
nos. 7074P2 and 7076P2; 1:5,000; Cell Signaling Technology, Inc.)
for 1 h at room temperature with slow shaking. The membranes were
washed again three times for 10 min in PBS with 0.1% Tween-20.
Protein bands were visualized by a ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc.) using an enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.). Protein expression was quantified using
the Image Lab software 5.2.1 (Bio-Rad Laboratories, Inc.). β-actin
was used as the loading control.
Histological analysis
Bladder tissue was fixed in 4% paraformaldehyde for
24 h at 4°C and subsequently embedded in paraffin. The
paraffin-embedded tissue samples were cut into 5-µm sections.
Histopathology was detected by Masson's trichrome staining for 15
min at room temperature. Samples were observed and imaged using an
ortho-fluorescence microscope (Nikon Corporation) at magnification,
×100 or ×200. The detrusor muscle and collagen content were
quantified using ImagePro Plus 6.0 software (Media Cybernetics,
Inc.).
Isolation and culture of BMSCs
Bladder tissue was cut into pieces and digested in
PBS containing 0.4 mg/ml type II collagenase (cat. no. 17101015;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 60 min with 5%
CO2 and 95% O2. Subsequently, the suspensions
were centrifuged at 1,300 × g for 5 min at 4°C and the supernatants
were discarded. MBSMCs were cultured in DMEM (cat. no. 12430054;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (cat. no. 16000044; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with
5% CO2 and 95% O2.
Cyclic hydrodynamic pressure
MBSMCs were cultured in scaffolds and subsequently
serum-starved for 12 h at 37°C. A total of ~1×105 cells
were seeded onto a silicone membrane and subjected to cyclic
hydrodynamic pressure to simulate the bladder cycle for up to 24 h
(2 h per cycle) at 37°C as follows: 1.75 h increasing from 0 to 10
cmH2O; rapid increase up to 200 or 400 cmH2O;
200 or 400 cmH2O maintained for 0.25 h; and returned to
0 cmH2O. No pressure was applied to the control
group.
Cell transfection
An siRNA (1 µl; cat. no. 18358; Shanghai GenePharma
Co., Ltd.) targeting SGK1 was transfected using Lipofectamine™ 2000
reagent (cat. no. 11668019; Thermo Fisher Scientific, Inc.),
Scrambled siRNAs (1 µl; cat. no. 0806; Shanghai GenePharma Co.,
Ltd.), according to the manufacturer's protocol. Mice BSMCs at 80%
density were transfected twice at an interval of 24 h with SGK1
siRNAs. Subsequent experiments was performed 72 h after
transfection. The siRNA sequences used were: SGK1 (A), sense:
5′-GUCCUUCUCAGCAAAUCAAUU-3′ and antisense:
5′-UUGAUUUGCUGAGAAGGACUU-3′; SGK1 (B), sense:
5′-CCUGAGCUUAUGAAUGCCAACCCUU-3′ and antisense:
5′-AAGGGUUGGCAUUCAUAAGCUCAGG-3′; scramble siRNA, sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense:
5′-ACGUGACACGUUCGGAGAATT-3′.
Cell cycle analysis via flow
cytometry
MBSMCs were seeded into scaffolds (5×106
cells/scaffolds) for 24 h at room temperature, collected and fixed
in 70% ethanol overnight at 4°C. After centrifugation at 1,300 × g
for 5 min at 4°C, the pellet was treated with PBS containing 50
mg/ml RNase A (cat. no. EN0531; Thermo Fisher Scientific, Inc.) and
incubated at 37°C for 1 h. The pellet was washed with ice-cold PBS
and resuspended in 1 ml propidium iodide 4°C for 1 h in the dark.
Cell cycle distribution was analyzed using an EPICS ELITE ESP flow
cytometer (Beckman Coulter, Inc.) and MultiCycle for Windows 32-bit
(Phoenix Flow Systems, Inc.). The number of cells used for
detection was ≥10,000 per sample. The cell proliferation index was
calculated as follows: proliferation index (%) = (S +
G2/M) / (G0/G1 + S +
G2/M) ×100%.
Statistical analysis
Data are presented as the mean ± SEM. Comparisons
among multiple groups were performed using one-way ANOVA followed
by the Least Significant Difference post hoc test. Statistical
analyses were performed using SPSS software version 22 (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
DBOO causes a stronger inflammatory
and proliferative response compared with GBOO during the early
stage of BOO
NFAT2 and PCNA protein expression levels were
significantly increased in the DBOO groups compared with the
control group at week 1 (P<0.05), but expression levels were not
significantly increased in the GBOO group compared with the control
group (P=0.194 and P=0.136, respectively). Whereas, protein
expression of SGK1 was significantly increased in both the DBOO and
GBOO group compared with the control (P<0.05). Furthermore, the
expression levels of NFAT2, SGK1 and PCNA were significantly higher
in the DBOO group compared with the GBOO group (P<0.05). No
significant differences were observed for IL-1β expression levels
between the GBOO and control groups (P=0.954). However, IL-1β
expression levels were significantly higher in the DBOO group
compared with the control and GBOO groups (P<0.05) (Fig. 1A and B).
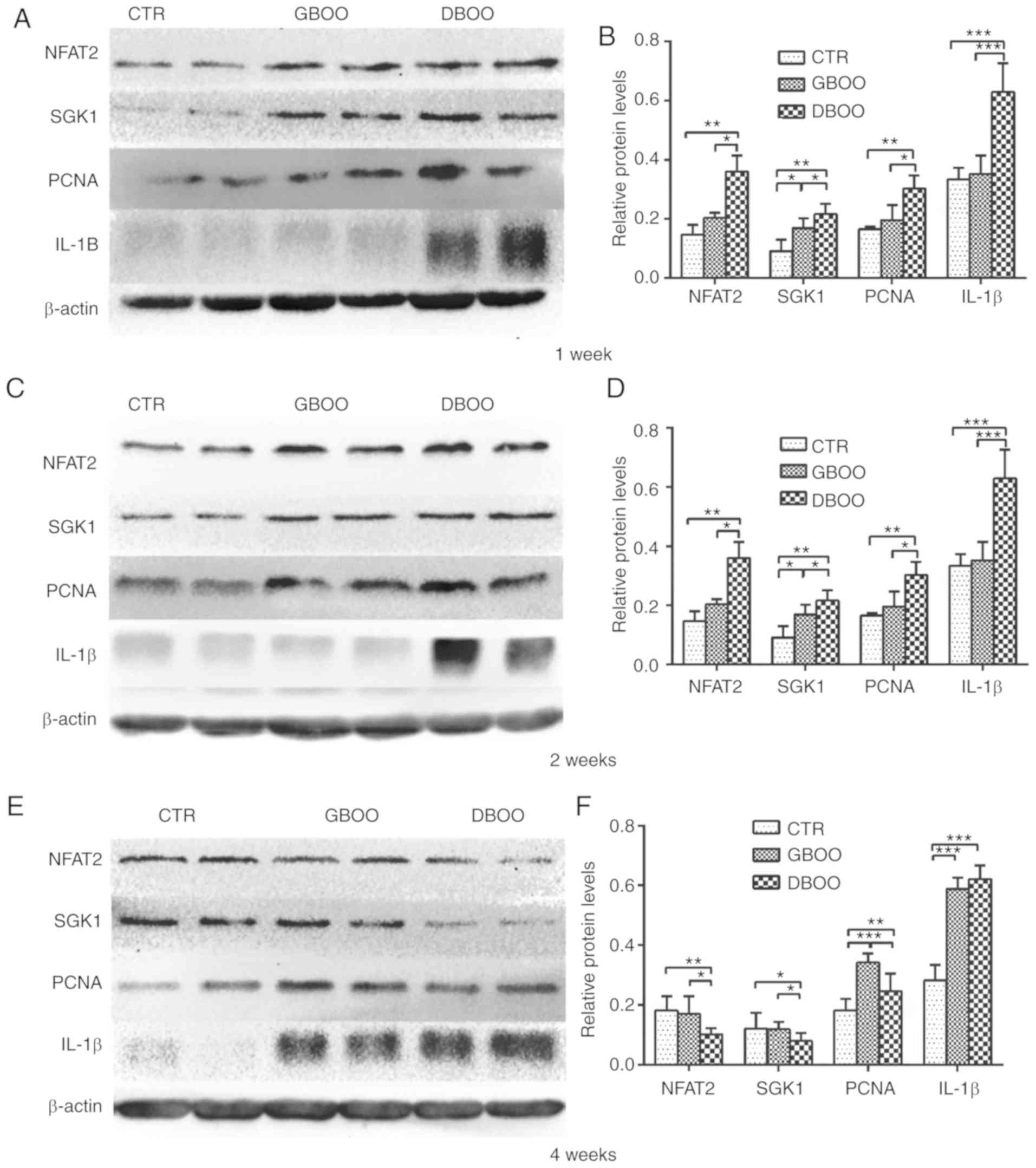 | Figure 1.Protein expression in the bladder at
week 1, 2 and 4 was determined by western blot analysis. Protein
expression in the bladder at week 1 was (A) determined by western
blotting and (B) quantified. Protein expression in the bladder at
week 2 was (C) determined by western blotting and (D) quantified.
Protein expression in the bladder at week 4 was (E) determined by
western blotting and (F) quantified. *P<0.05, **P<0.01 and
***P<0.005, as indicated. CTR, control; BOO, bladder outlet
obstruction; GBOO, gradually aggravated BOO; DBOO, directly
aggravated BOO; NFAT2, nuclear factor of activated T cells 2; SGK1,
serum/glucocorticoid regulated kinase 1; PCNA, proliferating cell
nuclear antigen; IL-1β, interleukin-1β. |
The expression levels of NFAT2, SGK1 and PCNA in the
DBOO groups were significantly higher compared with the control
group at week 2 (P<0.05), but expression of NFAT2 and PCNA was
not significantly increased in the GBOO group compared with the
control (P=0.095 and P=0.085, respectively). Whereas, SGK1
expression did significantly increase in the GBO group compared
with the control (P<0.05). Compared with the GBOO group, the
DBOO group showed increased NFAT2, SGK1 and PCNA expression levels
(P<0.05). The level of IL-1β expression in the DBOO group was
significantly increased compared with the control and GBOO groups
(P<0.05); however, IL-1β expression levels were not
significantly different between the control and GBOO groups
(P=0.180) (Fig. 1C and D).
At week 4, the control and GBOO groups showed
similar NFAT2 (P=0.323) and SGK1 (P=0.787) expression levels. NFAT2
and SGK1 expression levels were downregulated in the DBOO group
compared with the control and GBOO groups (P<0.05). PCNA
expression in the GBOO and DBOO groups was significantly higher
compared with the control group (P<0.05), and PCNA expression
was significantly decreased in the DBOO group compared with the
GBOO group (P<0.05). The level of IL-1β expression in the GBOO
and DBOO groups was significantly increased compared with the
control group (P<0.05). In addition, IL-1β expression levels
were not significantly different between the DBOO and GBOO groups
(P=0.076) (Fig. 1E and F).
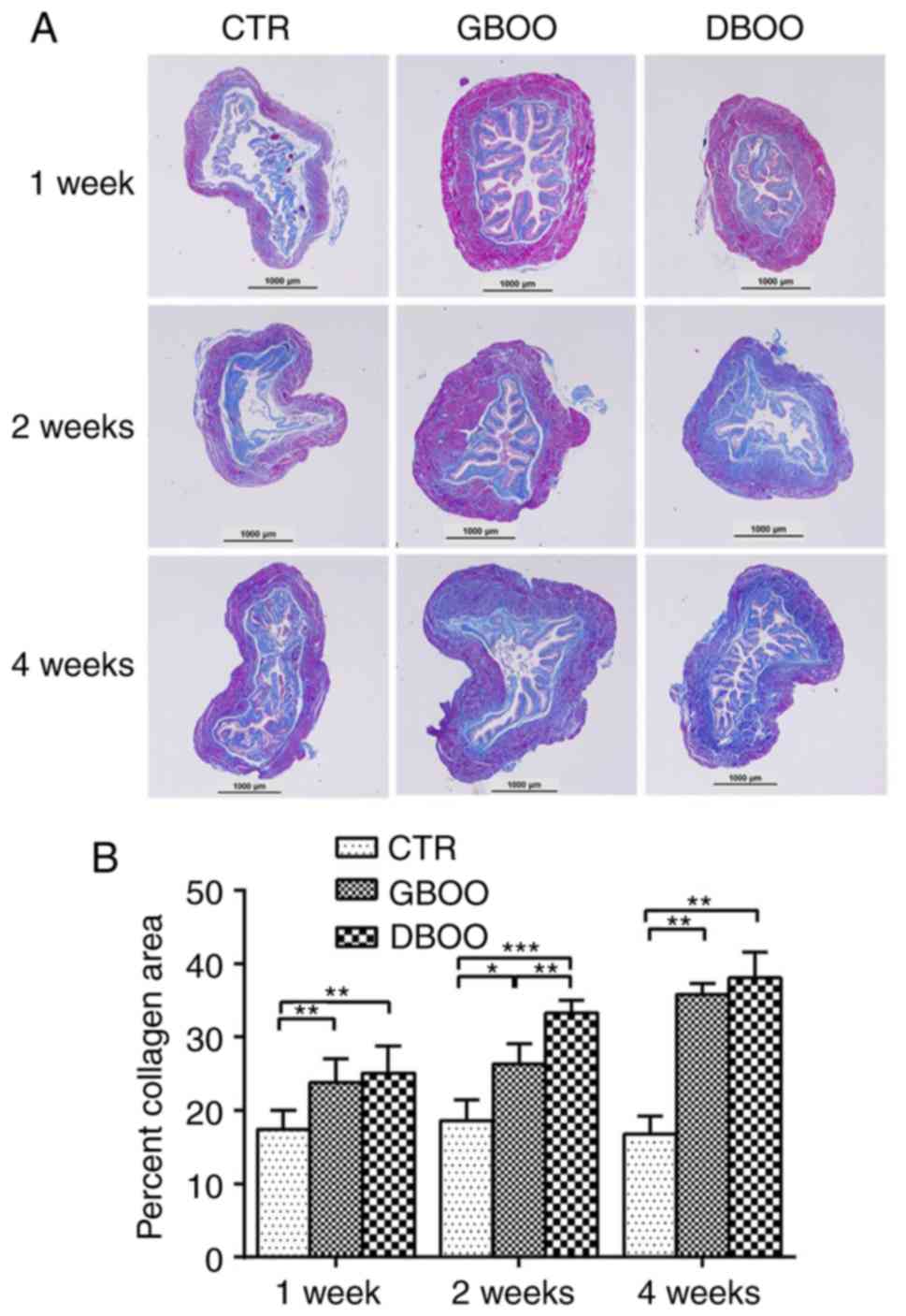
DBOO leads to increased collagen
deposition compared with GBOO during the early stage of BOO
At week 1, the area percentage of collagen in the
bladder tissues was higher in the GBOO (23.83±3.20%) and DBOO
groups (25.10±3.65%) compared with the control group (17.39±2.62%;
P<0.05). However, there was no significant difference between
the GBOO and DBOO groups (P=0.413). At week 2, the DBOO group
(33.31±1.71%) showed a significantly higher area percentage of
collagen compared with the GBOO (26.33±2.81%) and control
(18.66±2.77%) groups (P<0.05). At week 4, the area percentage of
collagen in the bladder was increased in the GBOO (35.80±1.50%) and
DBOO groups (38.12±3.48%) compared with the control group
(16.79±2.50%; P<0.05). The area percentage of collagen between
the GBOO and DBOO groups was not significantly different at week 4
(P=0.735) (Fig. 2A and B).
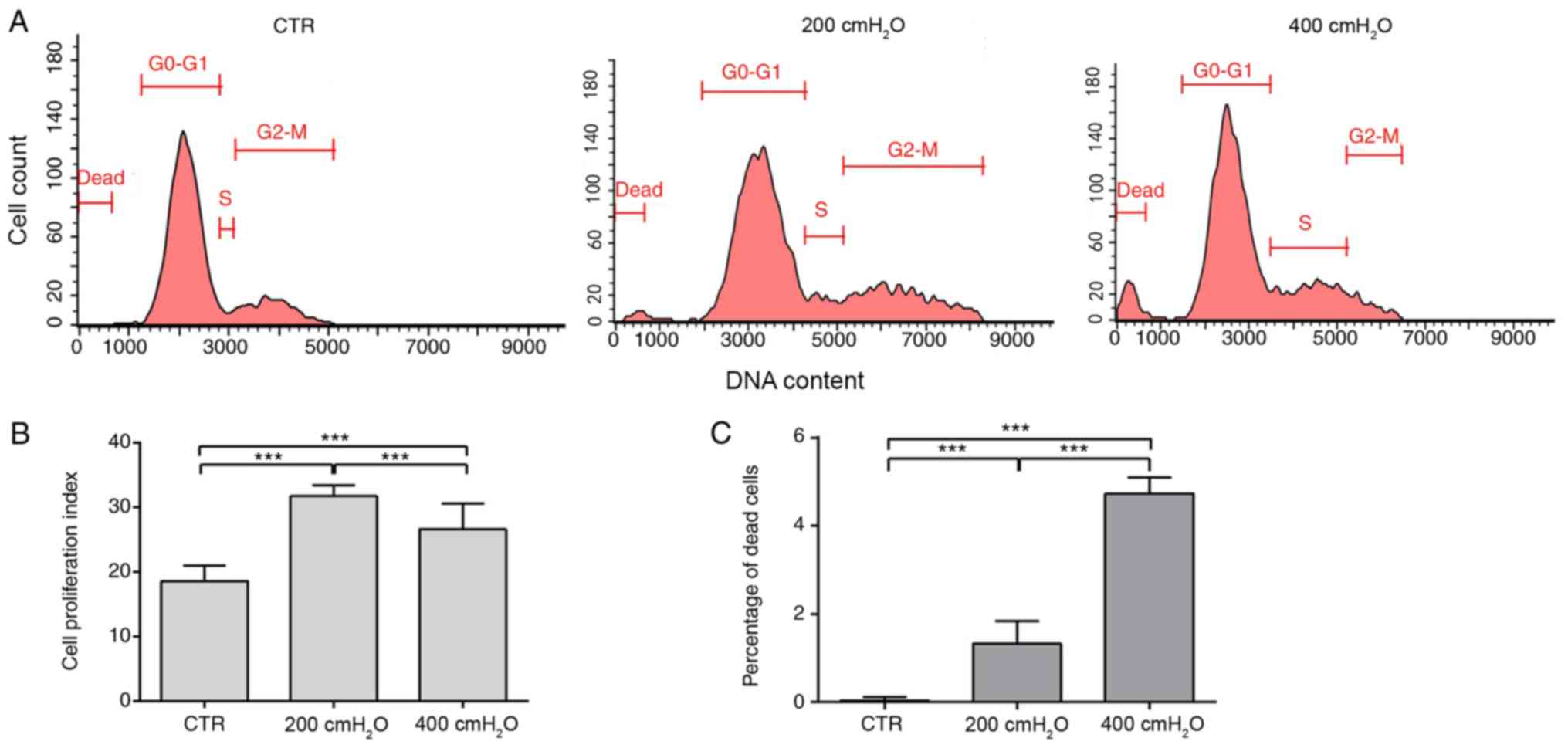
Low cyclic hydrodynamic pressure
promotes MBSMC proliferation and high cyclic hydrodynamic pressure
promotes cell death
The results indicated that 200 cmH2O
cyclic pressure significantly promoted cell proliferation compared
with the control group and 400 cmH2O group. The
proliferation index was significantly increased from 18.59±2.38% in
the control group to 31.79±1.58 and 26.64±3.99% in the 200 and 400
cmH2O pressure groups, respectively (P<0.05)
(Fig. 3A and B). The proportion of
dead cells was significantly increased in the 200 cmH2O
(1.32±0.11%) and 400 cmH2O (4.73±0.37%) pressure groups
compared with the control group (0.0333±0.01%) (Fig. 3A and C). The results suggested that
excessive stress promoted cell death in MBSMCs.
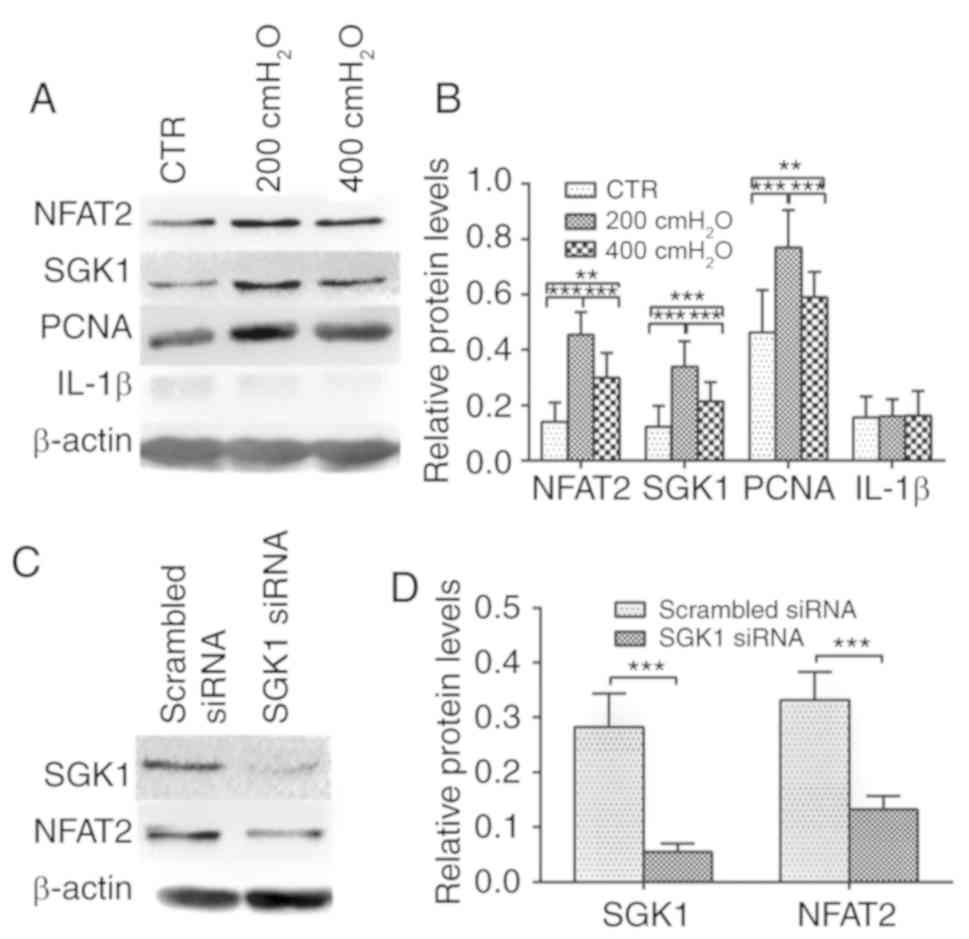
NFAT2, SGK1 and PCNA expression is
significantly increased under the stimulation of 200
cmH2O pressure, and SGK1 knockdown induces a decrease in
NFAT2 expression
The expression level of NFAT2 was significantly
decreased in the SGK1 siRNA group compared with the scrambled siRNA
group (Fig. 4C and D). The results
suggested that 200 and 400 cmH2O pressure increased
NFAT2, SGK1 and PCNA expression compared with the control group
(P<0.05) (Fig. 4A and B). In
addition, a significant increase in NFAT2, SGK1 and PCNA expression
was observed in the 200 cmH2O group compared with the
400 cmH2O group. IL-1β protein expression levels were
not significantly altered between the control, 200 and 400
cmH2O groups (Fig. 4A and
B).
Discussion
Previous studies have reported that pressure can
promote proliferation, inflammation and extracellular matrix (ECM)
remodeling in BSMCs (20–22). Obstructed bladder dysfunction
secondary to BPH is a slow and progressive disease (6). Ideally, an accurate model of
BPH-induced BOO would develop as gradually as possible (15). In the present study, a significant
difference in the pathology between the DBOO group and the GBOO
group was observed, which provided evidence that GBOO may serve as
a more appropriate model for studying BOO.
BOO significantly alters the structure and function
of the bladder (23,24), due to BMSC stimulation by increased
pressure (25,26). A previous study supported the
hypothesis that the natural progression of BOO is characterized by
three morphofunctional stages: an initial hypertrophy phase, a
subsequent compensatory phase and a late decompensation phase
(20). A number of studies have
reported that stressful stimuli promote the proliferation of BSMCs
via multiple pressure-dependent pathways (9,27–29).
In our previous study, pressure promoted HBSMC proliferation, which
may be caused by increased stress during the compensation stage of
BOO (3). During BOO, BSMCs are
stimulated by higher than normal hydrostatic pressure (30). In our previous study, cyclic
hydrodynamic pressure stimulated the proliferation of HBSMCs via
SGK1 (9). Previous findings have
also demonstrated that NFAT2 enhances the expression of cyclins,
particularly cyclin D1, in vascular smooth muscle cells to mediate
their proliferation (31,32). It was hypothesized that SGK1
promoted the proliferation of BSMCs under pressure, which may be
achieved by regulating NFAT2. Therefore, the expression levels of
SGK1 and NFAT2 in mice were investigated. In the present study,
SGK1 and NFAT2 expression levels were upregulated during the early
stages of BOO, and SGK1-siRNA treatment reduced NFAT2 expression.
The results indicated a relationship between SGK1 and NFAT2, and
suggested that pressure promoted MBSMC proliferation, potentially
by upregulating the expression of SGK1 and NFAT2. However, the
present study did not identify a direct relationship between the
two factors. To further investigate the interaction between SGK1
and NFAT2, coimmunoprecipitation was performed; however, the
experimental results were unsatisfactory (data not shown). A
limitation of the present study was that the interactions within
the SGK1-NFAT2 signaling pathway were not identified.
The expression of SGK1 and NFAT2 was upregulated in
the DBOO group compared with the GBOO group at week 1 and 2
post-obstruction. However, the expression of SGK1 and NFAT2 in the
DBOO group was lower compared with the GBOO group and the control
group at week 4 post-obstruction. PCNA is an indicator of cell
proliferation (33,34), and the PCNA expression pattern was
consistent with that of SGK1 and NFAT2 at week 1 and 2 in the
present study. The results indicated that the bladder displayed a
stronger proliferative response to acute obstruction compared with
chronic obstruction. In particular, 200 cmH2O cyclic
pressure significantly increased MBSMC proliferation; however, 400
cmH2O cyclic pressure stimulation resulted in a
significant decrease in cell proliferation compared with 200
cmH2O cyclic pressure stimulation. The results suggested
that pressure overload decreased MBSMC proliferation. The level of
BMSC proliferation increases during the compensation phase but
decreases during the decompensation phase (35,36).
The expression of SGK1 and NFAT2 in the DBOO group was lower
compared with the GBOO and control groups at week 4
post-obstruction, and the expression of PCNA in the DBOO group was
decreased compared with the GBOO group. As PCNA is a marker of
proliferation, this suggested that cell proliferation in the DBOO
group was decreased compared with the GBOO group. The results
indicated that mice in the DBOO group showed bladder decompensation
at week 4 post-obstruction. Therefore, the present study suggested
that chronic obstruction resulted in a longer compensatory period
in the bladder, whereas acute obstruction led to faster
decompensation of bladder function.
Pressure promotes BMSC proliferation and
inflammation (21,37). Recent studies have demonstrated
that inflammatory cytokines mediate the maturation of IL-1β and
lead to pyroptosis (38,39). In the present study, pressure
stimulation significantly increased cell death, and the proportion
of dead cells was increased in the 200 and 400 cmH2O
pressure groups compared with the control group. IL-1β expression
was assessed at different time points following BOO induction. The
GBOO and DBOO groups showed upregulated IL-1β expression compared
with the control group at the three different time points. The DBOO
group displayed a stronger inflammatory response at week 1 and 2
post-obstruction compared with the GBOO group; however, the
enhanced response was not observed at week 4 post-obstruction. The
bladder cannot accommodate a sudden increase in intravesical
pressure during acute obstruction (40), which results in the aggravation of
inflammation. As indicated by the expression levels of the
aforementioned biomarkers, the DBOO group displayed decreased
proliferation and increased inflammation at an earlier time point
compared with the GBOO group, suggesting that the DBOO group may
have experienced bladder function decompensation at an earlier time
point. Therefore, it was hypothesized that a pressure
overload-induced decrease in proliferation and increase in
pyroptosis of BSMCs could play an important role during the bladder
decompensation phase of BOO. The results suggested that acute
obstruction may cause rapid decompensation of bladder function by
aggravating inflammation-mediated pyroptosis and decreasing the
proliferation of BSMCs.
Bladder remodeling induced by BOO leads to impaired
storage and emptying of the bladder, which is characterized by
increased collagen accumulation (41,42).
Increased collagen deposition in the ECM is a key reason for
decreased compliance (43,44). Previous studies have reported that
pressure promotes an increase in ECM production around BSMCs
(45,46). Although the DBOO group was
obstructed for a shorter period of time compared with the GBOO
group and both groups were subjected to the same degree of
obstruction, collagen deposition occurred earlier and more
significantly in the DBOO group. In addition, the collagen
deposition in the DBOO group was more pronounced compared with the
GBOO group at week 2 post-obstruction. The bladders of mice in the
GBOO group were obstructed prior to constructing 1/2 UMS;
therefore, the bladder could adapt to the obstruction, suggesting
that the chronically obstructed bladder indicated an increased
ability to adapt to obstruction and a longer compensatory period.
High levels of collagen deposition induce a decrease in bladder
compliance, leading to bladder decompensation; therefore, acute
obstruction may cause rapid decompensation of bladder function
(47,48).
In conclusion, the chronically obstructed bladder
displayed a greater ability to adapt to obstruction and a longer
compensatory period. The results suggested that the bladder did not
adapt to a sudden increase in intravesical pressure caused by acute
obstruction, which resulted in increased proliferation,
inflammation and fibrosis. The present study indicated that acute
obstruction may lead to faster decompensation of bladder
function.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500577), the
Research Project from the Department of Science and Technology of
Sichuan Province (grant nos. 2017JY0097 and 19YYJC1427) and the
Research Projects of Chengdu Science and Technology (grant no.
2015-HM01-00580-SF).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WK, HPL and LX conceived and designed the study
protocol, collected data and performed data analysis. CL, YJ, AB,
SA, XSS, LX and CS designed the study. YJ was involved in designing
the study, data analysis and writing/editing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of the Affiliated Hospital of Chengdu University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singla S, Garg R, Singla A, Sharma S,
Singh J and Sethi P: Experience with uroflowmetry in evaluation of
lower urinary tract symptoms in patients with benign prostatic
hyperplasia. J Clin Diagn Res. 8:NC01–NC03. 2014.PubMed/NCBI
|
|
2
|
Hughes FM Jr, Hill HM, Wood CM, Edmondson
AT, Dumas A, Foo WC, Oelsen JM, Rac G and Purves JT: The NLRP3
Inflammasome Mediates Inflammation Produced by Bladder Outlet
Obstruction. J Urol. 195:1598–1605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niederhoff RA, Manson SR, Tawfik A and
Austin PF: The physiological significance of p27(KIP1) expression
in detrusor function. J Urol. 184 (Suppl):1686–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanno Y, Mitsui T, Kitta T, Moriya K,
Tsukiyama T, Hatakeyama S and Nonomura K: The inflammatory cytokine
IL-1β is involved in bladder remodeling after bladder outlet
obstruction in mice. Neurourol Urodyn. 35:377–381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Q, Luo D, Yang T, Liao B, Li H and
Wang KJ: Protective Effects of Antimuscarinics on the Bladder
Remodeling After Bladder Outlet Obstruction. Cell Physiol Biochem.
44:907–919. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levin R, Chichester P, Levin S and Buttyan
R: Role of angiogenesis in bladder response to partial outlet
obstruction. Scand J Urol Nephrol Suppl. 215:37–47. 2004.
View Article : Google Scholar
|
|
7
|
Lou Y, Hu M, Mao L, Zheng Y and Jin F:
Involvement of serum glucocorticoid-regulated kinase 1 in
reproductive success. FASEB J. 31:447–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lang F, Stournaras C, Zacharopoulou N,
Voelkl J and Alesutan I: Serum- and glucocorticoid-inducible kinase
1 and the response to cell stress. Cell Stress. 3:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Wei TQ, Wang Y, Zhang J, Li H and
Wang KJ: Simulated bladder pressure stimulates human bladder smooth
muscle cell proliferation via the PI3K/SGK1 signaling pathway. J
Urol. 188:661–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Zhang L, Shi W, Zhang B, Liang X,
Liu S and Wang W: NFAT2 mediates high glucose-induced glomerular
podocyte apoptosis through increased Bax expression. Exp Cell Res.
319:992–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Pan CG and Luo ZQ: High expression
of NFAT2 contributes to carboplatin resistance in lung cancer. Exp
Mol Pathol. 110:1042902019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaeth M, Bäuerlein CA, Pusch T, Findeis J,
Chopra M, Mottok A, Rosenwald A, Beilhack A and Berberich-Siebelt
F: Selective NFAT targeting in T cells ameliorates GvHD while
maintaining antitumor activity. Proc Natl Acad Sci USA.
112:1125–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ram BM, Dolpady J, Kulkarni R, Usha R,
Bhoria U, Poli UR, Islam M, Trehanpati N and Ramakrishna G: Human
papillomavirus (HPV) oncoprotein E6 facilitates Calcineurin-Nuclear
factor for activated T cells 2 (NFAT2) signaling to promote
cellular proliferation in cervical cell carcinoma. Exp Cell Res.
362:132–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee MK, Lee SH, Hur N, Kim S, Kim S and
Choi B: Correlation between intravesical pressure and prostatic
obstruction grade using computational fluid dynamics in benign
prostatic hyperplasia. Proc Inst Mech Eng H. 225:920–928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitta T, Kanno Y, Chiba H, Higuchi M,
Ouchi M, Togo M, Moriya K and Shinohara N: Benefits and limitations
of animal models in partial bladder outlet obstruction for
translational research. Int J Urol. 25:36–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schröder A, Tajimi M, Matsumoto H,
Schröder C, Brands M and Andersson KE: Protective effect of an oral
endothelin converting enzyme inhibitor on rat detrusor function
after outlet obstruction. J Urol. 172:1171–1174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang YJ, Jin LH, Park CS, Shin HY, Yoon SM
and Lee T: Early sequential changes in bladder function after
partial bladder outlet obstruction in awake sprague-dawley rats:
Focus on the decompensated bladder. Korean J Urol. 52:835–841.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Jongh R, van Koeveringe GA, van
Kerrebroeck PE, Markerink-van Ittersum M, de Vente J and Gillespie
JI: Damage to the bladder neck alters autonomous activity and its
sensitivity to cholinergic agonists. BJU Int. 100:919–929. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Yang Y, Yang J, He P, Amend B,
Stenzl A, Hu J, Zhang Y and Wang Z: Suture causing urethral meatus
stricture: A novel animal model of partial bladder outlet
obstruction. Neurourol Urodyn. 37:2088–2096. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fusco F, Creta M, De Nunzio C, Iacovelli
V, Mangiapia F, Li Marzi V and Finazzi Agrò E: Progressive bladder
remodeling due to bladder outlet obstruction: A systematic review
of morphological and molecular evidences in humans. BMC Urol.
18:152018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang Z, Xin W, Qiang L, Xiang C, Bang-Hua
L, Jin Y, De-Yi L, Hong L and Kun-Jie W: Hydrostatic pressure and
muscarinic receptors are involved in the release of inflammatory
cytokines in human bladder smooth muscle cells. Neurourol Urodyn.
36:1261–1269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Luo D, Zhu Y and Wang K: MicroRNA
4323 induces human bladder smooth muscle cell proliferationunder
cyclic hydrodynamic pressure by activation of erk1/2signaling
pathway. Exp. Biol. Med. (Maywood),. 242:169–176. 2017. View Article : Google Scholar
|
|
23
|
Kitta T, Kakizaki H, Tanaka H, Sano H,
Furuno T, Mitsui T, Moriya K and Nonomura K: An
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
glutamate-receptor antagonist can inhibit premicturition
contractions in rats with bladder outlet obstruction. BJU Int.
100:181–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Metcalfe PD, Wang J, Jiao H, Huang Y, Hori
K, Moore RB and Tredget EE: Bladder outlet obstruction: Progression
from inflammation to fibrosis. BJU Int. 106:1686–1694. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McGuire EJ, Woodside JR, Borden TA and
Weiss RM: Prognostic value of urodynamic testing in myelodysplastic
patients. J Urol. 126:205–209. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Engel JD, Jacobs D, Konsur B, Megaridis CM
and Bushman W: Urodynamic evaluation of the human bladder response
to an increase in outlet resistance. Neurourol Urodyn. 21:524–528.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei TQ, Luo DY, Chen L, Wu T and Wang KJ:
Cyclic hydrodynamic pressure induced proliferation of bladder
smooth muscle cells via integrin alpha5 and FAK. Physiol Res.
63:127–134. 2014.PubMed/NCBI
|
|
28
|
Sun Y, Luo DY, Zhu YC, Zhou L, Yang TX,
Tang C, Shen H and Wang KJ: MiR 3180-5p promotes proliferation in
human bladder smooth muscle cell by targeting PODN under
hydrodynamic pressure. Sci Rep. 6:330422016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Luo D, Zhu Y and Wang K: MicroRNA
4323 induces human bladder smooth muscle cell proliferation under
cyclic hydrodynamic pressure by activation of erk1/2 signaling
pathway. Exp Biol Med (Maywood). 242:169–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reddy SVK and Shaik AB: Non-invasive
evaluation of bladder outlet obstruction in benign prostatic
hyperplasia: A clinical correlation study. Arab J Urol. 17:259–264.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karpurapu M, Wang D, Van Quyen D, Kim TK,
Kundumani-Sridharan V, Pulusani S and Rao GN: Cyclin D1 is a bona
fide target gene of NFATc1 and is sufficient in the mediation of
injury-induced vascular wall remodeling. J Biol Chem.
285:3510–3523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Jin P, Lin X, Zhou Q, Wang F, Liu S
and Xi S: Arsenite increases Cyclin D1 expression through
coordinated regulation of the Ca2+/NFAT2 and NF-κB
pathways via ERK/MAPK in a human uroepithelial cell line.
Metallomics. 10:486–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zölzer F, Basu O, Devi PU, Mohanty SP and
Streffer C: Chromatin-bound PCNA as S-phase marker in mononuclear
blood cells of patients with acute lymphoblastic leukaemia or
multiple myeloma. Cell Prolif. 43:579–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sirotkin AV, Benco A, Mlyncek M, Kotwica
J, Alwasel S and Harrath AH: Transcription factor p53 regulates
healthy human ovarian cells function. C R Biol. 342:186–191. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elmissiry MM, Ali AG, Abulfotooh A, Moussa
AA and Ali GA: Factors determining the amount of residual urine in
men with bladder outlet obstruction: Could it be a predictor for
bladder contractility? Arab J Urol. 12:214–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bosch R, Abrams P, Averbeck MA, Finazzi
Agró E, Gammie A, Marcelissen T and Solomon E: Do functional
changes occur in the bladder due to bladder outlet obstruction? -
ICI-RS 2018. Neurourol Urodyn. 38 (Suppl 5):S56–S65. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang R, Amir J, Liu H and Chaqour B:
Mechanical strain activates a program of genes functionally
involved in paracrine signaling of angiogenesis. Physiol Genomics.
36:1–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo Q, Wu Y, Hou Y, Liu Y, Liu T, Zhang H,
Fan C, Guan H, Li Y, Shan Z, et al: Cytokine Secretion and
Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3,
NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune
Thyroiditis. Front Immunol. 9:11972018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bullon P, Pavillard LE and de la
Torre-Torres R: Inflammasome and Oral Diseases. Exp Suppl. 108
(Suppl 108):153–176. 2018.PubMed/NCBI
|
|
40
|
Rom M, Waldert M, Klingler HC and Klatte
T: Bladder outlet obstruction in men with acute urinary retention:
An urodynamic study. World J Urol. 31:1045–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Imamura M, Kanematsu A, Yamamoto S, Kimura
Y, Kanatani I, Ito N, Tabata Y and Ogawa O: Basic fibroblast growth
factor modulates proliferation and collagen expression in urinary
bladder smooth muscle cells. Am J Physiol Renal Physiol.
293:F1007–F1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Averbeck MA, De Lima NG, Motta GA, Beltrao
LF, Abboud Filho NJ, Rigotti CP, Dos Santos WN, Dos Santos SKJ, Da
Silva LFB and Rhoden EL: Collagen content in the bladder of men
with LUTS undergoing open prostatectomy: A pilot study. Neurourol
Urodyn. 37:1088–1094. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang L, Liu R, Wang X and He D: Imbalance
between matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) contributes to bladder compliance
changes in rabbits with partial bladder outlet obstruction (PBOO).
BJU Int. 112:E391–E397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bellucci CHS, Ribeiro WO, Hemerly TS, de
Bessa J Jr, Antunes AA, Leite KRM, Bruschini H, Srougi M and Gomes
CM: Increased detrusor collagen is associated with detrusor
overactivity and decreased bladder compliance in men with benign
prostatic obstruction. Prostate Int. 5:70–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Backhaus BO, Kaefer M, Haberstroh KM, Hile
K, Nagatomi J, Rink RC, Cain MP, Casale A and Bizios R: Alterations
in the molecular determinants of bladder compliance at hydrostatic
pressures less than 40 cm. H2O. J Urol. 168:2600–2604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen S, Peng C, Wei X, Luo D, Lin Y, Yang
T, Jin X, Gong L, Li H and Wang K: Simulated physiological stretch
increases expression of extracellular matrix proteins in human
bladder smooth muscle cells via integrin α4/αv-FAK-ERK1/2 signaling
pathway. World J Urol. 35:1247–1254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gosling JA, Kung LS, Dixon JS, Horan P,
Whitbeck C and Levin RM: Correlation between the structure and
function of the rabbit urinary bladder following partial outlet
obstruction. J Urol. 163:1349–1356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Herz DB, Aitken K and Bagli DJ: Collagen
directly stimulates bladder smooth muscle cell growth in
vitro: Regulation by extracellular regulated mitogen activated
protein kinase. J Urol. 170:2072–2076. 2003. View Article : Google Scholar : PubMed/NCBI
|