Introduction
Nasopharyngeal carcinoma (NPC) is a type of head and
neck cancer endemic in Southeast Asia, and is closely related to
Epstein-Barr virus (EBV) infection (1). Despite the improvement in local tumor
control achieved by more precise imaging modalities and
radiotherapy, the 5-year survival rate of patients with NPC remain
unsatisfactory, primarily due to distant metastasis (2). Therefore, there is a requirement for
the elucidation of the molecular mechanisms underlying the
pathogenesis of NPC as well as the development of novel therapeutic
strategies.
MicroRNAs (miRNA/miR) are a class of small,
non-protein-coding RNAs that function in RNA silencing and
post-transcriptional regulation of gene expression (3). miRNAs are also known to play key
roles in cancer, where they serve as oncomirs or tumor suppressors
(4). miRNAs have been found to
regulate genes involved in multiple cellular processes, including
development, differentiation, proliferation and apoptosis (5). The modulation of miRNAs, based on two
major approaches (miRNA mimics and miRNA antagonists/inhibitors),
is currently being investigated for the clinical development of
therapeutic miRNAs (6,7).
miR-19b, also termed miR-19b-1 or miR-19b-1-5p, is
upregulated in several types of cancer and has been reported to
serve as an oncomir (8). miR-19b
serves as a prognostic biomarker for breast cancer and promotes
tumor progression through the PI3K/AKT signaling pathway (9). A previous study reported that miR-19b
decreases apoptosis, promotes proliferation and induces
tumorigenicity in multiple myeloma cells by targeting phosphatase
and tensin homolog (10). The
miR-17-92 cluster, which includes miR-17-5p, miR-17-3p, miR-18a,
miR-19a, miR-20a, miR-19b-1 and miR-92-1, was reported to be
upregulated in NPC (11). However,
the role of miR-19b in NPC has not been fully elucidated.
Therefore, the present study investigated the function of miR-19b,
as well as the therapeutic effect of miR-19b inhibitors, in NPC
cells.
Materials and methods
Cell lines and culture
EBV-positive cells C666-1 and HK1-EBV were kindly
provided by Professor Sai Wah Tsao (The University of Hong Kong,
Hong Kong, China) (12). 5-8F,
SUNE1 and SXSW-1489 were kindly provided by Professor Xiao Dong
(Southern Medical University, Guangzhou, China) (13) and Professor Weiyi Fang (Southern
Medical University) (14). NPC
cell lines (C666-1, HK1-EBV, 5-8F, SUNE1) and the immortalized
nasopharyngeal epithelial cell line (SXSW-1489) were cultured in
Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
C666-1 cells were cultured with the addition of 10 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The cells
lines were maintained in a humidified atmosphere at 37°C and 5%
CO2.
Reverse transcription-quantitative PCR
analysis for miR-19b expression
Total RNA from cultured (C666-1, HK1-EBV, 5-8F,
SUNE1, SXSW-1489) cells and lenses was extracted using
TRIzol® reagent (cat. no. 15596026, Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Genomic
DNA was subsequently removed using DNase I. cDNA was synthesized
using the Mir-X miRNA First-Strand Synthesis kit (Clontech
Laboratories, Inc.) and the following primers: U6,
AACGCTTCACGAATTTGCGT; and miR-19b,
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGT. The expression
levels of miR-19b and the internal control U6 were quantified by
qPCR using a SYBR Premix Ex Taq II kit (Takara Bio, Inc.) and an
ABI Prism 7,000 sequence detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following primer pairs were
used: U6 forward, CTCGCTTCGGCAGCACA and reverse,
AACGCTTCACGAATTTGCGT; and mir-19b forward, TGTGCAAATCCATGCAAA and
reverse, GTGCAGGGTCCGAGGTATTC. miRNA levels were quantified using
the 2−ΔΔCq method and normalized to the internal control
U6.
Transient transfection of miRNA-19b
inhibitors
The miRCURY LNA™ miRNA Inhibitor [consisting of an
miR-19b inhibitor and a negative control (NC)] was obtained from
Qiagen, Inc. The sequences of the miRNA are proprietary
information. The miRNAs were transiently transfected into C666-1
cells at a working concentration of 15 µM using X-tremeGENE siRNA
Transfection Reagent (Roche Diagnostics) following the
manufacturer's protocol.
Cell Counting Kit-8 (CCK-8)
proliferation assay
A total of 2×103 C666-1 cells per well
were seeded in a 96-well plate in a final volume of 100 µl and
transfected with miRNAs. The effect of the miR-19b inhibitor on
cell proliferation was subsequently determined using the CCK-8
assay at 6, 12, 24 and 48 h post-transfection. A total of 10 µl
CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to
each well and incubated for 4 h at 37°C. The optical densities of
the resultant purple solutions were measured at a wavelength of 450
nm (15).
Transwell migration assay
Transwell chambers (24-well insert; Corning, Inc.)
were used to analyze cell migration. At 48 h post-transfection, a
total of 2×104 C666-1 cells in serum-free RPMI-1640
medium were seeded into the upper chamber of the insert. RPMI-1640
medium containing 10% FBS was added to the lower chamber to serve
as a chemo-attractant. Cells were allowed to migrate for 24 h. The
cells on the upper membrane surface were removed using a cotton bud
and the cells on the lower membrane surface were fixed with 4%
formaldehyde. The cells were subsequently stained with 0.1% crystal
violet (Amresco, LLC) and the migrated cells were counted in three
random-selected fields. The result of migrated cells was observed
and photographed under light microscope (Olympus, Japan).
Flow cytometry assay
C666-1 cells were harvested 48 h post-transfection
by trypsin digestion without EDTA and stained using the APC-Annexin
V/7-AAD Dual Staining Cell Apoptosis Detection kit (BD Biosciences)
according to the manufacturer's instructions. The cells were
subsequently analyzed with a flow cytometer. The cells in Q2
(late-stage apoptosis) and Q4 (early-stage apoptosis) were
considered to be apoptotic cells.
Western blot analysis
At 48 h post-transfection, cell lysates were
harvested. The lysis buffer used RIPA buffer and PMSF (cat. no.
R0020, 1:100, Beijing Solarbio Science & Technology, Inc.).
Proteins were separated by 10% SDS-PAGE separating gel and 5%
SDS-PAGE stacking gel, and then electrophoretically transferred
onto a polyvinylidene difluoride membrane. The membrane was
subsequently incubated with primary antibodies against β-actin
(cat. no. TA-09; 1:5,000; OriGene Technologies, Inc.), STAT3 (cat.
no. sc-8019; 1:2,000; Santa Cruz Biotechnology, Inc.), suppressor
of cytokine signaling (SOCS) 1 (cat. no. PA5-27239; 1:2,000; Thermo
Fisher Scientific, Inc.), p-STAT3 (Tyr705; cat. no. G.374.10;
1:2,000; Thermo Fisher Scientific, Inc.), p-STAT3 (Ser727; cat. no.
PS727.2; 1:2,000; Thermo Fisher Scientific, Inc.), cyclin D1 (cat.
no. OTI1F7; 1:1,000; OriGene Technologies, Inc.), Bcl-2 (cat. no.
OTI2E5; 1:1,000; ZSGB-BIO), myeloid leukemia protein 1 (Mcl-1; cat.
no. OTI3A12; 1:1,000; OriGene Technologies, Inc.) overnight at 4°C
in Primary Antibodies Dilution Buffer (cat. no. P0023A; Beyotime
Institute of Biotechnology). Following primary antibody incubation,
the membrane was incubated with peroxidase-conjugated goat
anti-mouse IgG (H+L; cat. no. ZB-2305; 1:5,000; ZSGB-BIO) and
peroxidase-conjugated goat anti-rabbit IgG (H+L; cat. no. ZB-2301;
1:5,000; OriGene Technologies, Inc.) secondary antibodies for 1 h
at room temperature. The protein bands were visualized using the
ECL Western Blot Kit detection system (Thermo Fisher Scientific,
Inc.). Protein expression was quantified with β-actin as the
loading control at least three times and analyzed by Image-J
(version1.50i, National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SEM of three
independent experiments. One-way ANOVA test was used to analyze the
groups. Multiple comparisons were made using the Tukey's post hoc
test. Statistical analysis was performed using SPSS software
(version 20; IBM Corp). P<0.05 were considered to indicate a
statistically significant difference.
Results
miR-19b is upregulated in the majority
of NPC cell lines
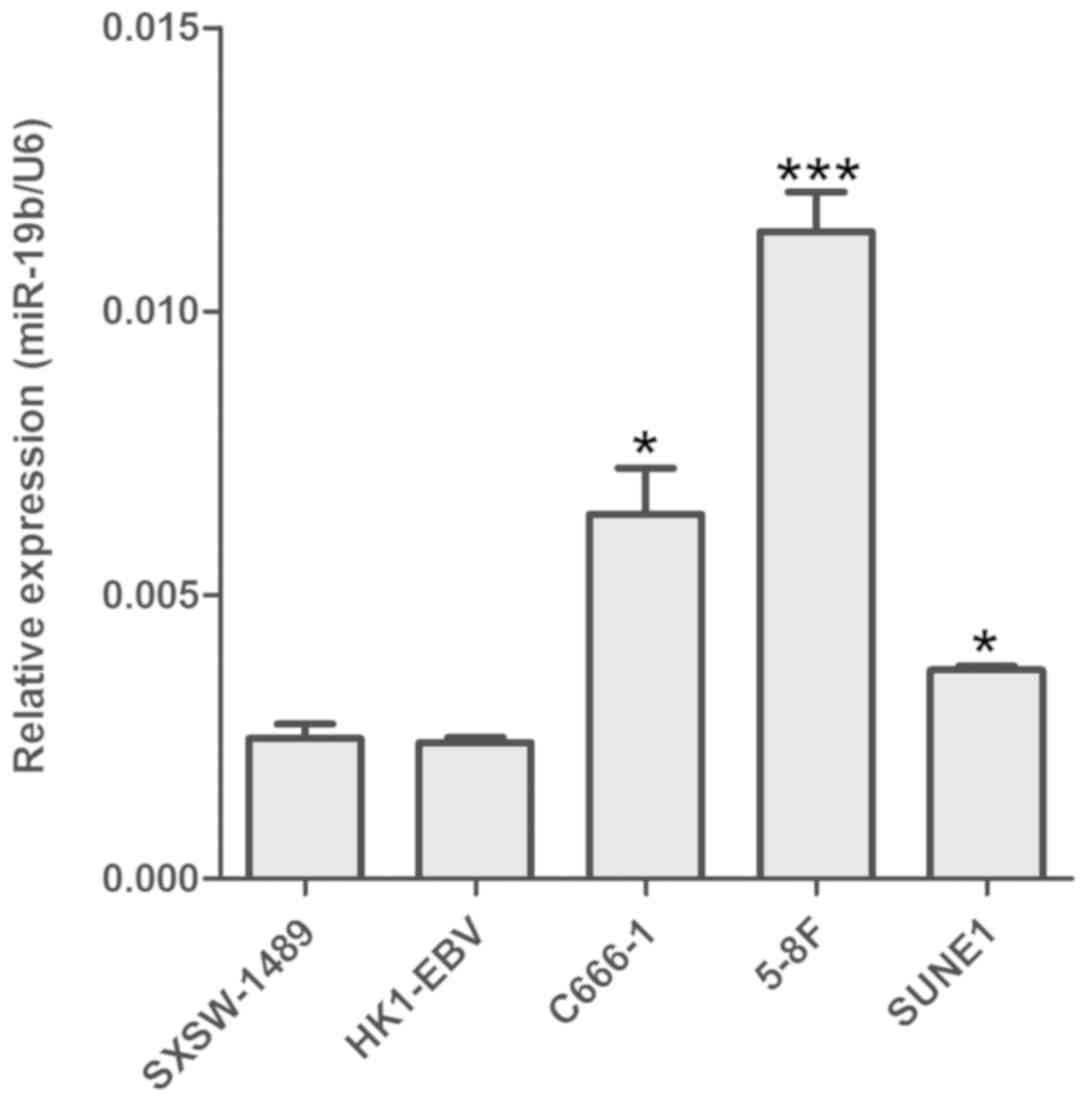
RT-qPCR revealed that miR-19b was upregulated NPC
cells (C666-1, 5-8F and SUNE1) compared with the nasopharyngeal
epithelial cell line SXSW-1489. However, no statistical difference
in miR-19b expression was observed between HK1-EBV and SXSW-1489
cells (Fig. 1). As C666-1 is the
only NPC cell line consistently harboring EBV during in
vitro propagation (16), this
cell line was selected for subsequent miR-19b interference.
miR-19b inhibitor inhibits the
proliferation of C666-1 cells
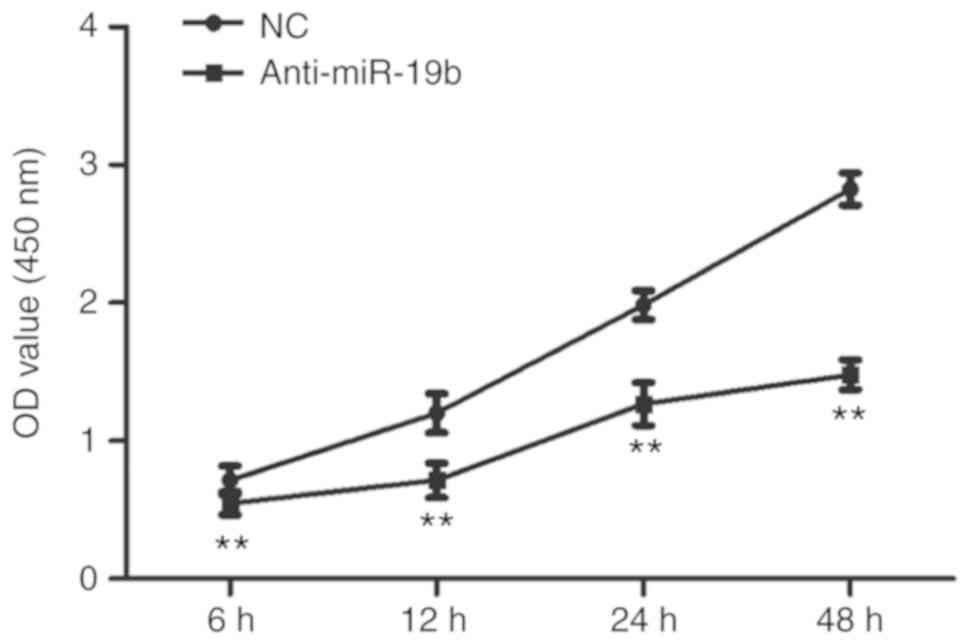
The miR-19b inhibitor or NC were transiently
transfected into C666-1 cells and the effect on proliferation was
subsequently investigated. As shown in Fig. 2, the miR-19b inhibitor inhibited
the proliferation of C666-1 cells compared with the NC.
miR-19b inhibitor promotes the
apoptosis of C666-1 cells
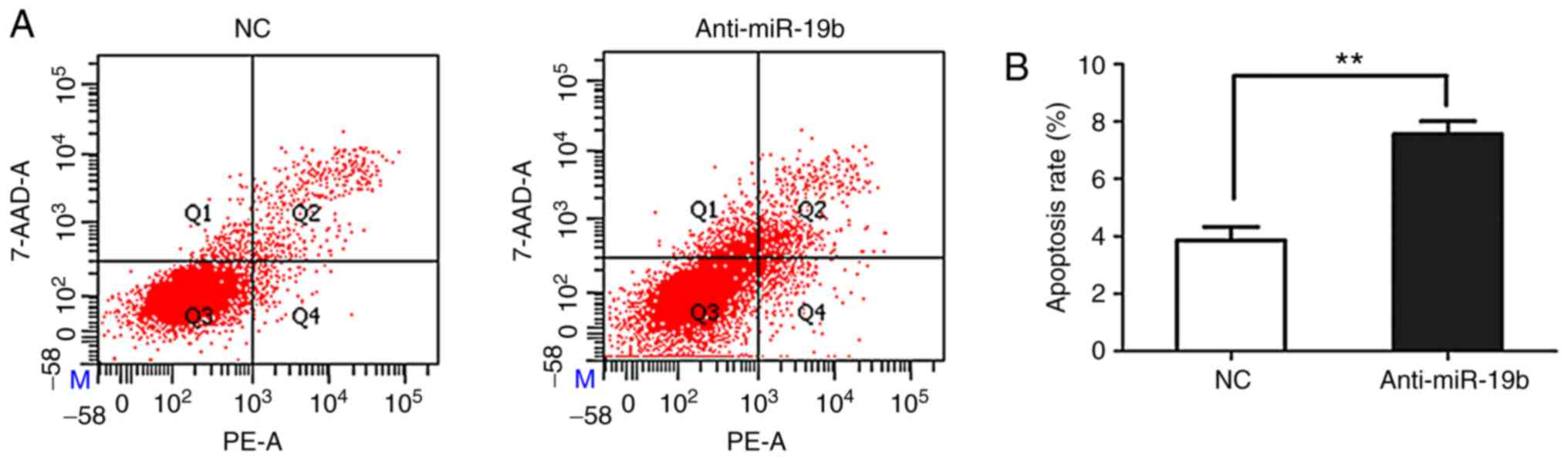
The miR-19b inhibitor or NC were transiently
transfected into C666-1 cells and the effect on apoptosis was
subsequently investigated. As shown in Fig. 3, flow cytometry revealed that the
miR-19b inhibitor promoted the apoptosis of C666-1 cells compared
with the NC.
miR-19b inhibitor inhibits the
migration of C666-1 cells
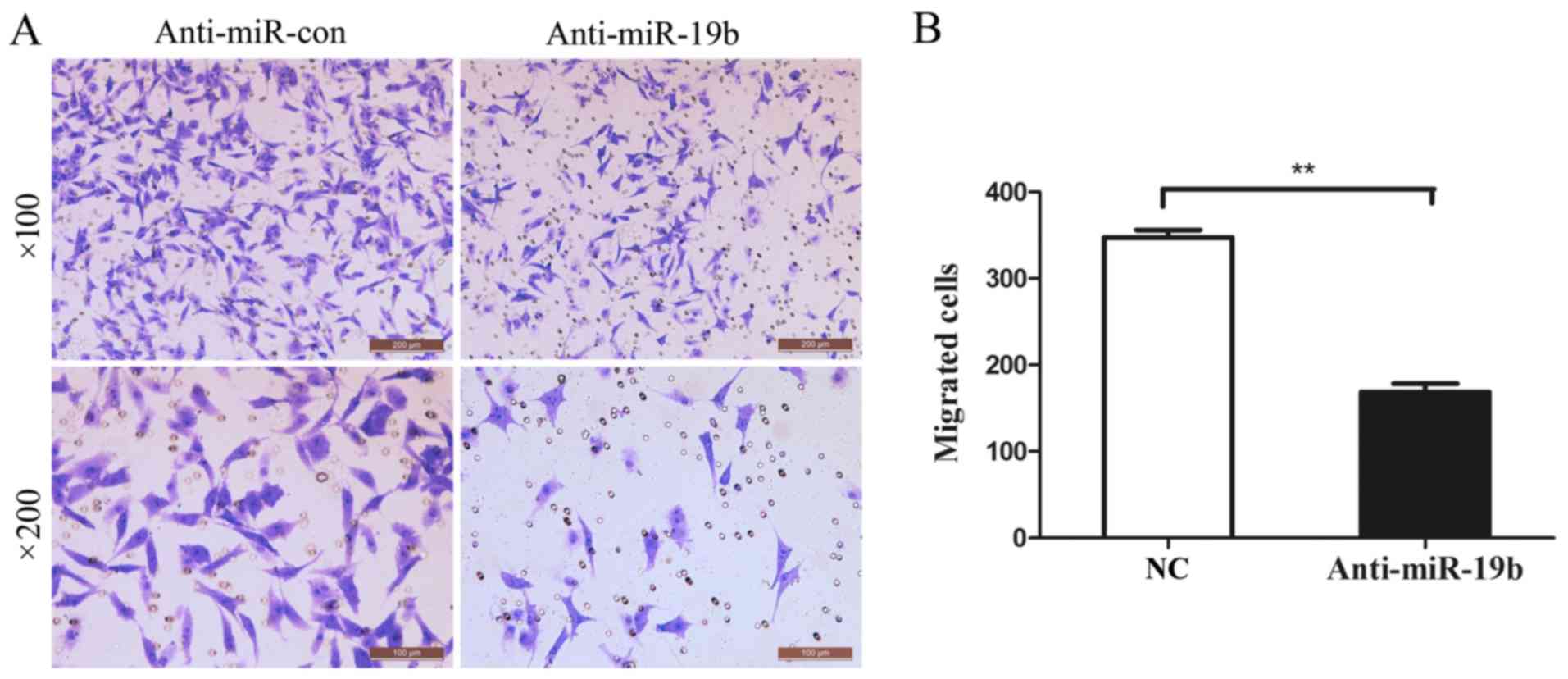
The effect on the migration of C666-1 cells was
investigated 48 h post-transfection using a Transwell assay. As
shown in Fig. 4, the migration of
C666-1 cells was significantly inhibited following transfection
with the miR-19b inhibitor, compared with the NC group.
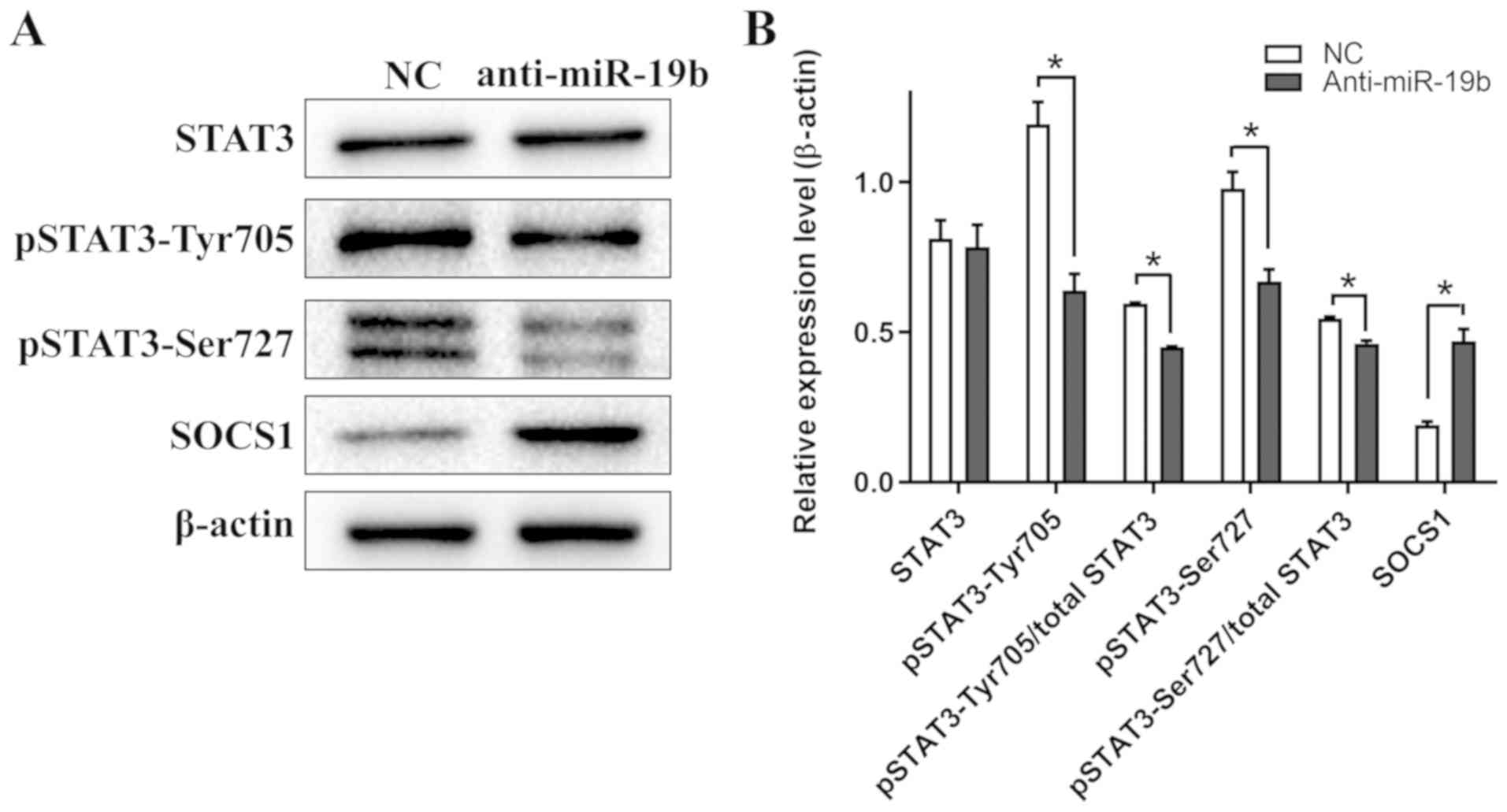
miR-19b inhibitor attenuates STAT3
signaling in C666-1 cells
Western blotting revealed that the expression levels
of pSTAT3-Tyr705 and pSTAT3-Ser727 in C666-1 cells decreased
following transfection with the miR-19b inhibitor compared with the
NC. Furthermore, the expression level of SOCS1, an endogenous
inhibitor of STAT3 phosphorylation (17), increased following transfection
with the miR-19b inhibitor compared with the NC (Fig. 5). Collectively, these results
suggested that the miR-19b inhibitor specifically targeted the
STAT3 signaling pathway.
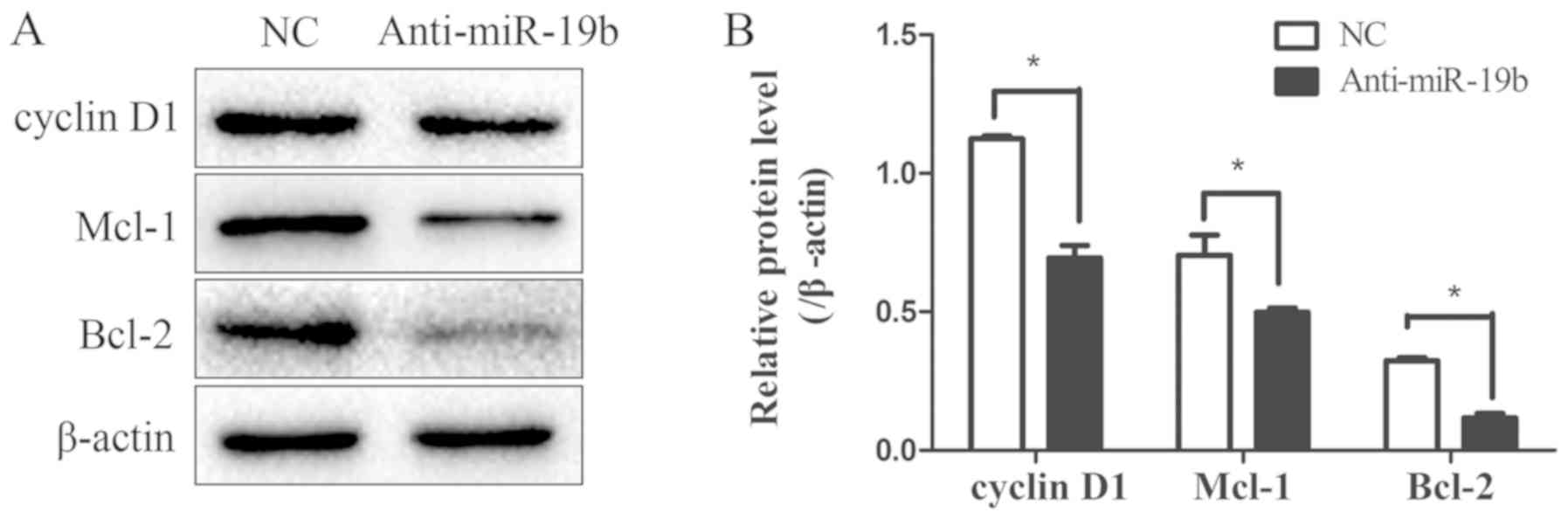
miR-19b inhibitor downregulates the
expression of the STAT3 signaling pathway downstream effectors
To explore the effect of the miR-19b inhibitor on
the expression of the downstream effector genes of the STAT3
signaling pathway, the expression levels of the
proliferation-associated gene cyclin D1 and the
apoptosis-associated genes Mcl-1 and Bcl-2 were detected by western
blotting. These three proteins were downregulated following
transfection with the miR-19b inhibitor compared with the NC
(Fig. 6), further suggesting that
the STAT3 signaling pathway was impaired. Furthermore, the change
in malignant biological behaviors such as proliferation, apoptosis
and migration may have been mediated by the downstream effectors of
the STAT3 signaling pathway.
Discussion
miR-19b, as a member of the miR-17-92 cluster, has
been revealed to serve as an oncomir in several types of tumors
(18,19). miR-19b promotes cell proliferation,
migration and angiogenesis and inhibits cell apoptosis in several
malignancies (20,21). The miR-17-92 cluster has been
reported to be upregulated in NPC tissues and cell lines (22). Furthermore, the miR-17-92 cluster
facilitates malignant biological processes and modulates
cancer-related pathways in NPC (11,21,22).
The results of the present study indicated that
miR-19b was upregulated in the majority of the NPC cell lines
investigated compared with the nasopharyngeal epithelial cell line
SXSW-1489. However, the role of miR-19b in NPC remains largely
unknown. Therefore, in order to elucidate the functions of miR-19b
in NPC cells, an miR-19b inhibitor was introduced into the NPC cell
line C666-1. This decreased the proliferation, increased apoptosis
and inhibited migration of C666-1 cells compared with the NC. These
data are consistent with studies in several other malignancies
(8,9,22).
STAT3 is a member of the STAT protein family. STAT3
is phosphorylated by receptor-associated Janus kinases (JAK) when
stimulated by cytokines and growth factors and forms homodimers or
heterodimers, which are translocated into the cell nucleus where
they act as transcriptional activators (23). STAT3 mediates the expression of a
variety of genes in response to extracellular or intracellular
stimuli (24), and thus plays a
key role in cell growth, apoptosis, invasion and metastasis, immune
escape and angiogenesis (25). The
STAT3 signaling pathway was revealed to be constitutively activated
in NPC (26). The involvement of
STAT3 in cancer cell growth and invasion has been previously
documented in NPC (27).
Furthermore, STAT3 has been identified as a therapeutic target in
NPC (27).
SOCS1 is a member of the STAT-induced STAT inhibitor
(SSI) family, also known as the SOCS family (28). SSI family members are
cytokine-inducible negative regulators of cytokine signaling
(29). SOCS1 takes part in a
negative feedback loop that involves the JAK/STAT3 signaling
pathway to attenuate cytokine signaling (30). Additionally, SOCS1 is reported to
be a target of miR-19b (31). The
aforementioned studies suggest that miR-19b positively modulates
the STAT3 signaling pathway by inhibiting SOCS1 expression. The
results obtained in the present study showed that SOCS1 expression
was upregulated following miR-19b inhibition in C666-1 cells. In
addition, upregulation of SOCS1 was accompanied by the
downregulation of pSTAT3, including both pSTAT3-Tyr705 and
pSTAT3-Ser727, in C666-1 cells. The data implied that miR-19b plays
a key role in STAT3 activation in NPC. STAT3 is activated through
phosphorylation of Tyr705 in response to a number of factors,
including interleukin-6 (32),
platelet derived growth factor (10) and epidermal growth factor (33). STAT3 Ser727 is phosphorylated by
various kinases (34).
Phosphorylation at Tyr-705 leads to an increase in the
transcriptional activity of STAT3. Serine phosphorylation is
important for the formation of stable DNA-binding STAT3 homodimers
and maximal transcriptional activity (35).
Phosphorylated STAT3 increases the expression of
multiple downstream genes, which include cyclin D1, Bcl-2 and Mcl-1
(36). Cyclin D1 is proto-oncogene
since it serves as a cell cycle regulator and is involved in the
G1/S transition (37). Bcl-2
encodes an integral outer mitochondrial membrane protein that
prevents apoptosis (38). Mcl-1 is
a member of the Bcl-2 family, and is involved in the regulation of
apoptosis and cell survival (39).
In the present study, the expression of cyclin D1, Bcl-2 and Mcl-1
was downregulated in C666-1 cells following transfection with the
miR-19b inhibitor. However, the downstream target genes of the
STAT3 signaling pathway requires further investigation.
In conclusion, the present study revealed that
inhibition of miR-19b attenuates the STAT3 signaling pathway and
decreases the malignant biological behavior of the NPC cell line
C666-1. Therefore, miR-19b may serve as potential therapeutic
target for patients with NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81560441
and 81760491 to S.J. Xiao), the Natural Science Foundation of
Guangxi Province of China (grant no. 2015GXNSFAA139131 to S.J.
Xiao), and Innovation Project of Guangxi Graduate Education (grant
no. YCSW2017211 to L.H. Bian).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX and XZ contributed to the conception and design
of the study. LB, XZ and SX drafted this manuscript. LB, JD, CZ,
GS, XW, YY and XZ performed the experiments and analyzed the data.
SX and XZ were involved in revising the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prawira A, Oosting SF, Chen TW, Delos
Santos KA, Saluja R, Wang L, Siu LL, Chan KKW and Hansen AR:
Systemic therapies for recurrent or metastatic nasopharyngeal
carcinoma: A systematic review. Br J Cancer. 117:1743–1752. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen HC, Chen GH, Chen YH, Liao WL, Liu
CY, Chang KP, Chang YS and Chen SJ: MicroRNA deregulation and
pathway alterations in nasopharyngeal carcinoma. Br J Cancer.
100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Gao DY and Huang L: In vivo
delivery of miRNAs for cancer therapy: Challenges and strategies.
Adv Drug Deliv Rev. 81:128–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braicu C, Calin GA and Berindan-Neagoe I:
MicroRNAs and cancer therapy-from bystanders to major players. Curr
Med Chem. 20:3561–3573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Yang S, Yan W, Yang J, Qin YJ, Lin
XL, Xie RY, Wang SC, Jin W, Gao F, et al: MicroRNA-19 triggers
epithelial-mesenchymal transition of lung cancer cells accompanied
by growth inhibition. Lab Invest. 95:1056–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Zhang J, Ma Z, Zhang F and Yu W:
miR-19b serves as a prognostic biomarker of breast cancer and
promotes tumor progression through PI3K/AKT signaling pathway. Onco
Targets Ther. 11:4087–4095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vij N, Sharma A, Thakkar M, Sinha S and
Mohan RR: PDGF-driven proliferation, migration, and IL8 chemokine
secretion in human corneal fibroblasts involve JAK2-STAT3 signaling
pathway. Mol Vis. 14:1020–1027. 2008.PubMed/NCBI
|
|
11
|
Ma F, Wang Z, Wang J, Liu X and Hu C:
MicroRNA-19a promotes nasopharyngeal carcinoma by targeting
transforming growth factor β receptor 2. Exp Ther Med.
14:1419–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CY, Yi YH, Chang KP, Chang YS, Chen SJ
and Chen HC: The epstein-barr virus-encoded MicroRNA MiR-BART9
Promotes Tumor Metastasis by Targeting E-Cadherin in nasopharyngeal
carcinoma. PLoS Pathog. 10:e10039742014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin TY, Chen Y, Jia JS, Zhou C, Lian M,
Wen YT, Li XY, Chen HW, Lin XL, Zhang XL, et al: Loss of Cirbp
expression is correlated with the malignant progression and poor
prognosis in nasopharyngeal carcinoma. Cancer Manag Res.
11:6959–6969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q,
Luo X, Chen Y, Deng X, Liang Z, et al: MiR-3188 regulates
nasopharyngeal carcinoma proliferation and chemosensitivity through
a FOXO1-modulated positive feedback loop with
mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 7:11309–11322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Zhao Z, Zhang X, Yang S, Lin X, Yang
X, Lin X, Shi J, Wang S, Zhao W, et al: Klf4 reduces stemness
phenotype, triggers mesenchymal-epithelial transition (MET)-like
molecular changes, and prevents tumor progression in nasopharygeal
carcinoma. Oncotarget. 8:93924–93941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao K, Yu Z, Li X, Li X, Tang K, Tu C, Qi
P, Liao Q, Chen P, Zeng Z, et al: Genome-wide analysis of
epstein-barr virus (EBV) integration and strain in C666-1 and raji
cells. J Cancer. 7:214–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davey GM, Heath WR and Starr R: SOCS1: A
potent and multifaceted regulator of cytokines and cell-mediated
inflammation. Tissue Antigens. 67:1–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Yang R, Urrehman U, Ye C, Yan X,
Cui S, Hong Y, Gu Y, Liu Y, Zhao C, et al: MiR-19b suppresses PTPRG
to promote breast tumorigenesis. Oncotarget. 7:64100–64108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Xiong M, Hu Y, Sun Y and Ma Q:
MicroRNA-19b inhibits proliferation of gastric cancer cells by
targeting B-cell CLL/lymphoma 3. Oncol Rep. 36:2079–2086. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Wang FS, Wu ZY, Lin JL, Lan WB and
Lin JH: MicroRNA-19b targets Mfn1 to inhibit Mfn1-induced apoptosis
in osteosarcoma cells. Neoplasma. 61:265–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan Y, Yin S, Hao Y, Yang J, Zhang H, Sun
C, Ma M, Chang Q and Xi JJ: miR-19b promotes tumor growth and
metastasis via targeting TP53. RNA. 20:765–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo Z, Dai Y, Zhang L, Jiang C, Li Z, Yang
J, McCarthy JB, She X, Zhang W, Ma J, et al: MiR-18a promotes
malignant progression by impairing microRNA biogenesis in
nasopharyngeal carcinoma. Carcinogenesis. 34:415–425. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng GZ, Zhang W, Sun M, Wang Q, Coppola
D, Mansour M, Xu LM, Costanzo C, Cheng JQ and Wang LH: Twist is
transcriptionally induced by activation of STAT3 and mediates STAT3
oncogenic function. J Biol Chem. 283:14665–14673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferguson SD, Srinivasan VM and Heimberger
AB: The role of STAT3 in tumor-mediated immune suppression. J
Neurooncol. 123:385–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAKSTAT. 3:e280862014.PubMed/NCBI
|
|
26
|
Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To
KF, Hayward DS, Chui YL, Lau YL, Takada K and Huang DP:
Epstein-barr virus infection alters cellular signal cascades in
human nasopharyngeal epithelial cells. Neoplasia. 8:173–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho Y, Tsao SW, Zeng M and Lui VW: STAT3 as
a therapeutic target for Epstein-Barr virus (EBV): Associated
nasopharyngeal carcinoma. Cancer Lett. 330:141–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naka T, Narazaki M, Hirata M, Matsumoto T,
Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et
al: Structure and functionof a new STAT-induced STAT inhibitor.
Nature. 387:924–929. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamanaka I, Saito Y, Yasukawa H, Kishimoto
I, Kuwahara K, Miyamoto Y, Harada M, Ogawa E, Kajiyama N, Takahashi
N, et al: Induction of JAB/SOCS-1/SSI-1 and CIS3/SOCS-3/SSI-3 is
involved in gp130 resistance in cardiovascular system in rat
treated with cardiotrophin-1 in vivo. Circ Res. 88:727–732. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben-Zvi T, Yayon A, Gertler A and
Monsonego-Ornan E: Suppressors of cytokine signaling (SOCS) 1 and
SOCS3 interact with and modulate fibroblast growth factor receptor
signaling. J Cell Sci. 2:380–387. 2006. View Article : Google Scholar
|
|
31
|
Mignacca L, Saint-Germain E, Benoit A,
Bourdeau V, Moro A and Ferbeyre G: Sponges against miR-19 and
miR-155 reactivate the p53-Socs1 axis in hematopoietic cancers.
Cytokine. 82:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang TQ, Willis MS and Meissner G:
IL-6/STAT3 signaling in mice with dysfunctional type-2 ryanodine
receptor. JAKSTAT. 4:e11583792016.PubMed/NCBI
|
|
33
|
Wang Y, van Boxel-Dezaire AH, Cheon H,
Yang J and Stark GR: STAT3 activation in response to IL-6 is
prolonged by the binding of IL-6 receptor to EGF receptor. Proc
Natl Acad Sci USA. 110:16975–16980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Parys JB: The multifaceted STAT3: How a
transcription factor regulates Ca2+ signaling via a
degradative pathway. Cell Calcium. 76:137–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yokogami K, Wakisaka S, Avruch J and
Reeves SA: Serine phosphorylation and maximal activation of STAT3
during CNTF signaling is mediated by the rapamycin target mTOR.
Curr Biol. 10:47–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pardee AB: G1 events and regulation of
cell proliferation. Science. 246:603–608. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lam M, Dubyak G, Chen L, Nuñez G, Miesfeld
RL and Distelhorst CW: Evidence that BCL-2 represses apoptosis by
regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl
Acad Sci USA. 91:6569–6573. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Q and Gehring K: Heterodimerization of
BAK and MCL-1 Activated by Detergent Micelles. J Biol Chem.
285:41202–41210. 2010. View Article : Google Scholar : PubMed/NCBI
|