Introduction
Diabetes is a serious and complex disease
characterized by chronic hyperglycemia, which aggravates a series
of metabolic reactions and increases the production of advanced
glycosylation end products (AGEs). It has been reported that in
diabetes the development of AGE-induced retinal inflammation
results in blood-retinal barrier damage, which in turn leads to
diabetic macular edema and retinal neovascularization (1,2).
Although blood glucose control has been reported to significantly
reduce the incidence of microvascular complications, some patients
still suffer from proliferative diabetic retinopathy (DR) for
>30 years (3). Therefore, it is
of vital importance to reveal the underlying mechanisms of retinal
impairment in DR. Several studies have shown that the endoplasmic
reticulum (ER) stress promotes inflammation and apoptosis in DR
(4,5). The pro-inflammatory cytokines,
including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and
IL-6, contribute to endothelial cell impairment and disruption of
blood-brain barrier, resulting in the development of macular edema
(6). Furthermore, ER stress and
inflammatory processes synergistically aggravate the progression of
DR (7,8).
ER stress is induced by several pathological
factors, and this is followed by aberrant accumulation of proteins.
At the early stages of ER, the unfolded-protein response pathway
upregulates the expression levels of transcriptases and chaperone
proteins, thereby increasing the volume and enhancing the ability
of protein folding in the ER for restoring its homeostasis. Protein
kinase R-like ER kinase (PERK), a member of the eukaryotic
initiation factor 2α (eIF2α) protein kinase family, is activated in
response to several stress stimuli. In addition, phosphorylated
(p)-PERK induces the protective stress mechanism by phosphorylating
eIF2α, which subsequently regulates the expression of activating
transcription factor (ATF) proteins. When severe or sustaining ER
stress causes irreversible cell damage, the apoptosis signaling
pathway may be activated by CCAAT/enhancer-binding protein
homologous protein (CHOP) upregulation (9). CHOP upregulation may result in the
activation of ATF4, promoting the expression of apoptosis-related
proteins, including Bax and caspase 12 (10–12).
Therefore, these ER stress-related proteins (p-PERK, ATF4, CHOP,
p-Eif2α) may reflect ER stress more accurately.
It has been demonstrated that the long non-coding
RNA growth arrest-specific 5 (lncRNA GAS5) regulates gene
expression and affects protein function. For example, a previous
study revealed that lncRNA GAS5 knockdown induced glucose uptake in
normal adipocytes and downregulated the expression of insulin
receptor genes by binding to the promoter region (13). Other studies have shown that GAS5
expression is abnormally altered and triggers specific pathological
processes in several diseases. For example, the expression of
lncRNA GAS5 was decreased in endometrial carcinoma tissue specimens
from patients with type 2 diabetes and in human endometrial
carcinoma cells exposed to high glucose (HG); however, lncRNA GAS5
overexpression inhibited the proliferation of these cells (14). Furthermore, gene expression
analysis based on the Gene Expression Omnibus database revealed
that GAS5 was downregulated in diabetic lymphatic endothelial cells
(15). These findings indicated
that GAS5 served a key role in diabetes. In addition, lncRNA GAS5
has been reported to be involved in other biological processes,
such as inflammation and apoptosis (16–18).
Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA)
is a type of ATPase that transports cations across the cell
membrane with 10 transmembrane domains. SERCA2b is located at the
sarcoplasmic reticulum or ER membrane of all types of cells and is
responsible for maintaining intracellular Ca2+
homeostasis. It has been reported that SERCA2b regulates ER stress
(19), which is attenuated in
pancreatic β-cells (20). A
previous study has indicated that SERCA2 exerts protective effects
by regulating HG-treated ER stress and apoptosis in podocytes
(19). Therefore, the present
study aimed to investigate the role and function of GAS5 and
SERCA2b in DR. The human adult retinal pigment epithelium 19
(ARPE-19) cell line was treated with a high concentration of
glucose and was used to mimic DR in vitro (21).
Materials and methods
Cells
The ARPE-19 cell line was purchased from the
American Type Culture Collection and cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 mg/ml streptomycin. Cells were incubated in a humidified
atmosphere containing 5% CO2 at 37°C. ARPE-19 cells were
detached following digestion with trypsin (2.5 g/l; Gibco; Thermo
Fisher Scientific, Inc.) until intercellular space enlarged,
cytoplasm retracted and the cells exhibited a round morphology.
These changes were observed by inverted microscopy (magnification,
×20). Subsequently, the trypsin solution was centrifuged at 250 × g
for 5 min to collect cells and then cells were resuspended in DMEM
medium for further experiments. Cells were divided into three
groups as follows: i) Normal glucose (NG) group, which were treated
with 5.5 mM glucose; ii) osmotic control mannitol group (MG), which
were treated with 5.5 mM glucose plus 27.5 mM mannitol, as
previously described (21–23); and iii) high glucose (HG) group,
which were treated with 33.3 mM glucose. Cells were cultured for 48
h at 37°C before further experiments. The transfection experiments,
described below, were performed in the HG group only. Following
transfection, cells were further incubated at 37°C for 48 h. The
transfection efficiency was evaluated by reverse
transcription-quantitative PCR (RT-qPCR). After transfection, cells
were treated with HG for 48 h at 37°C.
RT-qPCR
Total RNA was extracted from cells using
TRIzol® (Beyotime Institute of Biotechnology) and
reversely transcribed into cDNA using the High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.).
Subsequently, qPCR was performed using the Maxima SYBR Green qPCR
Master Mix (Thermo Fisher Scientific, Inc.) and the Real-Time PCR
System (Applied Biosystems, Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR: Initial
denaturation at 94°C for 5 min; and 40 cycles of 95°C for 5 sec,
65°C for 34 sec and 72°C for 30 sec. The following primers were
used for qPCR: SERCA2b, forward 5′-CGAACCCTTGCCACTCATCTTC3′,
reverse 5′-TGCCGAGAACGAGCAGGATTTG-3′; GAS5, forward
5′-CGACTCCTGTGAGGTATGGTG-3′, reverse 5′-ATCCTTCCTTGGGGACACAAC-3′;
for GADPH control, forward 5′-GGAGCGAGATCCCTCCAAAAT-3′, reverse
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative expression of GAS5 and
SERCA2b mRNA was quantified using 2−ΔΔCq method
(22) and normalized to the
internal reference gene GAPDH.
Western blot
Following treatment, ARPE-19 cells were lysed with
RIPA buffer (Beyotime Institute of Biotechnology) supplemented with
PMSF and total protein extracts were isolated. Protein
concentration was quantified using a bicinchoninic acid assay.
Subsequently, proteins were separated by SDS-PAGE (stacking gel 5%;
separation gel 12%) and transferred to PVDF membranes. The
membranes were blocked with 5% skim milk for 2 h at room
temperature and incubated with primary antibodies against SERCA2B
(1:1,000; cat. no. ab2861; Abcam), ATF4 (1:1,000; cat. no.
ab184909; Abcam), Bcl-2 (1:1,000; cat. no. ab32124; Abcam), Bax
(1:1,000; cat. no. ab32503; Abcam), total PERK [1:1,000; cat. no.
3192; Cell Signaling Technology (CST)], phosphorylated (p)-PERK
(1:1,000; cat. no. 3179; CST), eIF2α (1:1,000; cat. no. 2103; CST),
p-eIF2α (1:1,000; cat. no. 3398; CST), Bad (1:2,000; cat. no.
ab32445; Abcam), caspase 12 (1:1,000; cat. no. 55238-1-AP;
Proteintech), CHOP (1:1,000; cat. no. BS1136; Bioworld Technology),
GADPH (1:10,000; cat. no. ab181603; Abcam) at 4°C overnight. On the
following day, membranes were washed with tris-buffered saline +
Tween-20 (1X TBST) and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (1:1,000; cat. no. 31460; Thermo
Fisher Scientific, Inc.) or HRP-conjugated goat anti-mouse
(1:2,000; cat. no. ab47827; Abcam) secondary antibodies at room
temperature for 1 h, followed by three washes with TBST. The
immunoreactive proteins were visualized using the enhanced
chemiluminescence method (Advansta, America), and images captured
by a gel imaging system (Gene, America). ImageJ software (version
1.46r; National Intitutes of Health) was used to determine the mean
gray value, and calculate the gray value ratio between the target
protein and the internal reference protein (GADPH), to indicate the
relative protein expression levels.
Plasmid transfection
GAS5 recombinant overexpression plasmid pcDNA3.1
(OE-GAS5), SERCA2b recombination plasmid (OE-SERCA2b) and the empty
control plasmid (OE-NC) were purchased from Genomeditech. Short
hairpin RNA (shRNA) SERCA2b plasmids (pGPU6/GFP/Neo,
shRNA-SERCA2B-1 and shRNA-SERCA2B-2) and control plasmids with
scrambled sequences (pGPU6/GFP/Neo; shRNA-NC) were obtained from
GenePharma. Cells were seeded into the 6-well plate
(1×105/well). For transfection, 200 µl transfection
buffer, 4 µl Lipofectamine (Thermo Fisher Scientific, Inc) and 2 µg
plasmid were added into an eppendorf tube and mixed. Subsequently,
cells were transfected with the transfection mixture for 48 h at
37°C. Following the incubation, subsequent experiments were
performed.
ELISA
ARPE-19 cells were homogenized at 12,000 × g at 4°C
for 15 min and the supernatants were collected using a pipette. The
supernatant was analyzed using TNF-α (cat. no. SBJ-H0038), IL-1β
(cat. no. SBJ-H0417) or IL-6 (cat. no. SBJ-H0456) ELISA kits
(Nanjing SenBeiJia Biological Technology Co., Ltd.) according to
the manufacturer's instructions.
Flow cytometry
Cells were seeded into 6-well plates
(1×105/well) and treated with MG, HG, OE-GAS5 or
shRNA-SERCA2B-1. The Annexin V-FITC/PI Cell Apoptosis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect
apoptotic rates. Briefly, cells of each group were digested with
trypsin and washed with PBS, followed by centrifugation at 1,000 ×
g to discard the supernatant. Then, cells were resuspended in
binding buffer (500 µl) and subsequently, Annexin V-FITC was added
to the cells for 15 min at 37°C. Next, propidium iodide staining
solution was added to the cells at room temperature for 15 min in
the dark. Apoptotic rates were measured by flow cytometry. The
scatter plot quadrants were divided as: Upper left
(FITC−/PI+), cell fragments that have lost
their cell membranes, or dead cells from other causes; lower left
(FITC−/PI−), normal (living) cells; upper
right (FITC+/PI+), non-viable apoptotic
cells; lower right (FITC+/PI−), viable
apoptotic cells. The cell apoptotic rate was calculated by the
formula: Cell apoptotic rate = non-viable apoptotic cells + viable
apoptotic cells.
Statistical analysis
The experimental data were analyzed using GraphPad
Prism (version 6.0; GraphPad Software, Inc.) and presented as the
mean ± SEM. The difference among groups was analyzed with one-way
ANOVA. The comparison of two groups was analyzed with post-hoc
Tukey's test. P<0.05 was considered as statistically
significant. All experiments were repeated three times.
Results
GAS5 and SERCA2b expression levels
decrease in HG-treated ARPE-19 cells
RT-qPCR and western blotting were used to detect
lncRNA GAS5 and SERCA2b protein expression levels, respectively,
after varying glucose treatments in ARPE-19 cells. There was no
significant difference in GAS5 (Fig.
1A), SERCA2b protein and ER stress levels (Fig. 1B and C) between NG group and MG
group, suggesting that the influence of HG on ARPE-19 was not due
to osmotic pressure. By contrast, HG treatment reduced the levels
of GAS5 and SERCA2b compared with NG and MG, indicating that GAS5
and SERCA2b may serve vital roles in cells under HG exposure. In
addition, HG-induced ER stress markers were assessed by western
blotting in ARPE-19 cells. The results indicated that the ratios of
p-PERK/PERK and p-Eif2α/Eif2α were significantly increased in
HG-induced cells compared with NG and MG cells. Furthermore, ATF4
and CHOP levels were increased by HG compared with NG and MG cells
(Fig. 3C). The results suggested
that HG significantly increased ER stress compared with NG or MG
treatments (Fig. 1B and C).
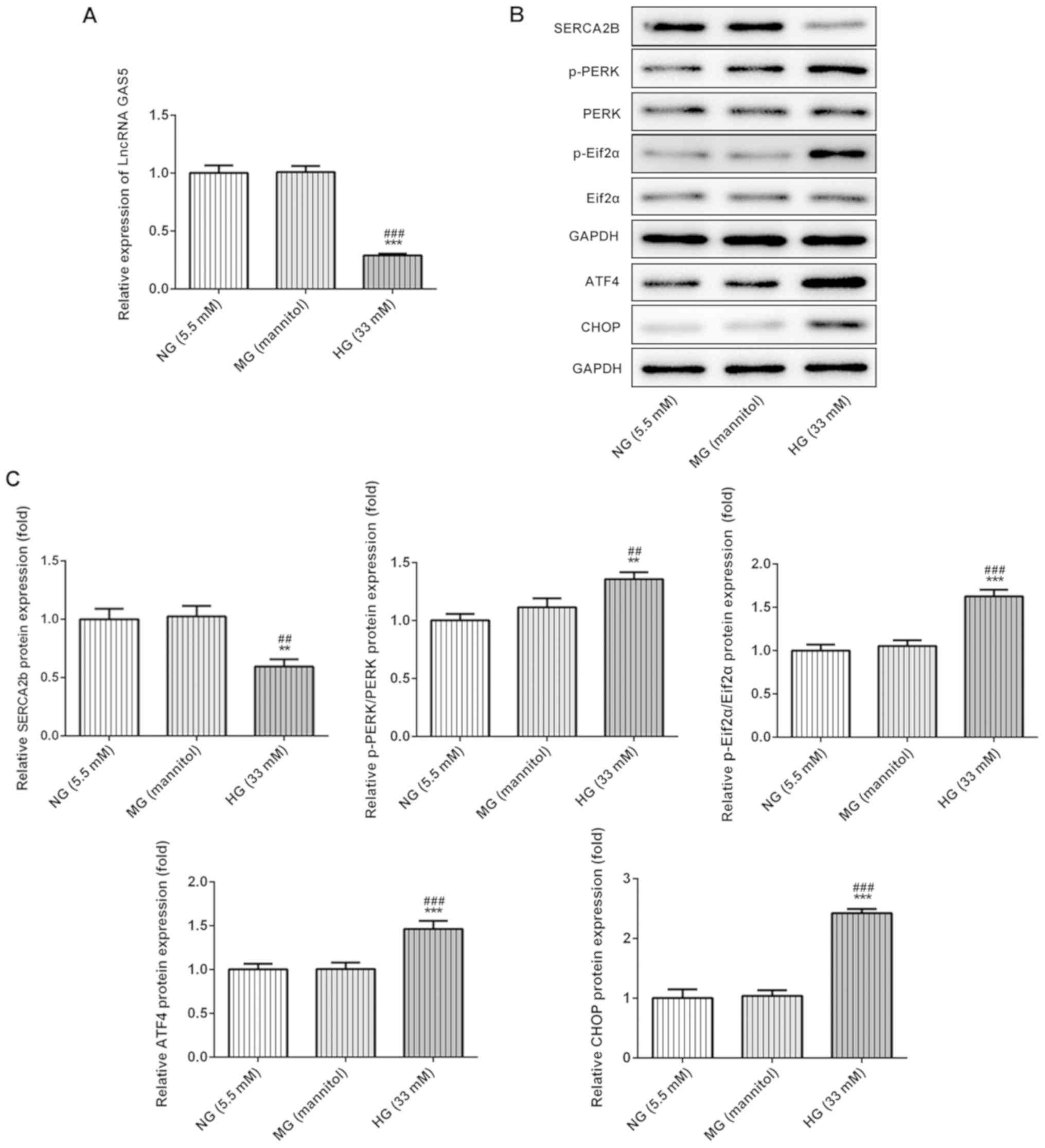 | Figure 1.HG treatment decreases lncRNA GAS5
levels and increases ER stress in ARPE-19 cells. (A) Reduced lncRNA
GAS5 levels in HG-treated ARPE-19 cells were determined by reverse
transcription-quantitative PCR. (B) ER stress-related protein
expression in HG-treated ARPE-19 cells was (B) determined by
western blotting and (C) semi-quantified. **P<0.01 and
***P<0.001 vs. NG; ##P<0.01 and
###P<0.001 vs. MG. ATF4, activating transcription
factor 4; CHOP, CCAAT/enhancer-binding protein homologous protein;
ER, endoplasmic reticulum; HG, high glucose; lncRNA GAS5, long
non-coding RNA growth arrest-specific transcript 5; MG, mannitol
control group; NG, normal glucose; PERK, protein kinase R-like ER
kinase; SERCA2b, sarcoplasmic/endoplasmic reticulum Ca2+
ATPase 2; eIF2α, eukaryotic initiation factor 2α; p,
phosphorylated. |
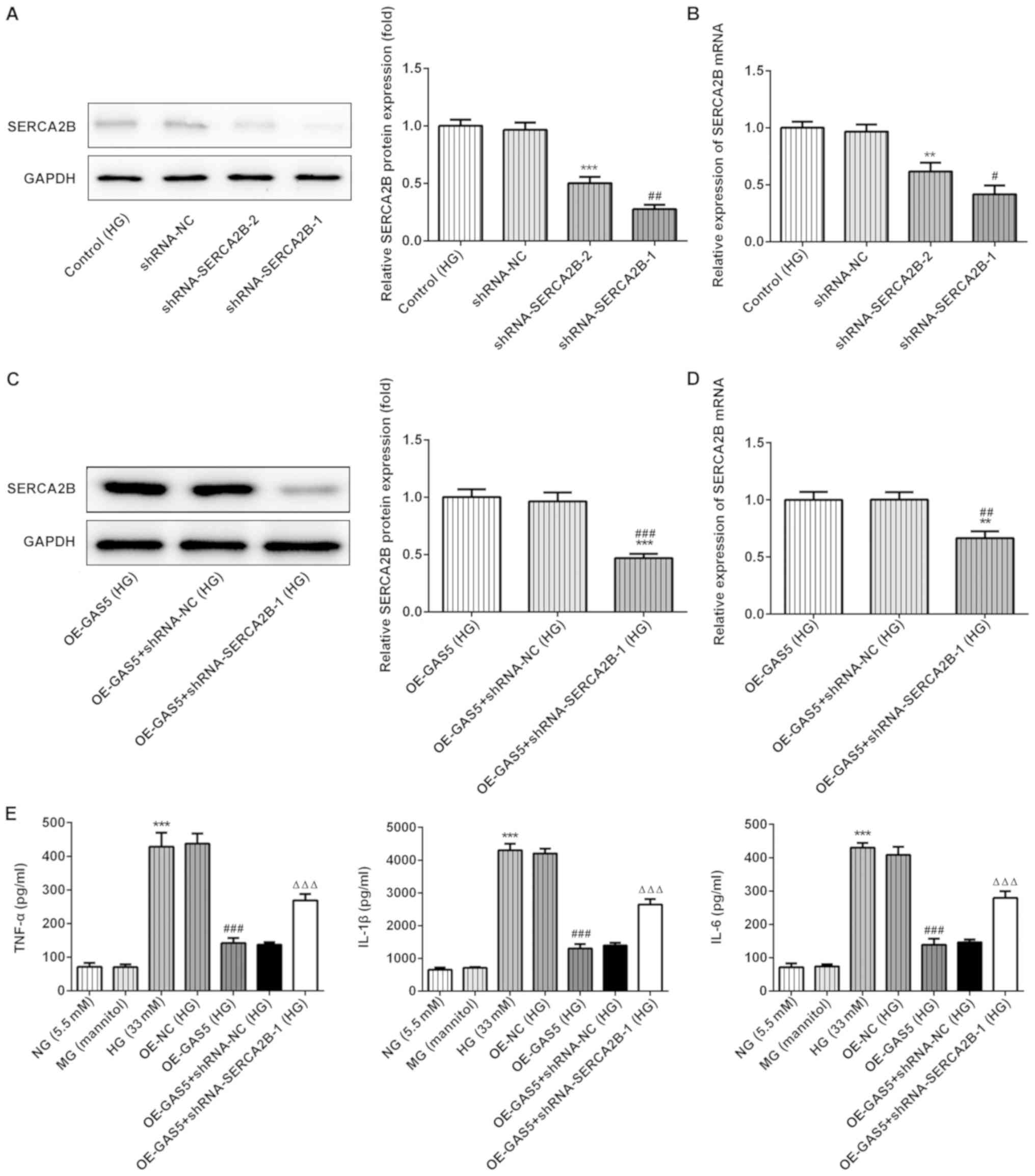 | Figure 3.SERCA2b knockdown reverses the
effects of GAS5 on inflammation in HG-treated ARPE-19 cells. (A and
B) Evaluation of the transfection efficacy of the SERCA2b knockdown
plasmids shRNA-SERCA2B-1 and shRNA-SERCA2B-2 by (A) western
blotting and (B) RT-qPCR. **P<0.01 and ***P<0.001 vs.
shRNA-NC. #P<0.05 and ##P<0.01 vs.
shRNA-SERCA2B-2 (C and D) SERCA2b was detected by (C) western
blotting and (D) RT-qPCR after the plasmids overexpressing GAS5 and
SERCA2b were co-transfected into HG-treated ARPE-19 cells.
##P<0.01 and ###P<0.001 vs. OE-GAS5;
**P<0.01 and ***P<0.001 vs. OE-GAS5 + shRNA-NC. (E) The
effects of GAS5 overexpression and SERCA2b knockdown on
inflammatory markers. ΔΔΔP<0.001 vs. OE-GAS5 +
shRNA-NC; ###P<0.001 vs. OE-NC; ***P<0.001 vs MG.
GAS5, growth arrest-specific transcript 5; HG, high glucose; IL,
interleukin; MG, mannitol control group; NC, negative control; NG,
normal glucose; OE, overexpression vector; RT-qPCR, reverse
transcription-quantitative PCR; SERCA2b, sarcoplasmic/endoplasmic
reticulum Ca2+ ATPase 2; shRNA, short hairpin RNA;
TNF-α, tumor necrosis factor-α. |
Overexpression of GAS5 and SERCA2b
inhibits HG-induced ER stress
Next, the role of GAS5 overexpression in ARPE-19
cells treated with HG was investigated. The results presented in
Fig. 2A show the increased
expression levels of GAS5 in OE-GAS5-transfected cells compared
with the OE-NC-transfected control group, which indicated
successful transfection of the overexpression vector (Fig. 2A). In addition, overexpressed GAS5
significantly increased SERCA2b protein expression levels,
suggesting that GAS5 affected the expression of SERCA2b (Fig. 2B). Furthermore, compared with the
control groups, upregulation of GAS5 significantly reduced the
total protein expression levels of ATF4 and CHOP, as well as the
relative protein phosphorylation ratio of p-PERK/PERK and
p-eIF2α/eIF2α in ARPE-19 cells exposed to HG (Fig. 2B).
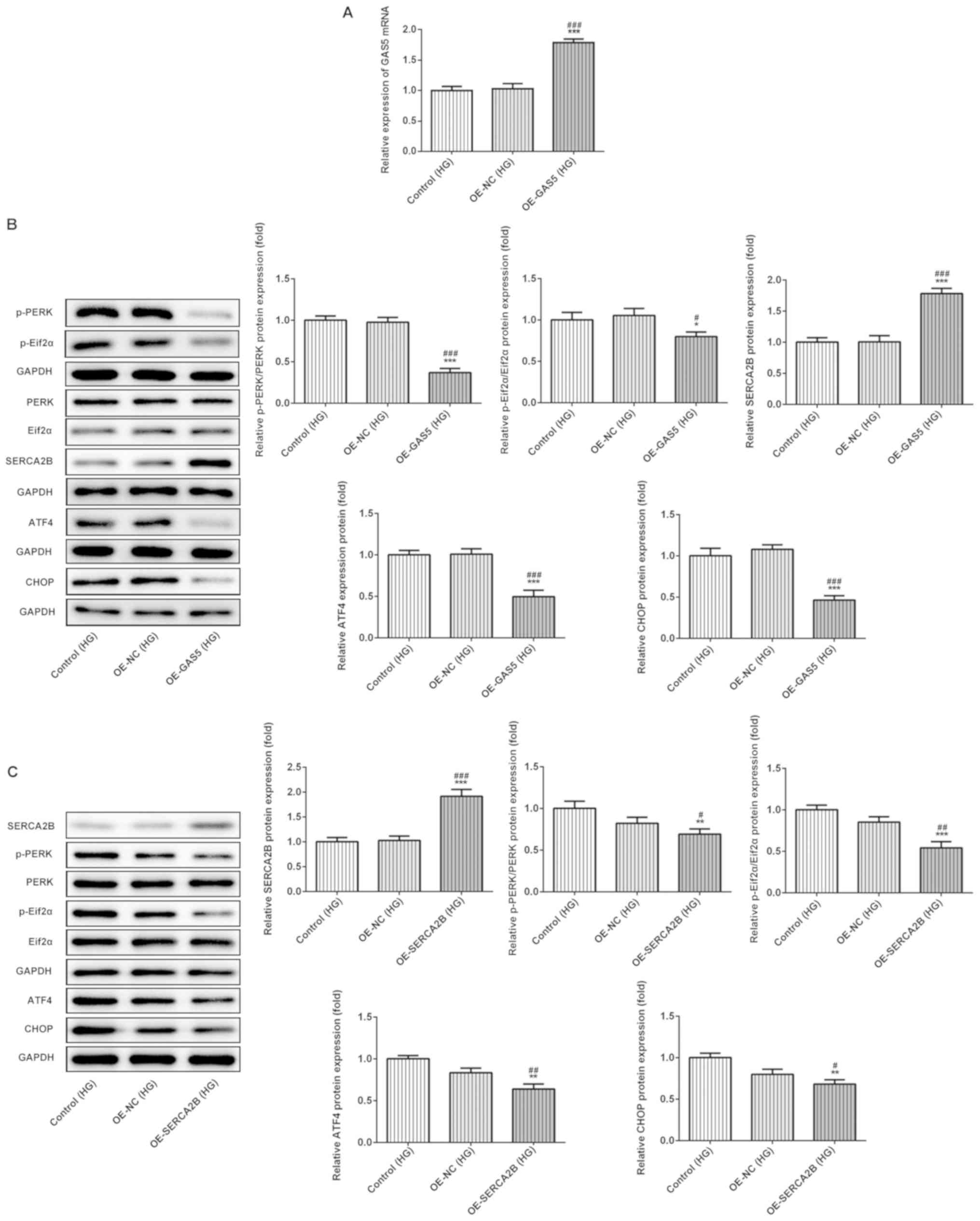 | Figure 2.Overexpression of GAS5 or SERCA2b
affects ER stress in HG-treated ARPE-19 cells. (A) lncRNA GAS5
expression levels were increased in cells transfected with
pcDNA-GAS5, as determined by reverse transcription-quantitative
PCR. (B) GAS5 overexpression upregulated SERCA2b protein expression
levels and reduced ER stress. (C) SERCA2b expression and ER stress
were assessed in cells transfected with pcDNA-SERCA2b. *P<0.05,
**P<0.01 and ***P<0.001 vs. OE-NC; #P<0.05,
##P<0.01 and ###P<0.001 vs. control.
ATF4, activating transcription factor 4; CHOP,
CCAAT/enhancer-binding protein homologous protein; eIF2α,
eukaryotic initiation factor 2α; ER, endoplasmic reticulum; GAS5,
growth arrest-specific transcript 5; HG, high glucose (33 mM); NC,
negative control; OE, overexpression vector; p-, phosphorylated;
PERK, protein kinase R-like ER kinase; SERCA2b,
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2. |
To study the role of SERCA2b in HG-treated ARPE-19
cells, a SERCA2b overexpression plasmid was used to transfect cells
(Fig. 2C). SERCA2b overexpression
also significantly decreased the total protein expression levels of
ATF4 and CHOP, as well as the ratios of p-PERK/PERK and
p-eIF2α/eIF2α. Thus, these data suggested that GAS5 and SERCA2b
could inhibit HG-induced ER stress in vitro (Fig. 2C).
Overexpression of GAS5 reduces
HG-induced inflammatory factors through SERCA2b
Considering that GAS5 was found to significantly
alter SERCA2b expression, two SERCA2b knockdown plasmids
(shRNA-SERCA2B-1 and shRNA-SERCA2B-2) were used to decrease
expression of endogenous SERCA2b in ARPE-19 cells (Fig. 3A and B). The results showed that
the effects of shRNA-SERCA2B-1 plasmid in reducing SERCA2b
expression was more efficient than shRNA-SERCA2B-2; thus,
shRNA-SERCA2B-1 plasmid was used to perform subsequent experiments.
To evaluate the effects of shRNA-SERCA2B-1 plasmid on SERCA2b
expression, ARPE-19 cells were co-transfected with OE-GAS5 and
shRNA-SERCA2b. Western blotting and RT-qPCR results indicated that
SERCA2b protein and mRNA expression levels, respectively, were
significantly reduced by shRNA-SERCA2b in the presence of GAS5
overexpression plasmid, compared with the control groups (Fig. 3C and D). TNF-α, IL-1β and IL-6
concentrations were detected by ELISA and used to assess
inflammation indexes in vitro (Fig. 3E). Overexpression of GAS5
significantly reduced proinflammation-related factor levels
compared with the OE-NC group. However, SERCA2b gene knockdown
significantly reversed the inhibitory effects of GAS5 upregulation
on inflammation. Thus, GAS5 may affect inflammation by regulating
SERCA2b.
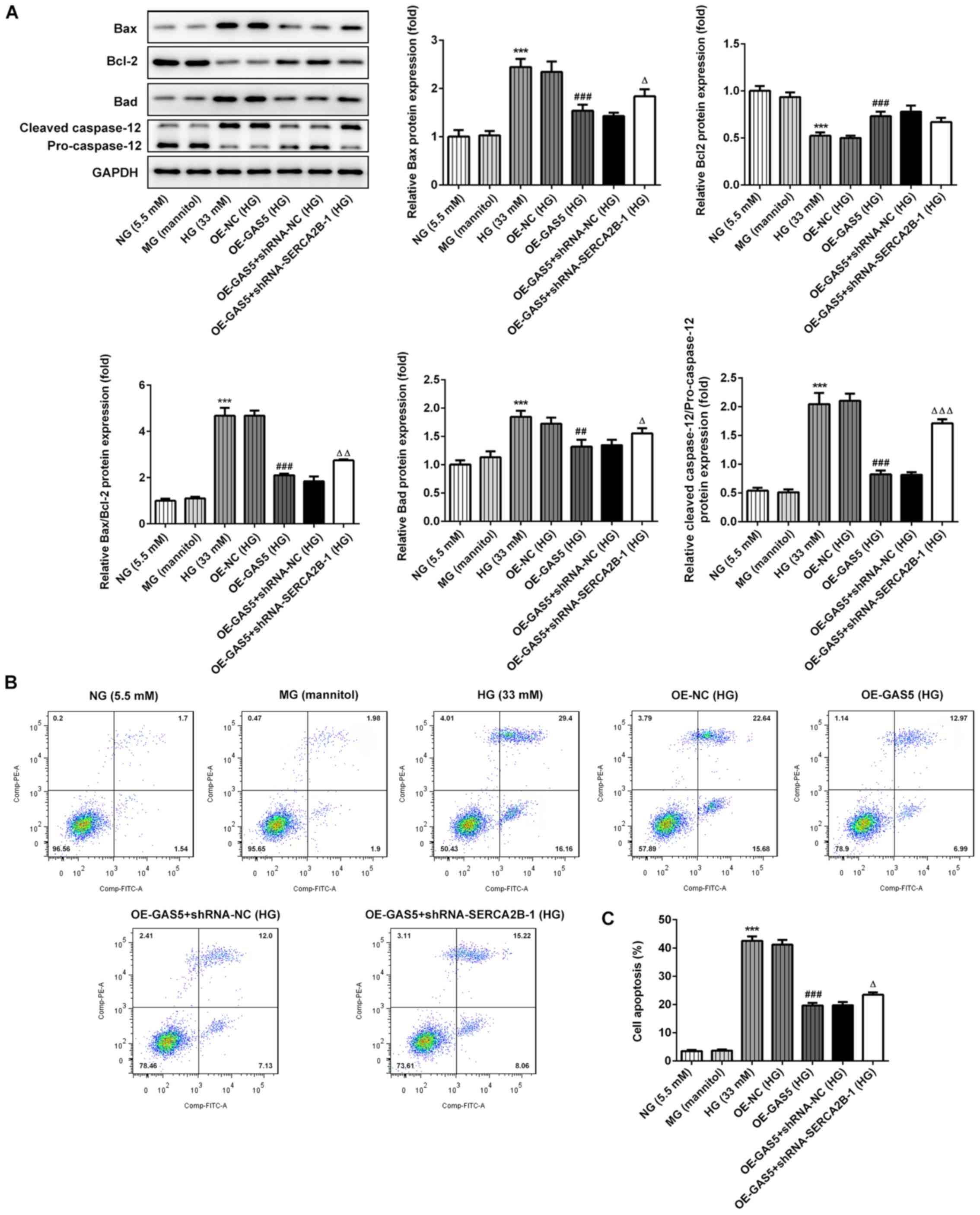
Overexpression of GAS5 reduces
HG-induced apoptosis through SERCA2b
The apoptosis levels were assessed by the
measurement of apoptosis-related proteins by western blotting and
flow cytometry (Fig. 4). Bcl-2,
Bad, Bax and caspase 12 were used as apoptosis markers. Apoptosis
levels were partially reduced by GAS5 overexpression compared with
the OE-NC group, as indicated by the decreased ratio of Bax/Bcl2
and cleaved caspase-12/pro-caspase 12, as well as the increased
levels of Bad expression (Fig.
4A). Moreover, flow cytometry results indicated that GAS5
overexpression reduced the percentage of cell apoptosis in HG
conditions (Fig. 4B and C).
However, SERCA2b gene knockdown was able to significantly reverse
the inhibitory effects of GAS5 overexpression on cell apoptosis
(Fig. 4A-C).
Discussion
In the present study lncRNA GAS5 expression levels
were decreased in ARPE-19 cells treated with HG, which is
consistent with previous studies that have demonstrated that HG
treatment induces decreased GAS5 expression in several tissues or
cells, such as endometrial carcinoma and adipocytes (13,14).
GAS5 overexpression significantly increased SERCA2b levels and
reduced ER stress in ARPE-19 cells treated with HG, indicating a
possible association between them. Several studies have provided
insights on GAS5 mechanism of action. For example, GAS5 can bind to
the promoter region of the insulin receptor gene to modulate its
expression in nondiabetic adipocytes (13), acting as a competing endogenous RNA
for microRNA (miRNA) involved in the regulation of the expression
of numerous proteins involved in cancer (23,24).
The findings of the present study suggest that GAS5 may serve a
role as a regulatory RNA or a miRNA sponge to regulate miRNA levels
and affect SERCA2b expressions in DR. It has been reported that
SERCA is involved in maintaining Ca2+ homeostasis in the
ER, by transferring Ca2+ from the cytosol into the ER
(25). Therefore, changes in
SERCA2b expression levels may affect the Ca2+
concentration, which in turn affects Ca2+-mediated
signaling pathways, resulting in abnormal metabolic processes, such
as lipid metabolism (26). The
eleventh transmembrane helix (TM11) of SERCA2b fine-tunes the
intramolecular interactions with other transmembrane regions to
modulate SERCA2b activity (27).
The modulation of TM11 plays a key role in maintaining
Ca2+ homeostasis in the ER. Recent studies have
suggested that ER-resident proteins can regulate SERCA2b activity
(28–30). The exact mechanism by which these
proteins affect the location of TM11 or its interaction with other
regions of SERCA2b have not yet been fully addressed (28–30).
These results indicate a possible mutual influence between ER
stress and SERCA2b activity; however, additional studies are
required to further confirm these findings. Calpain is activated in
response to abnormal Ca2+ levels in the cytosol and
subsequently promotes caspase 12 activation, inducing apoptosis
(31). Therefore, it is possible
that SERCA2b overexpression inhibited ER stress-induced apoptosis
by altering Ca2+ levels in HG-treated ARPE-19 cells in
the present study. However, this mechanism should be further
investigated.
lncRNA GAS5 overexpression increased anti-apoptotic
Bcl-2 and decreased pro-apoptotic Bad and Bax protein expression
levels. In addition, SERCA2b knockdown significantly reversed the
inhibitory effects of GAS5 during inflammation and apoptosis,
indicating that GAS5 affected the induction of these two processes
by HG partially through SERCA2b. Caspase 12 is located in the ER
and is a major protein involved in ER stress-induced apoptosis
(32,33). Caspase 12 is caused by specific
stimuli, such as higher calcium, hypoxia and lower glucose levels
(32,33). Moreover, downregulation of caspase
12 expression attenuated methylglyoxal-modified collagen-induced
apoptosis (34). The results of
the present study indicated that SERCA2b and GAS5 mediated a
decrease in CHOP protein expression. Previous studies have
indicated that the CHOP-mediated apoptotic rate was reduced
partially by increased Bcl-2 and decreased Bad, Bax and caspase 12
levels (11,35). These findings suggested that GAS5
attenuated ER stress-induced apoptosis through SERCA2b. The
imbalance of the expression of pro-apoptotic and anti-apoptotic
proteins of the Bcl-2 family may contribute to mitochondrial damage
and subsequently activate caspases, inducing cell apoptosis via the
intrinsic apoptosis pathway (36).
Among the Bcl-2 family members, Bcl-2 is involved in preserving
mitochondrial integrity, which may be disrupted by Bax (37). Furthermore, flow cytometry results
from the present study showed that apoptosis was significantly
reduced by GAS5 overexpression, suggesting that changes on these
apoptosis-related proteins might lead to reduced apoptosis levels.
There are certain mechanisms involved in cell apoptosis, one of
which was ER stress-mediated apoptosis. On the one hand,
phosphorylation of eIF2α induced by PERK could decrease protein
synthesis to reduce ER stress; on the other hand, it also could
mediate CHOP expression to induce apoptosis (38). The current study indicated that
GAS5 affected inflammatory markers partly through SERCA2b.
Previous studies showed that ER stress induced cell
apoptosis and inflammation (39,40).
Inflammation in DR is facilitated through increasing ER stress
(41), suggesting that HG-induced
ER stress may promote inflammatory response. Results from the
present study may aid our further understanding of the mechanism of
retinal pigment epithelium cell impairment induced by HG. Moreover,
shows obvious activation in retinal of mice with DR or in retinal
cell lines exposed to HG. Retinal cell damage can be alleviated
through inhibiting ER stress (42–44).
Thus, GAS5/SERCA2b could be potential targets for treating DR.
In conclusion, HG downregulated GAS5 expression,
followed by a decrease in SERCA2b expression levels., Furthermore,
HG enhanced ER stress-related apoptosis and pro-inflammatory
factors, and induced cell apoptosis in ARPE-19 cells. Therefore,
the results indicated that GAS5 regulated ER stress-related
apoptosis and inflammation in retinal epithelial cells via SERCA2b.
LncGAS5 and SERCA2b may serve as potential therapeutic targets for
DR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, CW and XS conceived and designed the study,
collected, analysed and interpreted the data, and revised the
manuscript. LJ wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumari N, Karmakar A and Ganesan SK:
Targeting epigenetic modifications as a potential therapeutic
option for diabetic retinopathy. J Cell Physiol. 235:1933–1947.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leal EC, Manivannan A, Hosoya K, Terasaki
T, Cunha-Vaz J, Ambrosio AF and Forrester JV: Inducible nitric
oxide synthase isoform is a key mediator of leukostasis and
blood-retinal barrier breakdown in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 48:5257–5265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diabetes Control and Complications
Trial/Epidemiology of Diabetes Interventions and Complications
(DCCT/EDIC) Research Group, . Nathan DM, Zinman B, Cleary PA,
Backlund JY, Genuth S, Miller R and Orchard TJ: Modern-day clinical
course of type 1 diabetes mellitus after 30 years' duration: The
diabetes control and complications trial/epidemiology of diabetes
interventions and complications and pittsburgh epidemiology of
diabetes complications experience (1983–2005). Arch Intern Med.
169:1307–1316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elmasry K, Ibrahim AS, Saleh H, Elsherbiny
N, Elshafey S, Hussein KA and Al-Shabrawey M: Role of endoplasmic
reticulum stress in 12/15-lipoxygenase-induced retinal
microvascular dysfunction in a mouse model of diabetic retinopathy.
Diabetologia. 61:1220–1232. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Liu Y, Tan JW, Hu T, Zhang HF,
Sorenson CM, Smith JA and Sheibani N: Tunicamycin-induced
photoreceptor atrophy precedes degeneration of retinal capillaries
with minimal effects on retinal ganglion and pigment epithelium
cells. Exp Eye Res. 187:1077562019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubsam A, Parikh S and Fort PE: Role of
inflammation in diabetic retinopathy. Int J Mol Sci. 19:E9422018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Diiorio P, Jurczyk A,
O'Sullivan-Murphy B, Urano F and Bortell R: Pathological
endoplasmic reticulum stress mediated by the IRE1 pathway
contributes to pre-insulitic beta cell apoptosis in a virus-induced
rat model of type 1 diabetes. Diabetologia. 56:2638–2646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo HL, Hassan HM, Ding PP, Wang SJ, Chen
X, Wang T, Sun LX, Zhang LY and Jiang ZZ: Pyrazinamide-induced
hepatotoxicity is alleviated by 4-PBA via inhibition of the
PERK-eIF2α-ATF4-CHOP pathway. Toxicology. 378:65–75. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Yuan X, Chen Y, Zheng Q, Xu L and
Wu Y: Endoplasmic reticulum stress mediated MDRV p10.8
protein-induced cell cycle arrest and apoptosis through the
PERK/eIF2α pathway. Front Microbiol. 9:13272018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Y, Parag S, Patel R, Lui A, Murr M,
Cai J and Patel NA: Stabilization of lncRNA GAS5 by a small
molecule and its implications in diabetic adipocytes. Cell Chem
Biol. 26:319–330.e316. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Yu Z, Meng X, Zhou S, Xiao S, Li X,
Liu S and Yu P: Long noncoding RNA GAS5 impairs the proliferation
and invasion of endometrial carcinoma induced by high glucose via
targeting miR-222-3p/p27. Am J Transl Res. 11:2413–2421.
2019.PubMed/NCBI
|
|
14
|
Qi M, Zhou Q, Zeng W, Shen M, Liu X, Luo
C, Long J, Chen W, Zhang J and Yan S: Analysis of long non-coding
RNA expression of lymphatic endothelial cells in response to type 2
diabetes. Cell Physiol Biochem. 41:466–474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie X, Dai J, Huang X, Fang C and He W:
MicroRNA-145 inhibits proliferation and induces apoptosis in human
prostate carcinoma by upregulating long non-coding RNA GAS5. Oncol
Lett. 18:1043–1048. 2019.PubMed/NCBI
|
|
16
|
He X, Wang S, Li M, Zhong L, Zheng H, Sun
Y, Lai Y, Chen X, Wei G, Si X, et al: Long noncoding RNA GAS5
induces abdominal aortic aneurysm formation by promoting smooth
muscle apoptosis. Theranostics. 9:5558–5576. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salemi M, Cannarella R, Condorelli RA,
Cimino L, Ridolfo F, Giurato G, Romano C, La Vignera S and Calogero
AE: Evidence for long noncoding RNA GAS5 up-regulationin patients
with Klinefelter syndrome. BMC Med Genet. 20:42019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo H, Wang Y, Zhang X, Zang Y, Zhang Y,
Wang L, Wang H, Wang Y, Cao A and Peng W: Astragaloside IV protects
against podocyte injury via SERCA2-dependent ER stress reduction
and AMPKα-regulated autophagy induction in streptozotocin-induced
diabetic nephropathy. Sci Rep. 7:68522017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zickri MB, Aboul-Fotouh GI, Omar AI,
El-Shafei AA and Reda AM: Effect of stem cells and gene transfected
stem cells therapy on the pancreas of experimentally induced type 1
diabetes. Int J Stem Cells. 11:205–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Capellades J, Navarro M, Samino S,
Garcia-Ramirez M, Hernandez C, Simo R, Vinaixa M and Yanes O:
geoRge: A computational tool to detect the presence of stable
isotope labeling in LC/MS-based untargeted metabolomics. Anal Chem.
88:621–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Civan MM, Marano CW, Matschinsky FW and
Peterson-Yantorno K: Prolonged incubation with elevated glucose
inhibits the regulatory response to shrinkage of cultured human
retinal pigment epithelial cells. J Membr Biol. 139:1–13. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei J, Zhao L, Zhang Y, Wu Y and Liu Y:
High glucose-induced podocyte injury involves activation of
mammalian target of rapamycin (mTOR)-induced endoplasmic reticulum
(ER) stress. Cell Physiol Biochem. 45:2431–2443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ran Z, Zhang Y, Wen X and Ma J: Curcumin
inhibits high glucose-induced inflammatory injury in human retinal
pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling
pathway. Mol Med Rep. 19:1024–1031. 2019.PubMed/NCBI
|
|
24
|
Liu B, Wu S, Ma J, Yan S, Xiao Z, Wan L,
Zhang F, Shang M and Mao A: lncRNA GAS5 reverses EMT and tumor stem
cell-mediated gemcitabine resistance and metastasis by targeting
miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids.
13:472–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Yang C, Xie S and Cheung E: Long
non-coding RNA GAS5 and ZFAS1 are prognostic markers involved in
translation targeted by miR-940 in prostate cancer. Oncotarget.
9:1048–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ali ES, Rychkov GY and Barritt GJ:
Deranged hepatocyte intracellular Ca(2+) homeostasis and the
progression of non-alcoholic fatty liver disease to hepatocellular
carcinoma. Cell Calcium. 82:1020572019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue M, Sakuta N, Watanabe S, Zhang Y,
Yoshikaie K, Tanaka Y, Ushioda R, Kato Y, Takagi J, Tsukazaki T, et
al: Structural basis of sarco/endoplasmic reticulum Ca(2+)-ATPase
2b regulation via transmembrane helix interplay. Cell Rep.
27:1221–1230.e1223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marino M, Stoilova T, Giorgi C, Bachi A,
Cattaneo A, Auricchio A, Pinton P and Zito E: SEPN1, an endoplasmic
reticulum-localized selenoprotein linked to skeletal muscle
pathology, counteracts hyperoxidation by means of redox-regulating
SERCA2 pump activity. Hum Mol Genet. 24:1843–1855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raturi A, Gutierrez T, Ortiz-Sandoval C,
Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D,
Lou PH, Lucchinetti E, et al: TMX1 determines cancer cell
metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux.
J Cell Biol. 214:433–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ushioda R, Miyamoto A, Inoue M, Watanabe
S, Okumura M, Maegawa KI, Uegaki K, Fujii S, Fukuda Y, Umitsu M, et
al: Redox-assisted regulation of Ca2+ homeostasis in the
endoplasmic reticulum by disulfide reductase ERdj5. Proc Natl Acad
Sci USA. 113:E6055–E6063. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sundar Rajan S, Srinivasan V,
Balasubramanyam M and Tatu U: Endoplasmic reticulum (ER) stress
diabetes. Indian J Med Res. 125:411–424. 2007.PubMed/NCBI
|
|
32
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nowotny K, Castro JP, Hugo M, Braune S,
Weber D, Pignitter M, Somoza V, Bornhorst J, Schwerdtle T and Grune
T: Oxidants produced by methylglyoxal-modified collagen trigger ER
stress and apoptosis in skin fibroblasts. Free Radic Biol Med.
120:102–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du Z, Xu S, Hu S, Yang H, Zhou Z, Sidhu K,
Miao Y, Liu Z, Shen W, Reiter RJ, et al: Melatonin attenuates
detrimental effects of diabetes on the niche of mouse
spermatogonial stem cells by maintaining Leydig cells. Cell Death
Dis. 9:9682018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Campbell GR, To RK and Spector SA: TREM-1
Protects HIV-1-infected macrophages from apoptosis through
maintenance of mitochondrial function. Mbio. 10:2019. View Article : Google Scholar
|
|
37
|
Donovan M and Cotter TG: Control of
mitochondrial integrity by Bcl-2 family members and
caspase-independent cell death. Biochim Biophys Acta. 1644:133–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li K, Zhang L, Xiang X, Gong S, Ma L, Xu
L, Wang G, Liu Y, Ji X, Liu S, et al: Arsenic trioxide alleviates
airway hyperresponsiveness and promotes apoptosis of CD4+ T
lymphocytes: Evidence for involvement of the ER stress-CHOP
pathway. Ir J Med Sci. 182:573–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Dong B, Kim KH, Choi S, Sun Z, Wu
N, Wu Y, Scott J and Moore DD: Vitamin D receptor activation in
liver macrophages protects against hepatic endoplasmic reticulum
stress in mice. Hepatology. 71:1453–1466. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang L, Wang Y, Zhang L, Xia X, Chao Y,
He R, Han C and Zhao W: ZBTB7A, a miR-663a target gene, protects
osteosarcoma from endoplasmic reticulum stress-induced apoptosis by
suppressing LncRNA GAS5 expression. Cancer Lett. 448:105–116. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Wang L, Guo H, Peng Y, Nie D, Mo J
and Ye L: Knockdown of MALAT1 attenuates high-glucose-induced
angiogenesis and inflammation via endoplasmic reticulum stress in
human retinal vascular endothelial cells. Biomed Pharmacother.
124:1096992020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu J, Wei L, Wang Z, Song S, Lin Z, Zhu
J, Ren X and Kong L: Protective effect of Liraglutide on diabetic
retinal neurodegeneration via inhibiting oxidative stress and
endoplasmic reticulum stress. Neurochem Int. 133:1046242020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu S, Zhu X, Guo B, Zheng T, Ren J, Zeng
W, Chen X and Ke M: Unfolded protein response pathways
correlatively modulate endoplasmic reticulum stress responses in
rat retinal muller cells. J Ophthalmol. 2019:90284832019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lenin R, Nagy PG, Alli S, Rao VR, Clauss
MA, Kompella UB and Gangaraju R: Critical role of endoplasmic
reticulum stress in chronic endothelial activation-induced visual
deficits in tie2-tumor necrosis factor mice. J Cell Biochem.
119:8460–8471. 2018. View Article : Google Scholar : PubMed/NCBI
|