Introduction
Reperfusion is the most effective treatment for
decreasing myocardial infarct size and myocardial apoptosis
following ischemic injury (1).
However, acting as a double-edged sword, reperfusion may initiate
additional damage, such as reperfusion injury, which may counter
the benefits of reperfusion and cause fatal damage in patients
(2). Previous studies have shown
that Toll-like receptor 2 (TLR2) triggers an inflammatory cascade
and serves an important role in ischemic/reperfusion (I/R) injury
(3–5). As part of the innate immune defense,
TLR2 is able to recognize danger signals and can regulate
endogenous or exogenous inflammatory responses (6). Furthermore, TLR2 serves a vital role
in the activation of the inflammatory immune response during
myocardial I/R injury by regulating pro-inflammatory cytokine
induction. Cytokines such as interleukin (IL)-1β and IL-6 can act
on cardiomyocyte membrane receptors and initiate a series of signal
cascades, which in turn causes the activation of TLR2, thus leading
to myocardial inflammatory damage (7). Moreover, inhibition of TLR2 with its
ligands (e.g. peptidoglycan, Pam3CSK4) or receptor antagonists
(OPN-305) has been shown to significantly decrease neutrophil
infiltration, cytokine production and cardiomyocyte apoptosis
(8,9). Therefore, targeting TLR2 may be a
promising therapy for I/R injury.
Ischemic postconditioning (IPostC) refers to
transient, repetitive I/R in the early stage of reperfusion and has
been revealed to alleviate myocardial I/R injury in a number of
animal and human models (10,11).
Moreover, previous studies (12,13)
have demonstrated that the protective effect of IPostC is closely
associated with the inhibition of TLR2-induced inflammation
(13); however, the underlying
mechanism has not been fully elucidated. Our previous study
identified that IPostC exerts a cardioprotective effect via protein
kinase C (PKC) signaling (14),
but the association between TLR2 and PKC signaling in IPostC
remains unknown.
MicroRNAs (miRs) are small non-coding RNAs that
serve an important role in the regulation of the natural immune
response and inflammation (15,16),
and are involved in cardiovascular diseases (17,18).
miR-499, a cardiac-enriched small RNA molecule, is involved in
regulating cardiac function, the pathogenesis of I/R injury and has
cardioprotective effects (19,20).
Furthermore, a previous study (21) demonstrated that miR-499 is involved
in the anti-inflammatory and anti-apoptotic effects of IPostC.
Therefore, based on the important role of miR-499 in
IPostC-induced cardioprotection, the present study investigated the
relevance of the regulation of TLR2 during IPostC. A myocardial I/R
rat model was established to examine the effects of IPostC on
infarct size and cardiomyocyte apoptosis. Moreover, the mRNA
expression level of miR-499, the serum and protein expression
levels of TLR2 and PKC and inflammatory cytokines, including IL-1β
and IL-6, were detected. In addition, the protein expression levels
of anti- and pro-apoptotic markers, and the effects of miR-499
overexpression and inhibition on IPostC were investigated.
Materials and methods
Experimental animals
The Experimental Animal Center of Guangxi Medical
University provided 90 adult male Sprague-Dawley rats ~8 weeks
(weight, 240–280 g). The experimental environment was maintained at
a constant room temperature (temperature, 22–24°C; relative
humidity, 35–65%; 12:12-h light: dark cycle) and rats were allowed
ad libitum access to food and water. The experimental
protocols were performed in strict accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals and were approved by the Animal Protection and Use
Committee of Guangxi Medical University.
Myocardial I/R model
Sodium pentobarbital (2%, 50 mg/kg) was
intraperitoneally injected to anesthetize the rats. The rats were
mechanically ventilated with oxygen-enriched room air using a small
animal respirator. A metal syringe needle was subcutaneously
inserted into all four limbs of each rat. The needle was connected
to an electrocardiograph with an electric clip that recorded the
cardiac electrical activity of the rat. An incision was made in the
5th intercostal space on the left sternal border to fully expose
the heart. The left anterior descending coronary (LAD) artery was
ligated ~2 mm below the left atrial appendage and the angle of the
articular cone with a suture with a nylon 6-0 needle. In the LAD
artery, the end of the suture was passed through a small plastic
tube to form a reversible snare LAD occlusion. Myocardial ischemia
was induced by tightening the ligature around the plastic tube; the
relaxation of the ligation caused reperfusion of the myocardial
tissue. The monitoring of the changes in the ST-T segment of the
electrocardiogram confirmed that ligation was successful, as
indicated when the ST-T segment of the anterior region of the heart
increased. Cervical dislocation of anesthetized rats was conducted
at the end of reperfusion, and 3 ml blood samples and the anterior
wall of the left ventricle near the apex were collected for
analysis.
Experimental groups
Rats were randomized into six groups (n=15): i) Sham
group, in which the ligature was passed, but not tied, and
maintained for 150 min; ii) I/R group, in which rats were subjected
to 30 min of ischemia followed by 2 h of reperfusion; iii) IPostC
group, in which rats underwent 3 cycles of 30 sec of reperfusion
and 30 sec of ischemia immediately after the onset of reperfusion;
iv) IPostC + negative control (NC) group, which received a miR-449
NC adeno-associated virus (AAV) vector (Hanbio Biotechnology Co.,
Ltd.; dose=1×1011 v.g./rat) injected into the tail vein,
the operation was performed after 2 weeks of routine feeding, as in
the IPostC group; v) IPostC + mimics group, which received a
miR-499-overexpressing AAV vector (Hanbio Biotechnology Co., Ltd.;
1×1011 v.g./rat) injected intravenously, with routine
feeding allowed for 2 weeks, as in the IPostC group; and vi) IPostC
+ inhibitor group, which received miR-499 inhibitor AAV vector
(Hanbio Biotechnology Co., Ltd.; dose, 1×1011 v.g./rat)
injected into the tail vein, followed by operation after 2 weeks,
as in the IPostC group. In total, three of the 90 rats used in this
study were excluded: One in the I/R group due to ventricular
fibrillation; one in the IpostC + NC group due to cardiogenic shock
during reperfusion; and one in the IPostC + inhibitors group due to
viral transfection failure. The results described are for the
remaining 87 rats.
AAV vector transfection
The miR-499 miR-499-overexpression AAV vector
(5′-GCTGTTAAGACTTGCAGTGATGTTTAGCTCCTCTCCATGTGAACATCACAGCAAGTCTGTGCTGC-3′),
miR-499 inhibitor AAV vector
(5′-AAACATCACTGCAAGTCTTAATATACAAACATCACTGCAAGTCTTAAACATCAAACATCACTGCAAGTCTTAATCTTCAAAACATCACTGCAAGTCTTAA-3′)
and miR-499 NC AAV vectors (Hanbio Biotechnology Co., Ltd.) were
constructed. The viral vector (1×1011 v.g./rat) was
injected into the tail vein of rats, and the expression level of
miR-499 in the myocardial tissue was detected by reverse
transcription-quantitative (RT-q) PCR following 2 weeks of routine
feeding to confirm that the transfection was successful.
Myocardial infarction size
analysis
Following reperfusion, the LAD was re-ligated and 3%
Evans blue dye at room temperature was injected into the inferior
vena cava and reacted for ~5 min to identify areas of risk for
myocardial infarction. The stained heart was removed, frozen, cut
into 2-mm sections and then incubated for 15 min at 37°C in 1%
2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich; Merck KGaA) to
examine the size of the infarct. The infarct and risk zones were
quantified using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.), as previously described (14). The infarct size was expressed as a
percentage of the risk area, and the risk area was calculated as
the percentage of the left ventricle.
TUNEL staining
The histochemical detection of apoptotic cells was
performed as previously reported (14). Apoptotic cells were identified
using a TUNEL detection kit (cat. no. 116848179; Roche Diagnostics
GmbH) according to the manufacturer's protocol. The dewaxed
myocardial tissue sections were immersed in 3% hydrogen peroxide in
methanol for 10 min at room temperature, and washed 3 times with
PBS; then the tissue sections and proteinase K working solution
(cat. no. G1205; WuHan Servicebio Technology Co., Ltd.) incubated
at 37C for 30 min, which the tissue sections and TdT reaction
mixture are incubated at 37C for 2 h. After washing 3 times with
PBS, counterstain the nuclei with DAPI solution (cat. no. D1306;
1:1,000; Thermo Fisher Scientific, Inc.) at room temperature for 5
min, and use 50 µl of anti-fade mounting medium (cat. no. G1401;
WuHan Servicebio Technology Co., Ltd.) TUNEL-positive cells were
observed in five randomly selected areas using a fluorescence
microscope (magnification, ×200). The apoptotic index was expressed
as the number of TUNEL-positive cells/total number of
cardiomyocytes ×100%.
Levels of TLR2, PKC, IL-1β and IL-6 in
serum
Prior to the removal of the heart, 5-ml blood
samples were collected and centrifuged at 4C, 2,000 × g for 10 min,
and the supernatant was stored in a refrigerator at −80C. The serum
levels of TLR2, PKC, IL-1β and IL-6 were assessed with an automatic
analyzer 7600 (Hitachi Ltd.) using rat TLR2 ELISA kit (cat. no.
0663R1; Enzyme immunoassay Co.), rat PKC ELISA kit (cat. no.
0684R1; Enzyme immunoassay Co.), rat IL-1β ELISA kit (cat. no.
0047R1; Enzyme immunoassay Co.) and rat IL-6 ELISA kit (cat. no.
0190R1; Enzyme immunoassay Co.). The experiment was repeated four
times.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. First-strand cDNA was synthesized
from 1 µg total RNA using a RevertAid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.) and reverse transcribed using
the following temperature protocol: 37C for 15 min, 85C for 5 sec
and stored at 4C. RT-qPCR was performed using 2X SYBR Green qPCR
ProMix (EnzyValley) in an ABI 7300 RT PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixtures
were incubated in a 96-well plate and the RT-qPCR thermal cycling
conditions were as follows: Initial denaturation at 95°C for 2 min,
followed by 40 cycles of 95°C for 5 sec and final extension at 40
cycles of 60°C for 30 sec. RNU6B was used as the reference control.
Primer sequences are presented in Table I. The relative expression level of
miR-499 was determined using the 2−ΔΔCq method (22).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR analyses. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR analyses.
| Genes | Primers | Sequences |
|---|
| U6 | Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
|
| Reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-499 | Forward |
5′-TTAAGACTTGCAGTGATGTTT-3′ |
|
| Reverse |
5′-CAGTGCAGGGTCCGAGGTAT-3′ |
| miRT Random
Primer |
|
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCAC |
|
|
|
TGGATACGACNNNNN-3′ |
Western blotting
Western blotting was performed as described
previously (14). Freshly frozen
myocardial tissue samples were homogenized in RIPA buffer (Beyotime
Institute of Biotechnology). The protein concentration was
determined with a bicinchoninic acid assay. Equal amounts (30 µg)
of proteins were separated by 12% SDS-PAGE and were transferred to
nitrocellulose membranes that were blocked with 5% non-fat milk for
1 h at room temperature in TBST (150 mM NaCl, 20 mM Tris-HCl and
0.1% Tween 20; pH 7.4). All antibodies were acquired from Cell
Signaling Technology, Inc. Membranes were incubated with TLR2
rabbit monoclonal antibodies (cat. no. 12276S; 1:1,000), PKC rabbit
monoclonal antibodies (cat. no. 38938S; 1:1,000.), IL-1βrabbit
monoclonal antibodies (cat. no. 12703S; 1:1,000.), IL-6 rabbit
monoclonal antibodies (cat. no. 12912S; 1:1,000.), Bcl-2 rabbit
monoclonal antibodies (cat. no. 3498S; 1:1,000) and Bax rabbit
monoclonal antibodies (cat. no. 5023S; 1:1,000) overnight at 4C.
After washing 3 times with TBST, the membranes were exposed to
peroxidase-conjugated goat anti-rabbit IgG (H+L) antibodies (cat.
no. 14708S; 1:3,000) for 1 h at room temperature. Finally, using
Chemiluminescence substrate (Pierce; Thermo Fisher Scientific,
Inc.) and exposed on radiographic film. The results were
quantitatively analyzed using ImageJ v1.8.0 software (National
Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD. One-way ANOVA
was performed using Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 23.0 (SPSS,
Inc.).
Results
Effect of IPostC on the expression
levels of inflammatory cytokines
The inhibition of inflammatory cytokine activation
serves an important role in mitigating myocardial I/R injury
(23). In order to determine
whether IPostC exerts cardioprotective effects by inhibiting the
activation of inflammatory cytokines, the present study examined
the serum and the protein expression levels of TLR2, IL-1β and
IL-6. It was identified that, when comparing the I/R group with the
IPostC group, the serum and protein expression levels of TLR2
(serum: 23.1±3.3 vs. 28.4±3.9; protein: 0.57±0.03 vs. 0.80±0.04,
respectively; P<0.05), IL-1β (serum: 51.9±5.0 vs. 60.6±5.8;
protein: 0.51±0.03 vs. 0.75±0.03, respectively; P<0.05) and IL-6
(serum: 108.7±8.9 vs. 156.9±10.5; protein: 0.46±0.03 vs. 0.70±0.03,
respectively; P<0.05) were significantly decreased in the IPostC
group (Table II; Fig. 1). Therefore, the results indicated
that IPostC may alleviate myocardial I/R damage by inhibiting the
activation of inflammatory cytokines.
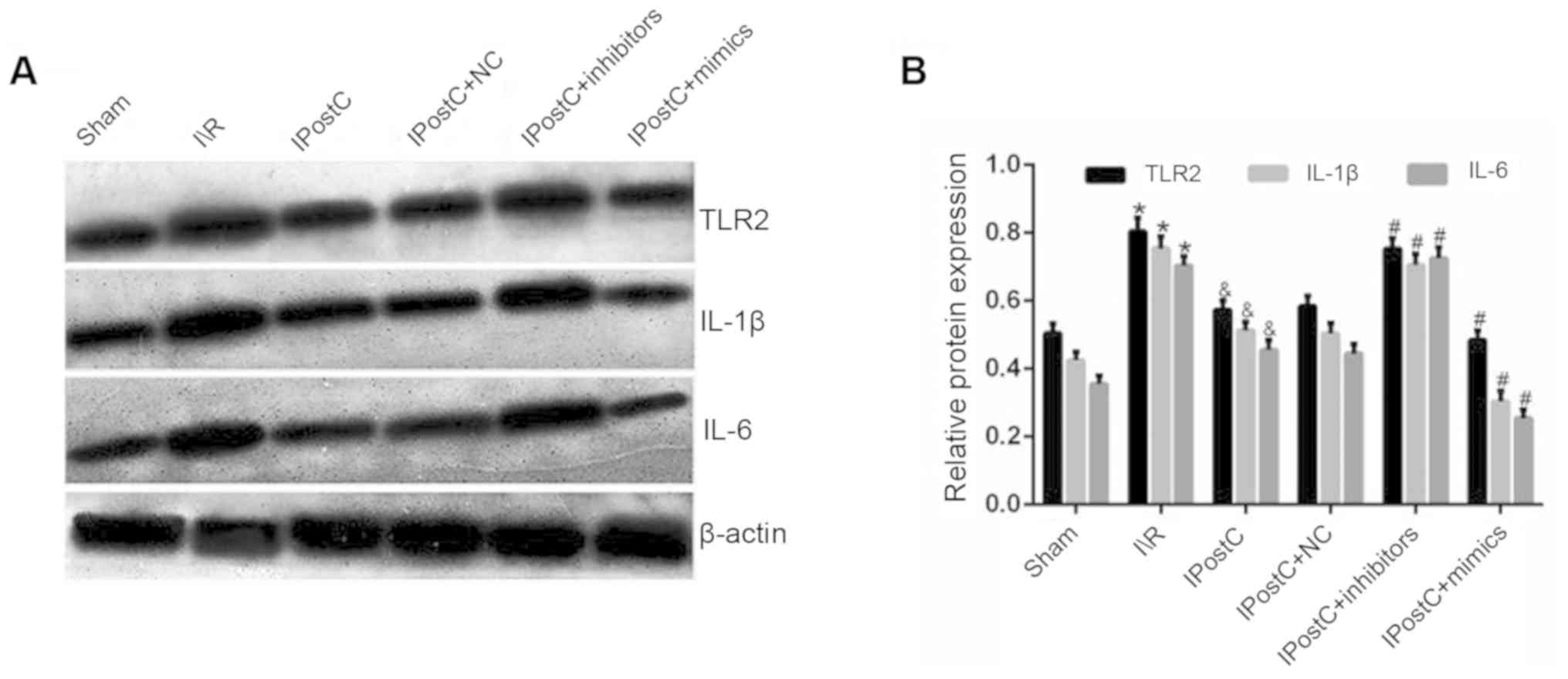 | Figure 1.Effect of IPostC on the expression
levels of inflammatory cytokines. (A) Effects of IPostC with or
without treatment with the miR-499-5p mimic, inhibitor and NC AAV
vectors on TLR2, IL-1β and IL-6 protein expression levels following
myocardial I/R. (B) Quantitative analysis of TLR2, IL-1β and IL-6
protein expression levels. Data are presented as the mean ± SD.
*P<0.05 vs. sham group. &P<0.05 vs. I/R group.
#P<0.05 vs. IPostC group. n=6 per group. NC, negative
control; miR, microRNA; TLR, Toll-like receptor; IL, interleukin;
IPostC, ischemic postconditioning; IR, ischemia/reperfusion; AAV,
adeno-associated virus. |
 | Table II.Effects of IPostC with the miR-499
mimic, inhibitor and negative control AAV vectors on the levels of
TLR2, PKC, IL-1β and IL-6 in the serum following myocardial
I/R. |
Table II.
Effects of IPostC with the miR-499
mimic, inhibitor and negative control AAV vectors on the levels of
TLR2, PKC, IL-1β and IL-6 in the serum following myocardial
I/R.
| Groups | TLR2, pg/ml | PKC, pg/ml | IL-1β, pg/ml | IL-6, pg/ml |
|---|
| Sham | 16.7±2.8 | 320.4±17.2 | 35.5±4.2 | 68.4±7.3 |
| I/R |
28.4±3.9a |
1036.5±30.1a |
60.6±5.8a |
156.9±10.5a |
| IPostC |
23.1±3.3b |
720.1±24.9b |
51.9±5.0b |
108.7±8.9b |
| IPostC + NC | 22.4±3.2 | 717.2±26.0 | 52.1±3.0 | 107.9±8.0 |
| IPostC +
inhibitors |
32.8±5.5c |
987.7±28.7c |
68.5±3.1c |
178.5±7.8c |
| IPostC +
mimics |
13.6±2.9c |
505.2±19.7c |
37.0±1.8c |
77.1±4.9c |
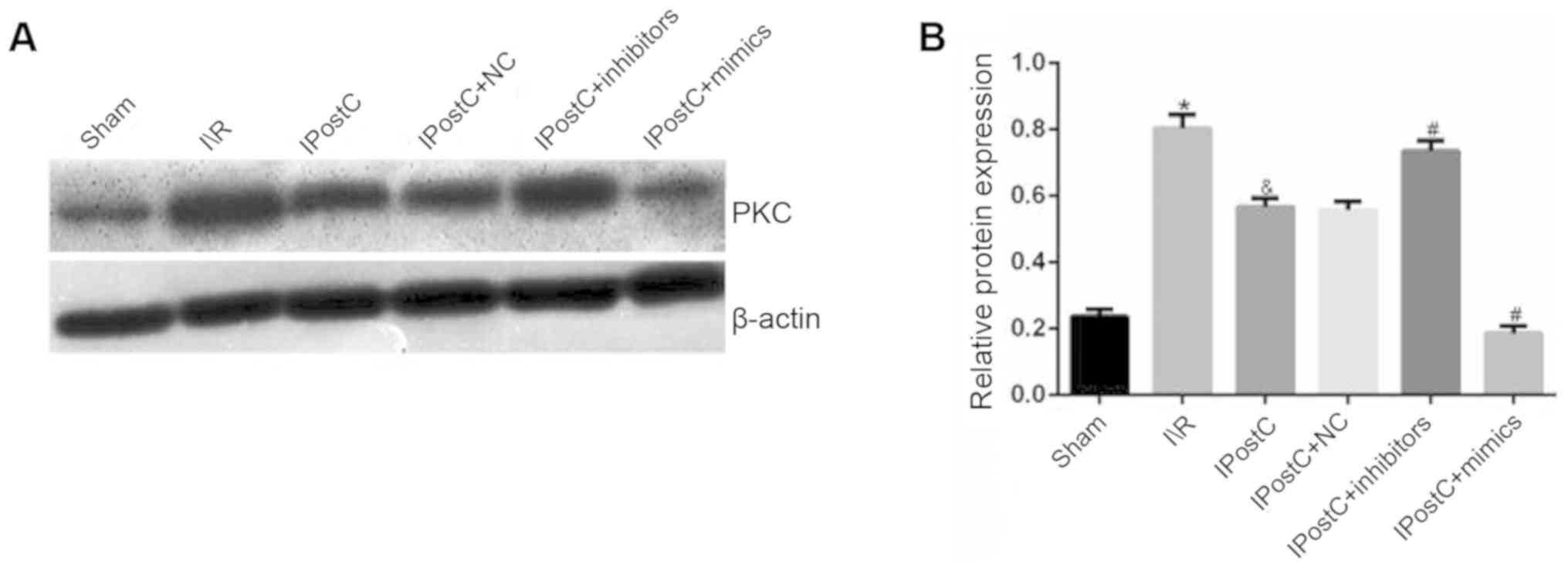
Effect of IPostC on the expression of
PKC
To determine the signal transduction mechanism via
which IPostC may serve a role in cardioprotection, the serum and
the protein expression levels of PKC were measured. It was
identified that the serum and the protein expression levels of PKC
were significantly decreased in the IPostC group compared with the
I/R group (serum: 720.1±24.9 vs. 1,036.5±30.1; protein: 0.57±0.03
vs. 0.80±0.04, respectively; P<0.05; Table II; Fig. 2). Thus, it was hypothesized that
IPostC may have a cardioprotective role by inhibiting the PKC
signal transduction pathway.
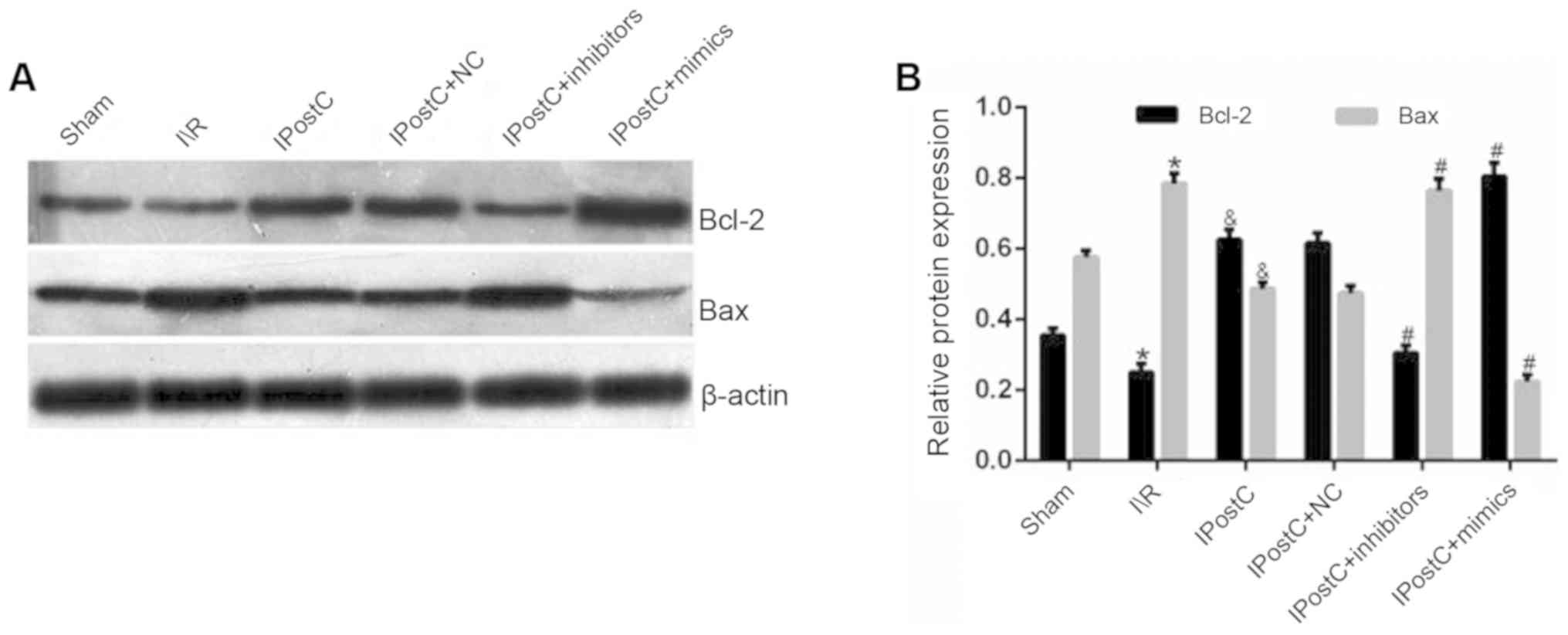
Effect of IPostC on the protein
expression levels of Bcl-2 and Bax
The results demonstrated that the IPostC group
exhibited an increased expression of Bcl-2 (0.63±0.03 vs.
0.25±0.02; P<0.05) and a decreased expression level of Bax
(0.49±0.02 vs. 0.78±0.03; P<0.05) in the myocardium compared
with the I/R group (Fig. 3).
Moreover, these results suggested that IPostC protected the heart
from I/R-induced apoptosis by increasing Bcl-2 and decreasing Bax
protein expression levels.
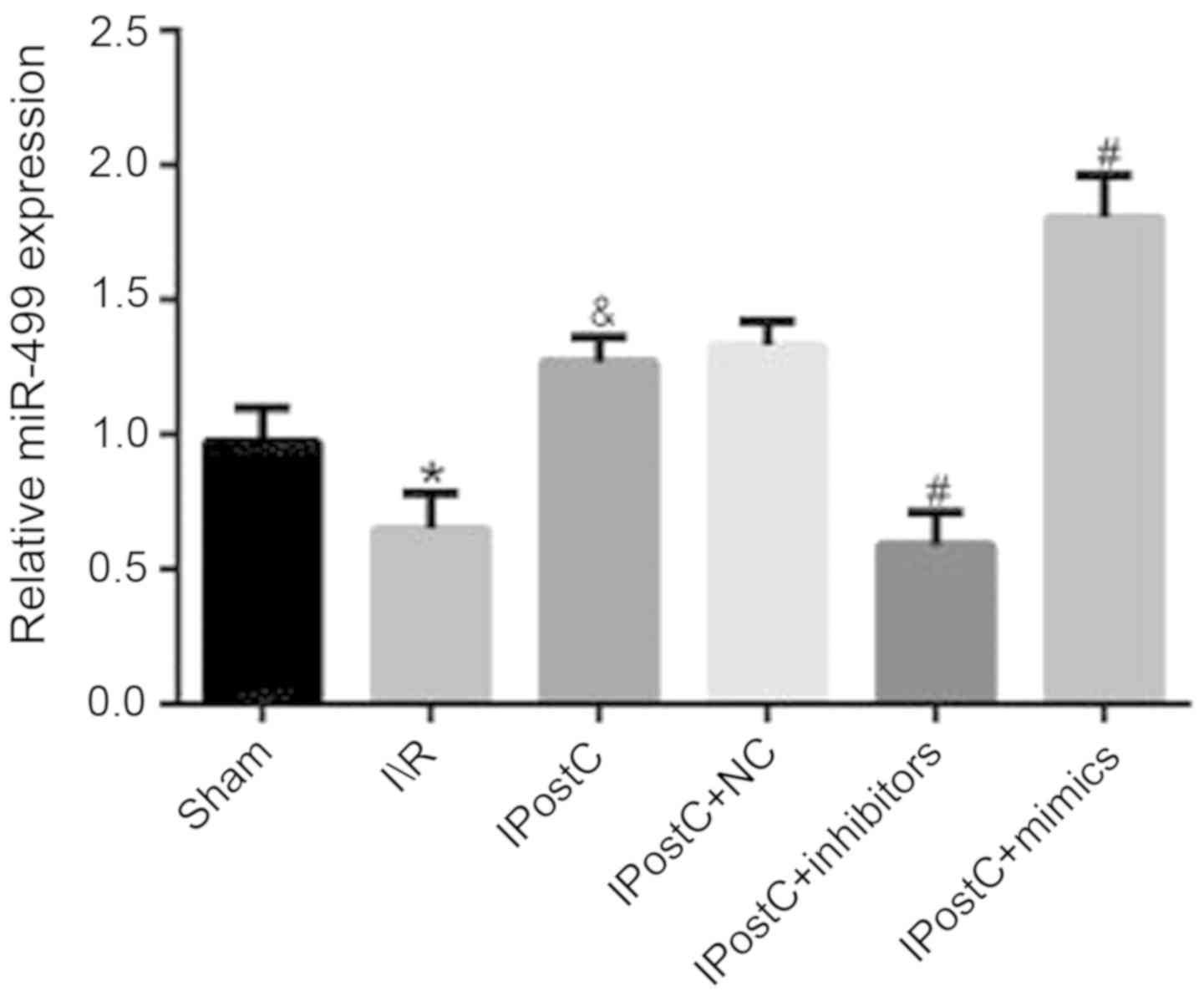
Effect of IPostC on the mRNA
expression level of miR-499
To determine whether miR-499 was associated with the
cardioprotective effects of IPostC, the mRNA expression level of
miR-499 was examined in myocardial tissue. It was identified that
the mRNA expression level of miR-499 in the IPostC group was
significantly increased compared with the I/R group (1.27±0.09 vs.
0.65±0.13; P<0.05; Fig. 4).
Expression levels of miR-499 in the
myocardium following viral transfection
In order to further investigate the effect of
miR-499 on the cardioprotective mechanism of IPostC, the rat
myocardium was transfected with miR-499 mimic, inhibitor and NC AAV
vectors and then the mRNA expression of miR-499 was measured. It
was identified that there was no significant difference in mRNA
expression level of miR-499 between the IPostC + NC and IPostC
groups (1.33±0.09 vs. 1.27±0.09; Fig.
4). However, compared with the miR-499 expression levels in the
IPostC group, the level in the IPostC + inhibitors group was
significantly decreased (0.59±0.12 vs. 1.27±0.09; P<0.05), while
that in the IPostC + mimics group was significantly increased
(1.80±0.16 vs. 1.27±0.09; P<0.05). Therefore, these experimental
results identified that the transfection of the miR-499 virus was
successful.
Effect of miR-499 on the expression
levels of inflammatory factors
The results demonstrated that the protein expression
levels of TLR2 (0.58±0.03 vs. 0.57±0.03; P>0.05), IL-1β
(0.50±0.03 vs. 0.51±0.03; P>0.05) and IL-6 (0.44±0.03 vs.
0.46±0.03; P>0.05) were not significantly different between the
IPostC + NC and IPostC groups (Fig.
1). Moreover, compared with the IPostC group, the expression
levels of TLR2 (0.75±0.03 in IPostC + inhibitors vs. 0.57±0.03 in
IPostC; P<0.05), IL-1β (0.71±0.03 in IPostC + inhibitors vs.
0.51±0.03 in IPostC; P<0.05) and IL-6 (0.73±0.03 in IPostC +
inhibitors vs. 0.46±0.03 in IPostC; P<0.05) were significantly
increased in the IPostC + inhibitors group, while the expression
levels of TLR2 (0.48±0.03 in IPostC + mimics vs. 0.57±0.03 in
IPostC; P<0.05), IL-1β (0.30±0.03 in IPostC + mimics vs.
0.51±0.03 in IPostC; P<0.05) and IL-6 (0.25±0.02 in IPostC +
mimics vs. 0.46±0.03 in IPostC; P<0.05) were significantly
decreased in the IPostC + mimics group. Collectively, these results
suggested that the upregulation of miR-499 can exert
cardioprotective effects by inhibiting the expression level of
inflammatory cytokines.
Effect of miR-499 on the expression
level of PKC
It was identified that there was no significant
difference in PKC expression levels between the IPostC + NC group
and IPostC groups (Table II;
Fig. 2). Furthermore, the serum
and the protein expression levels of PKC were significantly
increased in the IPostC + inhibitor group (987.7±28.7 vs.
720.1±24.9 and 0.74±0.03 vs. 0.57±0.03, respectively; P<0.05)
and significantly decreased in the IPostC + mimics group
(505.2±19.7 vs. 720.1±24.9 and 0.19±0.02 vs. 0.57±0.03,
respectively; P<0.05) compared with those in the IPostC group.
Therefore, it was speculated that the upregulation of miR-499 may
exert anti-inflammatory effects by inhibiting the PKC signal
transduction pathway.
Effect of miR-499 on the expression
level of circulating inflammatory cytokines
To further assess the effect of miR-499 on the
systemic inflammatory status of IPostC, the rat myocardium was
transfected with miR-499 mimic, inhibitor and NC AAV vectors and
the serum levels of inflammatory cytokines were measured. The
results demonstrated that the serum levels of TLR2, IL-1β and IL-6
were not significantly different between the IPostC + NC and IPostC
groups (Table II). In comparison
with the IPostC + NC and IPostC groups, the serum levels of TLR2,
IL-1β and IL-6 were significantly increased in the IPostC +
inhibitors group and were all significantly decreased in the IPostC
+ mimics group.
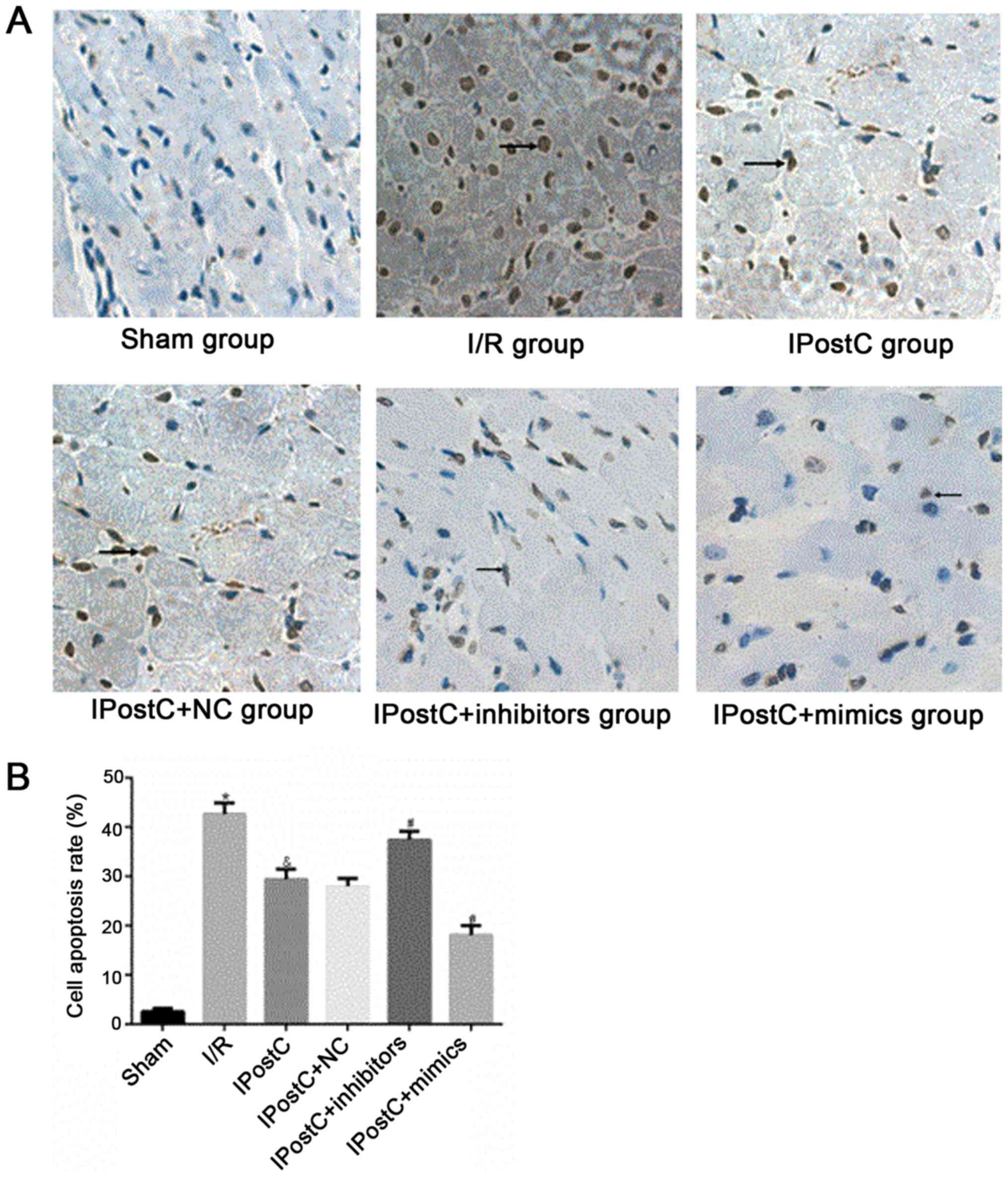
Effect of miR-499 on cardiomyocyte
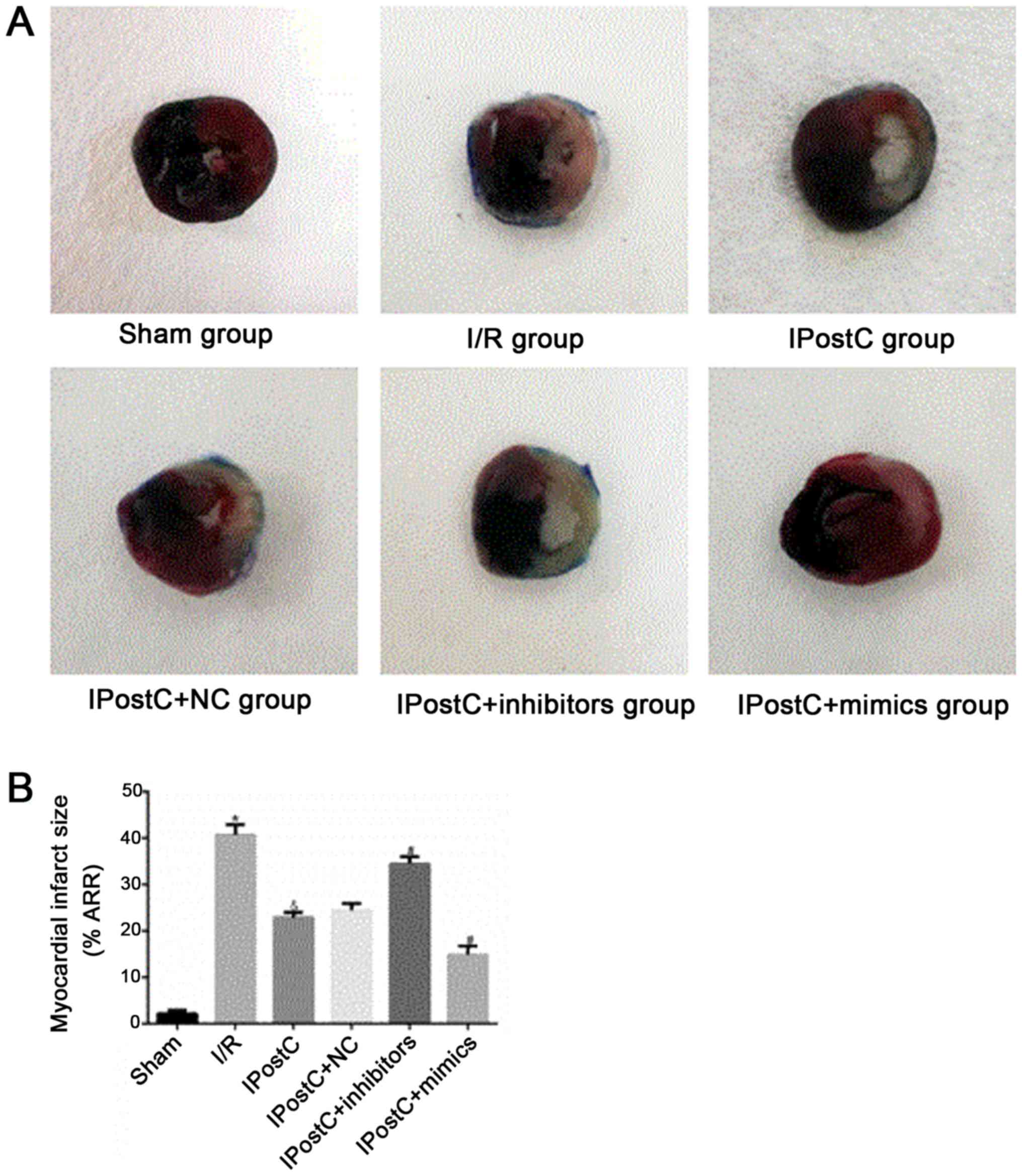
apoptosis and myocardial infarct size
To assess whether miR-499 serves a role in the
cardioprotective effects of IPostC, the apoptotic index of
cardiomyocytes (Fig. 5) and
myocardial infarct size (Fig. 6)
were examined. Relative to the I/R group, the IPostC group
exhibited a significantly decreased myocardial apoptotic index
(29.46±2.03% vs. 42.64±2.27%; P<0.05) and significantly
decreased myocardial infarct size (48.53±2.49% vs. 66.52±3.1%;
P<0.05). Furthermore, the cardiomyocyte apoptotic index
(28.00±1.54% vs. 29.46±2.03%; P>0.05) and myocardial infarct
size (47.53±2.47% vs. 48.53±2.49%; P>0.05) were not
significantly different between the IPostC + NC group and the
IPostC group. However, compared with those in the IPostC group, the
myocardial apoptotic index (37.45±1.73% vs. 29.46±2.03%; P<0.05)
and myocardial infarct size (60.18±2.81% vs. 48.53±2.49%;
P<0.05) were significantly increased in the IPostC + inhibitors
group, but significantly decreased in the IPostC + mimics group
(cardiomyocyte apoptotic index, 18.13±1.83% vs. 29.46±2.03%;
P<0.05; myocardial infarct size, 26.73±2.45% vs. 48.53±2.49%;
P<0.05). Collectively, the results suggested that the
upregulation of miR-499 could effectively alleviate myocardial I/R
damage and may be involved in the ischemic myocardial protection
mechanism of IPostC.
Effect of miR-499 on the protein
expression levels of Bcl-2 and Bax
It was found that the protein expression levels of
Bcl-2 and Bax were not significantly different between the IPostC +
NC and IPostC groups (Fig. 3). In
comparison with the IPostC group, the IPostC + mimics group
exhibited an increased expression level of Bcl-2 (0.80±0.04 vs.
0.63±0.03; P<0.05) and a decreased expression level of Bax
(0.22±0.02 vs. 0.49±0.02; P<0.05) in the myocardium; however,
these effects were reversed by miR-499 inhibitors.
Discussion
The present results suggested that IPostC
significantly increased miR-499 mRNA expression level, but
decreased the I/R-induced infarct size, cardiomyocyte apoptosis and
the release of the inflammatory mediators, including TLR2, PKC,
IL-1β and IL-6. To the best of our knowledge, the present study was
the first to demonstrate that miR-499 exerted an ischemic
cardioprotective effect in IPostC, possibly by inhibiting TLR2 and
PKC signals, thereby decreasing cardiomyocyte necrosis and
apoptosis via the induction of the anti-apoptotic protein Bcl-2 and
suppression of the pro-apoptotic Bax. The present results
identified a potentially novel mechanism of IPostC-induced
protection.
The pattern recognition receptor TLR2 is an emerging
therapeutic target in I/R injury and cardioprotection. Sakata et
al (24) revealed that TLR2 is
released from mouse myocardium and mediates subsequent injury
during I/R. Furthermore, TLR2 upregulation is observed in the
monocytes of patients with acute myocardial infarction, which
contributes to the severity of myocardial damage (25). By contrast, the application of TLR2
monoclonal antibody has been shown to result in significant
decreases in myocardial infarction size and local and systemic
inflammatory cells (26). It has
also been shown that preconditioning attenuates myocardial injury
by inhibiting the activation of TLR2 (27–29).
However, whether IPostC has a similar effect on the inhibition of
TLR2 signaling remains unknown. In the present study, low local and
circulating levels of TLR2 were identified in the IPostC group and
significantly increased levels in the I/R group, indicating that
IPostC-induced cardioprotection may be closely associated with the
inhibition of TLR2.
The interaction between TLR2 and inflammatory
cytokines is an important component of myocardial I/R injury
(30). As a potent inflammatory
mediator, activated TLR2 promotes the accumulation of inflammatory
cells and the release of inflammatory cytokines, such as IL-1β and
IL-6, from necrotic cardiomyocytes, triggering a wide range of
inflammation (31). Moreover, the
concentrations of these cytokines during I/R partially reflect the
severity of myocardial damage (32,33).
Furthermore, treatment strategies that inhibit these cytokines have
been shown to be effective for reducing infarct size and
cardiomyocyte apoptosis (34,35).
Consistent with previous studies, the present results indicated
that 30-min ischemia followed by 120-min reperfusion significantly
increased myocardial infarct size, cardiomyocyte apoptosis and the
release of IL-1β and IL-6, while IPostC prevented these effects.
Therefore, the results suggested that the beneficial effect of
IPostC may be due to the inhibition of the inflammatory cytokines
IL-1β and IL-6.
The PKC family is a class of signaling proteins that
are often associated with the regulation of I/R (36). Moreover, PKC may mediate
I/R-induced cardiomyocyte apoptosis via interactions with the
mitochondria (37). PKC is also an
important component of the TLR2 signal transduction pathway
(38). Park et al (39) showed that TLR2 can mediate
inflammatory responses by adjusting downstream PKC signaling.
However, while a previous study (40) has shown that PKC signaling is
linked to TLR2, to the best of our knowledge, no previous study has
investigated their association in IPostC. Therefore, the aim of the
present study was to determine whether TLR2 and PKC signaling
pathways were involved in I/R-induced injury and IPostC-mediated
cardioprotection. The present results identified high expression
levels of TLR2 and PKC in serum and heart in the I/R group, but
significantly decreased expression levels in the IPostC group,
suggesting that inhibition of PKC signaling in response to TLR2
inhibition may be associated with the protective effects of IPostC.
Collectively, the present study identified a potentially novel
protective mechanism of IPostC.
Previous studies have shown that miR-499 prevents
myocardial I/R injury. miR-499 is a small non-coding RNA that
regulates the expression levels of numerous target genes in heart
disease, particularly in acute myocardial infarction (41,42).
Qin et al (43) revealed
that the expression level of miR-499 in the left ventricular
myocardium is negatively correlated with troponin T and creatine
kinase-muscle/brain expression levels in a canine model of I/R. It
has also been shown that miR-499 is upregulated in IPostC, and
exerts anti-apoptotic and anti-inflammatory effects on the ischemic
myocardium (21). However, whether
the mechanisms underlying the cardioprotective effects of IPostC
involve miR-499 is not fully understood. The present study examined
whether the TLR2 and PKC signaling pathways were involved in these
cardioprotective effects by increasing and decreasing the
expression of miR-499 using miR-499 mimics and inhibitors. It was
demonstrated that miR-499, which was highly expressed in the IPostC
group, inhibits TLR2 and PKC signaling and the subsequent
inflammatory cascade in I/R. In addition, it was identified that
miR-499 inhibitors significantly increased the expression levels of
TLR2 and PKC and the proinflammatory cytokines, IL-1β and IL-6. By
contrast, the overexpression of miR-499 significantly reversed
these trends. To the best of our knowledge, the present study was
the first to identify the interaction between miR-499 and TLR2 in
IPostC, which suggested a possible mechanism of the
anti-inflammatory effect of IPostC. However, the underlying
mechanism via which miR-499 inhibits TLR2 expression remains
unknown.
In conclusion, the present results suggested that
miR-499 may be involved in IPostC cardioprotection, possibly by
inhibiting local and systemic TLR2 activation, inflammation and PKC
signaling. Therefore, the present study provided mechanistic
evidence to support the development of potential therapeutic
targets for providing IPostC-mediated cardioprotection against I/R
injury.
Acknowledgements
The authors would like to thank Professor Zhi-Yu
Zeng and Dr Hong Wen (both Department of Cardiology, First
Affiliated Hospital, Guangxi Medical University, Nanning, China)
for their collaboration.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant. no. 81560068)
and the Natural Science Foundation of Guangxi Province (grant. no.
2015GXNSFAA139198).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RT contributed to study conception and design,
manuscript revision and obtained funding. XZ contributed to study
design, data collection and analysis, writing of the manuscript,
and general feedback on the article. ZH contributed to data
interpretation and manuscript revision. QL contributed to data
acquisition and analysis. GZ contributed to data analysis and
interpretation. JM contributed to data analysis and writing of the
manuscript. DW contributed to data analysis and the writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were performed in strict
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals and were approved by the Animal
Protection and Use Committee of Guangxi Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ, Botker HE, Engstrom T,
Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM and
Garcia-Dorado D: Targeting reperfusion injury in patients with
ST-segment elevation myocardial infarction: Trials and
tribulations. Eur Heart J. 38:935–941. 2017.PubMed/NCBI
|
|
3
|
Yang Y, Yang J, Liu XW, Ding JW, Li S, Guo
X, Yang CJ, Fan ZX, Wang HB, Li Q, et al: Down-Regulation of
miR-327 alleviates ischemia/reperfusion-induced myocardial damage
by targeting RP105. Cell Physiol Biochem. 49:1049–1063. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pope MR and Fleming SD: TLR2 modulates
antibodies required for intestinal ischemia/reperfusion-induced
damage and inflammation. J Immunol. 194:1190–1198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arslan F, Keogh B, Mcguirk P and Parker
AE: TLR2 and TLR4 in ischemia reperfusion injury. Mediators
Inflamm. 2010:7042022010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen E, Bakr MM, Firth N and Love RM:
Inflammatory cell expression of Toll-like receptor-2 (TLR2) within
refractory periapical granuloma. F1000Res. 7:18192018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ward JR, Wilson HL, Francis SE, Crossman
DC and Sabroe I: Translational mini-review series on immunology of
vascular disease: Inflammation, infections and Toll-like receptors
in cardiovascular disease. Clin Exp Immunol. 156:386–394. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha T, Hu Y, Liu L, Lu C, McMullen JR,
Kelley J, Kao RL, Williams DL, Gao X and Li C: TLR2 ligands induce
cardioprotection against ischaemia/reperfusion injury through a
PI3K/Akt-dependent mechanism. Cardiovasc Res. 87:694–703. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arslan F, Houtgraaf JH, Keogh B, Kazemi K,
de Jong R, McCormack WJ, O'Neill LA, McGuirk P, Timmers L, Smeets
MB, et al: Treatment with OPN-305, a humanized anti-toll-like
receptor-2 antibody, reduces myocardial ischemia/reperfusion injury
in pigs. Circ Cardiovasc Interv. 5:279–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skyschally A, van Caster P, Iliodromitis
EK, Schulz R, Kremastinos DT and Heusch G: Ischemic
postconditioning: Experimental models and protocol algorithms.
Basic Res Cardiol. 104:469–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staat P, Rioufol G, Piot C, Cottin Y, Cung
TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, André-Fouët X
and Ovize M: Postconditioning the human heart. Circulation.
112:2143–2148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang DW, Zhang L, Liu JG, Wang CL, Shi DZ
and Chen KJ: Mechanisms of Chinese herbs combined with ischemic
postconditioning in protecting myocardium of rats from
ischemia-reperfusion injury. Zhong Xi Yi Jie He Xue Bao. 8:465–471.
2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Ge P, Yang L, Wu C, Zha H, Luo T
and Zhu Y: Protection of ischemic post conditioning against
transient focal ischemia-induced brain damage is associated with
inhibition of neuroinflammation via modulation of TLR2 and TLR4
pathways. J Neuroinflammation. 11:152014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong GQ, Tu RH, Zeng ZY, Li QJ, He Y, Li
S, He Y and Xiao F: Novel functional role of heat shock protein 90
in protein kinase C-mediated ischemic postconditioning. J Surg Res.
189:198–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheedy FJ and O'Neill LA: Adding fuel to
fire: MicroRNAs as a new class of mediators of inflammation. Ann
Rheum Dis. 67 (Suppl 3):iii50–iii55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CJ and Lu LF: MicroRNA in immune
regulation. Curr Top Microbiol Immunol. 410:249–267.
2017.PubMed/NCBI
|
|
17
|
Wojciechowska A, Braniewska A and
Kozar-Kamińska K: MicroRNA in cardiovascular biology and disease.
Adv Clin Exp Med. 26:865–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hajibabaie F, Kouhpayeh S, Mirian M,
Rahimmanesh I, Boshtam M, Sadeghian L, Gheibi A, Khanahmad H and
Shariati L: MicroRNAs as the actors in the atherosclerosis
scenario. J Physiol Biochem. 76:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Lu J, Bao X, Wang X, Wu J, Li X and
Hong W: MiR-499-5p protects cardiomyocytes against ischaemic injury
via anti-apoptosis by targeting PDCD4. Oncotarget. 7:35607–35617.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia Z, Wang J, Shi Q, Liu S, Wang W, Tian
Y, Lu Q, Chen P, Ma K and Zhou C: SOX6 and PDCD4 enhance
cardiomyocyte apoptosis through LPS-induced miR-499 inhibition.
Apoptosis. 21:174–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu J, Yao K, Wang Q, Guo J, Shi H, Ma L,
Liu H, Gao W, Zou Y and Ge J: Ischemic postconditioning-regulated
miR-499 protects the rat heart against ischemia/reperfusion injury
by inhibiting apoptosis through PDCD4. Cell Physiol Biochem.
39:2364–2380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheibani M, Faghir-Ghanesefat H, Dehpour
S, Keshavarz-Bahaghighat H, Sepand MR, Ghahremani MH, Azizi Y,
Rahimi N and Dehpour AR: Sumatriptan protects against myocardial
ischaemia-reperfusion injury by inhibition of inflammation in rat
model. Inflammopharmacology. 27:1071–1080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakata Y, Dong JW, Vallejo JG, Huang CH,
Baker JS, Tracey KJ, Tacheuchi O, Akira S and Mann DL: Toll-like
receptor 2 modulates left ventricular function following
ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol.
292:H503–H509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selejan S, Poss J, Walter F, Hohl M,
Kaiser R, Kazakov A, Böhm M and Link A: Ischaemia-induced
up-regulation of Toll-like receptor 2 in circulating monocytes in
cardiogenic shock. Eur Heart J. 33:1085–1094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arslan F, Smeets MB, O'Neill LA, Keogh B,
McGuirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA,
Pasterkamp G and de Kleijn DP: Myocardial ischemia/reperfusion
injury is mediated by leukocytic toll-like receptor-2 and reduced
by systemic administration of a novel anti-toll-like receptor-2
antibody. Circulation. 121:80–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mersmann J, Berkels R, Zacharowski P, Tran
N, Koch A, Iekushi K, Dimmeler S, Granja TF, Boehm O, Claycomb WC
and Zacharowski K: Preconditioning by toll-like receptor 2 agonist
Pam3CSK4 reduces CXCL1-dependent leukocyte recruitment in murine
myocardial ischemia/reperfusion injury. Crit Care Med. 38:903–909.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chatterjee PK: Cardiac preconditioning by
specific ligands of Toll-like receptors: Is it wither or whither?
Crit Care Med. 38:1003–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong JW, Vallejo JG, Tzeng HP, Thomas JA
and Mann DL: Innate immunity mediates myocardial preconditioning
through Toll-like receptor 2 and TIRAP-dependent signaling
pathways. Am J Physiol Heart Circ Physiol. 298:H1079–H1087. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vilahur G and Badimon L:
Ischemia/reperfusion activates myocardial innate immune response:
The key role of the toll-like receptor. Front Physiol. 5:4962014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin C, Cleveland JC, Ao L, Li J, Zeng Q,
Fullerton DA and Meng X: Human myocardium releases heat shock
protein 27 (HSP27) after global ischemia: The proinflammatory
effect of extracellular HSP27 through toll-like receptor (TLR)-2
and TLR4. Mol Med. 20:280–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jong WM, Ten Cate H, Linnenbank AC, de
Boer OJ, Reitsma PH, de Winter RJ and Zuurbier CJ: Reduced acute
myocardial ischemia-reperfusion injury in IL-6-deficient mice
employing a closed-chest model. Inflamm Res. 65:489–499. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Jesus NM, Wang L, Lai J, Rigor RR,
Francis Stuart SD, Bers DM, Lindsey ML and Ripplinger CM:
Antiarrhythmic effects of interleukin 1 inhibition after myocardial
infarction. Heart Rhythm. 14:727–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kharbanda RK, Mortensen UM, White PA,
Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K,
Redington AN and MacAllister R: Transient limb ischemia induces
remote ischemic preconditioning in vivo. Circulation.
106:2881–2883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Przyklenk K, Bauer B, Ovize M, Kloner RA
and Whittaker P: Regional ischemic ‘preconditioning’ protects
remote virgin myocardium from subsequent sustained coronary
occlusion. Circulation. 87:893–899. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Zhang F, Zhao G, Cheng Y, Wu T, Wu
B and Zhang YE: Mitochondrial PKC-εdeficiency promotes I/R-mediated
myocardial injury via GSK3β-dependent mitochondrial permeability
transition pore opening. J Cell Mol Med. 21:2009–2021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng H, Liu J, Liu C, Lu F, Zhao Y, Jin
Z, Ren H, Leng X, Jia J, Hu G, et al: Calcium-sensing receptor
activating phosphorylation of PKCδ translocation on mitochondria to
induce cardiomyocyte apoptosis during ischemia/reperfusion. Mol
Cell Biochem. 358:335–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cario E, Gerken G and Podolsky DK:
Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial
barrier integrity via protein kinase C. Gastroenterology.
127:224–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park DW, Lee HK, Lyu JH, Chin H, Kang SW,
Kim YJ, Bae YS and Baek SH: TLR2 stimulates ABCA1 expression via
PKC-η and PLD2 pathway. Biochem Biophys Res Commun. 430:933–937.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang SY, Wei CC, Shang TT, Lian Q, Wu CX
and Deng JY: High glucose induces inflammatory cytokine through
protein kinase C-induced toll-like receptor 2 pathway in gingival
fibroblasts. Biochem Biophys Res Commun. 427:666–670. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xin Y, Yang C and Han Z: Circulating
miR-499 as a potential biomarker for acute myocardial infarction.
Ann Transl Med. 4:1352016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Fan Z, Zhao T, Cao W, Zhang L, Li
H, Xie Q, Tian Y and Wang B: Plasma miR-1, miR-208, miR-499 as
potential predictive biomarkers for acute myocardial infarction: An
independent study of Han population. Exp Gerontol. 72:230–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin H, Chen GX, Liang MY, Rong J, Yao JP,
Liu H and Wu ZK: The altered expression profile of microRNAs in
cardiopulmonary bypass canine models and the effects of mir-499 on
myocardial ischemic reperfusion injury. J Transl Med. 11:1542013.
View Article : Google Scholar : PubMed/NCBI
|