Introduction
Throughout history, natural products have served an
important role in the treatment of human diseases. At present,
natural products are the major source of pharmaceutical agents,
particularly for cancer therapy. Natural products and their
derivatives account for ~80% of all drugs approved for cancer
therapy by the USA Food and Drug Administration during the last
three decades (1,2).
Daphne altaica Pall. (D. altaica) is a
medicinal herb used in traditional Kazakh medicine for the
treatment of numerous diseases, including cancer of the digestive
tract, tracheitis, common cold, sore throat, rheumatism and
snakebite (3). The plant is
endemically distributed in the North of the Jungar Basin of
Xinjiang (Tacheng and Habahe areas), the Altai, Manrak and
Tarbagatai Mountains of Kazakhstan, the Altai region of Russia and
Northwest Mongolia (3). The
medicinal use of D. altaica was first recorded in the Kazakh
medical classic work Shipagerlik Bayan (3). However, the anticancer effects of
D. altaica were reported for the first time by Kizaibek
et al (3), who demonstrated
that different D. altaica extracts, except for the aqueous
extract, displayed moderate to significant in vitro
cytotoxicity against several cancer cell lines, including Eca-109,
AGS, SMMC-7721 and HeLa. Kizaibek et al (4) also identified antiproliferative
activities of D. altaica in the human CCRF-CEM leukaemia and
MDA-MB-231 breast cancer cell lines, and identified the
constituents of the D. altaica CH2Cl2
extract using liquid chromatography (LC)-diode-array detection
(DAD)-mass spectrometry and LC-DAD-high resolution electrospray
ionization mass spectrometry in positive mode. Nugroho et al
(5) reported that three new
daphnane diterpenoids (Altadaphnans A-C) from the aerial parts of
D. altaica significantly inhibited the proliferation of A549
cancer cells. However, the mechanisms underlying the
antiproliferative activities of D. altaica have not been
previously reporteD. Therefore, the present study aimed to identify
the mechanism underlying the antiproliferative activity of an ethyl
acetate extract of D. altaica (Da-Ea) by assessing cell
apoptosis, cell cycle progression and the expression of peroxisome
proliferator-activated receptor γ (PPARγ) in the human Eca-109
oesophageal squamous cell carcinoma cell line.
Materials and methods
Cell culture
The human Eca-109 oesophageal cancer cell line was
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and maintained at 37°C with 5%
CO2 in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (HyClone;
GE Healthcare Life Sciences). At 90% confluency, cells were
harvested using 0.25% trypsin-EDTA (Gibco; Thermo Fisher
Scientific, Inc.). Cells in the exponential phase of growth were
used for subsequent experiments.
Plant materials
The D. altaica plant was collected from
Habahe County of Xinjiang Uyghur Autonomous Region, P.R. China in
July 2017. The plant was identified by Dr Omirshat Tahan (College
of Grassland and Environment Sciences, Xinjiang Agricultural
University, Urumqi, China). A voucher specimen (no. HB-2017001) was
deposited at the Traditional Kazakh Medicine Research Institute of
Traditional Chinese Medicine Hospital of Ili Kazakh Autonomous
Prefecture.
Extraction of Da-Ea
Da-Ea was extracted as previously described
(3). Briefly, dried bark of the
plant (150 g) was cut into small pieces and macerated with 95% EtOH
for 2 weeks at room temperature in the dark. The extraction process
was repeated twice. The extracted mixtures were combined,
concentrated by distillation under vacuum and freeze-dried to yield
the EtOH extract. Petroleum ether, chloroform and ethyl acetate
(Da-Ea; 0.8243 g) extracts were obtained from the EtOH extract
using a sequential liquid-liquid extraction with solvents of
increased polarity, including pure petroleum ether, pure chloroform
and pure ethyl acetate. The only extract used in subsequent
experiments was Da-Ea.
High performance liquid chromatography (HPLC) with
diode-array detection (DAD) was used to analyse the main
ingredients in the extracts. HPLC was conducted using a 1260 HPLC
system (Agilent Technologies, Inc.) equipped with a 1260 Infinity
Diode Array Detector (cat. no. G4212A; Agilent Technologies, Inc.)
in gradient elution mode. An XBridge™ C18 column (4.6×250 mm;
diameter, 5 µm; Waters Chromatography Europe BV) set at 25°C with a
0.5 ml/min flow rate was useD. For each experiment, a 5 µl
injection volume was useD. The mobile phase consisted of 0.1% (v/v)
acetic acid aqueous solution (solvent A) and acetonitrile (solvent
B). The gradient conditions used were as follows: 10% solvent B for
0 min; 62% solvent B for 37 min; 62% solvent B for 39 min; and 10%
solvent B for 40 min. The chromatogram was analysed using OpenLAB
CDS Chemstation software (version A.01.05; Agilent Technologies,
Inc.). To obtain stock solution, 22.7 mg Da-Ea was sonicated with
1,135 µl 80% MeOH-DMSO (1:1) at 40 MHz frequency and room
temperature for 10 min. Subsequently, the stock solution was
further diluted with methanol to generate 1.25 mg/ml sample
solution for HPLC analysis. Standard compounds
[daphnetin-7-O-β-D-glucoside, daphnetin,
demethyldaphnoretin-7-O-β-D-glucopyranoside and genkwanol A;
provided by Professor Zhengbing Gu (Jiangsu Yongjian Medical
Technology Ltd., Co.)] were dissolved in methanol. Compounds were
identified based on their retention time and UV spectra compared
with reference standards.
Evaluation of cell morphology
Eca-109 cells were seeded (2×105
cells/well) into 6-well culture plates and incubated with Da-Ea
(10, 20 or 50 µg/ml) at 37°C for 24, 48 or 72 h. Cells treated with
DMSO (10, 20 or 50 µg/ml) were used as the control. For each test
and control medium, the final concentration of DMSO was adjusted to
1%. Under ×100 magnification, cell morphology was assessed using an
IX71-12FL/PH phase contrast microscope (Olympus Corporation) with
Olympus cellSens Standard imaging software, version 1.0 (Olympus
Corporation).
Detection of cell apoptosis
Early cell apoptosis was examined by Annexin V-FITC
Apoptosis Detection kit (BD Bioscience) according to the
manufacturer's protocol. Cells (2×105 cells/well) were
seeded into 6-well culture plates and allowed to grow overnight.
Subsequently, cells were treated with Da-Ea (10, 20 or 50 µg/ml)
for 24, 48 and 72 h. Following treatment, cells were collected and
washed twice with PBS. Cells were incubated with 5 µl Annexin V and
5 µl PI (BD Biosciences; Becton, Dickinson and Company) for 15 min
at room temperature in the dark. Following incubation, each sample
was filtrated on a nylon membrane (pore size, 48 ìm). Flow
cytometry was performed using a FACSAriaII flow cytometer (Beckman
Coulter, Inc.) and FlowJo software, version 7.6 (Tree Star
Inc.).
Detection of cell cycle
For cell cycle analysis, cells were seeded
(2×105 cells/well) into 6-well culture plates and
maintained overnight. Subsequently, cells were treated with Da-Ea
(10, 20 or 50 µg/ml) for 24 or 48 h. Cells were washed twice with
PBS. Cells were fixed in 75% ethanol at 4°C for 2 h and
subsequently washed three times with PBS. Cells were stained with
500 µl PI/RNase staining buffer (BD Biosciences; Becton, Dickinson
and Company) at room temperature for 15 min in dark. Following
staining, cells were filtrated on a nylon membrane (pore size, 48
ìm). Cells were analysed using a FACSAriaII flow cytometer (Beckman
Coulter, Inc.) and ModFit LT software, version 3.2 (Verity Software
House, Inc.).
Assessment of PPARγ mRNA expression by
reverse transcription-quantitative PCR (RT-qPCR)
Eca-109 cells in the logarithmic phase of growth
were seeded (3×105 cells/ml) into 6-well culture plates.
Cells were incubated with Da-Ea (10, 20 or 50 µg/ml) for 24, 48 and
72 h at 37°C with 5% CO2. Subsequently, cells were
washed with PBS and total RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Subsequently, the cell
lysate was incubated at room temperature for 5 min and transferred
to a fresh 1.5 ml Eppendorf tube containing 200 µl chloroform. The
tubes were vortexed for 15 sec, incubated at room temperature for
2–3 min and centrifuged at 15,400 × g for 15 min at 4°C until the
liquid separated into three phases. The upper aqueous phase
(400-500 µl) was transferred to a new RNase-free Eppendorf tube,
mixed with an equal volume of isopropanol and incubated for 10 min
at room temperature. Subsequently, the sample was centrifuged for
10 min at 15,400 × g and 4°C to precipitate the RNA. The
supernatant was discarded and 75% ethanol was added to the RNA
prior to centrifugation at 3,800 × g for 15 min at 4°C.
Subsequently, the supernatant was discarded and the RNA was
air-dried at room temperature for 5–10 min. Total RNA was stored at
−20°C until further analysis. RNA purity was assessed by measuring
the A260/A280 absorbance ratio using a Nanodrop spectrophotometer
(Thermo Fisher Scientific, Inc.). RNA with an A260/A280 ratio of
1.7–2.0 was considered high quality. RNA quality and integrity were
verified by visualising 28S and 18S RNA bands on an ethidium
bromide-stained 1.5% agarose gel using the Gel DOC XR imaging
system (Bio-Rad Laboratories, Inc.). RNA samples with a clear and
sharp 28S band that were twice as intense as the 18S band were used
for reverse transcription. Total RNA was reverse transcribed to
cDNA using RevertAid First strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). Reverse transcription was performed for a total
volume of 20 µl consisting of 1 µg total RNA, 1 µl Oligo (dT)18
primer, 4 µl 5X Reaction Buffer, 1 µl Ribolock Rnase Inhibitor (20
U/µl), 2 µl dNTP mix (10 mm) and 1 µl RevertAid M-MuLV RT (200
U/µl). The following thermocycling conditions were used for reverse
transcription: 3 min at 65°C; 60 min at 4°C; and 5 min at 70°C.
Subsequently, qPCR was performed using Fast Start
Universal SYBR Green Master (ROX) kit (Thermo Fisher Scientific,
Inc.) and a 7500 Fast Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The reaction system (20 µl)
consisted of 10 µl Fast Start Universal SYBR Green Master (ROX), 2
µl cDNA, 8 µl ddH2O, 0.5 µl forward primer and 0.5 µl
reverse primer. The following primer pairs were used for qPCR:
PPARγ forward, 5′-TACTGTCGGTTTCAGAAATGCC-3′ and reverse,
5′-GTCAGCGGACTCTGGATTCAG-3′; and β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation for 3 min at
95°C; followed by 40 cycles of denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec and elongation at 72°C for 10 min; and
melt curve analysis from 65–95°C at a heating rate of 20°C/sec.
PPARγ mRNA levels were quantified using the 2−ΔΔCq
method and normalized to the internal reference gene β-actin
(6).
Assessment of PPARγ protein expression
by western blotting
Cells were treated with Da-Ea (10, 20 or 50 µg/ml)
for 24, 48 or 72 h. Total protein was extracted from cells using
RIPA buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
containing PMSF. Subsequently, samples were centrifuged at 12,000 ×
g for 10 min at 4°C. Total protein was quantified using a
bicinchoninic acid assay. Protein (20 µg per lane) was separated
via 10% SDS-PAGE and transferred to PVDF membranes. Ponceau S
staining was performed to confirm successful transfer of the
proteins to the membrane, for 5 min at room temperature.
Subsequently, the membranes were blocked with 5% non-fat milk in
TBST (Tris-buffered saline containing 0.1% Tween-20) for 1 h at
room temperature. The membranes were incubated overnight at 4°C
with primary antibodies targeted against: PPARγ (1:5,00; cat. no.
sc-7273; Santa Cruz Biotechnology, Inc.) and β-actin (1:5,000; cat.
no. 60008–1-Ig; ProteinTech Group, Inc.) After washing with TBST,
the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
SA00001-1; ProteinTech Group, Inc.) for 1 h at 37°C. Subsequently,
the membranes were washed three times with TBST for 5 min. Protein
bands were visualized using an ECL detection kit (7Sea Biotech;
http://7seapharmtech.com/) and imaged using the
ChemiScope 5300 Pro (Clinx Science Instruments Co., Ltd.) imaging
system. β-actin was used as the loading control. The bands were
then quantified using image analysis software (ImageJ2×; version
2.1.4.5; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc.). Comparisons
among groups were analysed using one-way ANOVA followed by
Dunnett's post hoc test. Data are expressed as the mean ± standard
deviation of three replicates. P<0.05 was considered to indicate
a statistically significant difference.
Results
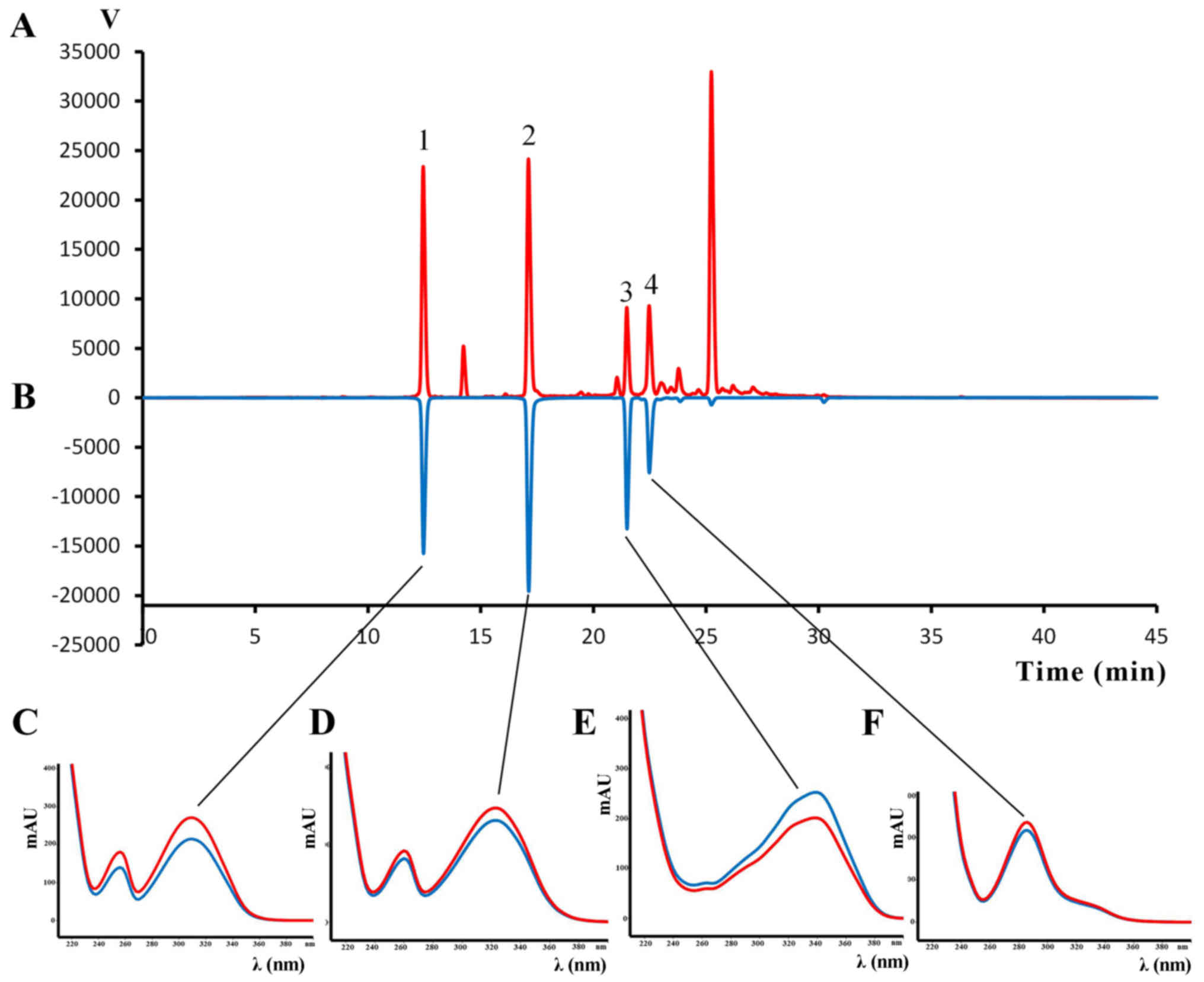
HPLC-DAD analysis
The main components of Da-Ea were identified by
HPLC-DAD. The HPLC chromatogram recorded at a wavelength of 320 nm
is presented in Fig. 1. Based on
their retention time and UV spectra, daphnetin-7-O-β-D-glucoside
(Fig. 1C), daphnetin (Fig. 1D),
demethyldaphnoretin-7-O-β-D-glucopyranoside (Fig. 1E) and genkwanol A (Fig. 1F) were detected in Da-Ea. The
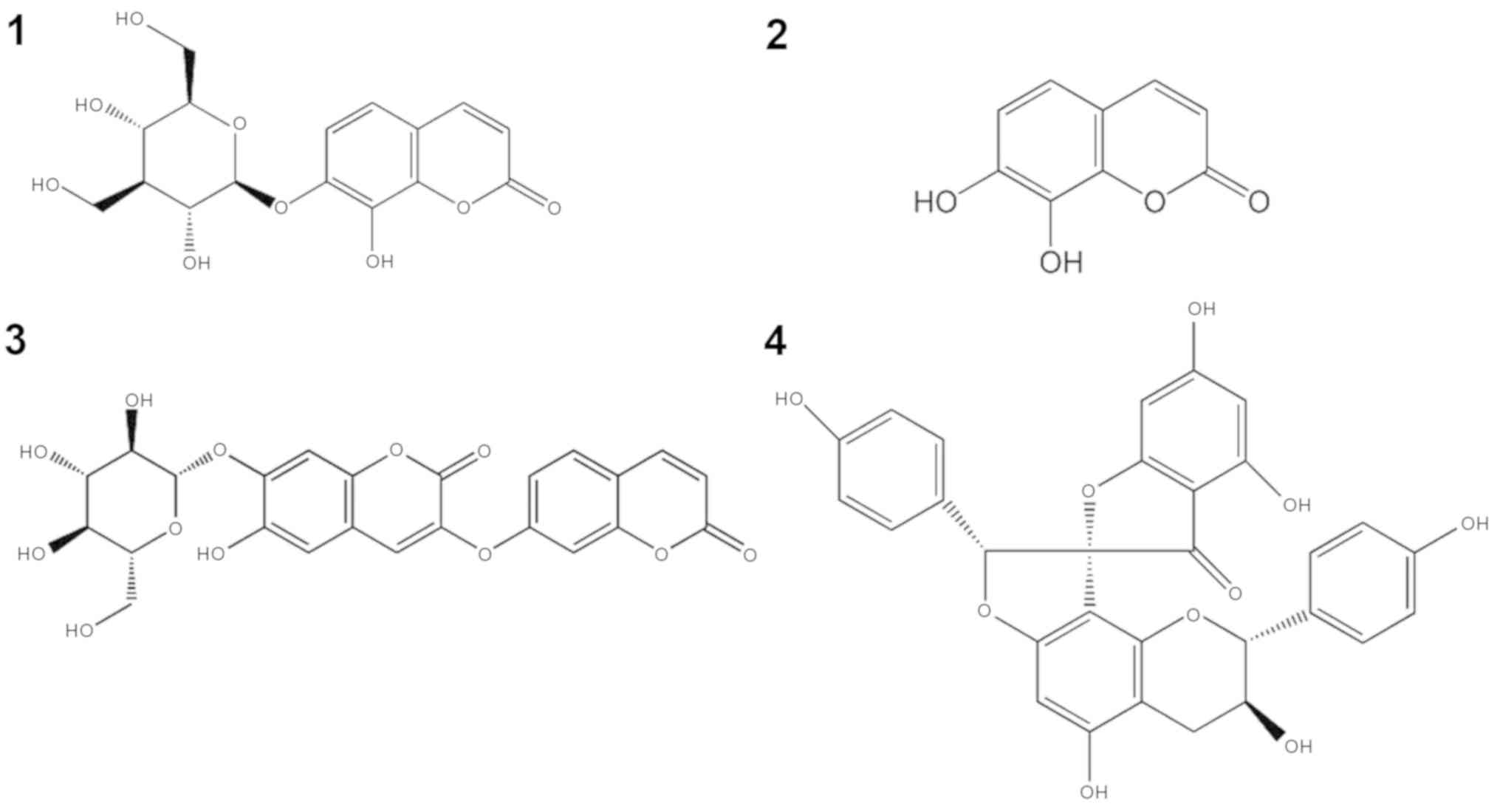
chemical structures of the four compounds are presented in Fig. 2.
Cell morphological observation
Cell morphology was examined using an inverted
fluorescence microscope. Microscopic examination indicated that
Eca-109 cells exposed to various concentrations of Da-Ea for 24–72
h underwent notable morphological alterations (Fig. 3). For example, round or
polygonal-shaped cells became elongated, cells appeared to shrink,
nuclei were destroyed and the number of floating cells increased in
a time- and concentration-dependent manner. In addition, cellular
integrity was increasingly damaged with longer incubations with
Da-Ea.
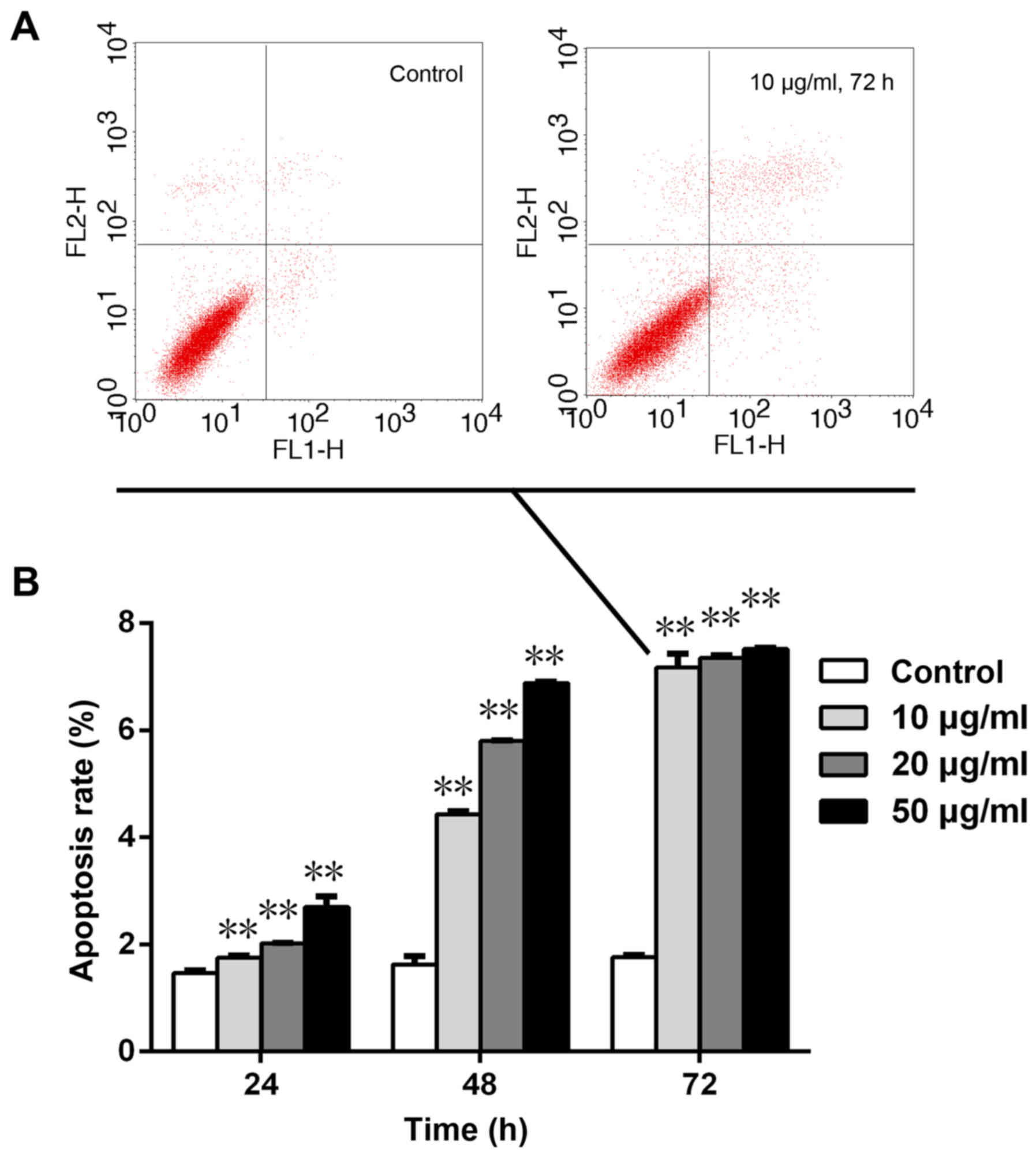
Cell apoptosis detection
Cell apoptosis was assessed by Annexin-V and PI
double staining using flow cytometry (Table I and Fig. 4). Following treatment with 10, 20
and 50 µg/ml Da-Ea, the percentage of apoptotic cells at 24 h was
1.756±0.040, 2.016±0.015 and 2.700±0.200%, respectively, which was
significantly increased compared with that of the control group
(1.463±0.055%; P<0.05). At 48 h, the percentage of apoptotic
cells was 4.430±0.060, 5.800±0.010 and 6.876±0.025% in the 10, 20
and 50 µg/ml Da-Ea groups, respectively, which was also
significantly increased compared with that of the control group
(1.63±0.155%; P<0.05). Following incubation for 72 h, the
percentage of apoptotic cells was significantly increased by
~4-fold to 7.173±0.251, 7.350±0.043 and 7.516±0.015% in the 10, 20
and 50 µg/ml Da-Ea groups, respectively, compared with the control
group (1.763±0.045%; P<0.05). The results suggested that Da-Ea
induced Eca-109 cell apoptosis in a dose- and time-dependent
manner.
 | Table I.Rate of Eca-109 cell apoptosis
following treatment with ethyl acetate extract of Daphne
altaica for 24, 48 or 72 h. |
Table I.
Rate of Eca-109 cell apoptosis
following treatment with ethyl acetate extract of Daphne
altaica for 24, 48 or 72 h.
|
| Rate of apoptosis
(%) |
|---|
|
|
|
|---|
| Concentration
(µg/ml) | 24 h | 48 h | 72 h |
|---|
| Control | 1.463±0.055 | 1.630±0.155 | 1.763±0.045 |
| 10 |
1.756±0.040a |
4.430±0.060a |
7.173±0.251a |
| 20 |
2.016±0.015a |
5.800±0.010a |
7.350±0.043a |
| 50 |
2.700±0.200a |
6.876±0.025a |
7.516±0.015a |
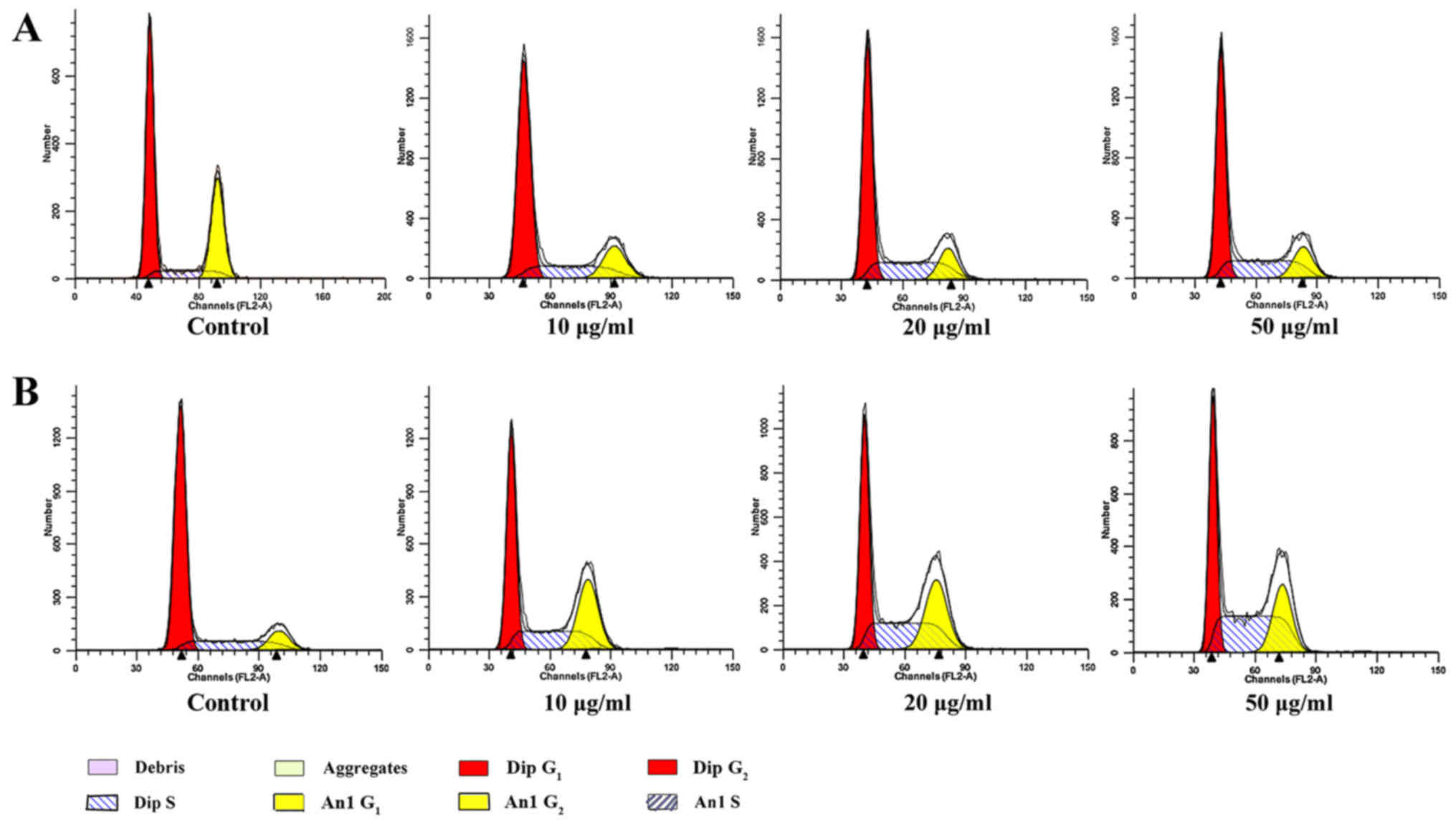
Cell cycle detection
Cell cycle distribution was detected by PI staining
and flow cytometry (Table II and
Fig. 5). Following treatment with
Da-Ea for 24 h, the percentage of S phase cells significantly
increased from 17.043±0.912% in the control group to 33.070±0.592%
in the 50 µg/ml Da-Ea group, whereas the percentage of
G0/G1 phase cells significantly decreased
from 74.956±0.912% in the control group to 58.926±0.592% in the 50
µg/ml Da-Ea group. When exposed to Da-Ea for 48 h, the percentage
of S phase cells significantly increased from 18.495±1.716% in the
control group to 40.298±7.949% in the 50 µg/ml group, whereas the
percentage of G0/G1 phase cells significantly
decreased from 72.053±0.458% in the control group to 44.476±1.017%
in the 50 µg/ml group. These effects were also observed in the 10
and 20 µg/ml Da-Ea groups. Da-Ea-induced alterations to the cell
cycle distribution occurred in a time- and dose-dependent manner,
which suggested that Da-Ea inhibited cell proliferation by inducing
S phase cell cycle arrest in Eca-109 cells.
 | Table II.Proportion of cells in the G0/G1 and
S phases of the cell cycle. |
Table II.
Proportion of cells in the G0/G1 and
S phases of the cell cycle.
|
| 24 h | 48 h |
|---|
|
|
|
|
|---|
| Concentration |
G0/G1 phase | S phase |
G0/G1 phase | S phase |
|---|
| Control | 74.956±0.912 | 17.043±0.912 | 72.053±0.458 | 18.495±1.716 |
| 10 |
69.096±0.382b |
24.570±2.542b |
57.857±0.236b | 29.390±5.520 |
| 20 |
61.410±1.260b |
30.590±1.260b |
50.843±0.458b |
35.873±5.849a |
| 50 |
58.926±0.592b |
33.070±0.592b |
44.476±1.017b |
40.298±7.949b |
PPARγ mRNA expression level
Agarose gel electrophoresis demonstrated that the
bands corresponding to 28S RNA and 18S RNA were sharp and clear.
Furthermore, the intensity of the 28S RNA band was approximately
twice as intense as the 18S RNA band, demonstrating that the total
RNA was intact and not degradeD. The A260/A280 ratios of the
extracted RNA were 1.9–2.0, suggesting that the obtained RNA had a
high purity. Additionally, only a single sharp peak was generated
during the melting curve analysis, indicating high specificity of
the products and absence of non-specific amplification products
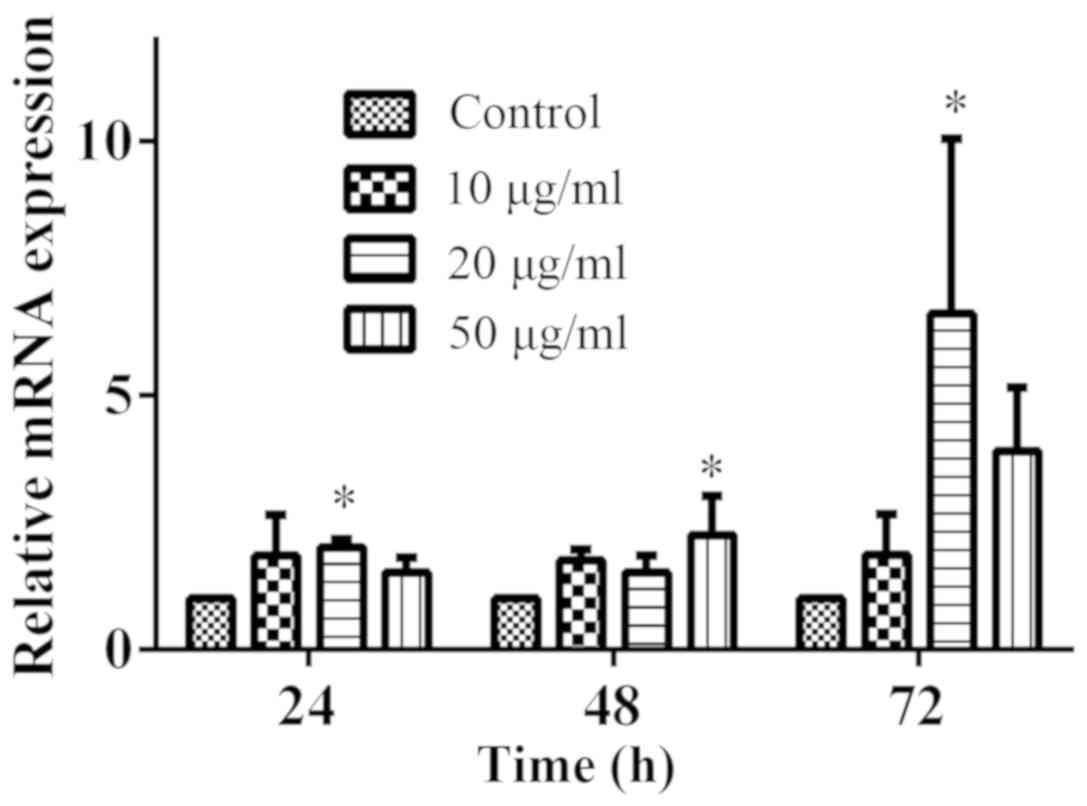
(data not shown). The mRNA expression levels of PPARγ were analysed
by RT-qPCR. At 24, 48 and 72 h, PPARγ mRNA expression levels were
increased in the 10, 20 and 50 µg/ml Da-Ea groups compared with the
control group; however, the increases in expression were only
significant for the 20 µg/ml Da-Ea group at 24 h, the 50 µg/ml
group at 48 h and the 20 µg/ml Da-Ea group at 72 h (Fig. 6). The results suggested that Da-Ea
increased the mRNA expression levels of PPARγ in Eca-109 cells in a
time-dependent manner.
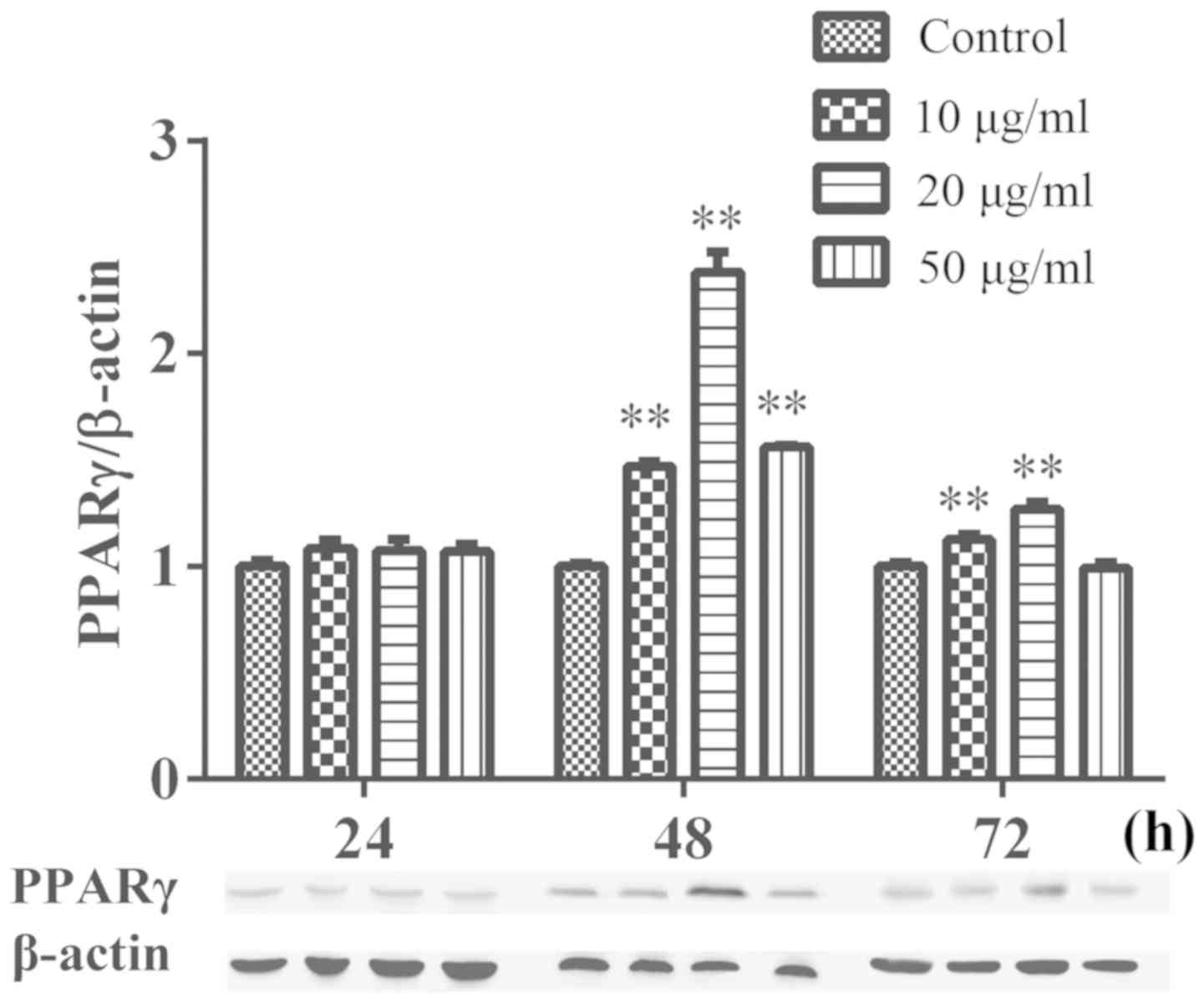
PPARγ protein expression level
Western blotting was performed to investigate the
effect of DA-Ea on the protein expression levels of PPARγ in
Eca-109 cells. A calibration curve was generated by plotting the
absorbance of protein standards against their concentration and the
linearity of the method was evaluated by analysing six working
solutions. The binary linear equation for the calibration curve was
determined as y=0.1056×-0.0018, with an R2 value of 0.9916,
suggesting the method displayed good linearity (data not shown).
Da-Ea (10, 20 and 50 µg/ml) treatment for 48 h significantly
increased the protein expression level of PPARγ in Eca-109 cells
compared with the control group. Following treatment for 72 h,
Da-Ea (10 and 20 µg/ml) significantly increased PPARγ protein
expression levels in Eca-109 cells compared with the control group
(Fig. 7).
Discussion
In the present study, the effects of Da-Ea on
Eca-109 cell apoptosis and cell cycle distribution were analysed,
and the underlying mechanisms were investigated by detecting the
expression levels of PPARγ.
A previous study reported that petroleum ether,
chloroform, DA-Ea and n-butanol extracts of D. altaica
display moderate to significant in vitro cytotoxicity
against several cancer cell lines (Eca-109, AGS, SMMC-7721 and
HeLa). Moreover, Da-Ea inhibited Eca-109 cell proliferation to the
greatest extent out of the four extracts; therefore, DA-Ea was used
in the present study (3).
The present study demonstrated that the main
constituents of Da-Ea were daphnetin-7-O-β-D-glucoside, daphnetin,
genkwanol A and demethyldaphnoretin-7-O-β-D-glucopyranoside.
Demethyldaphnoretin-7-O-β-D-glucopyranoside has been reported to
display potent cytotoxicity against HepG2 and Hep3B cells (7), which indicates that this compound
could be at least partially responsible for the antiproliferative
activity of Da-Ea. To the best of our knowledge, the effects of the
four identified compounds on PPARγ expression have not been
previously reporteD. However, a previous study demonstrated that
D. gnidium, which contains daphnetin-7-O-β-D-glucoside and
daphnetin, activates PPARγ (8,9).
Therefore, the effect of Daphne-derived chemical compounds,
including the four compounds identified in the present study, on
PPARγ expression requires further investigation.
In the present study, Da-Ea-treated cells displayed
characteristic morphological features of apoptotic cells, including
cell shrinkage, membrane blebbing, pyknotic cells with broken
nuclei and floating cell formation. In addition, the rate of
apoptosis increased in a time- and dose-dependent manner in
Da-Ea-treated Eca-109 cells. Cancer is a disease that is associated
with uncontrolled cell proliferation, which is mediated by
antiapoptotic mechanisms. When cancer cells undergo apoptosis, no
additional damage to surrounding normal cells and tissues is
induced; therefore, enhancing apoptosis may serve as an effective
therapeutic strategy for cancer (10). The results of the present study
suggested that the anticancer effects of Da-Ea on Eca-109 cells
were partly due to apoptosis induction. However, the rate of
Da-Ea-induced apoptosis was not as high as expected, which may have
been caused by low purity of the extract. Therefore, future studies
investigating the active principles isolated from D. altaica
are required.
Cell cycle progression dysregulation is also a
common characteristic of cancer. The cell cycle is separated into
four sequential phases, G1, S, G2 and M,
which are regulated by a series of proteins, including
cyclin-dependent kinases and cyclins, at a number of checkpoints.
Cells can be arrested at a cell cycle checkpoint for a number of
reasons, including DNA damage, which can ultimately result in
apoptosis induction (10).
Uncontrolled cell cycle progression is one of the most common
causes of the transformation of normal cells to cancer cells
(7); therefore, components of the
cell cycle machinery may serve as molecular therapeutic targets for
cancer (11). In the present
study, Eca-109 cell S phase arrest was increased following
treatment with Da-Ea for 24 and 48 h compared with the control
cells, as determined by flow cytometry.
Additionally, the effects of D. altaica on
the expression level of PPARγ in Eca-109 cells were investigateD.
PPARs are ligand-activated transcription factors that regulate the
expression of genes involved in lipid metabolism, glucose
homeostasis, cell proliferation, differentiation and survival.
PPARs are divided into three subfamilies: PPARα, PPARβ/δ and PPARγ,
with the PPARγ subfamily being the most intensively investigated
(12,13). A number of studies have
demonstrated that natural bioactive compounds can exert
chemopreventive effects by modulating PPARγ (14–16).
According to the literature, triterpenoids, flavonoids, carotenoids
and linoleic acid are cancer chemoprotective compounds that
effectively activate PPARγ (13).
Among these compounds, triterpenoids (17), flavonoids (18) and linoleic acid (19), which display antitumor activities,
have been identified in Daphne species (20); therefore, investigating whether the
bioactive extract of D. altaica can activate PPARγ
expression is important.
In the present study, Da-Ea treatment for 48 h
increased the protein expression level of PPARγ in Eca-109 cells
compared with the control cells. Similarly, the mRNA expression
levels of PPARγ in Eca-109 cells were increased following treatment
with Da-Ea compared with the control cells, which indicated that
D. altaica extract may inhibit cell proliferation and induce
cell apoptosis by upregulating PPARγ gene expression. However,
Da-Ea-induced PPARγ protein expression was not time- or
concentration-dependent, which may be associated with the
complexity of components present in the D. altaica extract.
Daphne species contain various coumarins (21), diterpenes (22), triterpenes (23), flavonoids (24), biflavionoids (25), lignans (22,26),
norlignans (27), simple
phenylpropanoids (28) and
steroids (26). Interactions,
including synergism or antagonism, among the Daphne-derived
components have been suggested (29); therefore, further investigation
into the effects of D. altaica extract-derived purified
compounds on PPARγ is required.
In summary, phase contrast microscopy was used to
observe Da-Ea induced morphological alterations in Eca-109 cells.
Flow cytometry was performed to investigate cell apoptosis and cell
cycle arrest in the Eca-109 cells. RT-qPCR and western blotting
were performed to detect the mRNA and protein expression levels of
PPARγ, respectively. The results suggested that Da-Ea induced
apoptosis and S phase cell cycle arrest, and also upregulated the
mRNA and protein expression levels of PPARγ in Eca-109 cells. In
conclusion, the results suggested that Da-Ea inhibited Eca-109 cell
proliferation by inducing cell cycle arrest and apoptosis via
PPARγ-mediated pathways.
Acknowledgements
The authors would like to thank Mrs Aerziguli
Tuerxun and Mrs Xue Zhang (Clinical Medical Research Institute, The
First Affiliated Hospital of Xinjiang Medical University, Urumqi,
P.R. China) for their assistance in performing the cell biology
assays.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360499) and the
Training Project for Scientific and Technological Talents of
Xinjiang Uighur Autonomous Region (grant no. QN2016YX0759).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK analysed the chemical constituents, performed the
data analysis and drafted and revised the manuscript. AW performed
the cell-based assays and data analysis. ZG provided the standard
compounds and interpreted HPLC-DAD data. DB, LT and KN prepared the
extract. DB also cooperated with AW on performing the cell-based
assays. BC and JW provided the related materials and performed the
HPLC-DAD assay. OT identified the plant taxonomically and
collaborated with MK and AW on statistical analyses of the data. PC
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Da-Ea
|
ethyl acetate extract of Daphne
altaica
|
|
PPARγ
|
peroxisome proliferator-activated
receptor-γ
|
|
HPLC-DAD
|
high-performance liquid
chromatography-diode-array detector
|
References
|
1
|
Cragg GM and Pezzuto JM: Natural products
as a vital source for the discovery of cancer chemotherapeutic and
chemopreventive agents. Med Princ Pract. 25 (Suppl 2):41–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bishayee A and Sethi G: Bioactive natural
products in cancer prevention and therapy: Progress and promise.
Semin Cancer Biol. 40:1–3. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kizaibek M, Daniar M, Li L and Upur H:
Antiproliferative activity of different extracts from Daphne
altaica Pall. On selected cancer cells. J Med Plants Res.
5:3448–3452. 2011.
|
|
4
|
Kizaibek M, Pferschy-Wenzig EM, Kretschmer
N, Hamburger M and Bauer R: LC-MS-based phytochemical
characterization of an antiproliferative Daphne altaica stem
bark extract. Planta Med. 81:PM–128. 2015. View Article : Google Scholar
|
|
5
|
Nugroho AE, Chin-Piow W, Hirasawa Y, Janar
J, Kaneda T, Shirota O and Morita H: Daphnane diterpenoids from
Daphne altaica. Nat Prod Commun. 11:1073–75. 2016.PubMed/NCBI
|
|
6
|
Kong TC, Xiang L, Wang X, Jun EL, Xi LF
and Schweinfurth JM: High level expression of human epithelial
β-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions.
Virol J. 3:752006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Huang M, Zheng S, Ma X, Wan D and
Feng Y: Liquid chromatography with mass spectrometry and NMR
spectroscopy based discovery of cytotoxic principles from Daphne
tangutica Maxim. J Sep Sci. 39:21792016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang MH, Avula B, Smillie T, Khan IA and
Khan SI: Screening of medicinal plants for PPARα and PPARγ
activation and evaluation of their effects on glucose uptake and
3T3-L1 adipogenesis. Planta Medica. 79:1084–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deiana M, Rosa A, Casu V, Cottiglia F,
Bonsignore L and Dessì MA: Chemical composition and antioxidant
activity of extracts from Daphne gnidium L. J Am Oil
Chemists' Society. 80:65–70. 2003. View Article : Google Scholar
|
|
10
|
Chen Y, Zhu L, Yang X, Wei C, Chen C, He Y
and Ji Z: Ailanthone induces G2/M cell cycle arrest and apoptosis
of SGC-7901 human gastric cancer cells. Mol Med Report.
16:6821–6827. 2017. View Article : Google Scholar
|
|
11
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ota K, Ito K, Suzuki T, Saito S, Tamura M,
Hayashi S, Okamura K, Sasano H and Yaegashi N: Peroxisome
proliferator-activated receptor gamma and growth inhibition by its
ligands in uterine endometrial carcinoma. Clin Cancer Res.
12:4200–4208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sainis I, Vareli K, Karavasilis V and
Briasoulis E: PPARgamma: The portrait of a target ally to cancer
chemopreventive agents. PPAR Res. 2008:4364892008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mora FD, Jones DK, Desai PV, Patny A,
Avery MA, Feller DR, Smillie T, Zhou YD and Nagle DG: Bioassay for
the identification of natural product-based activators of
peroxisome proliferator-activated receptor-gamma (PPARgamma): The
marine sponge metabolite psammaplin A activates PPARgamma and
induces apoptosis in human breast tumor cells. J Nat Prod.
69:547–552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edwards IJ and O'Falherty JT: Omega-3
fatty acids and PPARgamma in cancer. PPAR Res. 2008:3580522008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Josep BR, Kathryn R, Susan MC, Yongzhi C,
Lothar H, Frank G, Jurg R, Alejandro UB and Raquel H: Activation of
PPAR gamma and delta by conjugated linoleic acid mediates
protection from experimental inflammatory bowel disease.
Gastroenterology. 127:777–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ullah N, Ahmed Z, Ahmed S, Muhammad P and
Malik A: A pentacyclic triterpene from Daphne oleoides.
Phytochemistry. 50:839–841. 1999. View Article : Google Scholar
|
|
18
|
Xu WC, Shen JG and Jiang JQ: Phytochemical
and biological studies of the plants from the genus Daphne.
Chem Biodivers. 42:1215–1233. 2011. View Article : Google Scholar
|
|
19
|
Pang NN, Yong YU, Bi KS, Yan BQ and Chen
XH: Simultaneous determination of the contents of palmitic acid and
linoleic acid in Genkwa Flos by GC. J Shenyang Pharmaceutical
University. 28:47–50. 2011.(In Chinese).
|
|
20
|
Riaz M, Saleem A, Siddique S, Khan BA,
Nurealam M, Shahzadulhussan S, Miana GA and Khan MQ: Phytochemistry
of Daphne oleoides. Nat Prod Res. 30:880–897. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cabrera E, Garcia-Granados A and Maqueda
M: Antibacterial activity of coumarins isolated from Daphne
gnidium L. Microbios Letters. 37:153–159. 1988.
|
|
22
|
Pan L, Zhang XF, Deng Y, Zhou Y, Wang H
and Ding LS: Chemical constituents investigation of Daphne
tangutica. Fitoterapia. 81:38–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ulubelen A, Tan N and Tuzlaci E:
Constituents of Daphne mucronata. Fitoterapia.
61:2811990.
|
|
24
|
Park BY, Min BS, Oh SR, Kim JH, Bae KH and
Lee HK: Isolation of flavonoids, a biscoumarin and an amide from
the flower buds of Daphne genkwa and the evaluation of their
anti-complement activity. Phytother Res. 20:610–613. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baba K, Takeuchi K, Doi M, Inoue M and
Kozawa M: Chemical studies on the constituents of the
thymelaeaceous plants. II. Stereochemistry of daphnodorin A and
daphnodorin B. Chem Pharm Bull. 34:1540–1545. 1986. View Article : Google Scholar
|
|
26
|
Yuan XH, Xu CX, Zhou M, Zhang XY and Li
BG: Chemical constituents of Daphne tangutica. Tianran
Chanwu Yanjiu Yu Kaifa. 19:55–58. 2007.
|
|
27
|
Zhang W, Zhang WD, Liu RH, Shen YH, Zhang
C, Cheng HS, Fu P and Shan L: Two new chemical constituents from
Daphne odora Thunb. var. marginata. Nat Prod Res.
20:1290–1294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ullah N, Ahmad S and Malik A:
Phenylpropanoid glycosides from Daphne oleoides. Chem Pharm
Bull. 47:114–115. 1999. View Article : Google Scholar
|
|
29
|
Boik JC, Kirakosyan A, Kaufman PB, Seymour
EM and Spelman K: Interactions of bioactive plant metabolites:
Synergism, antagonism, and additivity. In: Recent advances in plant
biotechnology. Springer US. (Boston, MA). 213–230. 2009.
|