Introduction
Although endometriosis is considered to be a benign
disease, its characteristics (including ectopic invasion and high
recurrence) endow it with the biological behavior of malignant
tumors (1,2), of which ovaries and uterosacral
ligaments are the most common locations. Symptoms of endometriosis
include chronic pelvic pain, infertility and adnexal cystic mass
(3,4). Adhesion-invasion-implantation is the
pathophysiological process underlying endometriosis and involves
early cell detachment from the primary lesion and dissemination. At
the later stages, circulating cells anchor and proliferate in
distant tissues to form heterotopic lesions (5).
Mesenchymal stem cells (MSCs) are located in a
number of tissues, such as adipose tissue and bone marrow (6). MSCs can migrate from the original
tissue to the site of pathophysiological changes in response to
inducing factors, such as intercellular adhesion molecule-1
(7). Human umbilical cord MSCs
(Huc-MSCs) are active throughout pregnancy. Exosomes are membrane
bound extracellular vesicles that are produced in the endosomal
compartment of eukaryotic cells, they serve as important biomarkers
to identify different diseases and as therapeutic targets for
diseases (8). Exosomes derived
from MSCs contain abundant biological information, including
proteins, such as membrane receptors, ribosomes and genetic
information. These regulate tissue regeneration and repair,
immunoregulation, cell growth and differentiation regulation
(9–11). To the best of our knowledge,
however, the effects of Huc-MSCs on the biological activities of
endometrial glandular epithelial cells derived from patients with
endometriosis have not previously been reported.
Epithelial-mesenchymal transition (EMT) refers to
morphological and phenotypic transformation of epithelial cells to
mesenchymal cells in response to stimulation of certain
physiological or pathological changes, such as the loss of cell
polarity, decreased contact with surrounding cells and the
extracellular matrix, and increased migration and mobility
(12). EMT is involved in early
embryonic development and organogenesis, as well as wound healing
(13). In addition, the metastasis
of numerous types of malignant tumors, including lung cancer, is
associated with EMT (14). EMT is
a complex process by which epithelial cells acquire the
characteristics of invasive mesenchymal cells (15).
The present study aimed to investigate whether
exosomes derived from MSCs affect the migratory ability of
endometrial glandular epithelial cells. In order to identify the
underlying mechanism, the present study evaluated the effects of
exosomes on EMT in endometrial glandular epithelial cells.
Materials and methods
Tissue samples
The in situ endometrial tissue from five
patients with endometriosis (age range, 31–42 years) and human
umbilical cord tissue from six normal delivery woman (age range,
25–32 years) were provided by The Second Affiliated Hospital of
Nanchang University (Nanchang, China) between June 2018 and July
2018. The experimental protocols were approved by the Ethics
Committee of Nanchang University (China). All patients agreed to
the use of their samples in scientific research and written
informed consent was obtained from all patients.
Preparation of endometrial glandular
epithelial cells
Ectopic endometrial tissue from patients with
endometriosis was collected and cleaned using D-Hank's solution
(cat. no. H1045; Beijing Solarbio Science & Technology Co.,
Ltd.) containing antibiotics, and blood vessels and impurities in
the tissue were shaved using a scalpel. Ophthalmic scissors were
used to cut tissues into 1 mm3 blocks in a sterile Petri
dish and the samples were transferred to a sterile centrifugal
tube. Collagenase IV (0.1%; Sigma-Aldrich; Merck KGaA) was added to
the centrifugal tube and incubated at 37°C for 40–60 min. The
digestion was terminated by adding DMEM with 20% FBS (Hyclone;
Cytiva) after intermittent oscillation. The solution was filtered
through 100- and 400-mesh screens to remove large tissue fragments.
The large tissue fragments on the surface of 100-mesh screen were
transferred into a centrifugal tube, and collagenase (0.1%) was
added for a secondary digestion at 37°C for 5 min. Cell suspension
was collected, centrifuged (1,000 × g at room temperature for 10
min), and suspended cells were inoculated on the culture plate. The
cells were incubated at 37°C with 5% CO2.
Preparation of Huc-MSCs-derived
exosomes (Huc-MSCs-exo)
The blood vessels in the Huc tissue were removed
using a scalpel under a stereomicroscope, and tissues were washed
with PBS, cut into 1 mm3 blocks and digested with
diluted trypsin (PBS mixed with trypsin; 1:1) overnight at 4°C.
Tissue in the Petri dish was transferred to a 50 ml Eppendorf tube
and placed in a 37°C water bath for 15 min. DMEM with 20% FBS was
added to the Petri dish and cultured in an incubator for 15 min at
37°C and 5% CO2. The cells were incubated with the
following antibodies: Isothiocyanate (FITC) anti-CD34 (1:100; cat.
no. 343604; BioLegend, Inc.), FITC anti-CD44 and FITC anti-CD45
(1:100; cat. nos. ab46793 and ab134199, respectively; both
purchased from Abcam) at room temperature in the dark for 10 min
and detected using a NovoCyte™ flow cytometer (NovoCyte 2060R; ACEA
Bioscience, Inc; Agilent).
Extraction of Huc-MSCs-exo
Following 48 h starvation with FBS-free medium at
37°C, exosome extraction kit was used to extract exosomes (cat. no.
E1310; Bioruo) according to the manufacturer's instructions. Cells
and debris were removed by centrifugation (2,000 × g; 4°C; 10 min).
The isolated exosomes were observed using transmission electron
microscopy. The exosomes were fixed using 2.5% glutaraldehyde,
phosphoric acid buffer preparation for 2 h at room temperature.
After embedding, sections were cut (thickness, 70 nm) and stained
with 3% uranium acetate and lead citrate for 10 min at room
temperature. The slides were observed using transmission electron
microscopy [JEM-1230 (80KV); JEOL, Ltd.], at magnification ×1,000.
A total of 250 µg Huc-MSCs-exo was labeled using PKH Lipophilic
Membrane Dyes (cat. no. PKH67GL; Sigma-Aldrich, Merck KGaA)
according to the manufacturer's instructions. PKH67-labeled
Huc-MSCs-exo were centrifuged (40,000 × g; 4°C; 70 min) and
suspended in PBS (50 µl). Then, 2 µl PKH67-labeled Huc-MSCs-exo
solution was added to cells, which were incubated at 37°C for 0.5,
2.0 and 4.0 h. The cells were imaged using Zeiss confocal laser
scanning microscopy (LSM710; Zeiss GmbH), at magnification
×200.
Experimental groups
The samples were divided into two groups (n=6):
Control and Huc-MSCs-exo treatment (10 µg/ml) group. Subsequent
experiments were performed 24 h after treatment.
Transwell assay
Following treatment with Huc-MSCs-exo for 24 and 48
h, 3×103 endometrial glandular epithelial cells were
seeded in the upper chamber of Transwell plates (HyClone; GE
Healthcare Life Sciences) with serum-free DMEM. The lower chamber
contained DMEM with 10% FBS (Hyclone; Cytiva). One day after
seeding, the cells in the lower chamber were fixed with 4%
paraformaldehyde for 30 min at room temperature and stained with 1%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) for 5 min at room temperature. Images were captured using a
light microscope (magnification, ×200).
Wound healing assay
Endometrial glandular epithelial cells were seeded
in 6-well plates (3×103 cells/well) in DMEM with 20%
FBS, and treated with Huc-MSCs-exo for 24 and 48 h. The cell
monolayer was scratched. After 24 and 48 h incubation at 37°C,
images were captured using a light microscope (Olympus
Corporation), at magnification ×100.
Reverse transcription-quantitative
(RT-q)PCR
At 24 h post-treatment, total RNA was extracted from
endometrial glandular epithelial cells using an Ultrapure RNA
extraction kit (CoWin Biosciences), according to the manufacturer's
protocols. The purity of RNA was assessed by measuring optical
density at 280/260 nm using a spectrometer (LASPEC). RNA (1 µg) was
reverse transcribed into cDNA using an Avian Myeloblastosis Virus
Reverse-Transcriptase kit (cat. no. KL041; Shanghai Kang Lang
Biological Technology Co., Ltd.). The reaction system included 9.5
µl RNase-free dH2O, 1.0 µl cDNA/DNA, 2.0 µl primers and
12.5 µl UltraSYBR mixture (cat. no. 00081405; CoWin Biosciences)
and PCR was performed using the following thermocycling conditions:
Initial denaturation at 95°C 10 min; followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing at 58°C for 30 sec and
extension at 72°C for 30 sec; and final extension at 72°C for 3
min. GAPDH was used as a reference gene. The following primers were
used: E-cadherin forward, 5′-CTCACATTTCCCAACTCCTCT-3′ and reverse,
5′-TGTCACCTTCAGCCATCCT-3′; Vimentin forward,
5′-GGATTCACTCCCTCTGGTTG-3′ and reverse,
5′-TGATGCTGAGAAGTTTCGTTG-3′; N-cadherin forward,
5′-GCTTATCCTTGTGCTGATGTTT-3′ and reverse,
5′-GTCTTCTTCTCCTCCACCTTCT-3′; and GAPDH forward,
5′-CAATGACCCCTTCATTGACC-3′ and reverse,
5′-GAGAAGCTTCCCGTTCTCAG-3′.
Western blotting
At 24 h post-treatment with Huc-MSCs-exo, protein
was extracted from cells using the triplePrep kit (cat. no.
28-9425-44; ReadyPrep; GE Healthcare Life Sciences). The protein
levels were quantified using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. A total of 25 µg/lane protein was
separated via SDS-PAGE on a 10% gel and transferred onto
nitrocellulose membranes. The membranes were blocked using 5%
skimmed milk at room temperature for 2 h and incubated overnight at
4°C with the following primary antibodies: Rabbit polyclonal
anti-E-cadherin (1:1,500; cat. no. AF0131; Affinity Biosciences),
anti-GAPDH (1:1,000; cat. no. AG019; Beyotime Institute of
Biotechnology), anti-Vimentin and anti-N-cadherin (1:3,000 and
1:1,000, respectively; cat. nos. ab92547 and ab76057, respectively;
both Abcam). After washing with 0.1 M PBS, the membranes were
incubated with the secondary antibody (HRP-labeled goat anti-rabbit
IgG; cat. no. A16104; Thermo Fisher Scientific, Inc.) at 4°C for 2
h. The blots were visualized using an electrochemiluminescence kit
(Thermo Fisher Scientific, Inc.). The densities of the blots were
quantified using the Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc.).
Immunohistochemistry
The cells were fixed in paraformaldehyde for 30 min
at room temperature. Staining was performed using monoclonal
antibodies against N-cadherin, Vimentin (both 1:100; cat nos.
ab76057 and ab92547, respectively; both Abcam) and E-cadherin
(1:200; cat. no. AF0131; Affinity Biosciences). Endogenous
peroxidase activity was blocked with 3% (v/v)
H2O2 for 5 min at room temperature. The
slides were incubated with the aforementioned primary antibodies
overnight at 4°C, followed by incubation with horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. A16104SAMPLE; Thermo Fisher Scientific, Inc.)
for 30 min at room temperature. Cells were stained with
3,3′-diaminobenzidine chromogen for 3 min at room temperature.
Images were captured using a light microscope (BX51; Olympus
Corporation), at magnification, ×200.
Statistical analysis
Data are expressed as the mean ± standard deviation
of six independent experiments. All statistical analysis was
performed using unpaired Student's t-test using GraphPad Prism
(version 7.0; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of Huc-MSCs and
endometrial glandular epithelial cells
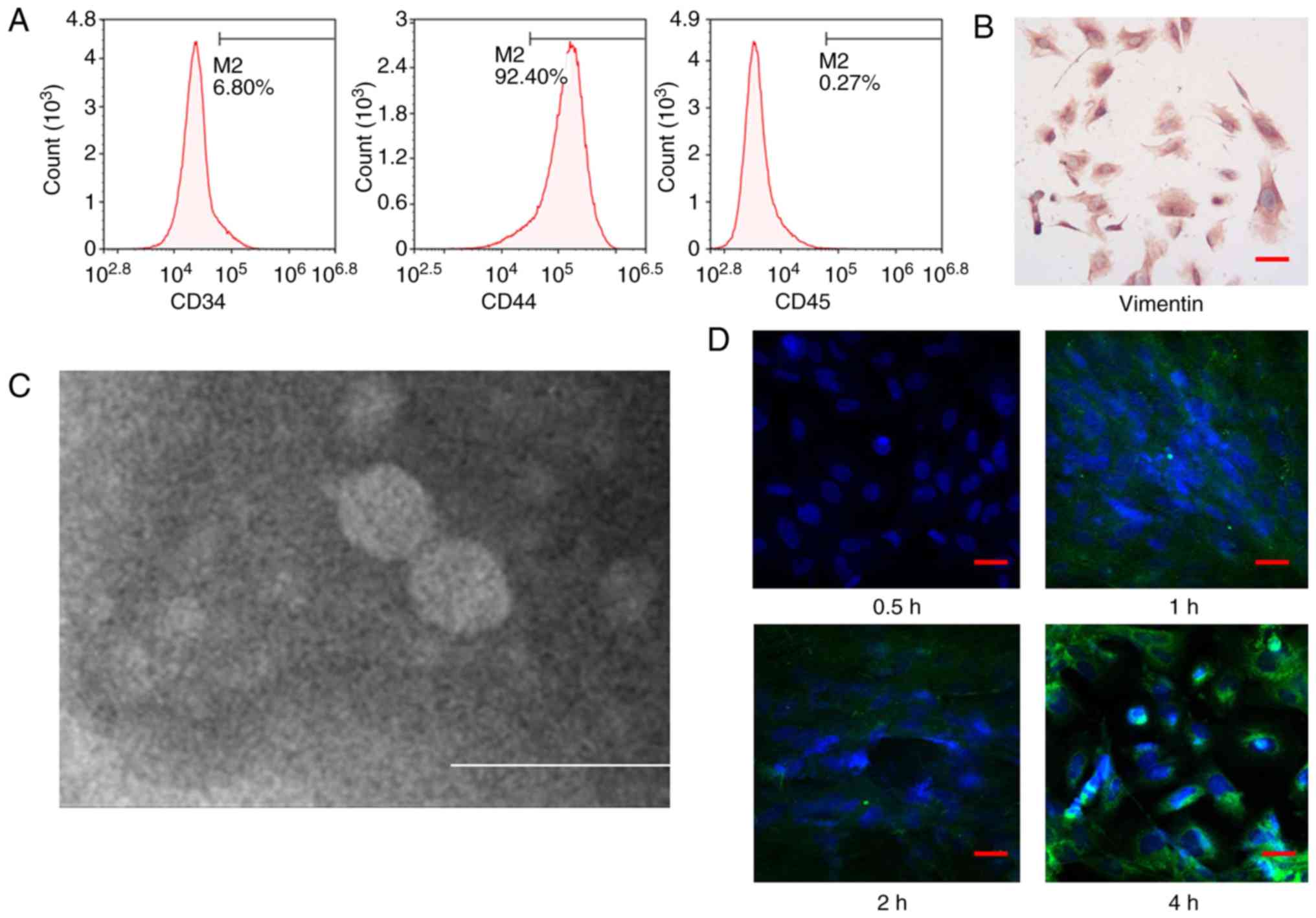
CD44, CD34 and CD45 expression levels in the
isolated cells were 92.40, 6.80 and 0.27%, respectively (Fig. 1A). These data indicated that the
isolated cells were CD44+, CD34− and
CD45−, which revealed the successful isolation of
umbilical cord-derived MSCs. Cells expressed vimentin (dark brown),
which indicated that endometrial glandular epithelial cells were
successfully isolated (Fig.
1B).
Labeling of Huc-MSCs-exo in
endometrial glandular epithelial cells via transmission electron
microscopy
Exosomes exhibited typical round or oval cup-shaped
structures with complete morphology (Fig. 1C). Huc-MSCs-exo entered endometrial
glandular epithelial cells over time (Fig. 1D). At 4 h, the labeled exosomes
were notably expressed in the cytoplasm.
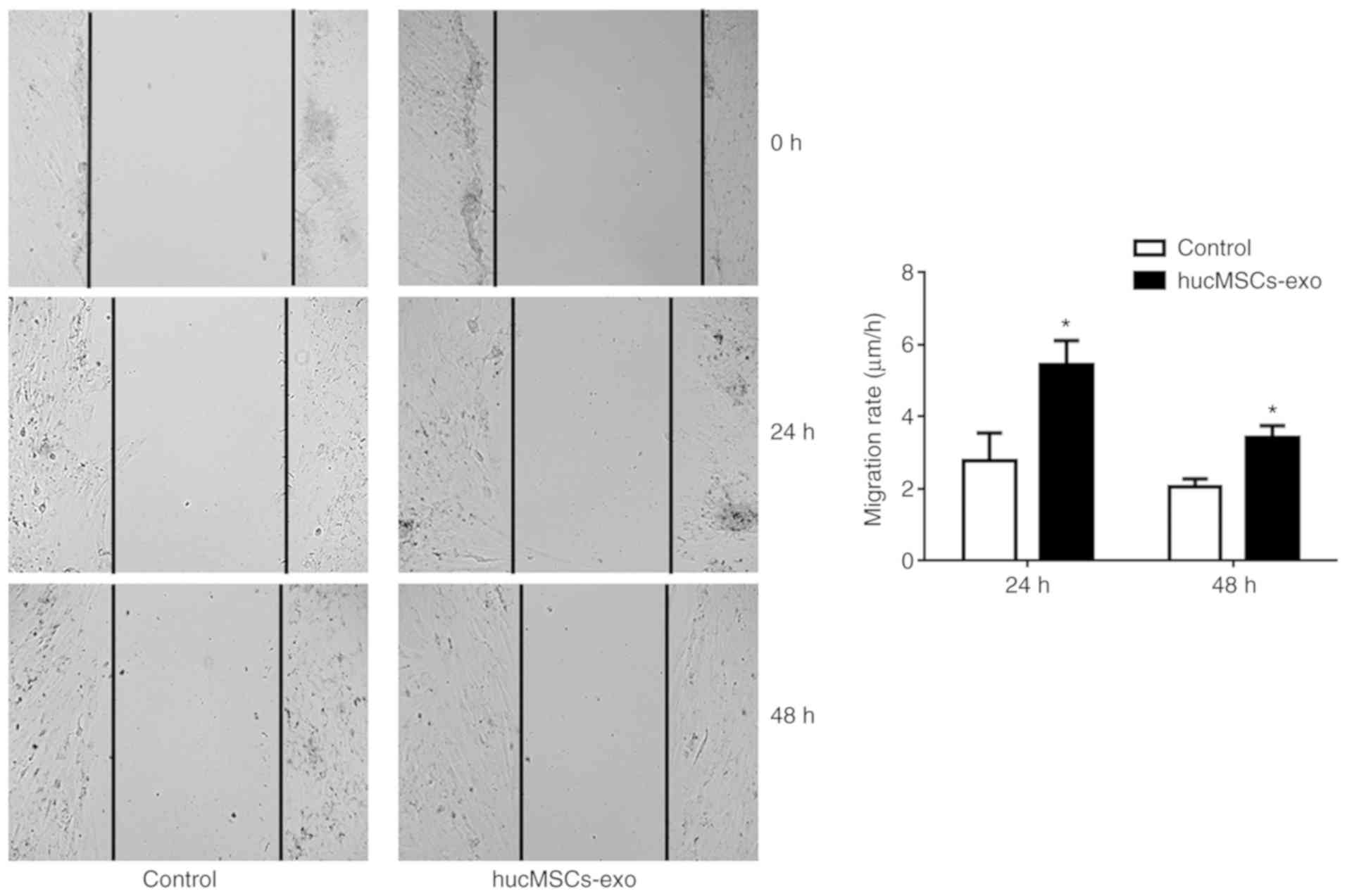
Huc-MSCs-exo facilitate the wound
healing ability of endometrial glandular epithelial cells
As shown in Fig. 2,
the migratory ability of endometrial glandular epithelial cells
treated with Huc-MSCs-exo was significantly enhanced at 24 and 48 h
compared with that of the control group (P<0.05), which
demonstrated that Huc-MSCs exosomes promote the migration of
endometrial glandular epithelial cells.
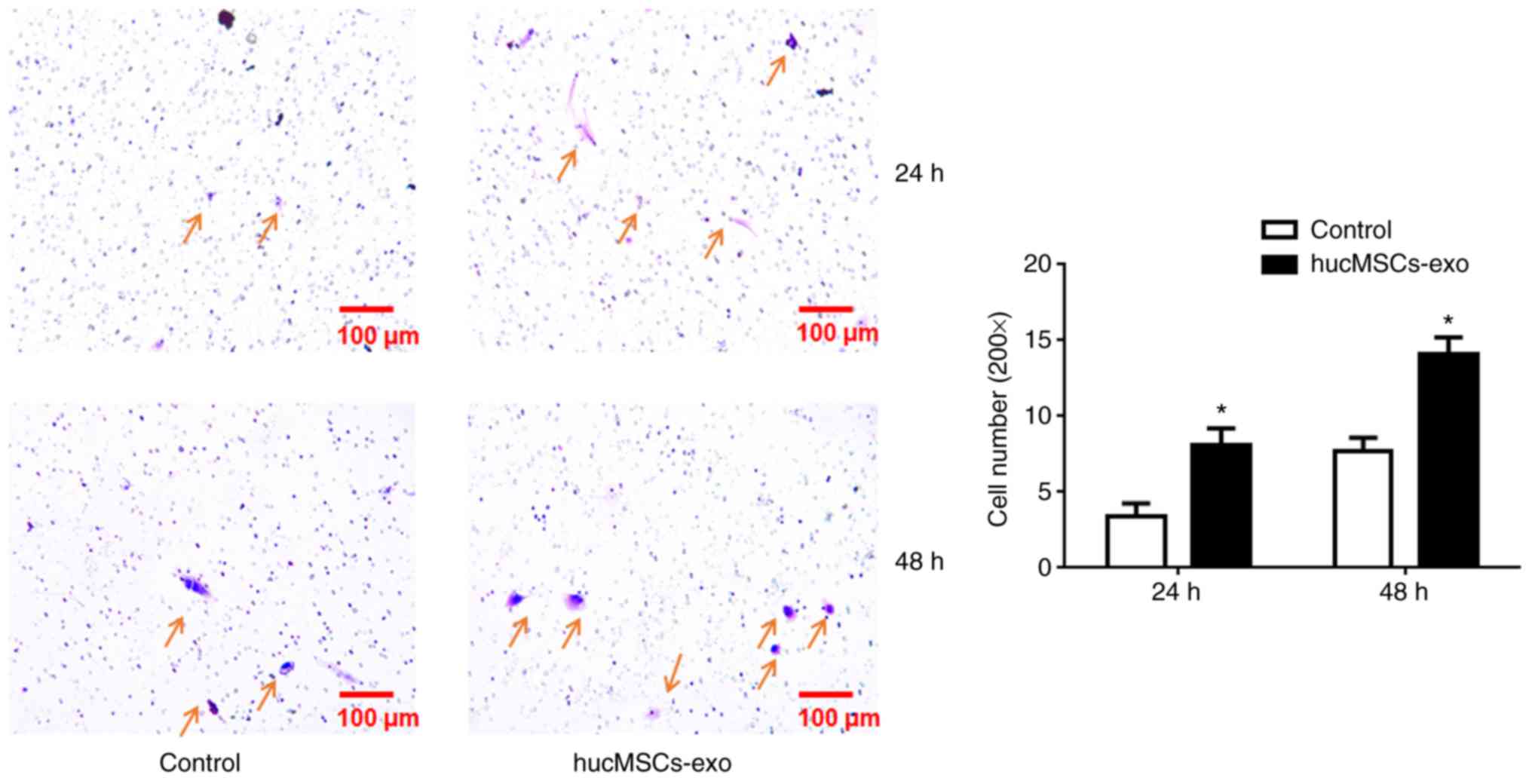
Huc-MSCs-exo facilitate the migratory
ability of endometrial glandular epithelial cells
Compared with control group, the migratory ability
of HucMSCs-exo-treated cells significantly increased at 24 and 48 h
(P<0.05; Fig. 3), which
demonstrated that Huc-MSCs-exo promote the migratory ability of
endometrial glandular epithelial cells.
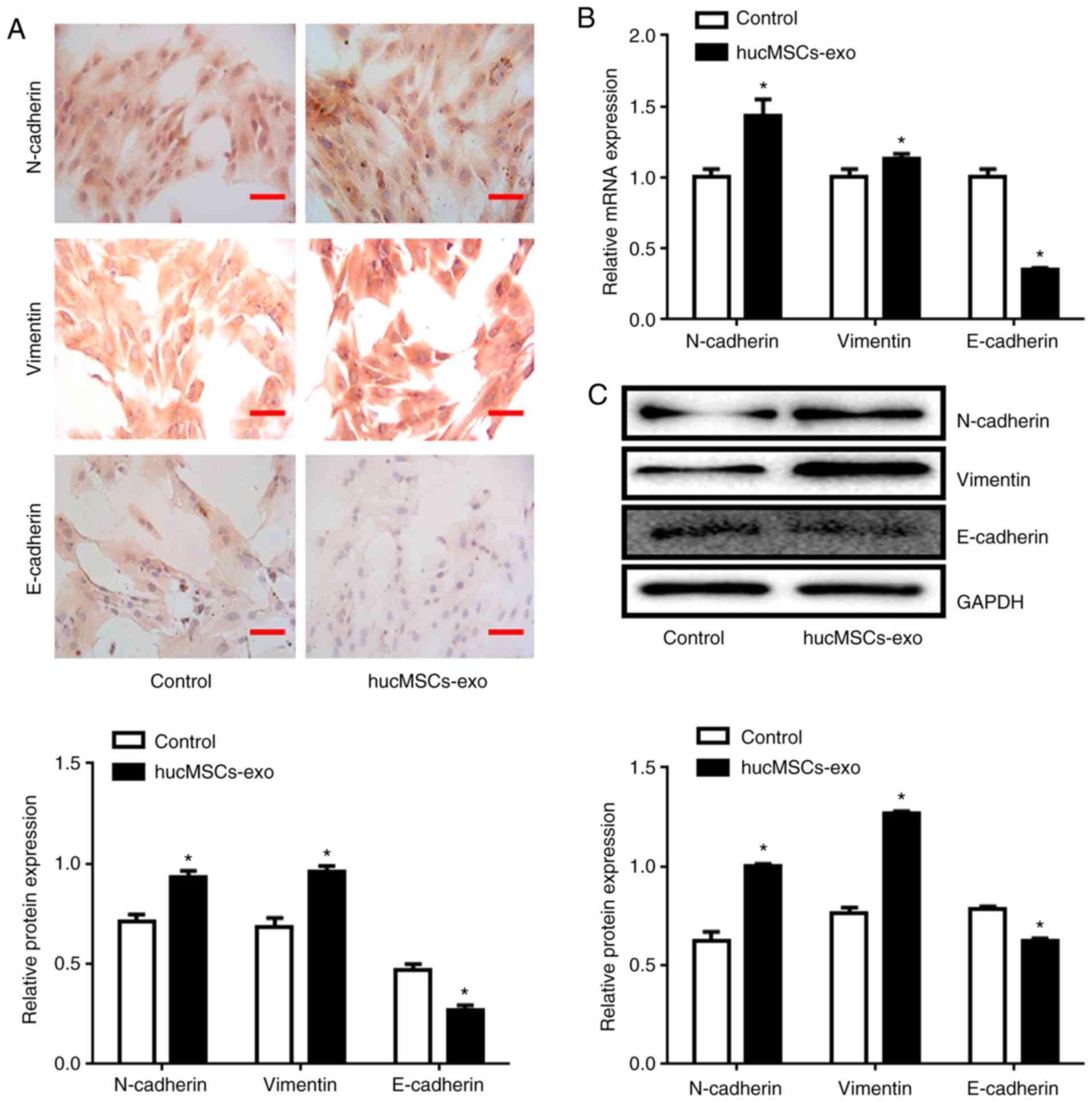
Huc-MSCs-exo increase expression
levels of N-cadherin and Vimentin, and decrease E-cadherin
expression levels
Immunohistochemical staining demonstrated that the
relative expression levels of N-cadherin and Vimentin increased and
the expression level of E-cadherin decreased in the Huc-MSCs-exo
group compared with the control group (P<0.05; Fig. 4A). RT-qPCR also demonstrated that
Huc-MSCs-exo treatment promoted expression levels of N-cadherin and
Vimentin and decreased expression levels of E-cadherin at the mRNA
level (Fig. 4B). This was
consistent with the results of the western blotting analysis
(Fig. 4C).
Discussion
The present study demonstrated that Huc-MSCs-exo
promoted the migration of endometrial glandular epithelial cells
from patients with endometriosis at both 24 and 48 h. It was also
demonstrated that treatment with Huc-MSCs-exo inhibited expression
levels of E-cadherin and promoted expression levels of Vimentin and
N-cadherin. The results of the present study indicate the potential
function of Huc-MSCs-exo in patients with endometriosis.
Endometriosis is a common gynecological disease,
primarily occurring in women of child-bearing age (25–45 years). It
is estimated that 10.8 million individuals were affected globally
as of 2015 (16). The primary
clinical symptoms include dysmenorrhea, increased menstrual volume
and infertility, which may adversely affect the quality of life of
patients (17). In the present
study, endometrial glandular epithelial cells were isolated and
identified via immunohistochemistry. MSCs are adult stem cells that
originate from the embryonic mesoderm and possess strong
self-renewal ability and multi-directional differentiation
potential (18). Numerous studies
have demonstrated that MSCs can serve as the host of primary or
metastatic tumors and promote the formation of tumor
microenvironment (19,20). Studies have demonstrated that MSCs
can either promote or inhibit the growth of tumors (21,22).
MSCs may promote the development of cancer via EMT, which is
characterized by the transformation of cells from epithelial to
mesenchymal phenotype (23). In
the present study, Huc-MSCs were isolated from Huc tissues and
identified via flow cytometry using specific antibodies as
previously described (23).
Exosomes from numerous cell sources contain
biologically active components, such as cell membrane molecules and
cytoplasmic proteins (24).
Intercellular information is transferred via fusion with the cell
membranes of adjacent cells (25).
Herrera et al (26)
demonstrated that exosomes derived from human stem cells accelerate
liver regeneration in hepatectomized rats. Exosomes are considered
to be potential mediators for inducing peripheral tolerance and
regulating the immune response (27). Exosomes are considered to be
mediators of intercellular communication, and influence target
receptor cells by inducing intracellular signal transduction or
endowing donor cells with novel substances, such as protein or mRNA
(9–11). In the present study, exosomes were
identified using transmission electron microscopy. It was
demonstrated that the exosomes were round or elliptical in shape
and conformed to typical morphological characteristics. The
labeling of endometrial glandular epithelial cells by exosomes
demonstrated that the majority of the exosomes entered cells,
allowing them to exert their function.
Endometriosis is a non-cancerous disease with
invasive ability. The invasiveness of endometrial cells serves a
key role in the occurrence and development of endometriosis.
Huc-MSCs-exo promote the migratory and invasive abilities of A549
cells (23). Numerous studies have
demonstrated that MSC-exo exerts both anti-tumor and pro-tumor
effects in human breast, ovarian, gastric and nasopharyngeal
cancer, as well as in multiple myeloma, osteosarcoma and rat liver
cancer (28–36). MSC-exo also promotes migration and
invasion of human lung cancer cells (23). The results of the present study
demonstrated that Huc-MSCs-exo promote the migration of endometrial
glandular epithelial cells.
EMT refers to the loss of epithelial characteristics
and acquisition of mesenchymal phenotypes. When cells undergo EMT,
they detach from other cells, decrease expression levels of
epithelial markers and acquire mesenchymal characteristics, such as
enhanced mobility and invasiveness (37,38).
Zhu et al demonstrated that human MSC-conditioned medium
induces EMT in cancer cells (39,40).
The active component of conditioned medium is exosome from
mesenchymal stem cells (39,40).
A previous study demonstrated that expression levels of EMT markers
are altered by exosomes in vivo (36). Bartley et al (41) demonstrated that expression levels
of E-cadherin are absent in certain glandular epithelial cells in
ectopic lesions of patients with endometriosis, which indicates
that EMT may also play a key role in the formation and progression
of endometriosis. EMT-associated transcription factors can directly
or indirectly inhibit expression levels of E-cadherin and promote
expression levels of N-cadherin and Vimentin in mesenchymal
phenotypes, thereby promoting EMT (42). The results of the present study
demonstrated that Huc-MSCs-exo promote EMT and upregulate
expression levels of N-cadherin and Vimentin, while inhibiting
expression levels of E-cadherin. This is consistent with the
aforementioned results and supports the hypothesis that
Huc-MSCs-exo alter expression levels of EMT-associated genes, thus
enhancing the migratory and invasive abilities of endometrial
glandular epithelial cells.
In the present study, Huc-MSCs-exo promoted the
migratory ability of endometrial glandular epithelial cells from
patients with endometriosis. The current results indicate the
potential application of Huc-MSCs-exo in the treatment of
endometriosis. Further research is required to quantify the dose
effect of Huc-MSCs-exo and to investigate the underlying mechanism.
Huc-MSCs-exo function should also be further evaluated in an in
vivo model.
In conclusion, Huc-MSCs-exo promote the migratory
ability of endometrial glandular epithelial cells, potentially via
promoting EMT in endometrial glandular epithelial cells.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF, FZ and YZ performed the experiments and analyzed
the data. YF and BT designed the study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of Nanchang University (approval no.
2018071401).
Patient consent for publication
All patients agreed to the use of their samples in
scientific research and written informed consent was obtained from
all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vercellini P, Viganò P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mehedintu C, Plotogea MN, Ionescu S and
Antonovici M: Endometriosis still a challenge. J Med Life.
7:349–357. 2014.PubMed/NCBI
|
|
3
|
Peiris AN, Chaljub E and Medlock D:
Endometriosis. JAMA. 320:26082018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berkley KJ, Rapkin AJ and Papka RE: The
pains of endometriosis. Science. 308:1587–1589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuzaki S and Darcha C: Co-operation
between the AKT and ERK signaling pathways may support growth of
deep endometriosis in a fibrotic microenvironment in vitro. Hum
Reprod. 30:1606–1616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orbay H, Tobita M and Mizuno H:
Mesenchymal stem cells isolated from adipose and other tissues:
Basic biological properties and clinical applications. Stem Cells
Int. 2012:4617182012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Wang Q, Ding L, Wang YX, Zhao ZD,
Mao N, Wu CT, Wang H, Zhu H and Ning SB: Intercellular adhesion
molecule-1 enhances the therapeutic effects of MSCs in a dextran
sulfate sodium-induced colitis models by promoting MSCs homing to
murine colons and spleens. Stem Cell Res Ther. 10:2672019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samanta S, Rajasingh S, Drosos N, Zhou Z,
Dawn B and Rajasingh J: Exosomes: New molecular targets of
diseases. Acta Pharmacol Sin. 39:501–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen
L, Wang M, Zhou Y, Zhu W, Li W and Xu W: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate liver
fibrosis. Stem Cells Dev. 22:845–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma
Y, Jiang J, Wang J, Zhong L, Chen M, et al: Exosomes derived from
human umbilical cord mesenchymal stem cells alleviate acute liver
failure by reducing the activity of the NLRP3 inflammasome in
macrophages. Biochem Biophys Res Commun. 508:735–741. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia
D, Fang S and Xu S: Exosomes from human umbilical cord mesenchymal
stem cells enhance fracture healing through HIF-1α-mediated
promotion of angiogenesis in a rat model of stabilized fracture.
Cell Prolif. 52:e125702019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barriere G, Fici P, Gallerani G, Fabbri F
and Rigaud M: Epithelial mesenchymal transition: A double-edged
sword. Clin Transl Med. 4:142015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasion-metastasis in serous ovarian cancer by competitively
binding miR-101-3p to regulate ZEB1 expression. Mol Cancer.
17:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son H and Moon A: Epithelial-mesenchymal
transition and cell invasion. Toxicol Res. 26:245–252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
GBD 2015 Disease and Injury Incidence and
Prevalence Collaborators, . Global, regional, and national
incidence, prevalence, and years lived with disability for 310
diseases and injuries, 1990–2015: A systematic analysis for the
global burden of disease study 2015. Lancet. 388:1545–1602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mboi N, Murty Surbakti I, Trihandini I,
Elyazar I, Houston Smith K, Bahjuri Ali P, Kosen S, Flemons K, Ray
SE, Cao J, et al: On the road to universal health care in
Indonesia, 1990–2016: A systematic analysis for the global burden
of disease study 2016. Lancet. 392:581–591. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marrazzo P, Crupi AN, Alviano F, Teodori L
and Bonsi L: Exploring the roles of MSCs in infections: Focus on
bacterial diseases. J Mol Med (Berl). 97:437–450. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Droujinine IA, Eckert MA and Zhao W: To
grab the stroma by the horns: From biology to cancer therapy with
mesenchymal stem cells. Oncotarget. 4:651–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kidd S, Spaeth E, Dembinski JL, Dietrich
M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M and Marini FC:
Direct evidence of mesenchymal stem cell tropism for tumor and
wounding microenvironments using in vivo bioluminescent imaging.
Stem Cells. 27:2614–2623. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III: Concise review: Dissecting a discrepancy in the
literature: do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhee KJ, Lee JI and Eom YW: Mesenchymal
stem cell-mediated effects of tumor support or suppression. Int J
Mol Sci. 16:30015–30033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Wu X, Qian M, Song Y, Wu D and
Zhang W: Knockdown of TGF-β1 expression in human umbilical cord
mesenchymal stem cells reverts their exosome-mediated EMT promoting
effect on lung cancer cells. Cancer Lett. 428:34–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schneider A and Simons M: Exosomes:
Vesicular carriers for intercellular communication in
neurodegenerative disorders. Cell Tissue Res. 352:33–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herrera MB, Fonsato V, Gatti S, Deregibus
MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C and
Camussi G: Human liver stem cell-derived microvesicles accelerate
hepatic regeneration in hepatectomized rats. J Cell Mol Med.
14:1605–1618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mokarizadeh A, Delirezh N, Morshedi A,
Mosayebi G, Farshid AA and Mardani K: Microvesicles derived from
mesenchymal stem cells: Potent organelles for induction of
tolerogenic signaling. Immunol Lett. 147:47–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol
(Dordr). 40:457–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ono M, Kosaka N, Tominaga N, Yoshioka Y,
Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K and Ochiya
T: Exosomes from bone marrow mesenchymal stem cells contain a
microRNA that promotes dormancy in metastatic breast cancer cells.
Sci Signal. 7:ra632014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reza AMMT, Choi YJ, Yasuda H and Kim JH:
Human adipose mesenchymal stem cell-derived exosomal-miRNAs are
critical factors for inducing anti-proliferation signalling to
A2780 and SKOV-3 ovarian cancer cells. Sci Rep. 6:384982016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC,
Huang CC, Ng SH and Lin JW: Adipose-derived mesenchymal stem cell
exosomes suppress hepatocellular carcinoma growth in a rat model:
Apparent diffusion coefficient, natural killer T-cell responses,
and histopathological features. Stem Cells Int. 2015:8535062015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Hendrix A, Hernot S, Lemaire M, De
Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken
K and Menu E: Bone marrow stromal cell-derived exosomes as
communicators in drug resistance in multiple myeloma cells. Blood.
124:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian
H, Chen Y, Jiang P and Xu W: Exosomes derived from human
mesenchymal stem cells promote gastric cancer cell growth and
migration via the activation of the Akt pathway. Mol Med Rep.
14:3452–3458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H and Xu W: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y and
Li Y, Chen H, Yang L, Zhu H and Li Y: Exosomes derived from human
bone marrow mesenchymal stem cells promote tumor growth through
hedgehog signaling pathway. Cell Physiol Biochem. 42:2242–2254.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi S, Zhang Q, Xia Y, You B, Shan Y, Bao
L, Li L, You Y and Gu Z: Mesenchymal stem cell-derived exosomes
facilitate nasopharyngeal carcinoma progression. Am J Cancer Res.
6:459–472. 2016.PubMed/NCBI
|
|
37
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan
Y, Mao F, Wu X and Xu WR: Mesenchymal stem cell-secreted soluble
signaling molecules potentiate tumor growth. Cell Cycle.
10:3198–3207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan
Y, Xu X, Wang M, Qian H and Xu W: Exosomes derived from human bone
marrow mesenchymal stem cells promote tumor growth in vivo. Cancer
Lett. 315:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bartley J, Jülicher A, Hotz B, Mechsner S
and Hotz H: Epithelial to mesenchymal transition (EMT) seems to be
regulated differently in endometriosis and the endometrium. Arch
Gynecol Obstet. 289:871–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells. 8(pii):
E11182019. View Article : Google Scholar : PubMed/NCBI
|