Introduction
Acute liver failure (ALF) is a rapidly developing
disease, with a rapid onset of symptoms, that is associated with
multi-organ failure (1). The main
causative factors of ALF include drug toxicity and hepatitis virus
infection (2). D-galactosamine
(D-GalN) and lipopolysaccharide (LPS) are common biochemical
reagents that may be used to establish fulminant hepatic failure
(FHF) injury models, which effectively simulate the FHF clinical
state (3). During ALF development,
one of the key pathological traits is the associated immoderate
immune cascade response, which leads to extensive liver cell
apoptosis and defective liver cell proliferation (4). However, little is known regarding the
mechanism of action of microRNAs (miRNAs) in the process of ALF
(5).
miRNAs are a class of endogenous non-coding RNAs,
measuring 19–22 nucleotides in length, that regulate the expression
of target genes by interacting with the 3′-untranslated (UTR)
regions of these genes at the post-transcriptional level (6). Furthermore, miRNAs have been
identified as potential biomarkers in the pathological processes of
several life-threatening diseases, including ALF (7). Abnormal regulation of miRNAs has also
been observed in various liver diseases, and miRNAs serve a key
role in regulating hepatocyte proliferation (8) and liver development (9,10).
For example, miR-122 is one of the most common miRNAs in the liver,
and is involved in appropriate proliferation and differentiation of
liver cells (11). In addition,
miR-122 has been implicated in viral hepatitis and liver disease
(12). Moreover, it has been
reported that miR-125b-5p could inhibit ALF (13). It has also been suggested that
miR-214 exerts anti-fibrotic effects in chemically induced liver
fibrosis and cirrhosis (14).
However, the role of miR-214 in human ALF remains unknown.
Therefore, the aims of the present study were to
investigate the role of miR-214 in ALF and to elucidate its
mechanism of action.
Materials and methods
Experimental animals and study
design
A total of 30 male BALB/c mice (age, 6–8 weeks;
weight, 20–22 g) were purchased from Shanghai SLAC Laboratory
Animal Co. Ltd., and were housed in a standard animal housing
facility (temperature, 22–24°C; humidity, 60–65%) with ad
libitum access to food and water under a 12-h light/12-h dark
cycle. The mice were randomly divided into two groups (control and
ALF model groups; n=15/group). To establish the mouse model of ALF,
the mice were administered D-GalN [800 mg/kg body weight
intraperitoneal (i.p.); Sigma-Aldrich; Merck KGaA] and LPS (10
µg/kg body weight, i.p.; Sigma-Aldrich; Merck KGaA) as described
previously (15). Mice in the
control group were treated with 500 µl saline by i.p. injection.
Mice were anesthetized with pentobarbital (50 mg/kg) by i.p.
injection and sacrificed by cervical dislocation to collect blood
samples (1 ml) at 0, 1, 3, 5, 7 and 9 h after D-GaIN/LPS treatment
for aspartate aminotransferase (AST) or alanine aminotransferase
(ALT) detection. Animal death was defined as the lack of heartbeat
or respiration. The blood (1 ml) of mice at 7 h after D-GaIN/LPS
treatment was collected for interleukin (IL)-6 and tumor necrosis
factor (TNF)-α detection.
All animal care and experimental protocols were
performed strictly according to the recommendations in the Guide
for the Care and Use of Laboratory Animals by the National
Institutes of Health and the Animal Ethics Committee of The First
Affiliated Hospital of Suzhou University. The present study was
approved by the Animal Ethics Committee of The First Affiliated
Hospital of Suzhou University. Moreover, there was no mouse
mortality during the aforementioned experimental procedures. The
experimental end-point was when mice lost >15% of their body
weight.
Cell culture and treatment
Normal murine embryonic liver cells (BNLCL2) were
provided by Wuhan Procell Life Technology Co., Ltd. (https://www.procell.com.cn/view/537.html) and cultured
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 4 mM glutamate and
1% penicillin/streptomycin (Gibco/Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified chamber with 5%
CO2.
BNLCL2 cells were treated with 1 mg/ml D-GalN
(Sigma-Aldrich; Merck KGaA) and 100 ng/ml TNF-α (Sigma-Aldrich;
Merck KGaA) at 37°C for 36 h to induce the hepatocyte injury model
in vitro.
For miR-214 mimic treatment, BNLCL2 cells were
transfected with 100 nM mimic control (sense,
5′-UUUGUACUACACAAAAGUACUG-3′ and anti-sense,
5′-CAGUACUUUUGUGUAGUACAAA-3′; Guangzhou RiboBio Co., Ltd.), 100 nM
miR-214 mimic (sense, 5′-ACAGCAGGCACAGACAGGCAGU-3′ and anti-sense,
5′-ACUGCCUGUCUGUGCCUGCUGU-3′; Guangzhou RiboBio Co., Ltd.)
or 100 nM miR-214 mimic + 1 µg Bax CRISPR activation plasmid (cat
no. sc-419292-ACT; Santa Cruz Biotechnology, Inc.) for 24 h using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequently, cells were treated with D-GalN (1 mg/ml) and TNF-α
(100 ng/ml) at 37°C for 36 h and used for further analysis.
Transfection of miR-214 mimic in
cells
miRNA mimic is small double-stranded RNA
oligonucleotide, which can simulate endogenous mature miRNA
molecules (16). The synthesized
miR-214 mimic was purchased from Guangzhou RiboBio Co., Ltd. BNLCL2
cells were transfected with miR-214 mimic, mimic control, Bax
plasmid, control-plasmid or miR-214 mimic + Bax plasmid using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. Then, 24 h after cell transfection, the efficiency of
transfection was analyzed using reverse transcription-quantitative
PCR (RT-qPCR).
Luciferase reporter assay
miRNA.org software (http://www.microrna.org/microrna/getMirnaForm.do;
August 2010 Release) was used to predict the potential target of
miR-214. To assess the association between miR-214 and Bax,
wild-type (WT) and mutant (MUT) 3′-UTR of Bax containing the
miR-214 binding sites, were amplified by RT-PCR using a
Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics),
incubating for 5 min at 25°C followed by 60 min at 42°C, from total
RNA preparations extracted from BNLCL2 cells and cloned into the
psiCHECKTM-2 vector (Promega Corporation). The following primer
sequences were used: Bax forward, 5′-GGACGAACTGGACAGTAACATGG-3′ and
reverse, 5′-GCAAAGTAGAAAAGGGCGACAAC-3′. Then, 100 ng psiCHECK-2
luciferase reporter plasmids containing WT and MUT 3′-UTR of Bax
were co-transfected into BNLCL2 cells with miR-214 mimic (100 nM)
or mimic control (100 nM) for 48 h using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, a
Dual Luciferase Assay system (Promega Corporation) was used to
detect luciferase activity in the transfected cells. Renilla
luciferase activity was used as the control.
ALT and AST detection assay
The levels of AST and ALT were detected in the blood
of mice to assess liver injury. Blood (0.1 ml) was collected from
each mouse to analyze the serum levels of ALT and AST. The samples
were centrifuged at 8,000 × g for 8 min at 4°C and an automatic
biochemical analyzer (Hitachi Ltd.) was used to determine the serum
ALT and AST levels according to the manufacturer's protocol.
ELISA
Serum levels of TNF-α (Mouse TNF-α ELISA kit; cat
no. PT512) and IL-6 (Mouse IL-6 ELISA kit; cat no. PI326) in
D-GalN/LPS-treated mice, and those in the supernatant
(centrifugation at 500 × g at 4°C for 5 min) of BNLCL2 cells were
determined by ELISA kits (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions.
TUNEL staining
TUNEL staining was performed to determine cellular
apoptosis post-D-GalN/LPS challenge using the in situ cell
death detection kit (cat no. 11684817910; Roche Diagnostics)
following the manufacturer's instructions. Cells in each group were
fixed with 4% paraformaldehyde at room temperature for 15 min, and
dewaxed and hydrated liver tissue sections were permeabilized with
0.1% Triton X-100 solution for 15 min. Then, 50 µl TUNEL reaction
mixture (Roche Diagnostics) containing terminal deoxynucleotidyl
transferase and fluorescein-dUTP was added into the sample.
Subsequently, the cells were incubated at 37°C in the dark for 60
min, washed three times with PBS solution for 5 min. Then, 50 µl
Converter-POD (containing anti-fluorescein antibody conjugated with
horseradish peroxidase; 1:1,000; cat no. 11684817910; Roche
Diagnostics) was added on the sample and incubated at 37°C for 30
min. Substrate solution were added and incubated at room
temperature for 10 min. The sample was mounted under glass
coverslip with PBS and then (random five fields of view) analyzed
under a light microscope at ×20 magnification (Olympus
Corporation). Quantification of the percentage of TUNEL-positive
cells was performed using Image-Pro Plus software (version 6.0;
Media Cybernetics, Inc.).
Flow cytometry analysis
Flow cytometry was used to analyze apoptosis of
BNLCL2 cells. BNLCL2 cells (2×106 cells/well) were
seeded in 6-well culture plates and transfected with mimic control,
miR-214 mimic or miR-214 mimic + Bax plasmid for 24 h. Then, cells
were treated with D-GalN (1 mg/ml) and TNF-α (100 ng/ml) at 37°C
for 36 h. For each sample, BNLCL2 cells (1×106 cells/ml)
were trypsinized and re-suspended in binding buffer according to
the manufacturer's protocol (Sigma-Aldrich; Merck KGaA).
Subsequently, 10 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide (Beyotime Institute of Biotechnology) were added
to BNLCL2 cells, which were stained for 30 min at room temperature
in the dark. A FACSCalibur flow cytometer (BD Biosciences) was used
to quantify stained cells and data were analyzed by FlowJo software
(version 7.6.1; FlowJo LLC).
RT-qPCR
Total RNA was isolated from BNLCL2 cells or liver
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol.
PrimeScript™ RT reagent kit (cat no. DRR037A; Takara Bio, Inc.) was
used to synthesize cDNA. The reaction condition was as follows:
25°C for 5 min, 42°C for 60 min and 80°C for 2 min. RT-qPCR was
performed using SYBR Premix Ex Taq II (Takara Bio, Inc.) with a
TaqMan 7900 (ABI) RT PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
Initial denaturation for 5 min at 95°C, followed by 40 cycles of 30
sec at 95°C, 30 sec at 60°C and 30 sec at 72°C, and a final
extension step at 72°C for 10 min. The primer sequences were as
follows: miR-214 forward, 5′-AGCATAATACAGCAGGCACAGAC-3′ and
reverse, 5′-AAAGGTTGTTCTCCACTCTCTCAC-3′; Bax forward,
5′-GCAGAGGATTGCTGATG-3′ and reverse: 5′-CTCAGCCCATATTCTTCCAG-3′;
TNF-α forward, 5′-CCACCACGCTCTTCTGTCTAC-3′ and reverse,
5′-TGGCTACAGGCTTGTCACT-3′; IL-6 forward,
5′-CCACTTCACAAGTCGGAGGCTTA-3′ and reverse,
5′-GCAAGTGCATCATCGTTGTTCATAC-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
and GAPDH forward, 5′-CCATGGGGAAGGTGAAGGTC-3′ and reverse,
5′-GAAGGGGTCATTGATGGCAAC-3′. U6 and GAPDH were used as endogenous
control for miRNAs and mRNAs, respectively. Data were measured
using the 2−∆∆Cq method (17).
Western blotting
Total protein was extracted from liver tissue or
cells using lysis buffer [50 mM Tris (pH 8.0), 1% NP-40, 150 mM
NaCl, 0.1% SDS]. Quantitative analysis of the protein was performed
using a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific,
Inc.). Equal protein quantities (40 µg per lane) were separated by
12% SDS-PAGE and transferred to a PVDF membrane (EMD Millipore).
Subsequently, the membranes were blocked with 5% skimmed milk for 1
h at room temperature and probed with anti-Bax (cat no. SAB4502546;
1:1,000, Sigma Aldrich; Merck KGaA), anti-caspase-3 (cat no. 14220;
1:1,000; Cell Signaling Technology, Inc.) or anti-GAPDH (cat no.
G9545; 1:2,000; Sigma Aldrich; Merck KGaA) antibodies overnight at
4°C. After 4 washes in PBST (0.1% Tween-20), the membranes were
incubated with Horseradish Peroxidase-conjugated Goat Anti-Rabbit
IgG H&L pre-adsorbed (1:2,000; cat. no. ab7090; Abcam) for 2 h
at room temperature. The immunoreactive proteins were detected
using ECL reagent (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
Experiments were repeated three times. Statistical
analysis was performed using SPSS 17.0 (IBM Corp.). Data are
presented as the mean ± standard deviation. Differences between
groups were analyzed by Student's t-test or one-way analysis of
variance with a Bonferroni post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
D-GalN/LPS induces acute liver injury
in mice
Liver function and/or the extent of damage in the
liver tissues were evaluated by serum ALT and AST levels. Mice were
sacrificed at various time points (0, 1, 3, 5, 7 and 9 h) after
D-GalN/LPS challenge, and blood was collected for serum ALT and AST
analysis. It was identified that serum ALT and AST levels gradually
increased over the 9 h post-D-GalN/LPS stimulation, peaking at 7 h,
compared with the control group, suggesting the presence of liver
damage in the mice (Fig. 1A and
B). The results also suggested that the maximum level of liver
damage was reached at 7 h after D-GalN/LPS stimulation, thus mice
at 7 h after D-GalN/LPS stimulation was selected in subsequent
experimentations.
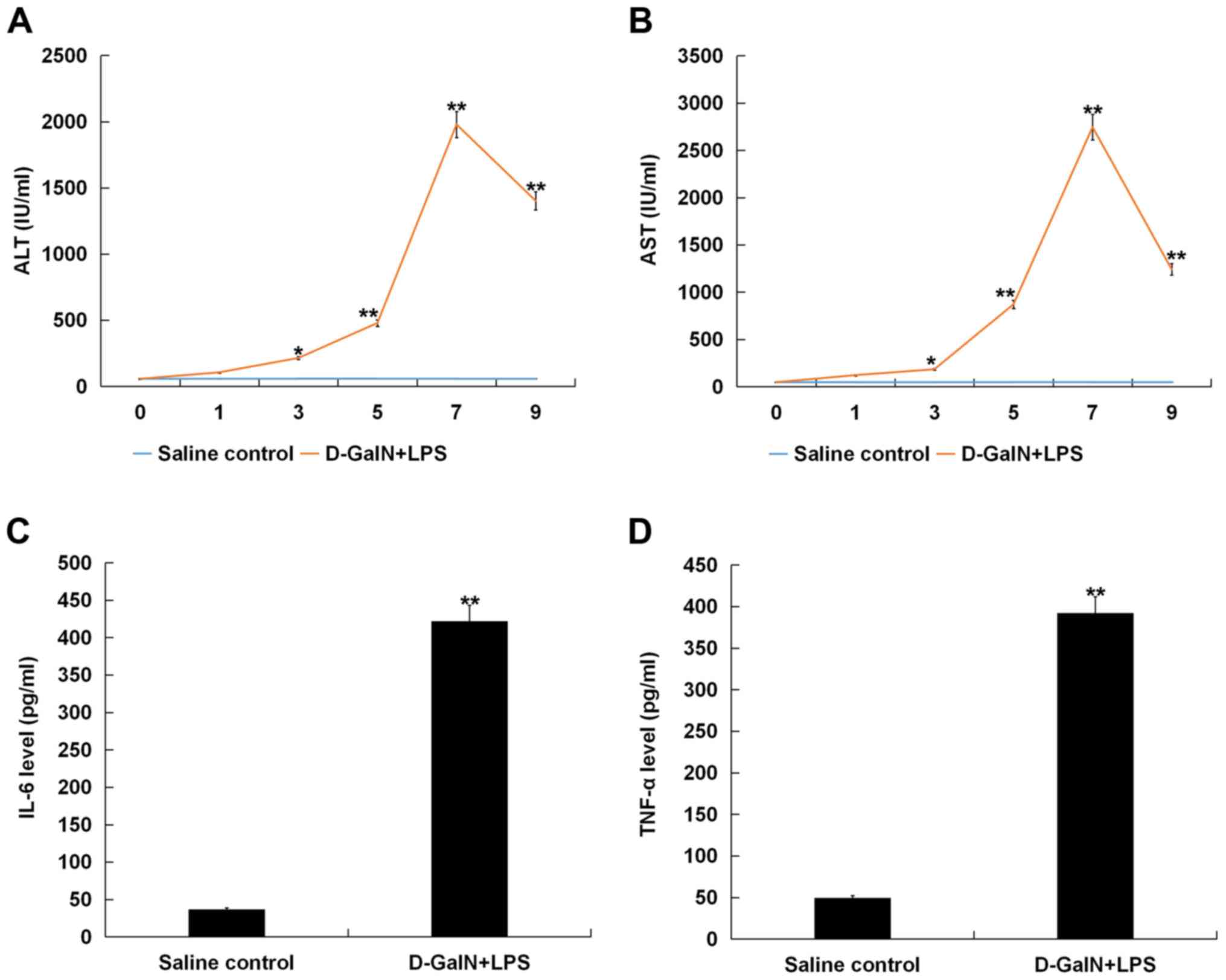 | Figure 1.D-GalN/LPS induces acute liver injury
in mice. Mice were sacrificed at various time points (0, 1, 3, 5, 7
and 9 h) after D-GalN/LPS challenge, and blood was collected for
serum ALT and AST analysis. Serum (A) ALT and (B) AST release
increased gradually and peaked at 7 h post-D-GalN/LPS challenge,
compared with the saline injection group. Serum concentrations of
(C) IL-6 and (D) TNF-α in D-GalN/LPS-treated mice increased
significantly at 7 h after D-GalN/LPS treatment compared with the
saline-treated group. Data are presented as the mean ± standard
deviation. *P<0.05 and **P<0.01 vs. saline control group.
D-GalN, D-galactosamine; LPS, lipopolysaccharide; TNF-α, tumor
necrosis factor-α; IL, interleukin; AST, aspartate
aminotransferase; ALT, alanine aminotransferase. |
IL-6 and TNF-α are involved in hepatocyte apoptosis
and regeneration (18,19). Therefore, the levels of IL-6 and
TNF-α in the serum of mice were measured after D-GalN/LPS
treatment. It was demonstrated that the levels of IL-6 and TNF-α
were significantly increased at 7 h post-D-GalN/LPS challenge
compared with the saline-treated group (Fig. 1C and D).
D-GalN/LPS stimulates hepatocyte
apoptosis in mice
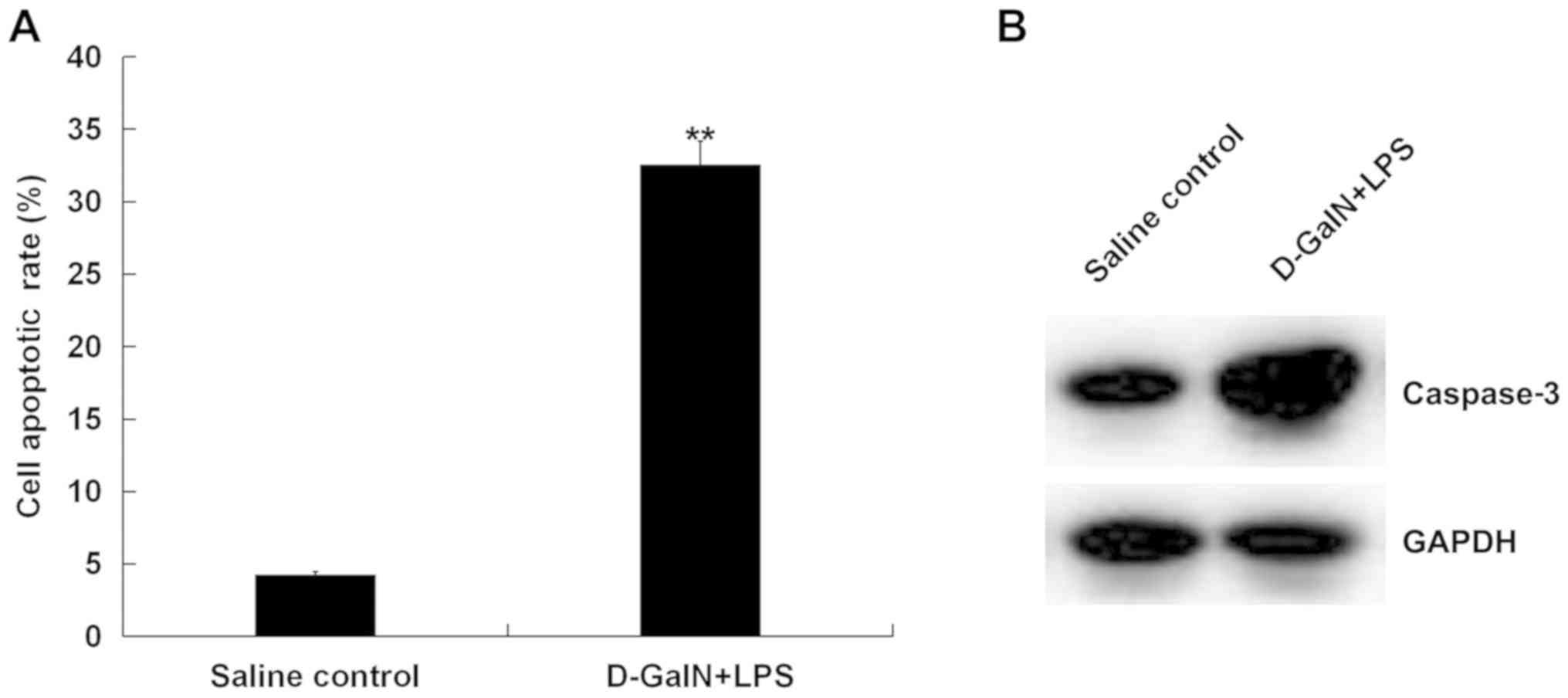
Apoptosis of hepatocytes was assessed by TUNEL
staining, and it was identified that the percentage of apoptotic
cells was significantly increased at 7 h post D-GalN/LPS challenge
compared with the saline-treated group (Fig. 2A). Furthermore, the expression of
the apoptotic-associated protein caspase-3 was analyzed, and
D-GalN/LPS-challenged mice exhibited increased caspase-3 protein
expression at 7 h post D-GalN/LPS challenge compared with
saline-treated mice (Fig. 2B).
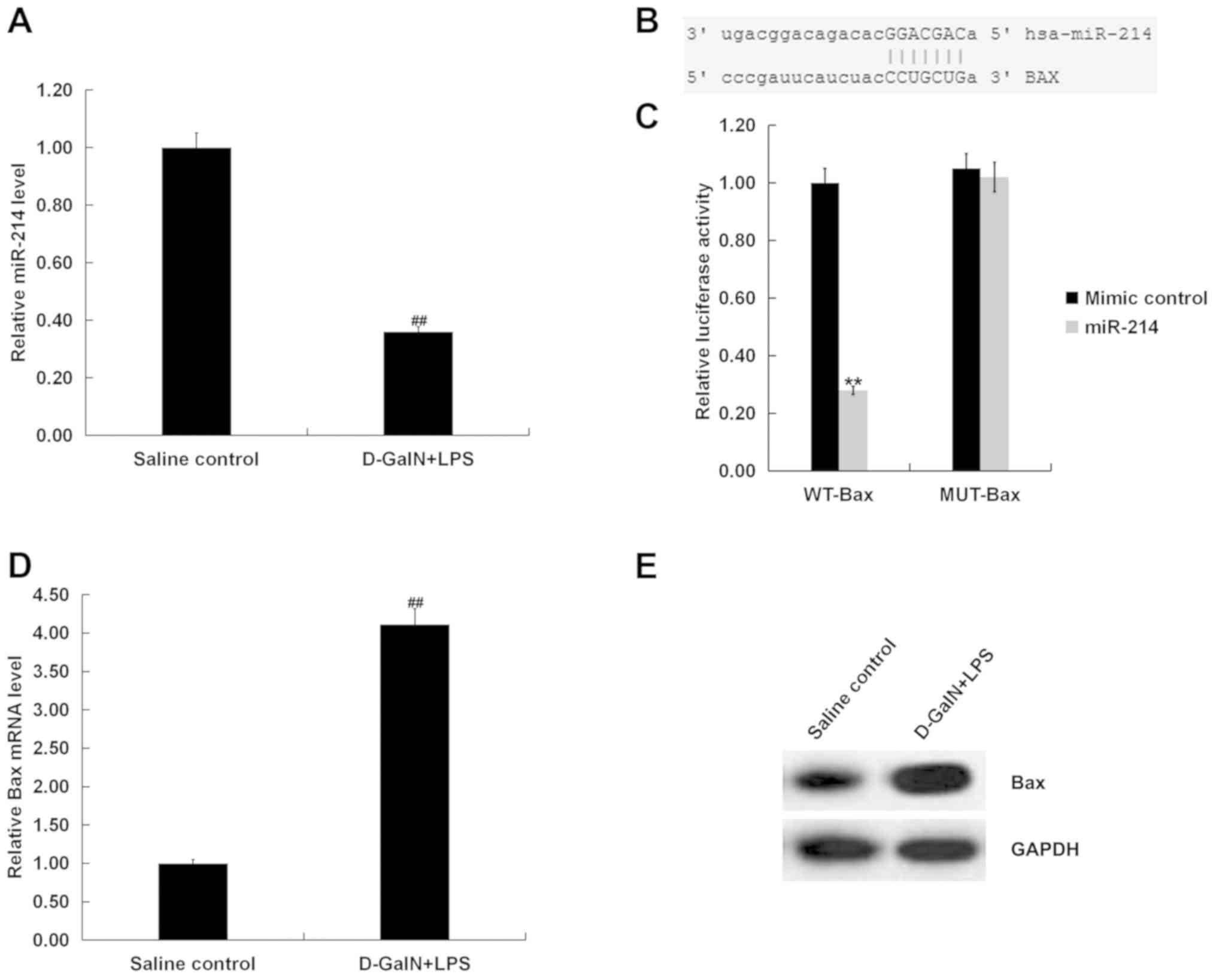
miR-214 is downregulated in
D-GalN/LPS-stimulated mice
The RT-qPCR results demonstrated that the mRNA
expression of miR-214 was significantly downregulated in the liver
tissue of D-GalN/LPS-stimulated mice compared with the saline
control group (Fig. 3A).
Bax is a target gene of miR-214 and is
upregulated in D-GalN/LPS-stimulated mice
miRNA.org software predicted the binding
sites between Bax and miR-214 (Fig.
3B), and a dual luciferase reporter assay was conducted to
examine the identified miR-214 binding sites in the 3′-UTR of Bax.
The results indicated that treatment with the miR-214 mimic
decreased the relative luciferase activity of Bax-WT, but had no
effect on Bax-MUT, compared with the mimic control group (Fig. 3C). Therefore, it was speculated
that Bax may be a target gene of miR-214. In addition, Bax mRNA
(Fig. 3D) and protein (Fig. 3E) expression levels were
significantly increased in the liver tissue of mice at 7 h post
D-GalN/LPS stimulation compared with the saline control group.
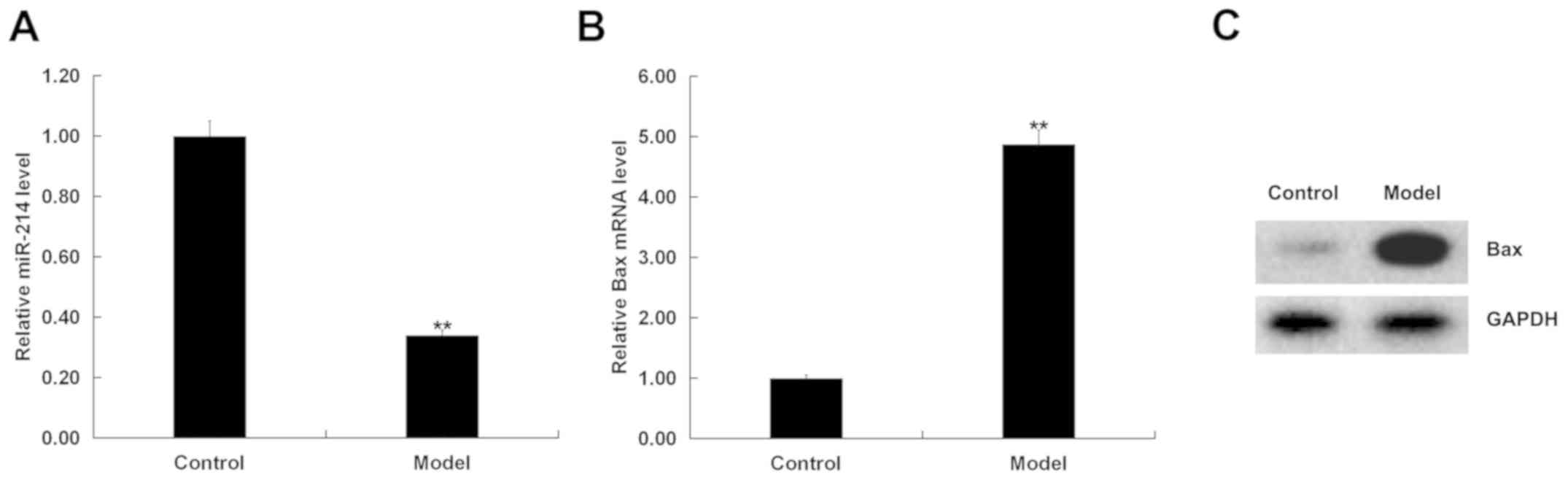
miR-214 is downregulated and Bax is
upregulated in D-GalN/TNF-α-stimulated hepatocytes
Subsequent experiments were conducted in an in
vitro model of BNLCL2 cells stimulated by D-GalN and TNF-α. The
expression of miR-214 was first detected in BNLCL2 cells treated
with (model) or without (control) D-GalN/TNF-α. The results
indicated that, compared with the control group, miR-214 expression
was significantly decreased in D-GalN/TNF-α-treated BNLCL2 cells
(Fig. 4A). In addition, compared
with the control group, Bax was significantly increased in
D-GalN/TNF-α-treated BNLCL2 cells at both the mRNA (Fig. 4B) and protein expression levels
(Fig. 4C).
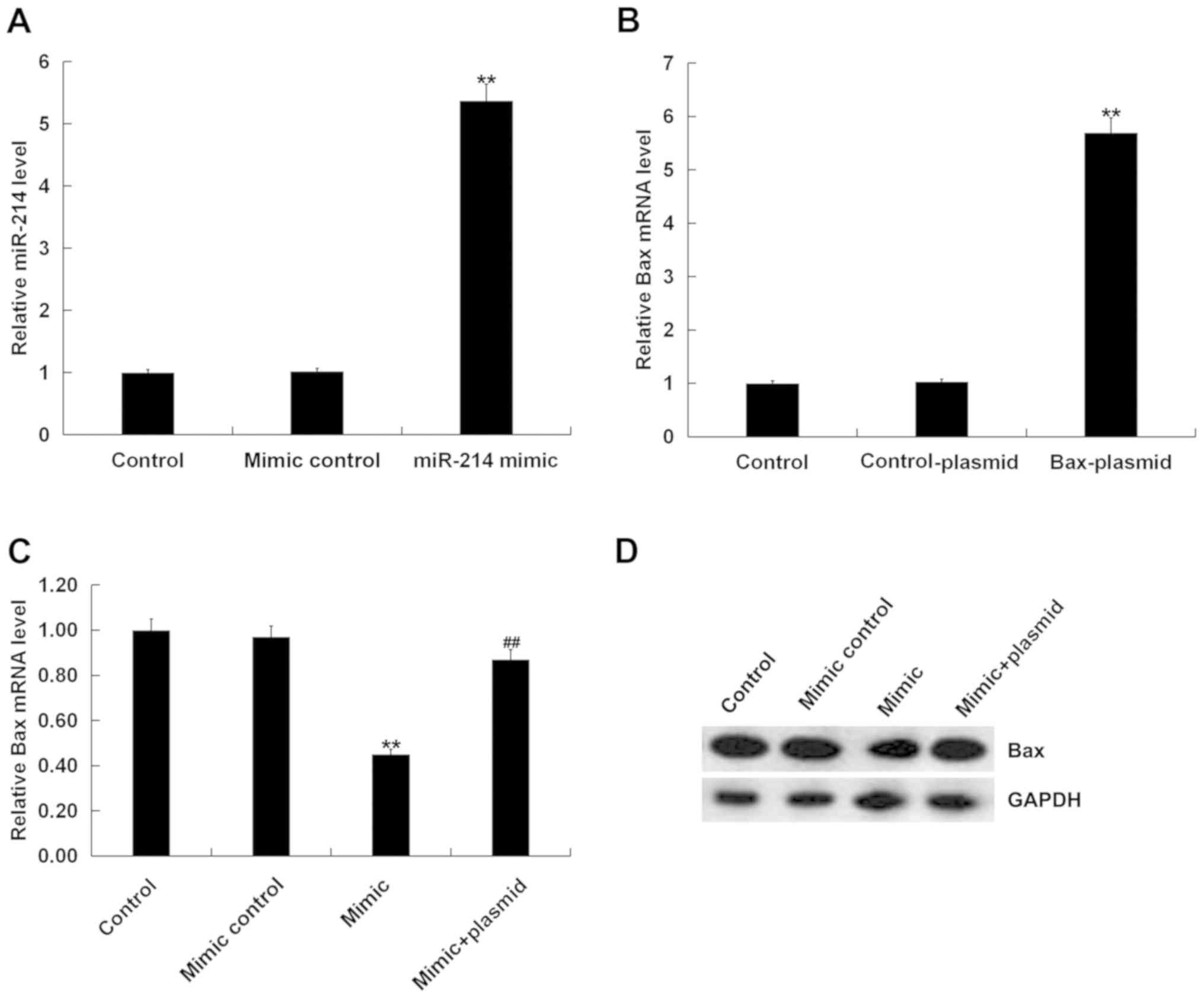
miR-214 mimic inhibit cell apoptosis
and inflammation in D-GalN/TNF-α-stimulated hepatocytes
To investigate the regulatory role of miR-214 in
D-GalN/TNF-α-stimulated hepatocytes, BNLCL2 cells were transfected
with mimic control, miR-214 mimic or miR-214 mimic + Bax plasmid
for 24 h; subsequently, cells were treated with D-GalN (1 mg/ml)
and TNF-α (100 ng/ml) for 36 h. Transfection efficiencies were
detected by RT-qPCR, and it was identified that miR-214 mimic
transfection caused a significant increase in miR-214 mRNA
expression (Fig. 5A), and that Bax
plasmid transfection caused a significant increase in Bax (Fig. 5B) in BNLCL2 cells. In addition,
miR-214 overexpression resulted in the downregulation of the mRNA
and protein expression levels of Bax in BNLCL2 cells, and this
downregulation was reversed by Bax plasmid transfection (Fig. 5C and D).
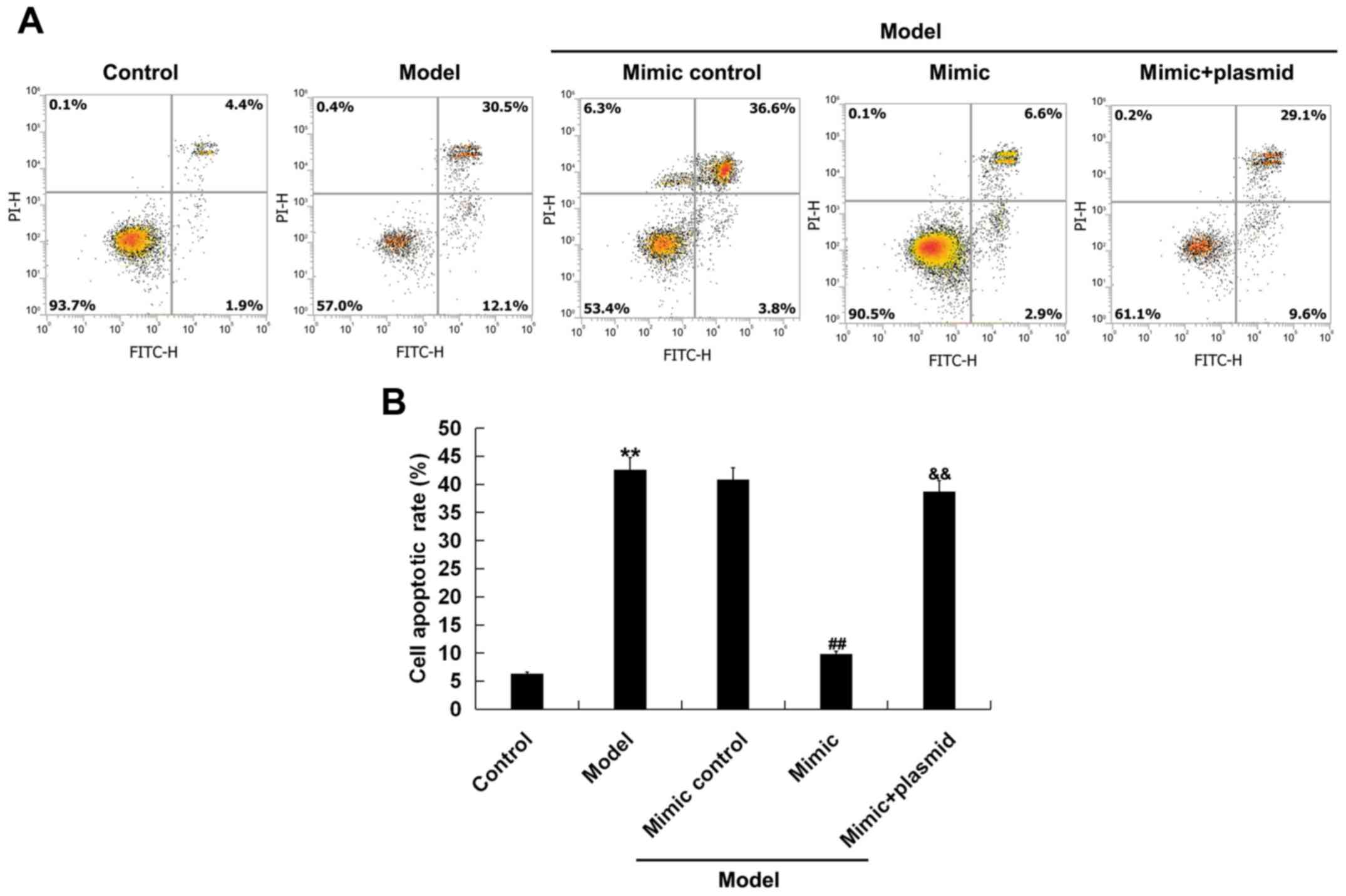
Furthermore, flow cytometry results demonstrated
that D-GalN/TNF-α treatment significantly enhanced BNLCL2 cell
apoptosis compared with the control group. Moreover, compared with
the D-GalN/TNF-α treatment alone group, the results indicated that
the miR-214 mimic significantly decreased BNLCL2 cell apoptosis,
which was reversed by Bax plasmid transfection (Fig. 6A and B).
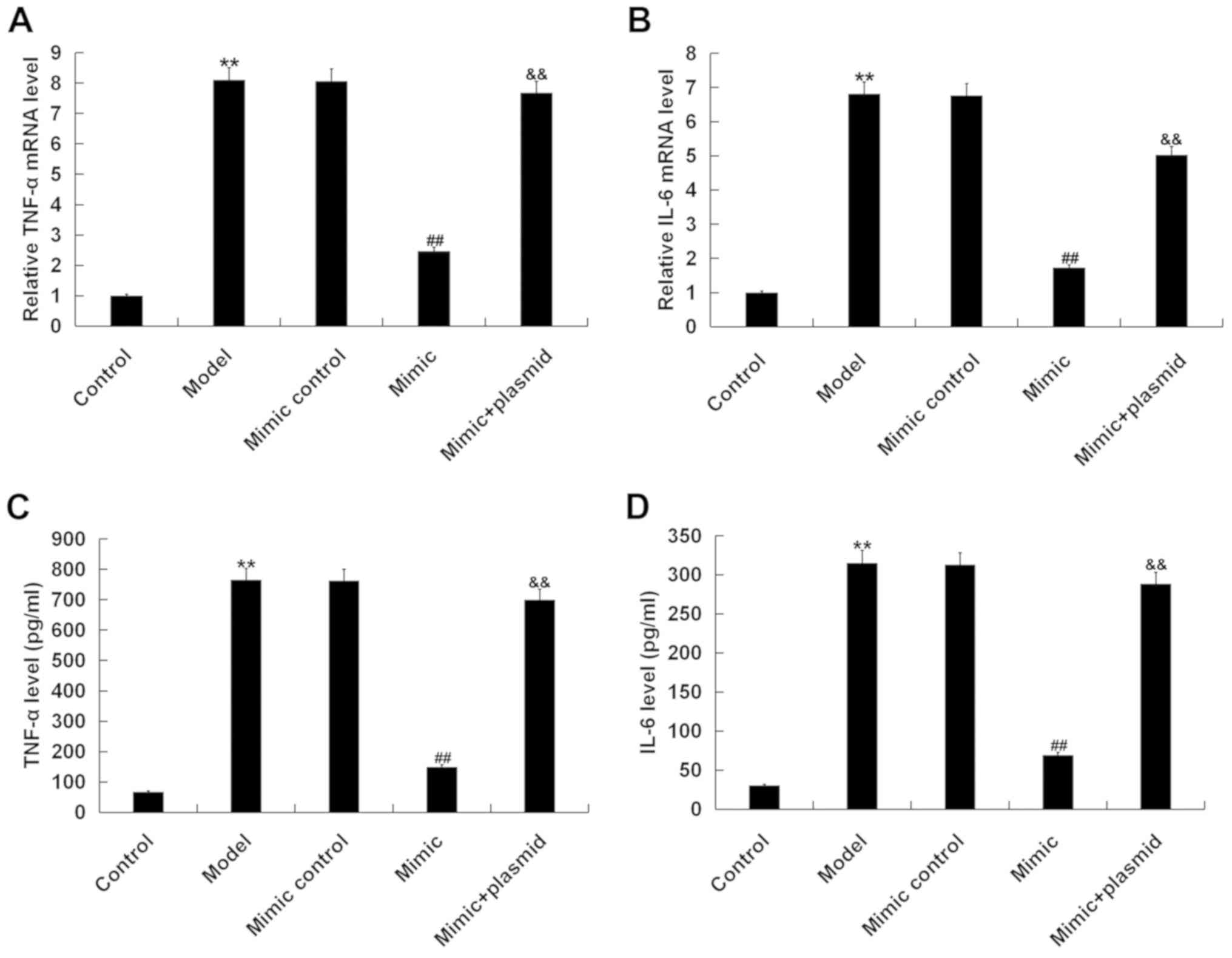
In concordance with the in vivo results, the
mRNA and protein expression levels of TNF-α (Fig. 7A and C) and IL-6 (Fig. 7B and D) in BNLCL2 cells
post-D-GalN/TNF-α challenge were significantly increased compared
with the control group. Furthermore, the results suggested that
miR-214 mimic transfection significantly decreased the mRNA
expression and protein levels of TNF-α and IL-6 in BNLCL2 cells
stimulated by D-GalN/TNF-α, and all of these changes were reversed
by the Bax plasmid.
Discussion
ALF is a condition associated with high mortality
(1), but its underlying
pathological mechanism remains largely unknown. Therefore, the
development of novel prognostic biomarkers and therapeutic targets
for ALF is crucial. miRNAs have been reported to regulate various
aspects of hepatic function, including cell proliferation,
metabolism and viral infection (20). The present results demonstrated
that miR-214 was downregulated and Bax was upregulated in a
D-GalN/LPS-induced murine ALF model. In addition, the results
indicated that miR-214 ameliorated ALF via the regulation of Bax
expression.
D-GalN/LPS has been widely used to induce hepatic
damage, accompanied by changes in hepatic apoptosis and necrosis,
which are similar to the changes observed in human viral hepatitis
(21). In the present study,
D-GalN and LPS, which are well known for ALF induction (15,21–23),
were used to induce an experimental acute liver injury model in
mice. Consistent with previous results (15), the present results suggested that
D-GalN/LPS significantly increased the levels of serum AST and ALT,
enhanced the expression levels of the pro-inflammatory factors
TNF-α and IL-6, and promoted hepatocyte apoptosis. Moreover, these
results indicated that the D-GalN/LPS-induced ALF model was
successfully established. In addition, miR-214 was identified to be
significantly decreased in the liver tissue of D-GalN/LPS-induced
mice. However, in the present study, groups of mice treated with
only D-GalN or only LPS were not conducted, which may be a
limitation, and thus further examination in future studies is
required. Moreover, Bax was identified to be a direct target of
miR-214 in BNLCL2 cells. However, the association between miR-214
and Bax in other hepatocyte cell lines was not investigated in the
present study. Therefore, this is a limitation of the present
study, and must be elucidated in the future.
ALF is characterized by extensive hepatocyte
apoptosis and necrosis (24).
Furthermore, 2 major mechanisms of cell death, namely the death
receptor pathway and the mitochondrial pathway, are involved in the
progression of ALF, in which TNF serves a crucial role (25). The caspase family is a key
contributor to cell apoptosis, and it has been reported that
caspase-3 is significantly activated in the ALF model (26). In addition, apoptotic-associated
proteins tightly control cell apoptosis (27). For example, members of the Bcl-2
family interact with members of the Bax subfamily to induce
apoptosis signals and cause apoptosis (28). In a GalN/LPS-treated mouse model,
Bcl-2 expression was significantly decreased, while Bax expression
was increased (29). Consistent
with previous studies (15,21–23),
the present results demonstrated that D-GalN/LPS treatment
significantly increased hepatocyte apoptosis and increased the
expression Bax in vivo and in vitro.
Ameliorated hepatocyte apoptosis has been shown to
be a key step in the mitigation of D-GalN/LPS-induced ALF (30). Previous studies have also reported
that miRNAs can regulate the expression of pro-apoptotic and
anti-apoptotic genes (31).
Moreover, it has been reported that miR-15b and miR-16 negatively
adjust TNF-α-mediated liver cell apoptosis via Bcl2 in severe liver
failure (32). In addition, miR-24
regulates the key apoptotic gene BCL2-like protein 11 during ALF
(15). The present results
indicated that miR-214 expression was downregulated in the liver
tissues from mice stimulated with D-GalN/LPS. It has also been
reported that miR-214 serves a suppressive role on extrinsic cell
death pathways, such as necrosis and autophagy (33). A previous study indicated that
miR-214 improves acute kidney injury in vivo by inhibiting
apoptosis (34). miR-214 has also
been demonstrated to protect cells from
hypoxia/reoxygenation-induced damage and attenuates
ischemia/reperfusion (I/R)-induced myocardial injury via
suppression of PTEN and Bcl-2 homology domain 3 (BH3)-only
Pro-Protein expression levels, leading to decreases in I/R-induced
myocardial apoptosis (35). The
results of the present study demonstrated that miR-214 ameliorated
D-GalN/TNF-α-induced cell apoptosis via targeting Bax. However, the
specific association between these miRNAs, including miR-214,
miR-24, miR-15b and miR-16, in liver injury requires further
research.
In conclusion, the present results suggested that
miR-214 was downregulated in a D-GalN/LPS-induced murine ALF model
and in D-GalN/TNF-α-stimulated hepatocytes. Moreover, it was
identified that miR-214 ameliorated D-GalN/TNF-α-induced
inflammation and apoptosis in hepatocytes via targeting Bax.
Therefore, it was hypothesized that miR-214 may serve as a novel
therapeutic strategy for ALF treatment. However, this is only a
preliminary study on the role of miR-214 in ALF, and the present
study had limitations; therefore, additional in-depth research is
required to establish the role of miR-214 in ALF. For example, the
effects of miR-214, on factors other than cell apoptosis, such as
hepatocyte function and stress markers in hepatocytes, should be
subsequently investigated. In addition, the present study focused
on the effect of miR-214 on normal murine embryonic liver cells.
However, due to the interspecies variation in hepatic responses, it
is necessary to study the effect of miR-214 on human hepatocytes;
this was also a limitation of the present study, which will be
addressed in future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
S&T Major Project (grant no. 2017ZX10203201-002-002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. XH, WS, LC, YH, YW, EL, AQ and WZ contributed to data
collection and statistical analysis. JG contributed to data
collection, statistical analysis and manuscript preparation. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal care and animal experimental protocols
were carried out strictly according to the recommendations in the
Guide for the Care and Use of Laboratory Animals by the National
Institutes of Health and the Animal Ethics Committee of The First
Affiliated Hospital of Suzhou University. The present study was
approved by the Animal Ethics Committee of The First Affiliated
Hospital of Suzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grek A and Arasi L: Acute liver failure.
AACN Adv Crit Care. 27:420–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernal W and Wendon J: Acute liver
failure. N Engl J Med. 369:2525–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li X, Gou C, Yang H, Qiu J, Gu T and Wen
T: Echinacoside ameliorates D-galactosamine plus
lipopolysaccharide-induced acute liver injury in mice via
inhibition of apoptosis and inflammation. Scand J Gastroenterol.
49:993–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong V, Nanchal R and Karvellas CJ:
Pathophysiology of acute liver failure. Nutr Clin Pract. 35:24–29.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Z, Han M, Chen T, Yan W and Ning Q:
Acute liver failure: Mechanisms of immune-mediated liver injury.
Liver Int. 30:782–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Antoine DJ, Dear JW, Lewis PS, Platt V,
Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, et
al: Mechanistic biomarkers provide early and sensitive detection of
acetaminophen-induced acute liver injury at first presentation to
hospital. Hepatology. 58:777–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song G, Sharma AD, Roll GR, Ng R, Lee AY,
Blelloch RH, Frandsen NM and Willenbring H: MicroRNAs control
hepatocyte proliferation during liver regeneration. Hepatology.
51:1735–1743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szabo G and Bala S: MicroRNAs in liver
disease. Nat Rev Gastroenterol Hepatol. 10:542–552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XW, Heegaard NH and Orum H: MicroRNAs
in liver disease. Gastroenterology. 142:1431–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Sun X, Wei Y, Liang H, Yuan M, Jin
F, Chen X, Liu Y, Zhang CY, Li L and Zen K: Nuclear miR-122
directly regulates the biogenesis of cell survival oncomiR miR-21
at the posttranscriptional level. Nucleic Acids Res. 46:2012–2029.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bandiera S, Pfeffer S, Baumert TF and
Zeisel MB: miR-122-a key factor and therapeutic target in liver
disease. J Hepatol. 62:448–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang D, Yuan Q, Balakrishnan A, Bantel H,
Klusmann JH, Manns MP, Ott M, Cantz T and Sharma AD:
MicroRNA-125b-5p mimic inhibits acute liver failure. Nat Commun.
7:119162016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izawa T, Horiuchi T, Atarashi M, Kuwamura
M and Yamate J: Anti-fibrotic role of miR-214 in
thioacetamide-induced liver cirrhosis in rats. Toxicol Pathol.
43:844–851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Z, Li Z, Zhu D, Ling W, Zheng L, Pu L
and Kong L: Mir-24 regulates hepatocyte apoptosis via BIM during
acute liver failure. Am J Transl Res. 9:4925–4935. 2017.PubMed/NCBI
|
|
16
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhla A, Eipel C, Siebert N, Abshagen K,
Menger MD and Vollmar B: Hepatocellular apoptosis is mediated by
TNFalpha-dependent Fas/FasLigand cytotoxicity in a murine model of
acute liver failure. Apoptosis. 13:1427–1438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan J, Benkdane M, Alons E, Lotersztajn S
and Pavoine C: M2 kupffer cells promote hepatocyte senescence: An
IL-6-dependent protective mechanism against alcoholic liver
disease. Am J Pathol. 184:1763–1772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerr TA, Korenblat KM and Davidson NO:
MicroRNAs and liver disease. Transl Res. 157:241–252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen J, Lin H, Zhao M, Tao L, Yang Y, Xu X,
Jia A, Zhang J and Weng D: Piceatannol attenuates
D-GalN/LPS-induced hepatoxicity in mice: Involvement of ER stress,
inflammation and oxidative stress. Int Immunopharmacol. 64:131–139.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bian X, Liu X, Liu J, Zhao Y, Li H, Zhang
L, Li P and Gao Y: Hepatoprotective effect of chiisanoside from
Acanthopanax sessiliflorus against LPS/D-GalN-induced acute liver
injury by inhibiting NF-κB and activating Nrf2/HO-1 signaling
pathways. J Sci Food Agric. 99:3283–3290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Chen L, Zhang X, Xu L, Xie B, Shi
H, Duan Z, Zhang H and Ren F: Kaempferol protects mice from
d-GalN/LPS-induced acute liver failure by regulating the ER
stress-Grp78-CHOP signaling pathway. Biomed Pharmacother.
111:468–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rutherford A and Chung RT: Acute liver
failure: Mechanisms of hepatocyte injury and regeneration. Semin
Liver Dis. 28:167–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwabe RF and Luedde T: Apoptosis and
necroptosis in the liver: A matter of life and death. Nat Rev
Gastroenterol Hepatol. 15:738–752. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shirozu K, Hirai S, Tanaka T, Hisaka S,
Kaneki M and Ichinose F: Farnesyltransferase inhibitor, tipifarnib,
prevents galactosamine/lipopolysaccharide-induced acute liver
failure. Shock. 42:570–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gómez-Fernández JC: Functions of the
C-terminal domains of apoptosis-related proteins of the Bcl-2
family. Chem Phys Lipids. 183:77–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang RZ, Qiu H, Wang N, Long FL and Mao
DW: Effect of rheum palmatum L. on NF-κB signaling pathway of mice
with acute liver failure. Asian Pac J Trop Med. 8:841–847. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Zheng X, Wang Y, Fan Q, Zhang M, Li
R, Ye J, Wu X, Zhao W and Zhang Y: Berberine protects acute liver
failure in mice through inhibiting inflammation and
mitochondria-dependent apoptosis. Eur J Pharmacol. 819:161–168.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakama T, Hirono S, Moriuchi A, Hasuike S,
Nagata K, Hori T, Ido A, Hayashi K and Tsubouchi H: Etoposide
prevents apoptosis in mouse liver with
D-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure resulting in reduction of lethality. Hepatology.
33:1441–1450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garofalo M, Condorelli GL, Croce CM and
Condorelli G: MicroRNAs as regulators of death receptors signaling.
Cell Death Differ. 17:200–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An F, Gong B, Wang H, Yu D, Zhao G, Lin L,
Tang W, Yu H, Bao S and Xie Q: miR-15b and miR-16 regulate TNF
mediated hepatocyte apoptosis via BCL2 in acute liver failure.
Apoptosis. 17:702–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghaderi S, Alidadiani N, SoleimaniRad J,
Heidari HR, Dilaver N, Heim C, Ramsperger-Gleixner M, Baradaran B
and Weyand M: DJ1 and microRNA-214 act synergistically to rescue
myoblast cells after ischemia/reperfusion injury. J Cell Biochem.
119:7192–7203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu X, Li W and Li H: miR-214 ameliorates
acute kidney injury via targeting DKK3 and activating of
Wnt/β-catenin signaling pathway. Biol Res. 51:312018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Ha T, Hu Y, Lu C, Liu L, Zhang X,
Kao R, Kalbfleisch J, Williams D and Li C: MicroRNA-214 protects
against hypoxia/reoxygenation induced cell damage and myocardial
ischemia/reperfusion injury via suppression of PTEN and Bim1
expression. Oncotarget. 7:86926–86936. 2016. View Article : Google Scholar : PubMed/NCBI
|