Introduction
Chronic inflammation is involved in the pathological
processes of various bone diseases including ankylosing
spondylitis, osteoporosis, periodontitis and peri-implantitis
(1–3). In these inflammatory bone diseases,
impaired osteoblast differentiation and bone formation
significantly contribute to the imbalanced bone metabolism
(4). As a pleiotropic
proinflammatory cytokine, tumor necrosis factor-α (TNF-α) is
regarded as one of the major factors in the regulatory network of
bone-related inflammatory diseases (5). On binding to its receptors, TNF-α
activates sequential signaling cascades and promotes the nuclear
translocation of NF-κB, which subsequently mediates the
transcription of the downstream target genes (6).
(E)3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile (BAY 11-7082), is
an irreversible inhibitor of TNF-α-induced IκB-α phosphorylation,
and is widely used to inactivate NF-κB signaling (7–10).
TNF-α displays contradictory and complex effects on osteoblast
differentiation and bone formation, depending upon the local
microenvironment and concentration of TNF-α (4,11).
The regulatory effect of TNF-α on bone formation can be, at least
in part, mediated by its inhibitory effect on the canonical Wnt
signaling (12). The canonical Wnt
signaling actively participates in the regulation of osteoblast
proliferation, apoptosis and differentiation, and the genetic
deletion of β-catenin results in the absence of skeletal structures
and the arrest of osteoblast differentiation (13). As one of the extracellular
antagonists of canonical Wnt signaling, Dickkopf WNT signaling
pathway inhibitor 1 (DKK1) inactivates canonical Wnt signaling and
disrupts osteogenic differentiation by binding to the receptors of
Wnt proteins (14). As a result,
DKK1 serves a pivotal role in sustaining proper skeletal
homeostasis and regulating bone remodeling. In estrogen
deficiency-induced osteoporosis, TNF-α was revealed to suppress
bone formation via suppression of Wnt/β-catenin signaling
(15). In addition, the inhibitory
effect of TNF-α on canonical Wnt signaling can be mediated by
enhanced expression of the Wnt antagonists DKK1 and sclerostin
(SOST) (16).
MicroRNAs (miRNAs) are a group of small non-coding
RNAs that post-transcriptionally regulate gene expression by
binding to specific sequences in the 3′UTR of target genes and
induce either translational repression or cleavage of the target
mRNAs (17). Studies have revealed
that miRNAs are key regulators in mediating TNF-α-induced
inflammatory responses. For example, miR-218 has been revealed to
target tumor necrosis factor receptor 1 (TNFR1) and suppress the
NF-κB signaling pathway (18,19).
TNF-α was also revealed to enhance the expression of miR-23b and
miR-33a-5p, which in turn downregulated the levels of Runt-related
transcription factor 2 (Runx2) and special AT-rich sequence-binding
protein 2 (SATB2), respectively, and therefore hindered osteogenic
differentiation (20,21). miRNAs also actively participate in
the crosstalk between canonical Wnt signaling TNF-α signaling
(2,22). In human fetal osteoblastic cells,
miR-29a activated the canonical Wnt signaling by targeting Wnt
antagonists DKK1 and GSK3β, and the downregulation of miR-29a by
TNF-α indicated that miR-29a plays a role in TNF-α-mediated
osteogenic inhibition (22). In
our previous studies, it was determined that miR-335-5p activated
Wnt signaling and promoted osteogenic differentiation by
downregulating the protein levels of DKK1 (13,23).
However, it remains to be elucidated whether the
post-transcriptional regulation of DKK1 by miR-335-5p also plays a
role in an inflammatory microenvironment.
To investigate the effect of TNF-α on the
post-transcriptional regulation of DKK1 by miR-335-5p in MC3T3-E1
murine osteoblast-like cells and primary calvarial osteoblasts, the
effect of TNF-α on the mRNA and protein levels of DKK1 in these
cells was investigated. The expression levels of miR-335-5p upon
TNF-α stimulation were then determined. Changes in the functions of
miR-335-5p in an inflammatory microenvironment were also evaluated.
It was determined that although TNF-α treatment exhibited
cell-specific effects on DKK1 mRNA expression, the stimulation of
TNF-α time- and concentration-dependently upregulated the protein
levels of DKK1, indicating that TNF-α treatment played an important
role in the post-transcriptional regulation of DKK1 expression.
Materials and methods
Animals
C57BL/6 mice (6 male and 6 female, 4–6-day-old,
weight 2–4 g), were obtained from Shandong University Laboratory
Animal Center (Jinan, China). The animals were immediately
sacrificed for getting primary calvarial osteoblasts. The animals
were used in accordance with the guidelines developed by the Animal
Care and Use Committee of the School of Dentistry, Shandong
University (https://www.qlyxb.sdu.edu.cn/info/1103/4118.htm;
SDYZ-[2018]-06) Jinan, China. Ethical approval for the animal
experiments was obtained by The Medical Ethics Committee of the
School of Stomatology, Shandong University (protocol no.
20180602).
Cell culture and reagents
MC3T3-E1 murine osteoblast-like cells were purchased
from the Cell Bank of Chinese Academy of Sciences. The cells were
maintained in α-minimum essential medium (α-MEM; HyClone; GE
Healthcare life Sciences) containing 10% (v/v) fetal bovine serum
(FBS; Biological Industries), 100 U/ml penicillin and 100 µg/ml
streptomycin (both from Beijing Solarbio Science & Technology
Co., Ltd.). As previously described (13), primary calvarial osteoblasts were
isolated from C57BL/6 mice by enzymatic digestion and cultured in
the same medium as that used for MC3T3-E1 cells.
Briefly, the calvaria bone was isolated from
4-6-day-old C57BL/6 mice, gently separated and cut into small
pieces. The tissue was then digested with 0.1% type I collagenase
and 0.25% trypsin at 37°C for 20 min. The digestion procedure was
repeated 5 times, and cell suspensions of the last 4 digestions
were collected. The cells were centrifuged (500 × g, 10 min, 20°C),
re-suspended and cultured in a 37°C sterile incubator containing 5%
CO2.
Recombinant murine TNF-α was purchased from
PeproTech, Inc. BAY 11-7082 was obtained from Beyotime Institute of
Biotechnology.
To assess whether DKK1 and miR-335-5p participate in
the effects of specific inflammatory stimuli, MC3T3-E1 cells and
primary calvarial osteoblasts were exposed to TNF-α for different
time-points and concentrations. For time-response assay, cells were
exposed to 15 ng/ml TNF-α for 0, 12, 24 and 48 h. For
concentration-response assay, cells were treated with TNF-α (0, 15
or 50 ng/ml) for 48 h. For signal pathway investigation, TNF-α (15
ng/ml) was added to the medium with or without the NF-κB signal
pathway inhibitor BAY11-7082 (1 µM) for 48 h.
Reverse transcription-quantitative
(RT-q)PCR
Following the manufacturer's instructions, total RNA
was extracted from cells (1×106) using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and reverse-transcribed into cDNA using PrimeScript™ RT
Reagent Kit with gDNA Eraser (Takara Bio, Inc.). RT-qPCR was
performed using the LightCycler480 SYBR Green I Master mix (Roche
Diagnostics) on a Roche 480 Lightcycler (Roche Diagnostics). The
thermocycling conditions were 94°C for 5 min, followed by 40 cycles
of 95°C for 10 sec, 60°C for 20 sec and 72°C for 20 sec. The primer
sequences were: DKK1, forward: 5′-CTCATCAATTCCAACGCGATCA-3′ and
reverse: 5′-GCCCTCATAGAGAACTCCCG-3′; GAPDH, forward:
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse:
5′-TGTAGACCATGTAGTTGAGGTCA-3′ (synthesized by Invitrogen; Thermo
Fisher Scientific, Inc.). The relative gene expression levels were
evaluated by the 2−ΔΔCq method (24), with GAPDH serving as an internal
control. Each sample was prepared in triplicate, and each
experiment was repeated at least three times.
Western blot analysis
Whole protein lysates were extracted from cells with
Radio Immunoprecipitation Assay (RIPA) lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) containing 1%
phenylmethanesulfonyl fluoride (PMSF; Beyotime Institute of
Biotechnology) The concentrations of the protein lysates were
determined using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology). The protein lysates were then mixed with 5X loading
buffer (Beyotime Institute of Biotechnology) at 100°C for 5 min,
and 25 µg protein from each sample were run on 10% sodium dodecyl
sulfate polyacrylamide electrophoresis gel (Beyotime Institute of
Biotechnology) and electrotransferred to polyvinylidene fluoride
membranes (Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at
100 V. After blocking in 5% nonfat-dried milk (BD Biosciences) for
1 h at room temperature, the membranes were then incubated with
primary antibodies overnight at 4°C according to the manufacturer's
instructions. Mouse monoclonal anti-DKK1 antibody was purchased
from Abcam (1:1,000; product code ab61275), and mouse monoclonal
anti-β-actin antibody was purchased from OriGene Technologies, Inc.
(1:1,000; cat. no. TA-09). Horseradish Peroxidase linked
(HRP-linked) anti-mouse GAPDH antibody (1:20,000; cat. no.
HRP-60004) was purchased from ProteinTech Group, Inc. Blots were
stripped and reprobed with HRP-labelled goat anti-mouse IgG (H+L)
antibody (1:10,000; cat. no. ZB-2305, OriGene Technologies, Inc.)
and HRP-linked anti-mouse GAPDH antibody (1:20,000; cat. no.
HRP-60004, ProteinTech Group, Inc) to monitor protein loading.
Protein bands were visualized using the Chemiluminescent HRP
Substrate (EMD Millipore) on an ECL detection system (SmartChemi
420; Beijing Sage Creation Science Co., Ltd.) with β-actin or GAPDH
serving as an internal control. The relative intensity of each
protein band was evaluated using the ImageJ software package
v.1.6.0 (National Institutes of Health).
RT-qPCR for miRNA analysis
Total RNA was extracted from cells
(1×106) using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and the miRNA was
reverse-transcribed into cDNA using the Mir-X™ miRNA First-Strand
Synthesis kit (Takara Bio, Inc.). RT-qPCR was conducted using the
SYBR Premix Ex TaqTMII kit (Takara Bio, Inc.). on the
aforementioned Roche 480 Lightcycler according to the
manufacturer's instructions. The thermocycling conditions were 95°C
for 15 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 20
sec and then 95°C for 5 sec, 55°C for 30 sec. The primer sequences
were: U6, 5′-GGAACGATACAGAGAAGATTAGC-3′ and
5′-TGGAACGCTTCACGAATTTGCG-3′; miR-335-5p:
5′-TCAAGAGCAATAACGAAAAATGT-3′ (synthesized by Invitrogen; Thermo
Fisher Scientific, Inc.). The mRQ universal primer was obtained
using the aforementioned Mir-X™ miRNA First-Strand Synthesis kit.
The relative miR-335-5p expression level was calculated using the
2−ΔΔCq method (24),
with U6 serving as an internal control. Each sample was prepared in
triplicate, and each experiment was repeated at least three
times.
Cell transfection and luciferase
assay
The luciferase reporter construct encoding the 3′UTR
of murine DKK1, pMIR-REPORT-DKK1 UTR, was a gift from Dr Jake Chen
(Tufts University, Boston, MA, USA) (13). For transient transfection, MC3T3-E1
cells were cultured in 12-well plates at a density of
5×104cells per ml overnight, and co-transfected with 900
ng pMIR-REPORT-DKK1 UTR and 100 ng pRL-TK Vector (Promega
Corporation) using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells co-transfected
with pMIR-REPORT (900 ng) and pRL-TK (100 ng) served as controls.
Then, 6 h after the co-transfection, the transfection complex was
removed and the cells were cultured with fresh, normal medium for
another 12 h. Then after exposure to TNF-α for different
time-points and concentrations, the cells were harvested and the
luciferase levels were monitored using the Dual-Luciferase Assay
System (Promega Corporation) on a Centro xs3 LB960 luminometer
(Berthold Technologies). The firefly luciferase activity was
normalized to the Renilla luciferase activity. Transfected
MC3T3-E1 cells were also treated with or without 15 ng/ml TNF-α
and/or 1 µM BAY 11-7082 for 48 h, with untreated cells serving as
negative controls, and then the cells were harvested and the
luciferase levels were also determined.
pMIR-REPORT-DKK1 UTR and pRL-TK were also
co-transfected into the MC3T3-E1 cells with the mmu-miR-335-5p
mimic or mirVana™ miRNA Mimic Negative Control #1 according to the
manufacturer's instructions (30 nM; Ambion; Thermo Fisher
Scientific, Inc.). Following treatments with TNF-α, the cells were
harvested and the luciferase levels were determined as previously
described.
Statistical analysis
All results are presented as the mean ± SEM for at
least three replicates. One-way analysis of variance (ANOVA) test
with post hoc contrasts by Student-Newman-Keuls test or Student's
t-test was used to test the statistical significance using the SPSS
16.0 Software Package (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
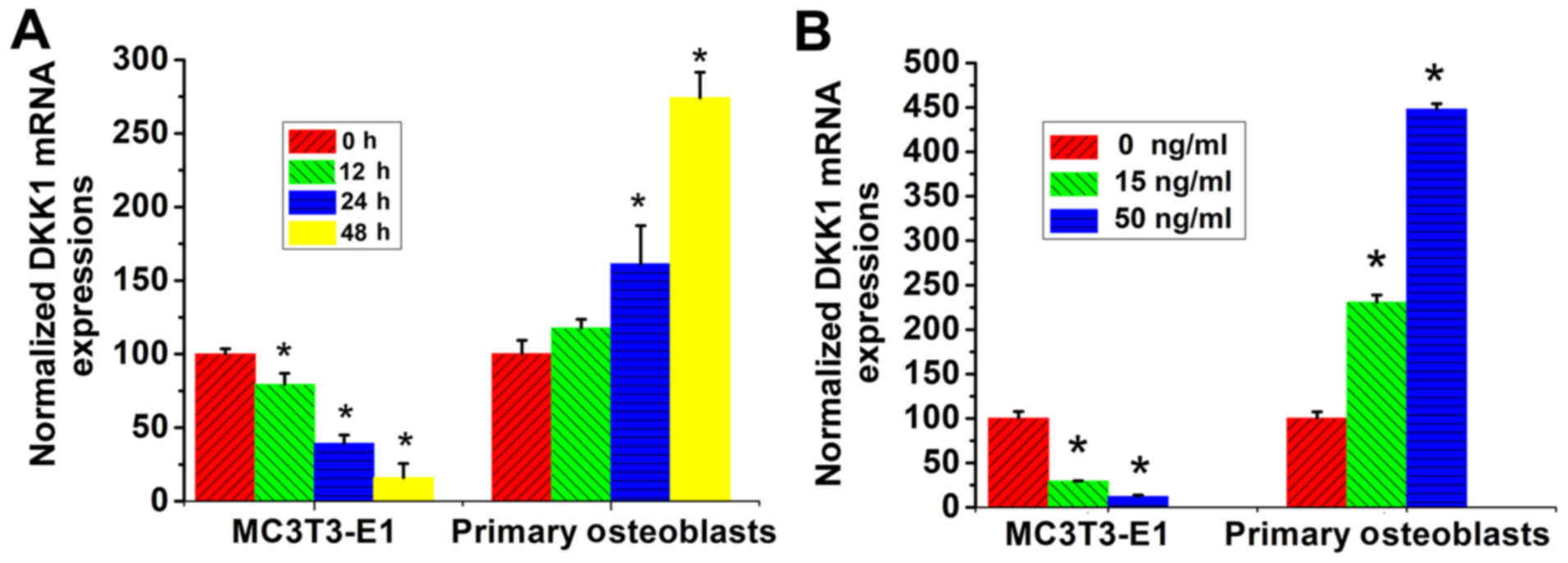
TNF-α exhibits different effects on
DKK1 mRNA expression in different osteoblast lineage cells
MC3T3-E1 cells and primary calvarial osteoblasts
were treated with TNF-α for different time durations, and the mRNA
levels of DKK1 were determined using RT-qPCR. It was determined
that the DKK1 mRNA levels exhibited a time-dependent decrease by
TNF-α treatment in MC3T3-E1 cells, while those in primary calvarial
osteoblasts exhibited a time-dependent increase (Fig. 1A).
MC3T3-E1 cells and primary calvarial osteoblasts
were cultured with different concentrations of TNF-α and the mRNA
expression of DKK1 then determined by RT-qPCR. It was revealed that
in the MC3T3-E1 cells, the DKK1 mRNA levels demonstrated a
concentration-dependent decrease upon TNF-α stimulation, while
those in the primary calvarial osteoblasts exhibited a
concentration-dependent increase (Fig.
1B).
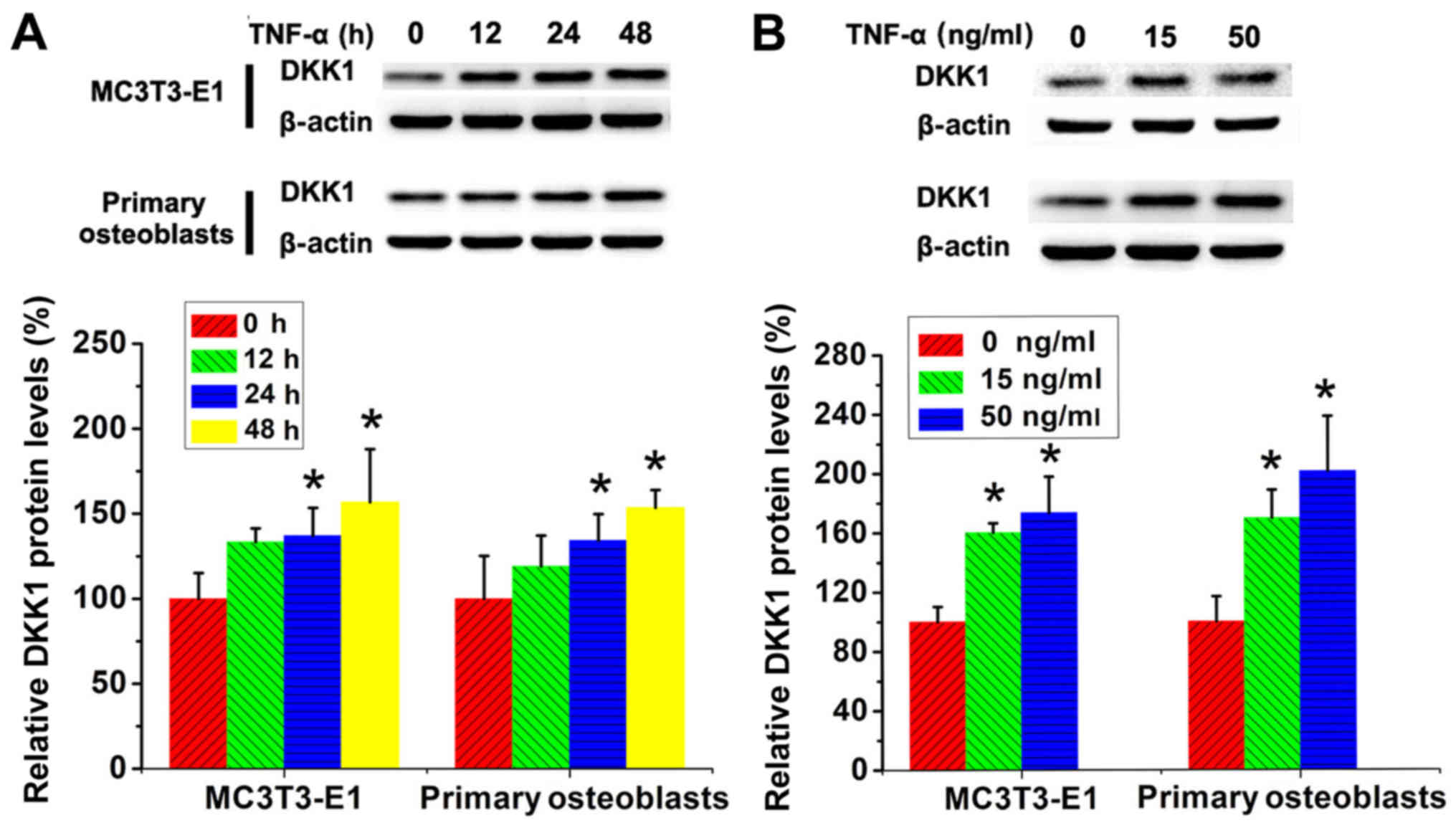
TNF-α plays a role in the
post-transcriptional regulation of DKK1 in both osteoblast lineage
cells
MC3T3-E1 cells and primary calvarial osteoblasts
were then treated with TNF-α for different concentrations and
duration, and the protein levels of DKK1 were determined using
western blotting. In contrast to the time- and
concentration-dependent decrease in DKK1 mRNA levels after TNF-α
treatment in MC3T3-E1 cells, DKK1 protein levels in these cells
exhibited a time- and concentration-dependent increase upon TNF-α
stimulation. As with the changes in DKK1 mRNA levels in primary
calvarial osteoblasts, DKK1 protein levels in these cells also
exhibited a time- and concentration-dependent increase. However,
the extent of the increase in DKK1 protein levels upon TNF-α
treatment was considerably lower than that in DKK1 mRNA levels
(Fig. 2A and B).
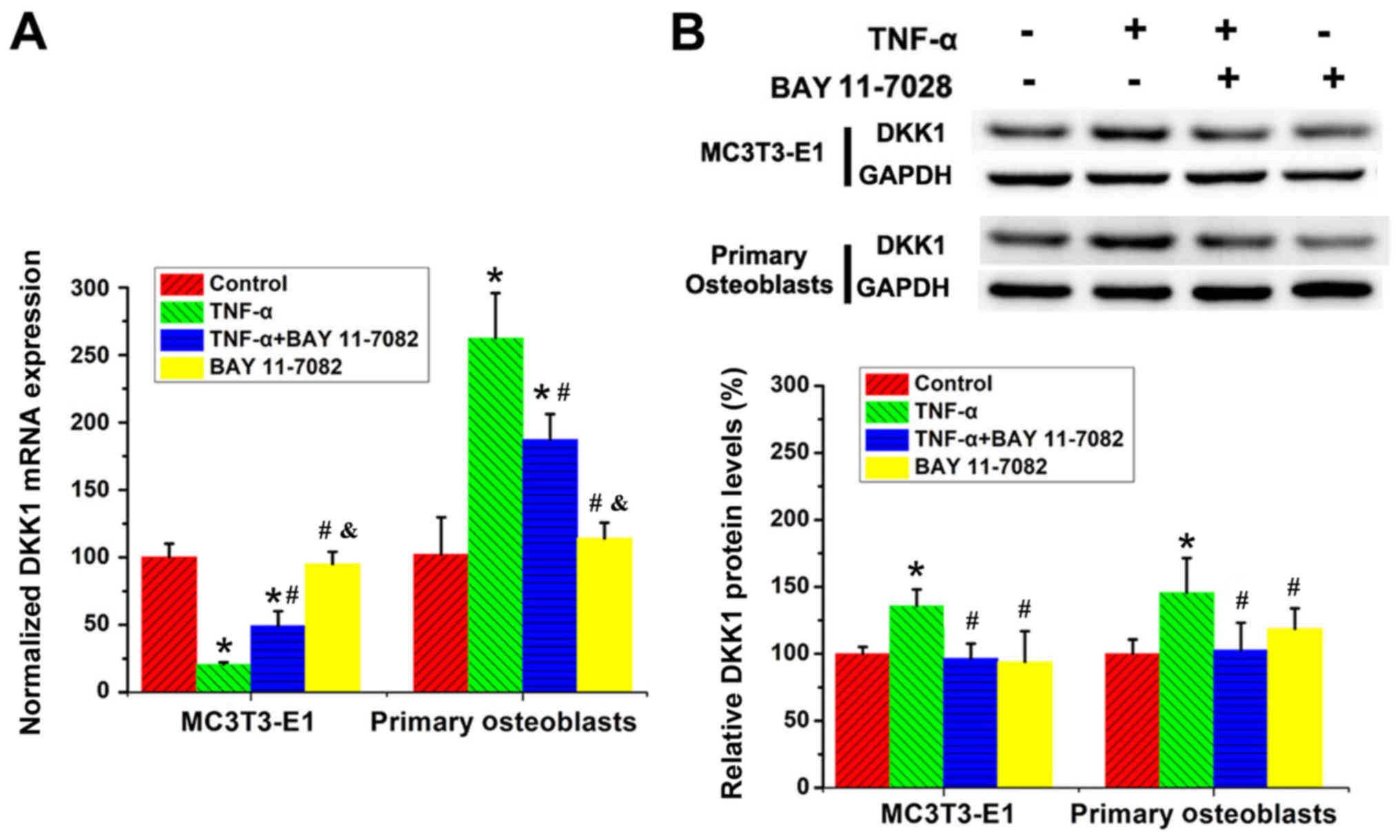
Regulatory effects of TNF-α treatment
on DKK1 protein levels are mediated by NF-κB signaling
MC3T3-E1 cells and primary calvarial osteoblasts
were treated with TNF-α and/or BAY 11-7082 for 48 h, with untreated
cells serving as negative controls, followed by the determination
of the mRNA and protein levels of DKK1. It was determined that BAY
11-7082 treatment partially reversed the regulatory effects of
TNF-α on DKK1 mRNA levels in both MC3T3-E1 cells and the primary
calvarial cells (Fig. 3A). In
addition, BAY 11-7082 treatment completely reversed the positive
effects of TNF-α on DKK1 protein levels in both osteoblast lineage
cells (Fig. 3B).
TNF-α treatment downregulates the
expression of miR-335-5p via different molecular mechanisms in
different osteoblast lineage cells
MC3T3-E1 cells and primary calvarial osteoblasts
were then treated with TNF-α for different concentrations and
duration and the levels of miR-335-5p were determined using
RT-qPCR. In both MC3T3-E1 cells and primary calvarial osteoblasts,
a time- and concentration-dependent decrease in miR-335-5p
expression upon TNF-α stimulation was observed (Fig. 4A and B).
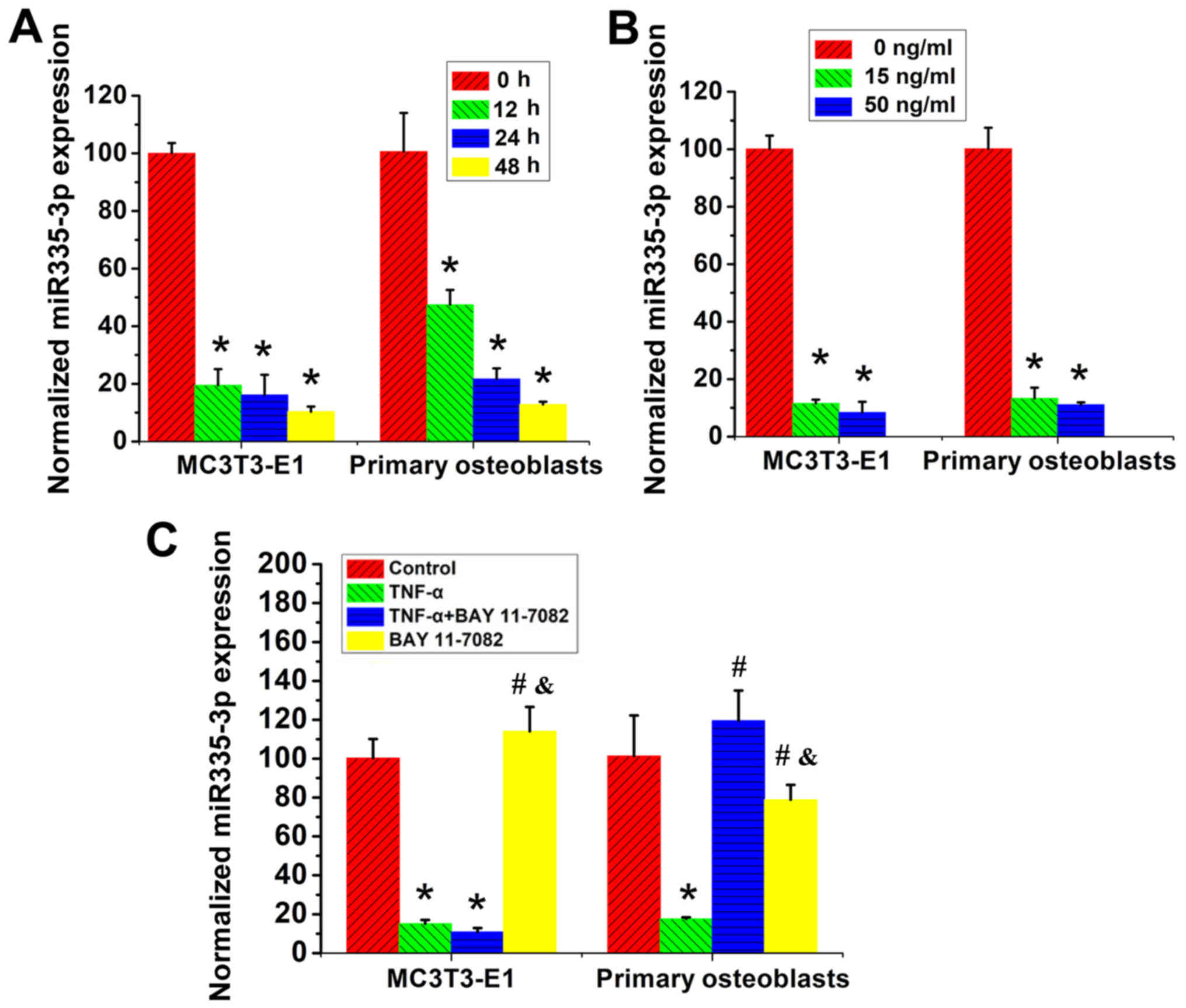 | Figure 4.Effects of TNF-α treatment on the
expression of miR-335-5p in MC3T3-E1 cells and primary calvarial
osteoblasts. (A) Levels of miR-335-5p in MC3T3-E1 cells and primary
calvarial osteoblasts treated with 15 ng/ml TNF-α for 0, 12, 24 and
48 h. *P<0.05, vs. the 0-h group, n=3. (B) Levels of miR-335-5p
in MC3T3-E1 cells and primary calvarial osteoblasts treated with 0,
15 and 50 ng/ml TNF-α for 48 h. *P<0.05 vs. the 0-ng/ml group,
n=3. (C) MC3T3-E1 cells and primary calvarial osteoblasts were
treated with 15 ng/ml TNF-α and/or 1 µM BAY 11-7082 for 48 h, and
the untreated cells served as controls. Levels of miR-335-5p in
these cells were determined. *P<0.05 vs. Control group;
#P<0.05 vs. TNF-α group; &P<0.05
vs. TNF-α + BAY 11-7082 group, n=3. TNF-α, tumor necrosis factor-α;
miR, microRNA. |
MC3T3-E1 cells and primary calvarial osteoblasts
were then treated with TNF-α and/or BAY 11-7082 for 48 h, with
untreated cells serving as negative controls, and the expression of
miR-335-5p was evaluated. Notably, BAY 11-7082 treatment exhibited
no effects on the expression of miR-335-5p in MC3T3-E1 cells, while
completely reversing TNF-α-induced inhibition of miR-335-5p
expression in the primary calvarial cells (Fig. 4C).
TNF-α treatment exhibits no effects on
the inhibitory action of miR-335-5p by targeting DKK1 3′UTR
MC3T3-E1 cells were then treated with TNF-α for
different concentrations and duration, and the luciferase
activities were determined using luciferase assays. The results
demonstrated that the insertion of DKK1 3′UTR resulted in a ~30%
reduction in the luciferase activity of MC3T3 cells. Furthermore,
TNF-α treatment time- and concentration-dependently reversed the
inhibitory effect of the DKK1 3′UTR insertion on luciferase
activities (Fig. 5A and B).
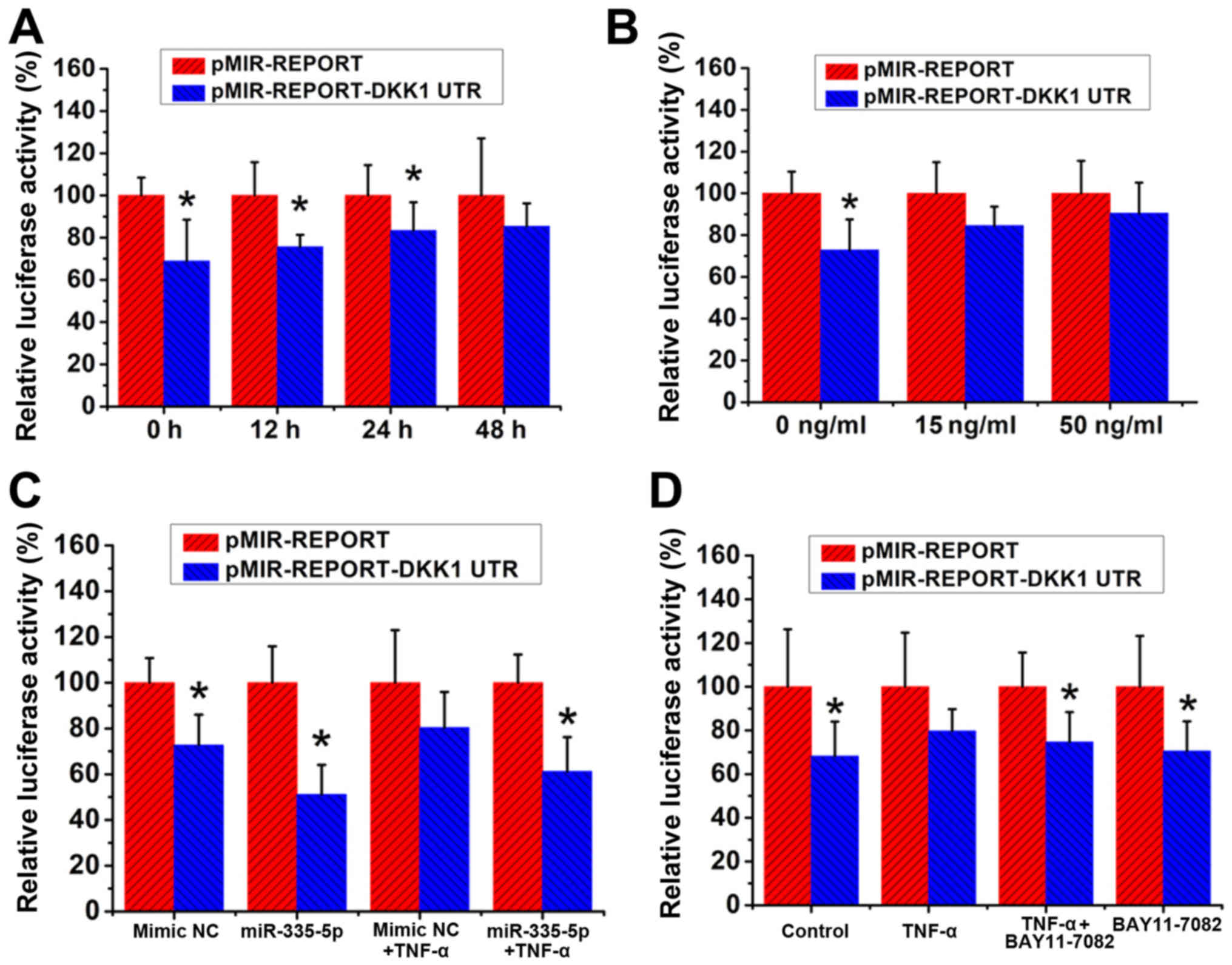 | Figure 5.TNF-α treatment exhibits no effects
on the inhibitory action of miR-335-5p via targeting of DKK1 3′UTR.
MC3T3-E1 cells were co-transfected with pMIR-REPORT-DKK1 UTR and
pRL-TK Vector, and cells co-transfected with pMIR-REPORT and pRL-TK
served as controls. (A) Transfected MC3T3-E1 cells were treated
with 15 ng/ml TNF-α for 0, 12, 24 and 48 h, and the luciferase
levels were determined. *P<0.05 vs. the pMIR-REPORT group, n=3.
(B) Transfected MC3T3-E1 cells were treated with 0, 15 and 50 ng/ml
TNF-α for 48 h, and the luciferase levels were determined.
*P<0.05, vs. the pMIR-REPORT group, n=3. (C) mmu-miR-335-5p
mimic or mirVana™ miRNA Mimic Negative Control #1 were transfected
into the MC3T3-E1 cells concurrently, and the luciferase levels
were determined. The transfected cells were treated with or without
15 ng/ml TNF-α for 48 h. The luciferase levels were determined.
*P<0.05, vs. the pMIR-REPORT group, n=3. mimic NC= miRNA Mimic
negative Control. (D) Transfected MC3T3-E1 cells were treated with
or without 15 ng/ml TNF-α and/or 1 µM BAY 11-7082 for 48 h.
*P<0.05 vs. the pMIR-REPORT group, n=3. TNF-α, tumor necrosis
factor-α; miR, microRNA; DKK1, Dickkopf WNT signaling pathway
inhibitor 1. |
pMIR-REPORT-DKK1 UTR and pRL-TK were then
co-transfected into the MC3T3-E1 cells with the mmu-miR-335-5p
mimic or mirVana™ miRNA Mimic Negative Control #1. pMIR-REPORT and
pRL-TK were also co-transfected into MC3T3-E1 cells with the
mmu-miR-335-5p mimic or mirVana™ miRNA Mimic Negative Control #1 to
serve as controls. Following transfection, cells were treated with
or without 15 ng/ml TNF-α for 48 h and the luciferase levels were
determined as described above. Consistent with the results
illustrated in Fig. 5A and B, the
insertion of DKK1 3′UTR resulted in a ~30% decrease in the
luciferase levels when compared with the corresponding control
cells. The co-transfection of pMIR-REPORT-DKK1 UTR with the
mmu-miR-335-5p mimic led to an additional 20% decrease in the
luciferase levels, resulting in a total 50% decrease in the
luciferase levels when compared with the control cells
co-transfected with pMIR-REPORT and the mmu-miR-335-5p mimic. After
48 h of TNF-α treatment, although MC3T3-E1 cells transfected with
pMIR-REPORT-DKK1 UTR exhibited a 20% decrease when compared with
the control cells, no statistically significant difference was
detected between these two groups. In addition, in the presence of
TNF-α, the luciferase levels in MC3T3-E1 cells co-transfected with
pMIR-REPORT-DKK1 UTR and the mmu-miR-335-5p mimic were 40% lower
than those in the control cells co-transfected with pMIR-REPORT and
the mmu-miR-335-5p mimic (Fig.
5C).
Following transient co-transfection with
pMIR-REPORT- DKK1 UTR and the pRL-TK Vector, MC3T3-E1 cells were
treated with or without 15 ng/ml TNF-α and/or 1 µM BAY 11-7082 for
48 h. MC3T3-E1 cells co-transfected with pMIR-REPORT and the pRL-TK
Vector served as controls. Luciferase activities were determined
using luciferase assays as aforementioned. It was determined that
TNF-α treatment alone resulted in a 20%, but not a statistically
significant, decrease in luciferase levels when compared with
pMIR-REPORT-DKK1 UTR-transfected MC3T3-E1 cells with the control
cells. By contrast, treatment with both TNF-α and BAY 11-7082
slightly enhanced the decrease in luciferase levels to 26%, which
showed statistical significance, when compared with
pMIR-REPORT-DKK1 UTR-transfected MC3T3-E1 cells with the control
cells (Fig. 5D).
Discussion
Bone-related inflammatory diseases are usually
associated with impaired osteogenic differentiation and bone
formation (25,26). It is of great importance to
investigate the underlying mechanisms and promote the development
of novel therapeutic techniques. The present study focused on the
changes in the regulatory effect of miR-335-5p on DKK1 in an
inflammatory microenvironment. Due to their ability to
differentiate into osteoblasts and because they are easy to
manipulate, together with their homogeneity, MC3T3-E1
osteoblast-like cells have been widely used in the research on
osteogenic differentiation and have provided useful information
concerning the molecular mechanisms underlying the regulation of
osteoblast differentiation (27,28).
However, established cell lines cannot truly reflect the in
vivo biological characteristics. Based on these reasons, both
MC3T3-E1 cells and primary calvarial cells were used in the present
study.
TNF-α is the most potent proinflammatory cytokine
ascribed to members of the TNF superfamily and has two different
receptors: TNF receptor-1 (TNFR1) and TNF receptor-2 (TNFR2)
(29,30). TNFR2 is mainly expressed in immune
cells, whereas TNFR1 is the functional receptor of TNF-α in both
osteoblasts and osteoclasts (31).
On binding to TNFR1, TNF-α activates the IκB kinase complex, which
in turn initiates the activation of NF-κB signaling to perform
various biological and pathological functions (19). Considerable research efforts have
been devoted to the investigation of the molecular mechanisms
underlying TNF-α-regulated bone metabolism (32). In contrast to its effect on
activating osteoclastogenesis and promoting bone loss, TNF-α
possesses a biphasic and complicated role in the osteogenic
differentiation of osteoprogenitor cells and bone formation
(33,34).
As a negative regulator of the canonical Wnt
signaling pathway, DKK1 prevents the binding of Wnt proteins to
LRP5/6 and therefore precludes the accumulation and nuclear
translocation of β-catenin (35)
Evidence has also emerged indicating that DKK1 is involved in
inflammation-mediated bone loss (26,36,37).
In TNF transgenic mice, neutralization of DKK1 using specific
antibodies was revealed to protect bone from inflammatory damage
(38). TNF-α upregulated DKK1 both
in vitro and in vivo to suppress osteogenic
differentiation by inhibiting the canonical Wnt signaling pathway
(22,38). Consistent with these findings, the
present study revealed that the protein levels of DKK1 were
significantly enhanced by TNF-α treatment in MC3T3-E1 murine
osteoblast-like cells and primary calvarial osteoblasts. In
MC3T3-E1 cells, the mRNA levels of DKK1 exhibited a time- and
concentration-dependent decrease by the stimulation of TNF-α. By
contrast, the DKK1 mRNA levels in the primary calvarial osteoblasts
were increased by TNF-α treatment, and the increase in the DKK1
mRNA levels after TNF-α treatment was more prominent compared with
the DKK1 protein levels. One reason for the difference in DKK1 mRNA
expression levels between these two cell types is that the
transcriptional regulation of DKK1 may have been changed in
MC3T3-E1 cells. Another possible reason is that MC3T3-E1 cells were
isolated from the calvaria of C57BL/6 mice within 24 h after birth
(27), while the primary calvaria
osteoblasts used in the present study were isolated from
4–6-day-old C57BL/6 mice. The difference in the developmental
stages may also contribute the difference in gene expression. These
findings demonstrated the existence of cell-specific molecular
mechanisms underlying TNF-α-regulated biological processes. In
addition, the post-transcriptional regulation played an important
role in the control of DKK1 protein expression in an inflammatory
environment. Notably, the results of the present study revealed
that the post-transcriptional regulation of the DKK1 protein
expression following TNF-α treatment was activated in MC3T3-E1
cells, while still suppressed in the primary calvarial
osteoblasts.
As a widely accepted NF-kB inhibitor, BAY 11-7082
selectively inhibits nucleotide oligomerization domain-,
leucine-rich repeat- and pyrin domain-containing protein 3 (NLRP3)
inflammasome activity in macrophages independent of their
inhibitory effect on NF-κB activity (39). In fact, a two-step mechanism is
necessary for NLRP3 activation. First, NLRP3 is transcriptionally
upregulated by activated NF-κB signaling. Second, NLRP3 is
activated via stimuli such as ATP, pore-forming toxins and reactive
oxygen species. Once activated, NLRP3 forms a molecular platform
and recruits the adaptor protein ASC, which eventually leads to the
activation of procaspase-1 and the upregulation of active
pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (39). Although NLRP3 is also affected by
BAY 11-7082 treatment, it has been reported that in bone marrow
mesenchymal stem cells and osteoblasts, NLRP3 acts downstream of
canonical Wnt signaling and the Wnt antagonist, DKK1 (40). In addition, although activated
caspase-1 by NLRP3 activation triggers pyroptosis, which is a form
of cell death, changes in cell survival/proliferation/apoptosis
only have limited effect on the final results in the present study
due to the application of various internal controls in all
experiments. Indeed, BAY 11-7082 was still used as a potent
inhibitor of NF-κB signaling in a number of studies (7–10).
Based on these previous investigations, the present study also used
BAY 11-7082 to inhibit TNF-α-activated NF-κB signaling.
BAY 11-7082 was then used to investigate the
molecular mechanisms underlying TNF-α-regulated DKK1 mRNA and
protein expression. It was revealed that in both MC3T3-E1 cells and
the primary calvarial osteoblasts, BAY 11-7082 partially reversed
the effects of TNF-α on DKK1 mRNA expression, while completely
reversing the effects of TNF-α on DKK1 protein expression. In
addition to NF-κB signaling, TNF-α has also been demonstrated to
activate p38, ERK1/2, and JNK1/2 mitogen-activated protein kinase
(MAPK) signaling pathways (41).
Furthermore, although TNF-α and BMP-2 have been revealed to
activate p38 and ERK1/2 signaling pathways, the p38 and ERK1/2
signaling activated by TNF-α and BMP-2 have opposing roles in
regulating osteoblastic differentiation (41). Together with these previous
findings, the results of the present study indicated that in
addition to the canonical NF-κB signaling, other signaling pathways
such as the MAPK signaling pathways also participate in
TNF-α-induced regulation of the DKK1 mRNA expression. However,
NF-κB signaling plays a pivotal role in TNF-α-induced regulation of
the DKK1 protein expression.
A previous study reported that miR-335-5p
post-transcriptionally downregulates the protein level of DKK1 by
specifically binding to the 3′UTR of the DKK1 mRNA, and thus
activating Wnt signaling and promoting osteogenic differentiation
in a cell- and developmental stage-specific way (13). To investigate the role of TNF-α in
the post-transcriptional regulation of DKK1 by miR-335-5p, the
expression levels of miR-335-5p upon TNF-α stimulation were first
determined. It was revealed that in both MC3T3-E1 cells and the
primary calvarial osteoblasts, the expression levels of miR-335-5p
exhibited a time- and concentration-dependent decrease after TNF-α
treatment. Further investigation revealed that inhibition of the
NF-κB signaling via BAY 11-7082 treatment in MC3T3-E1 cells
exhibited no effect on TNF-α-mediated downregulation of miR-335-5p
in these cells, demonstrating that the inhibitory effect of TNF-α
on the expression of miR-335-5p in MC3T3-E1 cells was not mediated
by NF-κB signaling. Considering that the positive effect of TNF-α
on DKK1 protein expression in MC3T3-E1 cells is completely reversed
by BAY 11-7082 treatment, it can be hypothesized that the decreased
expression of miR-335-5p in MC3T3-E1 cells, probably induced by
TNF-α-activated MAPK signaling pathways, has limited effects on the
regulation of DKK1 protein expression in these cells. By contrast,
BAY 11-7082 treatment in the primary calvarial cells fully reversed
the inhibitory effect of TNF-α on miR-335-5p expression. As
aforementioned, upon BAY 11-7082 treatment the enhanced DKK1
protein expression after TNF-α treatment was completely reversed in
the primary calvarial osteoblasts. Therefore it can be concluded
that following TNF-α treatment, the final upregulation of DKK1
protein expression in the primary calvarial osteoblasts was not
only based on the predominantly increased DKK1 mRNA level, but also
the results of the compromised post-transcriptional inhibition due
to, at least partly, the downregulated miR-335-5p expression.
As aforementioned, the decreased miR-335-5p level
induced by TNF-α treatment in MC3T3-E1 cells exhibited limited
positive effects on DKK1 protein levels, which contradicts a
previous study revealing that miR-335-5p specifically and
post-transcriptionally inhibited DKK1 protein expression (13). To investigate whether the
inhibitory effect of miR-335-5p on DKK1 protein expression by
binding to DKK1 3′UTR is compromised in MC3T3-E1 cells after TNF-α
treatment, pMIR-REPORT-DKK1 UTR encoding the 3′UTR of DKK1 was
transiently transfected into MC3T3-E1 cells and the changes in
luciferase levels upon TNF-α stimulation were evaluated. It was
determined that TNF-α treatment time- and concentration-dependently
reversed the inhibitory effects of the insertion of DKK1 3′UTR.
Exogenous miR-335-5p was then co-transfected with pMIR-REPORT-DKK1
UTR into the MC3T3-E1 cells. With or without TNF-α treatment, the
exogenous miR-335-5p resulted in an additional ~20% decrease in
luciferase levels. These results indicated that in MC3T3-E1 cells,
the binding and inhibitory effect of miR-335-5p on DKK1 3′UTR was
intact following TNF-α treatment. In addition, the effect of TNF-α
treatment on DKK1 3′UTR was mainly achieved by regulating the
expression levels of endogenous miRNAs specifically targeting DKK1
3′UTR. Further studies should be performed to investigate the
mechanisms underlying the compromised inhibitory effect of
miR-335-5p on DKK1 expression in MC3T3-E1 cells after TNF-α
treatment.
In conclusion, although TNF-α treatment exhibited
cell-specific effects on DKK1 mRNA expression, the stimulation of
TNF-α time- and concentration-dependently upregulated the protein
levels of DKK1, indicating the important role of
post-transcriptional regulation during this biological process. In
the primary calvarial osteoblasts, the predominantly increased DKK1
mRNA level and the compromised post-transcriptional inhibition by
specific miRNAs such as miR-335-5p both participated in the
enhanced expression of DKK1 induced by TNF-α treatment. By
contrast, the post-transcriptional regulation served a pivotal role
in TNF-α-stimulated DKK1 expression in MC3T3-E1 cells; the
molecular mechanisms underlying this post-transcriptional
regulation is more complex and requires further investigation in
the future. The results of the present study provide insight into
the molecular mechanisms underlying osteogenic differentiation in
bone-related inflammatory diseases. Furthermore, they could aid
researchers in finding a potential pharmaceutical target to treat
bone-related inflammatory diseases.
Acknowledgements
Not applicable.
Funding
This work was supported by Medical and Health
Science and Technology Development Project of Shandong Province
(grant no. 2018WSA01018) and the Dean's Research Assistance
Foundation of Ji Nan Stomatology Hospital (grant no. 2018-02).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL performed most of the experiments, collected and
analyzed the data, and prepared the figures. YY, LY, ZL and SS
performed some of the experiments. JZ provided advice on
experimental design and revised the manuscript for intellectual
content. XL designed the experiments, interpreted the experimental
results, and drafted the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Ethical approval for the animal experiments was
obtained by The Medical Ethics Committee of the School of
Stomatology, Shandong University (protocol no. 20180602).
Patient consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taurog JD, Chhabra A and Colbert RA:
Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med.
374:2563–2574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu X, Gu Q, Chen X, Mi W, Wu T and Huang
H: MiR-27a targets DKK2 and SFRP1 to promote reosseointegration in
the regenerative treatment of peri-implantitis. J Bone Miner Res.
34:123–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor α
suppresses the mesenchymal stem cell osteogenesis promoter miR-21
in estrogen deficiency-induced osteoporosis. J Bone Miner Res.
28:559–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Magrey MN and Khan MA: The paradox of bone
formation and bone loss in ankylosing spondylitis: Evolving new
concepts of bone formation and future trends in management. Curr
Rheumatol Rep. 19:172017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weitzmann MN: Bone and the immune system.
Toxicol Pathol. 45:911–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun SC, Chang JH and Jin J: Regulation of
nuclear factor-κB in autoimmunity. Trends Immunol. 34:282–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Litke JL and Jaffrey SR: Highly efficient
expression of circular RNA aptamers in cells using autocatalytic
transcripts. Nat Biotechnol. 37:667–675. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keller SA, Schattner EJ and Cesarman E:
Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary
effusion lymphoma cells. Blood. 96:2537–2542. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunkemoeller B, Bancroft T, Xing H, Morris
AH, Luciano AK, Wu J, Fernandez-Hernando C and Kyriakides TR:
Elevated thrombospondin 2 contributes to delayed wound healing in
diabetes. Diabetes. 68:2016–2023. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salahuddin S, Fath EK, Biel N, Ray A, Moss
CR, Patel A, Patel S, Hilding L, Varn M, Ross T, et al:
Epstein-barr virus latent membrane protein-1 induces the expression
of SUMO-1 and SUMO-2/3 in LMP1-positive lymphomas and cells. Sci
Rep. 9:2082019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osta B, Benedetti G and Miossec P:
Classical and paradoxical effects of TNF-α on bone homeostasis.
Front Immunol. 5:482014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell Res. 19:71–88. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Tu Q, Bonewald LF, He X, Stein G,
Lian J and Chen J: Effects of miR-335-5p in modulating osteogenic
differentiation by specifically downregulating Wnt antagonist DKK1.
J Bone Miner Res. 26:1953–1963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda Y, Farina NH, Matzelle MM, Fanning
PJ, Lian JB and Gravallese EM: Synovium-derived MicroRNAs regulate
bone pathways in rheumatoid arthritis. J Bone Miner Res.
32:461–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sang C, Zhang Y, Chen F, Huang P, Qi J,
Wang P, Zhou Q, Kang H, Cao X and Guo L: Tumor necrosis factor
alpha suppresses osteogenic differentiation of MSCs by inhibiting
semaphorin 3B via Wnt/β-catenin signaling in estrogen-deficiency
induced osteoporosis. Bone. 84:78–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diarra D, Stolina M, Polzer K, Zwerina J,
Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C,
et al: Dickkopf-1 is a master regulator of joint remodeling. Nat
Med. 13:156–163. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pritchard CC, Cheng HH and Tewari M:
MicroRNA profiling: Approaches and considerations. Nat Rev Genet.
13:358–369. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang
W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL,
et al: miR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Yang L, Zhang D, Gao C, Wu J, Zhu
Y and Zhang H: MicroRNA-218 negatively regulates osteoclastogenic
differentiation by repressing the nuclear factor-κB signaling
pathway and targeting tumor necrosis factor receptor 1. Cell
Physiol Biochem. 48:339–347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng L, Hu G, Jin L, Wang C and Niu H:
Involvement of microRNA-23b in TNF-alpha-reduced BMSC osteogenic
differentiation via targeting runx2. J Bone Miner Metab.
36:648–660. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mi W, Shi Q, Chen X, Wu T and Huang H:
miR-33a-5p modulates TNF-alpha-inhibited osteogenic differentiation
by targeting SATB2 expression in hBMSCs. FEBS Lett. 590:396–407.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Zhang P and Gu J: miR-29a modulates
tumor necrosis factor-α-induced osteogenic inhibition by targeting
Wnt antagonists. Dev Growth Differ. 57:264–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sui L, Wang M, Han Q, Yu L, Zhang L, Zheng
L, Lian J, Zhang J, Valverde P, Xu Q, et al: A novel
Lipidoid-MicroRNA formulation promotes calvarial bone regeneration.
Biomaterials. 177:88–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matzelle MM, Gallant MA, Condon KW, Walsh
NC, Manning CA, Stein GS, Lian J B, Burr DB and Gravallese EM:
Resolution of inflammation induces osteoblast function and
regulates the Wnt signaling pathway. Arthritis Rheum. 64:1540–1550.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walsh NC and Gravallese EM: Bone
remodeling in rheumatic disease: A question of balance. Immunol
Rev. 233:301–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: An in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arriero Mdel M, Ramis JM, Perelló J and
Monjo M: Differential response of MC3T3-E1 and human mesenchymal
stem cells to inositol hexakisphosphate. Cell Physiol Biochem.
30:974–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naismith JH and Sprang SR: Tumor necrosis
factor receptor superfamily. J Inflamm. 47:1–7. 1995.PubMed/NCBI
|
|
30
|
Idriss HT and Naismith JH: TNF alpha and
the TNF receptor superfamily: Structure-function relationship(s).
Microsc Res Tech. 50:184–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J
and Yang P: Dose-specific effects of tumor necrosis factor alpha on
osteogenic differentiation of mesenchymal stem cells. Cell Prolif.
44:420–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao B: TNF and bone remodeling. Curr
Osteoporos Rep. 15:126–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Zhang J, Wang C, Qi Y, Du M, Liu
W, Yang C and Yang P: Low concentrations of TNF-α promote
osteogenic differentiation via activation of the ephrinB2-EphB4
signalling pathway. Cell Prolif. 50:e123112017. View Article : Google Scholar
|
|
34
|
Karnes JM, Daffner SD and Watkins CM:
Multiple roles of tumor necrosis factor-alpha in fracture healing.
Bone. 78:87–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang FS, Chuang PC, Lin CL, Chen MW, Ke
HJ, Chang YH, Chen YS, Wu SL and Ko JY: MicroRNA-29a protects
against glucocorticoid-induced bone loss and fragility in rats by
orchestrating bone acquisition and resorption. Arthritis Rheum.
65:1530–1540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Redlich K and Smolen JS: Inflammatory bone
loss: Pathogenesis and therapeutic intervention. Nat Rev Drug
Discov. 11:234–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jing Y, Feng J ZH, Zhao X, Yang J, Bai D
and Han X: Role of DKK1 in periodontitis and innovative strategy
with its neutralizing antibody for periodontitis treatment. OHDM.
13:994–997. 2014.
|
|
38
|
Heiland GR, Zwerina K, Baum W, Kireva T,
Distler JH, Grisanti M, Asuncion F, Li X, Ominsky M, Richards W, et
al: Neutralisation of Dkk-1 protects from systemic bone loss during
inflammation and reduces sclerostin expression. Ann Rheum Dis.
69:2152–2159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Juliana C, Fernandes-Alnemri T, Wu J,
Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP and
Alnemri ES: Anti-inflammatory compounds parthenolide and Bay
11-7082 are direct inhibitors of the inflammasome. J Biol Chem.
285:9792–9802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu L, Zhang L, Wang Z, Li C, Li S, Li L,
Fan Q and Zheng L: Melatonin suppresses estrogen deficiency-induced
osteoporosis and promotes osteoblastogenesis by inactivating the
NLRP3 inflammasome. Calcif Tissue Int. 103:400–410. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5:e11872014. View Article : Google Scholar : PubMed/NCBI
|