Introduction
Osteosarcoma is the most common malignant bone
tumour in children and adolescents (1). Currently, the main treatment of
osteosarcoma is surgical resection combined with neoadjuvant
chemotherapy (2), but the
long-term survival rate of primary osteosarcoma is still only ~67%
(3). In recent years, microwave
ablation has been successfully applied to the clinical treatment of
bone tumours (3). To completely
eliminate the tumour, microwave ablation currently requires
ablation applied to the tumour safety boundary when treating
para-articular tumours (4).
However, this procedure has a risk of damaging the surrounding
normal tissue when the temperature induced by microwave ablation is
too high (5). Therefore, exploring
a method that can kill tumour cells and reduce the temperature of
the microwave ablation to protect surrounding normal tissues is of
great importance for the clinical application of microwave ablation
in osteosarcoma.
Tumours synthesize heat shock protein 90 (HSP90) in
response to microwave stimulation (6). High expression of HSP90 increases the
tolerance of tumour cells to heat and inhibits apoptotic signalling
pathways (7). Furthermore, HSP90
is more abundant in tumour cells than in normal cells (8); it can inhibit the apoptosis of tumour
cells by participating in various protective signalling pathways,
including inhibiting the mitochondrial release of cytochrome
c (cyto c), inhibiting the formation of apoptotic
bodies, further enhancing the tolerance of tumour cells to heat and
inhibiting tumour necrosis (9).
Previous studies have revealed that HSP90 inhibitors are novel and
effective anticancer drugs (10,11).
Therefore, suppressing the expression of HSP90 in tumours under
microwave ablation has the potential to enhance the therapeutic
effect of microwave treatment.
Another key signalling molecule in tumour cells is
transforming growth factor-β1 (TGF-β1), which can affect the
growth, differentiation, metastasis and apoptosis of tumour cells
(12). Animal experiments have
demonstrated that TGF-β1 is an effective target for tumour therapy
(13,14). Previous studies have reported that
small inhibitors of TGF-β1 can block SMAD phosphorylation and
nuclear translocation, as well as inhibiting tumour morphological
changes, cell proliferation, migration and angiogenesis (15,16).
To reinforce the therapeutic effect of microwave ablation, TGF-β1
inhibitors can be combined with it in the treatment of osteosarcoma
(17). It has been reported that
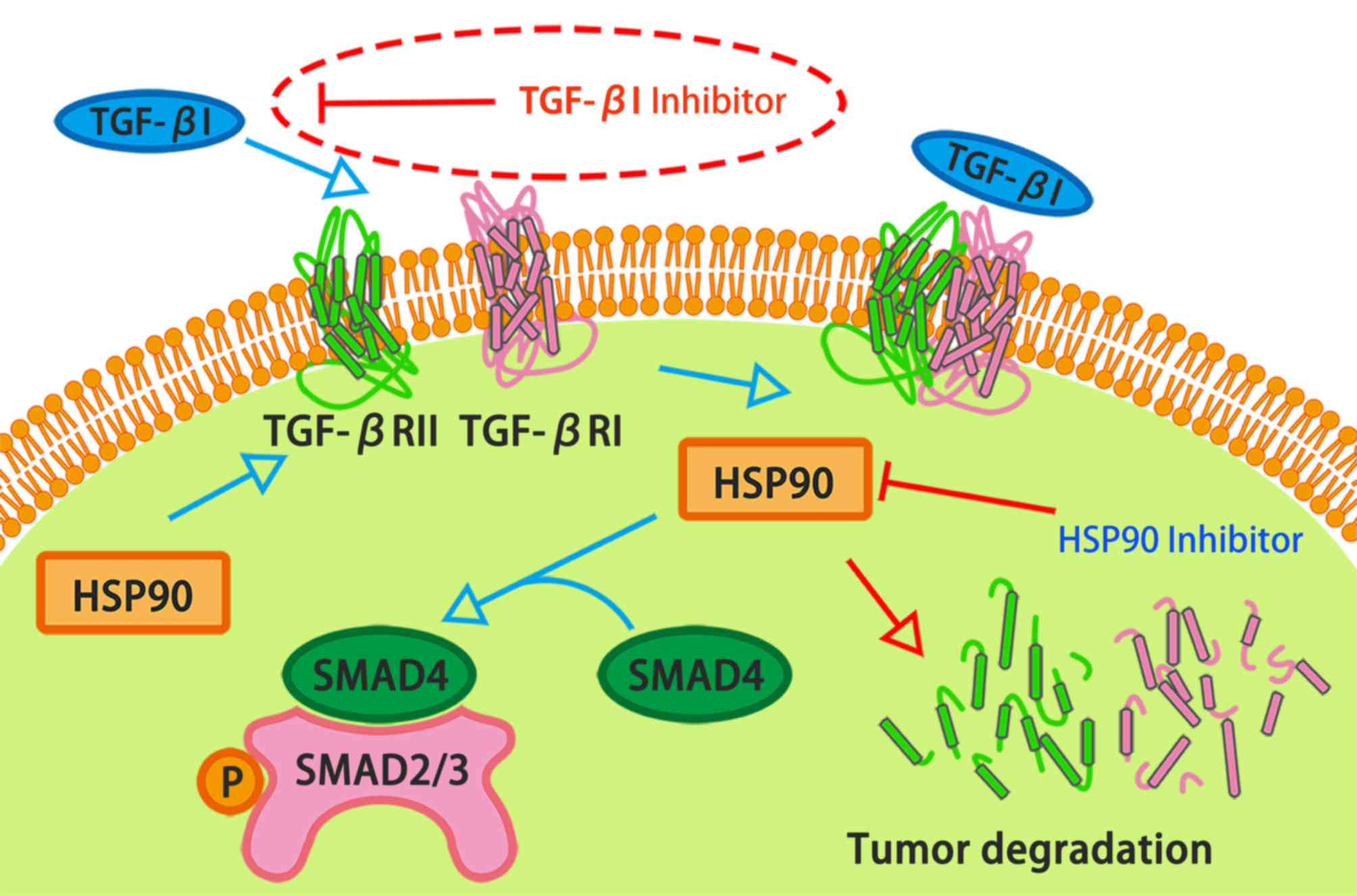
both HSP90 and TGF-β1 can bind to the same receptor to have
synergistic effects on tumour adhesion, migration and other
functions (17). In addition,
HSP90 inhibitors can suppress TGF-β1 signalling at the receptor
level (17) (Fig. 1).
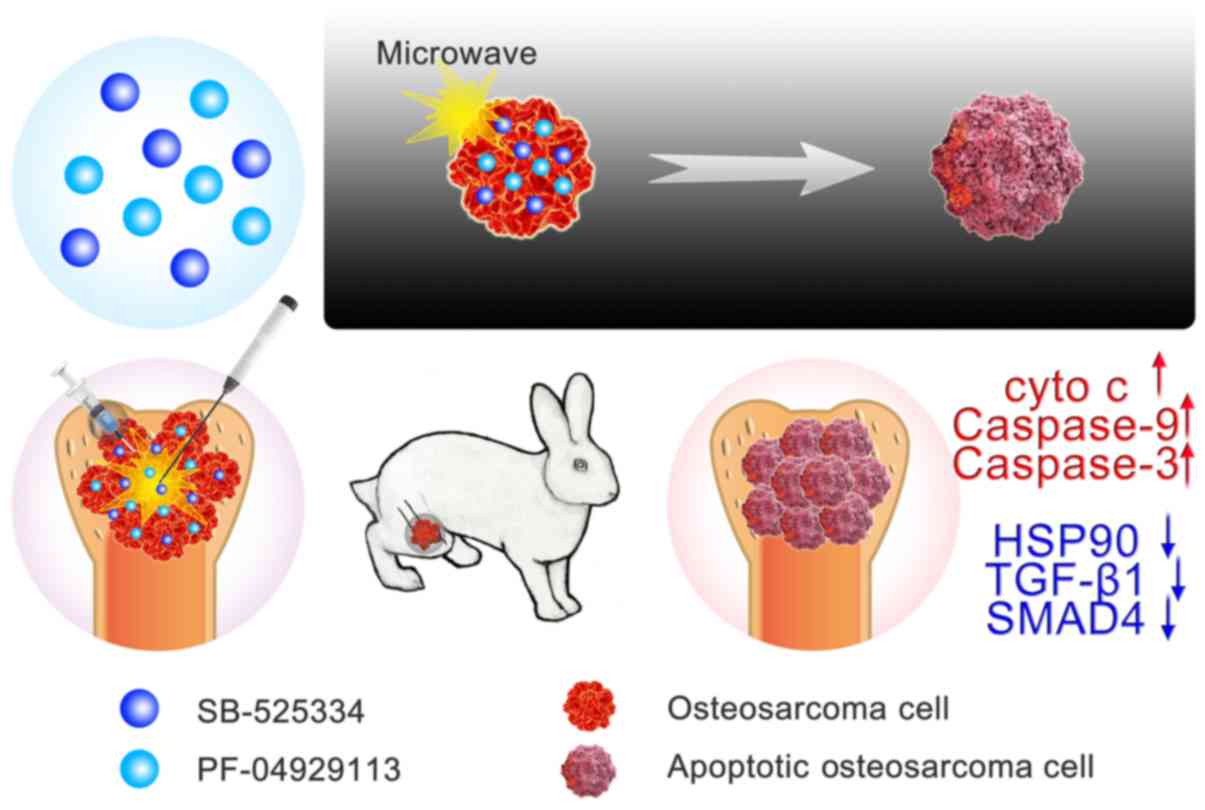
In the present study, microwave ablation was
combined with HSP90 and TGF-β1 inhibitors to treat osteosarcoma
(Fig. 2). To the best of our
knowledge, this is a rare method of applying microwave ablation in
the treatment of tumours. Based on previous findings concerning
cell tolerance of hyperthermia (18), four microwave ablation temperatures
(37, 41, 48 and 60°C) were set in this study. It was discovered
that the apoptotic rate of VX2 cells was significantly increased
after microwaving at 48°C combined with TGF-β1 and HSP90
inhibitors. The in vivo experiments also showed the same
results. In addition, it was demonstrated that the expression of
cytokines in the apoptotic signalling pathway were increased.
Collectively, these findings may provide a mild microwave ablation
method that can kill tumour cells while avoiding damage to the
surrounding normal tissue.
Materials and methods
Cell culture
The rabbit squamous cell carcinoma VX2 cells
(American Type Culture Collection) and the rabbit bone marrow
mesenchymal stem cells (R-BMSCs; iCell Bioscience, Inc., cat. no.
s018.) were cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.), 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution. The cells were cultured in an
incubator at 37°C and 5% CO2. When the cells occupied
90–95% of the of the bottom of the culture flask, they were
digested and prepared in suspension with a density of
1×104 cells.
After all cells were adhered to the plate for 20 h,
fresh culture medium was added with one of the following
conditions: 37 nM HSP90 inhibitor (PF-04929113; p) alone; 14.3 nM
TGF-β1 inhibitor (SB-525334; s) alone; or both 37 nM PF-04929113
and 14.3 nM SB-525334 (p+s). The inhibitors were purchased from
Gibco; Thermo Fisher Scientific, Inc. Culture medium with no
inhibitors was regarded as the control group.
Temperature rise curve
In each well of 48-well culture plates, 500 µl DMEM
was added, after which a microwave needle fixed with a temperature
measuring probe was inserted into the well; the temperature
measuring probe was the same type of microwave temperature
measuring probe used in the clinical treatment of bone tumours. The
microwave therapy device was adjusted to 15 W under physiotherapy
mode, and the starting microwave temperature of each well was 37°C.
The microwave time was set at 15, 20, 25, 30, 40, 50 and 60 sec.
The microwave treatment was repeated three times for each treatment
time, the temperature of the DMEM was recorded, and the
time-temperature curve was plotted.
MTT assay
The VX2 cell suspension density was 7×104
cells/ml and 500 µl suspension was added to each well of a 48-well
plate. After incubation for 20 h in a 37°C constant temperature
incubator, fresh culture medium was added with the inhibitors (as
aforementioned). After co-culture with inhibitors for 4 h, each
group was heated by microwave to 37, 41, 48 and 60°C. The R-BMSCs
were used to repeat the aforementioned procedure and were
microwaved to 48°C. After 4 h, the cells were incubated with 5
mg/ml MTT solution in a 37°C incubator for 4 h, and the crystal
violet was dissolved with DMSO solution. The optical density values
at 490 nm were measured using a microplate reader. The cell
survival rates were calculated by reference to the control group at
37°C. Each experimental condition was repeated three times.
Calcein/PI staining
After the cells were cultured with the inhibitors
and heated, the supernatant was discarded, and 200 µl calcein/PI
(Gibco; Thermo Fisher Scientific, Inc.) solution was added to each
well. After 30 min incubation at 37°C, the cell viability was
observed under a fluorescence inverted microscope (Olympus
Corporation) and images (magnification, ×200) were captured.
Flow cytometry
Apoptosis of cells was tested in the following
groups: Microwave alone (M); microwave + 37 nM PF-04929113 (M+p);
microwave + 14.3 nM SB-525334 (M+s); and microwave + 37 nM
PF-04929113 + 14.3 nM SB-525334 (M+p+s) groups. Following addition
of the inhibitors, the groups received microwave ablation with a
power of 15 W and a duration time of 40 sec. Subsequently, the
cells were collected, washed twice with cold PBS, stained with
Annexin V-FITC and PI (BD Biosciences, Inc.) for 30 min at 4°C in
the binding buffer and analysed by flow cytometry (FACSCalibur flow
cytometer; BD Biosciences) and FlowJo software 10.6 (FlowJo
LLC).
Cellular immunofluorescence assay
The cells in the control, M, M+p, M+s and M+p+s
groups were fixed with 4% paraformaldehyde for 15 min at room
temperature. After washing twice with PBS, cells were permeabilized
using a 0.2% Triton X-100 solution and subsequently blocked with 3%
BSA (neoFroxx GmbH) for 30 min at room temperature. Next, the cells
were incubated for 8 h at 4°C with primary antibodies against
TGF-β1 (cat. no. sc-130348, 1:1,000), SMAD4 (cat. no. sc-73040,
1:1,500), HSP90 (cat. no. sc-101494, 1:1,000), caspase-3 (cat. no.
sc-56046, 1:1,500), caspase-9 (cat. no. sc-56076, 1:1,000) and cyto
c (cat. no. sc-75806, 1:1,000 all from Santa Cruz
Biotechnology, Inc.), washed with PBS and incubated for 1 h at 37°C
with Alexa Fluor® 488 goat anti-rabbit IgG secondary
antibody (1:1,000; cat. no. A32731, Invitrogen, Thermo Fisher
Scientific, Inc.). Nuclei were stained using DAPI for 5 min at
37°C. Images (magnification, ×400) were acquired using a confocal
laser-scanning microscope (Olympus Corporation).
Western blotting analysis
The cells in the control, M, M+p, M+s and M+p+s
groups were lysed on ice with RIPA (Thermo Scientific, Inc.) for 30
min to extract the total protein. Protein concentration was
assessed using a BCA protein assay kit. Protein (60 µg) from each
sample were separated by SDS-PAGE using a 10% acrylamide gel and
transferred to PVDF transfer membranes (EMD Millipore). Membranes
were blocked for 1 h at room temperature with 5% non-fat dried milk
in TBS containing 0.05% Tween-20 (TBST) buffer and incubated
overnight at 4°C with primary antibodies against TGF-β1 (cat. no.
sc-130348, 1:2,000), SMAD4 (cat. no. sc-73040, 1:2,000), HSP90
(cat. no. sc-101494, 1:1,000), caspase-3 (cat. no. sc-56046,
1:1,100), caspase-9 (cat. no. sc-56076, 1:1,000) and cyto c
(cat. no. sc-75806, 1:1,000 all from Santa Cruz Biotechnology,
Inc.). After washing three times for 5 min in TBST buffer, the
membranes were incubated with the HRP-Goat Anti-Mouse IgG (Jackson
ImmunoResearch Laboratories, Inc., cat. no. 115-035-003) for 30 min
at room temperature. Protein bands were detected with Immobilon
Western Chemiluminescent HRP Substrate (EMD Millipore) and analysed
with the Bio-Image Analysis system: BOX F3 (Syngene) and ImageJ
v1.8.0 (National Institutes of Health).
Establishment of an osteosarcoma
model
All animal studies were approved by the
Institutional Animal Care and Use Committee of Guangzhou General
Hospital of Guangzhou Military Command of PLA (Guangzhou, China).
Rabbits (n=31) were housed individually in stainless steel cages
and maintained with free access to both food and water. The rabbits
were housed in an environmentally controlled breeding room, with at
least 10 air changes per hour. The animal feeding room was
maintained between 18–26°C and 30–70% relative humidity. Under the
control of a timer, a12-h light/dark cycle was maintained. VX2
cells were suspended at a concentration of 1×107
cells/ml. The tibial tuberosity of a New Zealand rabbit was
exposed, 1 ml bone marrow was extracted and then 1 ml VX2 cell
suspension was injected into the bone marrow cavity. After
formation of the tumours, the tumour tissue was removed and cut
into tumour tissue blocks (volume, 0.5 mm3).
Subsequently, the other 30 1.5 kg healthy male New Zealand rabbits
were anaesthetized by injecting 30 mg/kg 3% pentobarbital into the
ear vein. The tumour tissue blocks were transplanted into a femoral
condyles bone defect (5 mm in diameter and 5 mm in depth) and the
defect was closed with bone wax. The tumour model rabbits were
obtained 1 week after implantation.
In vivo treatment
The tumour model rabbits were anaesthetized with 30
mg/kg 3% pentobarbital in the ear vein. Tumor volume was measured
by Vernier calipers prior to surgery. The rabbits then received
injections of the inhibitors followed by microwave treatment. The
groups were: Control (no drugs or microwave treatment); M
(microwave only); M+p (microwave plus PF-04929113, 55.5 nM, 100
µl); M+s (microwave plus SB-525334, 21.45 nM, 100 µl); and M+p+s
(microwave plus the two inhibitors). Microwave ablation was
conducted at a power of 15 W with a duration time of 40 sec under
physiotherapy mode. The body weight and tumour sizes of the rabbits
were measured every other day for a period of 21 days. Tumour size
was measured with calipers and volume was calculated as follows:
V=ab2/2; where V (cm3) is tumour volume, and
a (cm) and b (cm) are tumour length and width, respectively. After
all experimental data and samples were collected, the rabbits were
euthanized by intravenous injection of 100 mg/kg pentobarbital
sodium (19), the tumor tissues
were <10% the weight of the rabbits at the time of
sacrifice.
Haematoxylin and eosin (H&E)
staining
The tumour tissues were fixed in 4% paraformaldehyde
for >30 min at room temperature, embedded in paraffin and cut
into 5-µm slices. The histological morphology of the tumours was
acquired with H&E staining for 15 min at room temperature. All
samples were observed under a fluorescence microscope
(magnification, ×400; BX51; Olympus Corporation).
Immunohistochemical staining
After deparaffinization and rehydration, the 5-µm
tumor sections were treated with 3% hydrogen peroxide for 15 min at
room temperature and blocked with 10% goat serum (Sigma-Aldrich;
Merck KGaA) for 20 min at room temperature. Subsequently, they were
incubated with primary antibodies in PBS for 8 h at 4°C. After
washing, secondary antibodies were added and incubated for 20 min
at room temperature. The antibodies used were identical to the
antibodies used in the cellular immunofluorescence assay.
Subsequently, HRP-conjugated streptavidin and 3,3′-diaminobenzidine
solution were added to the slides and developed for 5 min. After
rinsing with tap water for 10 min, they were counterstained with
haematoxylin for 2 min at room temperature and differentiated with
hydrochloric acid alcohol. All samples were observed under a
fluorescence microscope (magnification, ×400; BX51; Olympus
Corporation). Image-Pro Plus 6.0 image analysis software (Media
Cybernetics, Inc.) was used to analyse the integrated optical
density (IOD) value of each image.
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 (IBM Corp.). Data are expressed as the mean ± SD.
Statistical comparisons among multiple treatment groups were
measured using one-way ANOVA followed by a post-hoc multiple
comparison Dunnett's test.
Results
Cell survival rate of VX2 cells
decreases with microwave treatment combined with inhibitors
In the physiotherapy mode of the microwave therapy
device, the temperature and time are linearly related when the
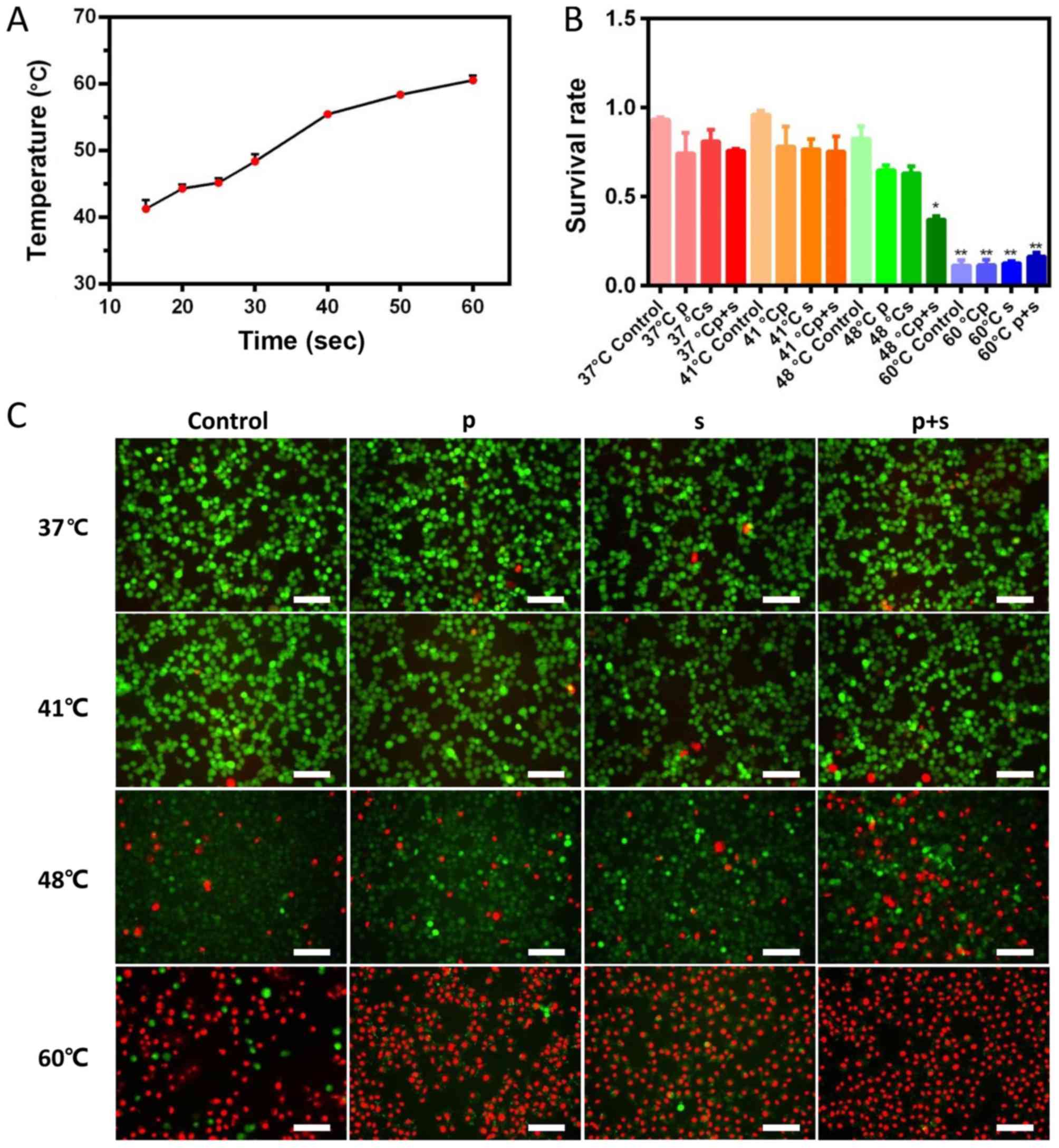
power is 15 W (Fig. 3A). The times
required to reach the temperatures of 41, 48 and 60°C were 15, 30
and 60 sec, respectively. MTT results showed that the survival rate
of VX2 cells decreased with increasing temperature in all groups.
The addition of inhibitors at 37 and 41°C had no significant effect
on the survival rate of VX2 cells compared with the 37°C control
group. When the temperature was 48°C, the survival rate of VX2
cells was 82.75% in the control group, 64.81% in the p group,
63.18% in the s group and 37.18% in the p+s group (Fig. 3B). The cell survival rate of the
48°C M+p+s group was significantly lower compared with the 48°C
control, M, M+s and M+p groups (P<0.05). The cell survival rate
in the 60°C group was significantly lower compared with the other
temperature groups (P<0.05; Fig.
3B).
As shown in Fig.
3C, most of the VX2 cells were viable at 37 and 41°C. A small
number of dead cells was observed in the groups treated with
inhibitors. When the temperature reached 48°C, an increased number
of dead cells was found and the number of dead cells in the p+s
group was greater than in the control group. At 60°C, only a few
live cells were observed in the control group. These results were
in accordance with the MTT results.
Treatment with inhibitors followed by
microwave ablation increases cell apoptotic rate
As shown in Fig.
4A, when cells were subjected to microwaving at 48°C, the
apoptotic rate was 3.4% in the control group, 3.2% in the M group,
6.7% in the M+p group, 6.8% in the M+s group and 38.4% in the M+p+s
group. The quantitative data revealed that the M+p+s group had a
significantly higher apoptotic rate compared with the control group
(Fig. 4C).
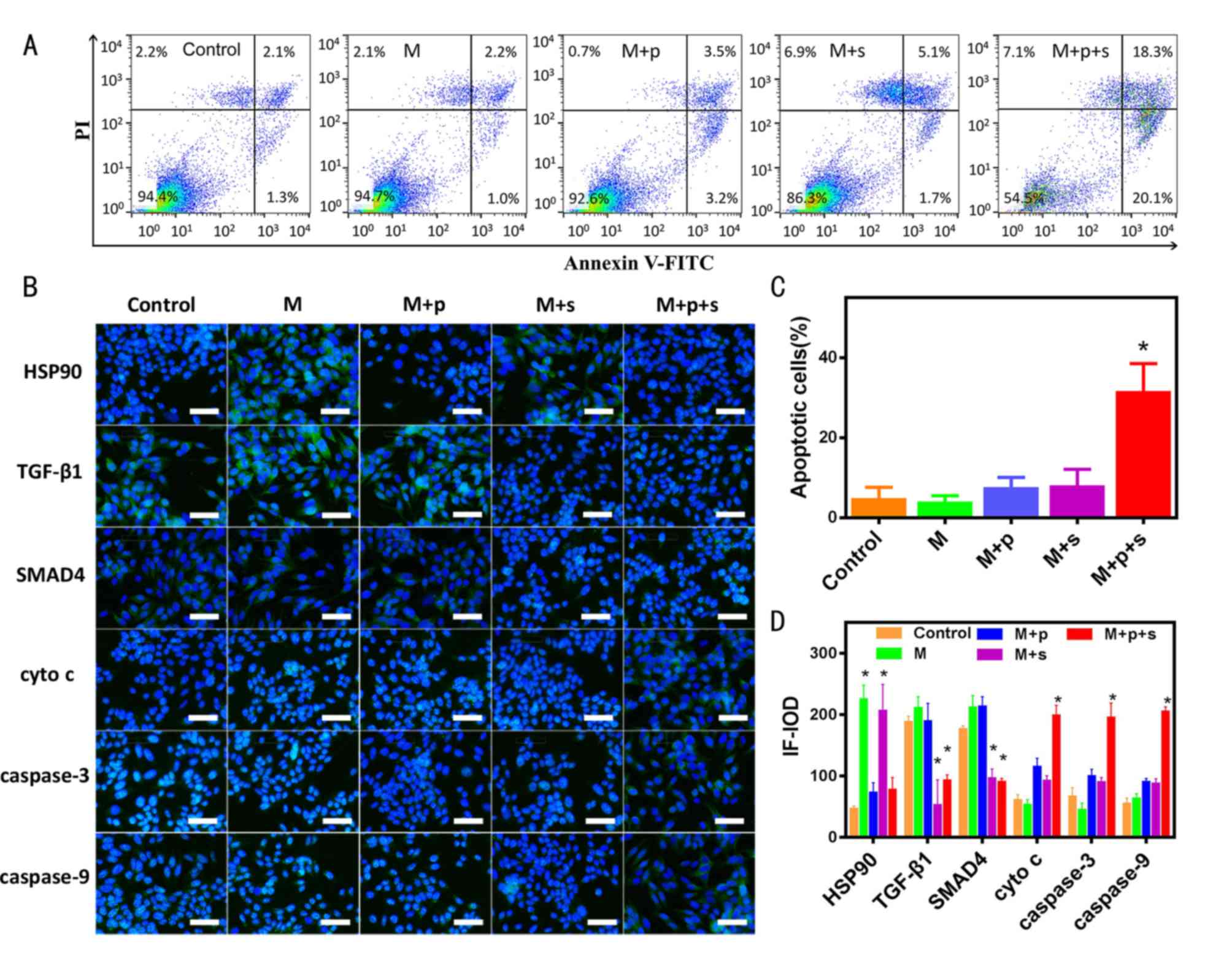 | Figure 4.Microwave to 48°C combined with HSP90
and TGF-β1 inhibitors promotes the apoptosis of VX2 cells. (A)
Proportion of apoptosis after microwave ablation combined with
inhibitors in VX2 cells. (B) Apoptosis-related signalling proteins
detected by immunofluorescence staining; blue, DAPI; green, Alexa
Fluor showing positively expressed protein; scale bar=50 µm. (C)
Corresponding statistical results of flow cytometry (n=3). (D)
Corresponding statistical results of cellular immunofluorescence
images (n=3). *P<0.05 vs. control group. cyto c,
cytochrome c; HSP90, heat shock protein 90; IF-IOD,
immunofluorescence-integrated optical density; M, microwave; p,
PF-04929113; PI, propidium iodide; s, SB-525334; TGF-β1,
transforming growth factor-β1. |
As determined by immunofluorescence, HSP90, TGF-β1
and SMAD4 expression was increased in the M group, whereas cyto
c, caspase-3 and caspase-9 expression was not altered in the
M group when compared with the control group (Fig. 4B and D). In the M+s group, the
expression of apoptotic downstream signalling pathway-related
proteins were similar to that of the M group. The expression of
cyto c, caspase-3 and caspase-9 was significantly increased
in the M+p+s group compared with the M group (Fig. 4B and D), indicating that the TGF-β1
inhibitor could promote the signalling pathway of apoptosis. In
addition, the IOD values of HSP90 in the M and M+s groups were
significantly increased, whereas those in the M+p and M+p+s groups
were similar to those in the control group. The IOD values of
TGF-β1 in the M+p and M+p+s groups were significantly decreased
when compared with the control group (Fig. 4B and D).
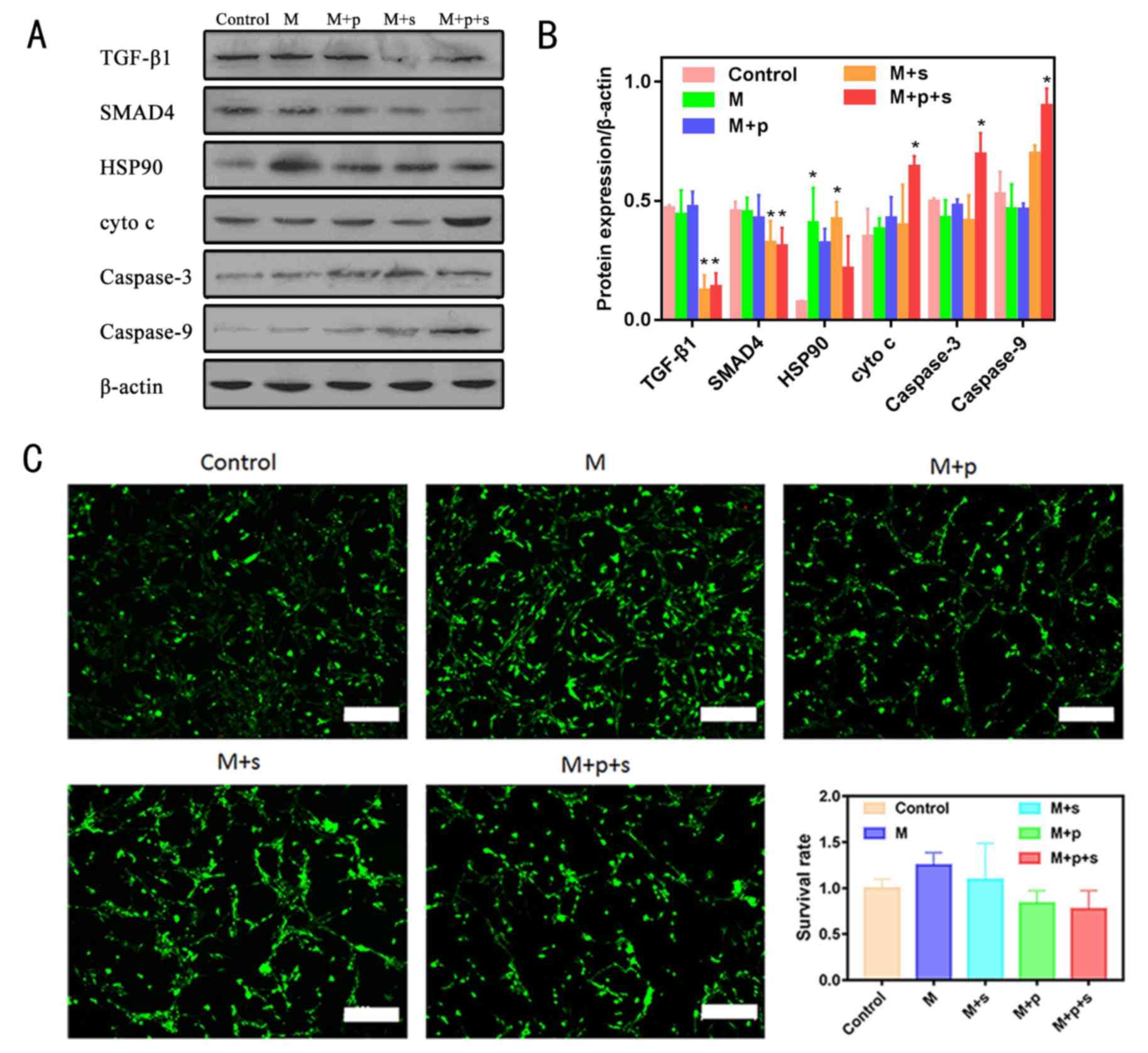
Western blotting analysis
Fig. 5A and B shows
the expression levels of proteins associated with the HSP90 and
TGF-β1/SMAD4 signalling pathways. In the M+p+s groups, the
expression levels of TGF-β1 and SMAD4 were significantly decreased.
HSP90 in the VX2 cells was significantly upregulated by microwaving
to 48°C, whereas the expression levels of the other proteins in the
M group were similar to that of the control group. When combined
with the HSP90 inhibitor (M+p group), the expression of HSP90 in
VX2 cells was reduced. The expression levels of cyto c,
caspase-3 and caspase-9 were markedly increased in the M+p+s group
when compared with the control group.
Microwave ablation has no significant
effects on the survival rate of R-BMSC cells
As shown in Fig. 5C
there was no significant difference in the survival rate of the
R-BMSCs after microwave treatment at 48°C compared with the control
group, even when inhibitors were used in combination.
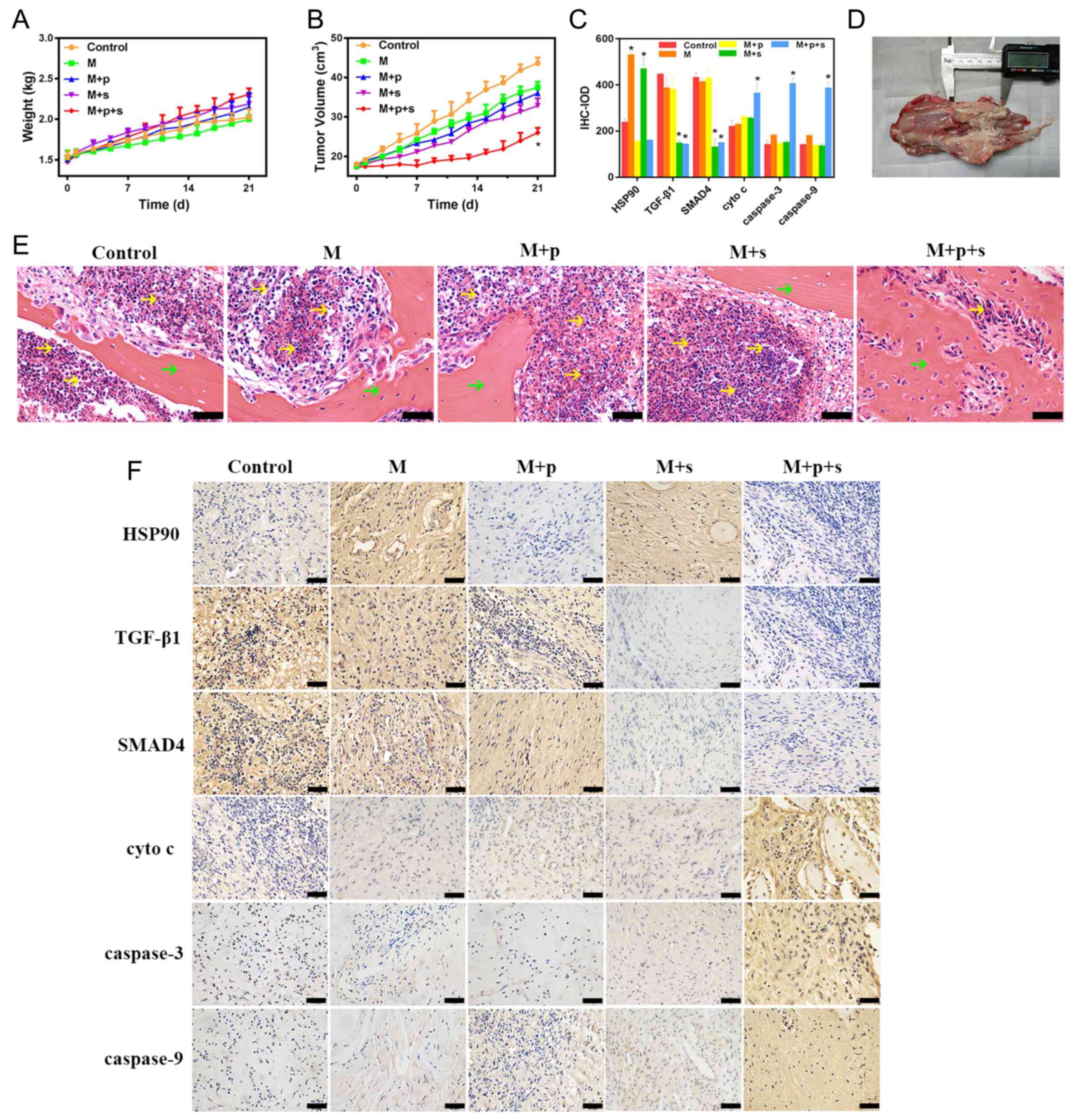
Changes in tumour volume and body
weight
The weight of the rabbits in each group increased
over 0–21 days, the rabbits were ~1.5 kg before surgery and ~2.0 kg
21 post-surgery (Fig. 6A).
However, there were no significant differences in body weight among
the groups at each time point. No rabbit was found to have multiple
tumours. As shown in Fig. 6B,
there was an increase in tumour volume in all groups over the 0–21
days after microwave ablation. The M+p+s group had the smallest
volume at 21 days post-surgery, the volume was ~58.05% of the
control group and was lower than that of the other groups. In
addition, there were a large number of tumour cells observed in the
control group, which decreased in the groups subjected to microwave
treatment; the M+p+s group had the lowest number of tumour cells
among these groups (Fig. 6E).
Immunohistochemistry
As shown in Fig. 6C and
F, HSP90 expression in VX2 tumour tissue was significantly
increased after microwaving at 48°C, whereas the expression levels
of the other proteins were similar to that of the control group.
When combined with the HSP90 inhibitor (M+p group), the expression
of HSP90 in VX2 tumour tissues decreased to close to that of the
control group. When combined with the TGF-β1 inhibitor (M+s and
M+p+s groups), the expression levels of TGF-β1 and SMAD4 were
significantly decreased compared with the control group. The
expression of cyto c, caspase-3 and caspase-9 markedly
increased in the M+p+s group when compared with the control
group.
Discussion
The use of microwave therapy on tumours is
associated with the risk of causing thermal damage to the
surrounding normal tissues due to the high temperatures generated
by the microwave thermal effect. A previous study reported the
effect of hyperthermic temperatures on cells, the findings were as
follows: Between 37 and 41°C, cells maintained a dynamic balance;
between 41 and 48°C for >60 min triggered irreversible damage to
the cells; between 48 and 60°C for only 4–6 min led to irreversible
damage to proteins, DNA and cells; >60°C caused the protein to
immediately coagulate, and the cells were immediately and
irreversibly damaged (18).
Based on the aforementioned studies, four microwave
ablation temperatures (37, 41, 48 and 60°C) were set in this
experiment, and the effect of microwave ablation combined with the
TGF-β1 and HSP90 inhibitors on the survival rate of osteosarcoma
tumours was investigated. The present study revealed that the
survival rate of VX2 cells after microwaving to 48°C combined with
TGF-β1 and HSP90 inhibitors, the rate of apoptosis was
significantly higher than the microwave or inhibitor treatment
groups alone. The results of the in vivo experiments also
demonstrated the same synergistic tumour treatment effect, without
any systemic effect on the experimental rabbits, as during the
experiment, there was no significant difference in the weight of
the rabbits in each group.
The effect of this combination therapy could be
attributed to the combination of two inhibitors blocking the
synergistic activity of TGF-β1 and HSP90. Although HSP90 is
essential for the survival of healthy cells, cancer cells have been
shown to be more dependent on HSP90, and therefore require
increased active HSP90 compared with normal tissues (20), which may provide a novel target for
tumour therapy. In the present study, live/dead staining and MTT
assays were used to verify that microwave ablation at 48°C combined
with inhibitors had no significant effect on the survival rate of
R-BMSCs. This may be because normal cells are less sensitive to
thermal stimulation than tumour cells (21). A previous study reported that the
anti-tumour effect of the HSP90 inhibitor may be mediated by a
significant decrease in TGF-β1 at the post-translational level
rather than the transcript level, followed by a decrease in SMAD2/3
(22). Moreover, TGF-β1 and HSP90
can bind to the same receptor (23), and previous studies have
investigated their synergistic effects on tumour cell adhesion,
migration and immunoglobulins (24,25).
TGF-β1 has no effect on the adhesion and migration levels of tumour
cells, but the addition of HSP90 can increase the adhesion of
metastatic tumour cells without affecting the migration of primary
tumour cells (26,27).
In the present study, the results of cellular
immunofluorescence and Immunohistochemistry demonstrated that
SB-525334 blocked the expression of TGF-β1 and SMAD4, and
PF-04929113 blocked the expression of HSP90 but significantly
increased the expression of apoptosis-related proteins cyto
c, caspase-3 and caspase-9. In Fig. 4D and B, HSP90 in the M+p group
decreased, but cyto c and caspases were also affected by other
signaling pathways, thus there was no significant decrease overall.
When an injury signal is transmitted to the mitochondria,
mitochondrial outer membrane permeabilization leads to the release
of cyto c into the cytoplasm (28), where it binds and activates
apoptotic peptidase activating factor 1 (APAF1) (29). APAF1 has a caspase activation and
recruitment binding domain (CARD), which is homologous to the
initiating apoptotic protease caspase-9, so that activated APAF1
can aggregate and activate caspase-9 in a CARD-CARD manner, forming
a cytochrome apoptotic body composed of cyto c, APAF1 and
caspase-9, which activates caspase-3 and ultimately induces
apoptosis (30).
In another signalling pathway associated with tumour
proliferation/apoptosis, TGF-β1 phosphorylates and activates TGF-β1
type receptors (31).
Phosphorylation of receptor-specific SMAD2/3 allows them to
oligomerize with the common mediator SMAD4, thereby mediating the
proliferation of tumour cells (32). Since TGF-β1 and HSP90 are able to
bind to the same receptors, HSP90 inhibitors can target TGF-β1
signalling at the receptor level (23). HSP90 inhibitors can induce caspase
activation by proteolysis (33).
Caspase activation is mainly carried out by hydrolysis (34), and thus an immunoblot analysis was
performed using an anti-lytic caspase-3/8/9 antibody. It was found
that the caspase-9 content of the M+p+s group cells was
significantly higher than that of untreated cells (33). The cleaved form of caspase-3 was
detected in untreated cells, and the caspase-3 content of the M+p+s
group cells was significantly increased compared with untreated
cells (33).
When tumour cells are damaged, hypoxia triggers an
increase in mitochondrial membrane permeability and cyto c
translocates from the mitochondrial matrix to the cytoplasmic
matrix (35). The resulting
apoptotic body, a complex composed of cyto c, APAF1 and
caspase-9, then activates the apoptosis-executing caspase-3 to
induce apoptosis (36). These
reports are consistent with the results of the present study, which
suggested that the combination treatment activated tumour cell
apoptosis by activating the mitochondrial apoptotic pathway. The
immunohistochemical results also revealed that the expression of
cyto c, caspase-3 and caspase-9 were enhanced when microwave
ablation was combined with TGF-β1 and HSP90 inhibitors, which
promoted osteosarcoma apoptosis. A previous study reported that the
HSP90 inhibitor 17-DMAG could enhance the apoptotic effect of
hyperthermic conditions on melanoma cells via melanoma-induced
apoptosis (37), which differs
from the inhibitor used of the present study. HSP90 can also
inhibit exogenous apoptotic signalling pathways (38); its related anti-tumor effects
require further study.
In summary, this study investigated the use of
microwaving to 48°C combined with HSP90 and TGF-β1 inhibitors. It
was demonstrated that this combination could decrease cell survival
rate and promote apoptosis in osteosarcoma tumours, providing a
novel strategy for osteosarcoma treatment by microwave ablation
while also offering a solution for the protection of surrounding
normal tissues.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province (grant nos.
2017B030314139 and 2012B031500014) and the Natural Science
Foundation of Guangdong Province (grant no. 2015A030312004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY, YZ and LC conceived and designed the study. LC,
MW, ZL, MY, SC, WW and BL performed the experiments and collected
the data. LC, WW and MW were major contributors in drafting and
revising the manuscript. All authors reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the
Institutional Animal Care and Use Committee of Guangzhou General
Hospital of Guangzhou Military Command of PLA.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Omer N, Le Deley MC, Piperno-Neumann S,
Marec-Berard P, Italiano A, Corradini N, Bellera C, Brugieres L and
Gaspar N: Phase-II trials in osteosarcoma recurrences: A systematic
review of past experience. Eur J Cancer. 75:98–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller BJ, Cram P, Lynch CF and Buckwalter
JA: Risk factors for metastatic disease at presentation with
osteosarcoma: An analysis of the SEER database. J Bone Joint Surg
Am. 95:e892013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nomura M, Rainusso N, Lee YC, Dawson B,
Coarfa C, Han R, Larson JL, Shuck R, Kurenbekova L and Yustein JT:
Tegavivint and the β-catenin/ALDH axis in Chemotherapy-resistant
and metastatic osteosarcoma. J Natl Cancer Inst. 111:1216–1227.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Guo Z, Wang Z, Fan H and Fu J: Does
microwave ablation of the tumor edge allow for joint-sparing
surgery in patients with osteosarcoma of the proximal Tibia? Clin
Orthop Relat Res. 473:3204–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogura Y, Naito H, Tsurukawa T,
Ichinoseki-Sekine N, Saga N, Sugiura T and Katamoto S: Microwave
hyperthermia treatment increases heat shock proteins in human
skeletal muscle. Br J Sports Med. 41:453–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moran LT, Mayer MP and Rüdiger SGD: The
Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol.
29:164–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lauwers E, Wang YC, Gallardo R, Van der
Kant R, Michiels E, Swerts J, Baatsen P, Zaiter SS, McAlpine SR,
Gounko NV, et al: Hsp90 mediates membrane deformation and exosome
release. Mol Cell. 71:689–702.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang DJ, An H, Kim KS, Kim HH, Jung J,
Lee JM, Kim NJ, Han YT, Yun H, Lee S, et al: Design, synthesis, and
biological evaluation of novel deguelin-based heat shock protein 90
(HSP90) inhibitors targeting proliferation and angiogenesis. J Med
Chem. 55:10863–10884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pezzulo AA, Tudas RA, Stewart CG,
Buonfiglio L, Lindsay BD, Taft PJ, Gansemer ND and Zabner J: HSP90
inhibitor geldanamycin reverts IL-13- and IL-17-induced airway
goblet cell metaplasia. J Clin Invest. 129:744–758. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neckers L, Kern A and Tsutsumi S: Hsp90
inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem
Biol. 14:1204–1206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maris P, Blomme A, Palacios AP, Costanza
B, Bellahcène A, Bianchi E, Gofflot S, Drion P, Trombino GE, Di
Valentin E, et al: Asporin is a fibroblast-derived TGF-β1 inhibitor
and a tumor suppressor associated with good prognosis in breast
cancer. PLoS Med. 12:e10018712015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu XF, Liu F, Xin JQ, Fan JW, Wu N, Zhu
LJ, Duan LF, Li YY and Zhang H: Respective roles of the
mitogen-activated protein kinase (MAPK) family members in
pancreatic stellate cell activation induced by transforming growth
factor-beta1 (TGF-β1). Biochem Biophys Res Commun. 501:365–373.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magnussen SN, Hadler-Olsen E, Costea DE,
Berg E, Jacobsen CC, Mortensen B, Salo T, Martinez-Zubiaurre I,
Winberg JO, Uhlin-Hansen L and Svineng G: Cleavage of the urokinase
receptor (uPAR) on oral cancer cells: Regulation by transforming
growth factor-β1 (TGF-β1) and potential effects on migration and
invasion. BMC Cancer. 17:3502017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CA, Chang JM, Chang EE, Chen HC and
Yang YL: Crosstalk between transforming growth factor-β1 and
endoplasmic reticulum stress regulates alpha-smooth muscle cell
actin expression in podocytes. Life Sci. 209:9–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Yang J, Ran B, Yang X, Zheng W,
Long Y and Jiang X: Small Molecular TGF-β1-inhibitor-loaded
electrospun fibrous scaffolds for preventing hypertrophic scars.
ACS Appl Mater Interfaces. 9:32545–32553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sibinska Z, Tian X, Korfei M, Kojonazarov
B, Kolb JS, Klepetko W, Kosanovic D, Wygrecka M, Ghofrani HA,
Weissmann N, et al: Amplified canonical transforming growth
factor-β signalling via heat shock protein 90 in pulmonary
fibrosis. Eur Respir J. 49(pii): 15019412017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaque D, Martinez ML, Del RB,
Haro-Gonzalez P, Benayas A, Plaza JL, Martin RE and Garcia SJ:
Nanoparticles for photothermal therapies. Nanoscale. 6:9494–9530.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altinsoy A, Dileköz E, Kul O, Ilhan SÖ,
Tunccan ÖG, Seven I, Bagriacik EU, Sarioglu Y, Or M and Ercan ZS: A
cannabinoid ligand, anandamide, exacerbates endotoxin-induced
uveitis in rabbits. J Ocul Pharmacol Ther. 27:545–552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang G, Liu Z, Ding H, Zhou Y, Doan HA,
Sin K, Zhu ZJ, Flores R, Wen Y, Gong X, et al: Tumor induces muscle
wasting in mice through releasing extracellular Hsp70 and Hsp90.
Nat Commun. 8:5892017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu J, Ding Y, Qian S and Tang X:
Simulations of adaptive temperature control with self-focused
hyperthermia system for tumor treatment. Ultrasonics. 53:171–177.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki S and Kulkarni AB: Extracellular
heat shock protein HSP90beta secreted by MG63 osteosarcoma cells
inhibits activation of latent TGF-beta1. Biochem Biophys Res
Commun. 398:525–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wrighton KH, Lin X and Feng XH: Critical
regulation of TGFbeta signaling by Hsp90. Proc Natl Acad Sci USA.
105:9244–9249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de la Mare JA, Jurgens T and Edkins AL:
Extracellular Hsp90 and TGFβ regulate adhesion, migration and
anchorage independent growth in a paired colon cancer cell line
model. BMC Cancer. 17:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, An YS, Kim MR, Kim YA, Lee JK,
Hwang CS, Chung E, Park IC and Yi JY: Heat shock protein 90
regulates subcellular localization of smads in Mv1Lu cells. J Cell
Biochem. 117:230–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomcik M, Zerr P, Pitkowski J,
Palumbo-Zerr K, Avouac J, Distler O, Becvar R, Senolt L, Schett G
and Distler JH: Heat shock protein 90 (Hsp90) inhibition targets
canonical TGF-β signalling to prevent fibrosis. Ann Rheum Dis.
73:1215–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Snigireva AV, Vrublevskaya VV, Afanasyev
VN and Morenkov OS: Cell surface heparan sulfate proteoglycans are
involved in the binding of Hsp90α and Hsp90β to the cell plasma
membrane. Cell Adh Migr. 9:460–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Fu R, Pan Y, Meza-Sosa KF, Zhang Z
and Lieberman J: PNPT1 Release from mitochondria during apoptosis
triggers decay of Poly(A) RNAs. Cell. 174:187–201.e12. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saita S, Nolte H, Fiedler KU, Kashkar H,
Venne AS, Zahedi RP, Kruger M and Langer T: PARL mediates Smac
proteolytic maturation in mitochondria to promote apoptosis. Nat
Cell Biol. 19:318–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bononi A, Giorgi C, Patergnani S, Larson
D, Verbruggen K, Tanji M, Pellegrini L, Signorato V, Olivetto F,
Pastorino S, et al: BAP1 regulates IP3R3-mediated Ca2+
flux to mitochondria suppressing cell transformation. Nature.
546:549–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Häger M, Pedersen CC, Larsen MT, Andersen
MK, Hother C, Grønbaek K, Jarmer H, Borregaard N and Cowland JB:
MicroRNA-130a-mediated down-regulation of Smad4 contributes to
reduced sensitivity to TGF-β1 stimulation in granulocytic
precursors. Blood. 118:6649–6659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lam CK, Zhao W, Cai W, Vafiadaki E, Florea
SM, Ren X, Liu Y, Robbins N, Zhang Z, Zhou X, et al: Novel role of
HAX-1 in ischemic injury protection involvement of heat shock
protein 90. Circ Res. 112:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tian C, Gao P, Zheng Y, Yue W, Wang X, Jin
H and Chen Q: Redox status of thioredoxin-1 (TRX1) determines the
sensitivity of human liver carcinoma cells (HepG2) to arsenic
trioxide-induced cell death. Cell Res. 18:458–471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Milasta S, Dillon CP, Sturm OE, Verbist
KC, Brewer TL, Quarato G, Brown SA, Frase S, Janke LJ, Perry SS, et
al: Apoptosis-inducing-factor-dependent mitochondrial function is
required for T cell but Not B cell function. Immunity. 44:88–102.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shin MK, Jeong KH, Choi H, Ahn HJ and Lee
MH: Heat shock protein 90 inhibitor enhances apoptosis by
inhibiting the AKT pathway in thermal-stimulated SK-MEL-2 human
melanoma cell line. J Dermatol Sci. 90:357–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tahmasbi V, Ghoreishi M and Zolfaghari M:
Investigation, sensitivity analysis, and multi-objective
optimization of effective parameters on temperature and force in
robotic drilling cortical bone. Proc Inst Mech Eng H.
231:1012–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Palacios-Rodriguez Y, Garcia-Lainez G,
Sancho M, Gortat A, Orzaez M and Perez-Paya E: Polypeptide
modulators of caspase recruitment domain (CARD)-CARD-mediated
protein-protein interactions. J Biol Chem. 286:44457–44466. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wagatsuma A, Takayama Y, Hoshino T,
Shiozuka M, Yamada S, Matsuda R and Mabuchi K: Pharmacological
targeting of HSP90 with 17-AAG induces apoptosis of myogenic cells
through activation of the intrinsic pathway. Mol Cell Biochem.
445:45–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ke X, Chen J, Peng L, Zhang W, Yang Y,
Liao X, Mo L, Guo R, Feng J, Hu C, et al: Heat shock protein 90/Akt
pathway participates in the cardioprotective effect of exogenous
hydrogen sulfide against high glucose-induced injury to H9c2 cells.
Int J Mol Med. 39:1001–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|