Introduction
Temporomandibular joint disorders (TMDs) are
diseases involving pain and dysfunction in the temporomandibular
joint (TMJ) and masticatory muscles (1). Osteoarthritis (OA) is a degenerative
TMD characterised by progressive cartilage degeneration,
subchondral bone remodelling, synovitis and chronic pain (2,3). OA
of the TMJ (TMJOA) often involves all soft and hard tissues of the
TMJ, resulting in pain, joint motion limitation and joint noises
(4). Physiotherapy, non-steroidal
anti-inflammatory drugs, arthroscopy and surgical treatment are
often used in the clinical treatment of TMJOA (5). These treatments can relieve the
symptoms; however, owing to the limited healing ability of
avascular cartilage, they do not completely restore joint function
or reverse the destruction of cartilage and other tissues (6,7).
Mesenchymal stem cell (MSC) treatment is a potential new
therapeutic strategy for TMJOA. Synovial-derived mesenchymal stem
cells (SMSCs) have been demonstrated to have osteogenic,
chondrogenic and adipogenic potential (8,9), and
are recognised for their proliferation efficiency and potential to
differentiate into cartilage (10).
Although TMJOA is defined as a low-grade
inflammatory joint condition (5),
degenerative changes in the synovium and disc of the TMJ can still
be caused by persistent inflammation (11). Interleukin (IL)-1β is one of the
most significant pro-inflammatory factors and has been demonstrated
to cause articular cartilage inflammation (12). IL-1β is significantly upregulated
in the synovial fluid, synovium and cartilage of patients with
TMJOA, where it stimulates chondrocytes and rheumatoid
fibroblast-like synoviocytes (RA-FLSs) to release matrix
metalloproteinases (MMPs), which cause excessive degeneration of
the cartilage extracellular matrix (ECM) (13–16).
IL-1β has also been demonstrated to inhibit the expression of type
II collagen in MSCs, resulting in unbalanced synthesis and
catabolism, which ultimately leads to cartilage destruction
(17). Additionally, IL-1β can
also increase the production of other inflammatory mediators such
as IL-6, IL-8, and tumour necrosis factor (TNF)-α (18). Previous studies have demonstrated
that IL-1β upregulates the expression of IL-6 in synovial
fluid-derived and synovial-derived mesenchymal stem cells by
activating the NF-κB pathway (8,9).
IL-6 is also considered an important inflammatory factor associated
with synovitis and OA of the TMJ and was demonstrated to be
upregulated in the synovial fluid of patients with OA and
correlated positively with MMPs (19–21).
In addition, IL-6 also impedes MSCs in the synovial fluid from
differentiating to cartilage, thus reducing the effectiveness of
stem cell-based TMJOA therapy (22,23).
Long non-coding RNAs (LncRNAs) are a class of
>200-nucleotide non-coding RNA molecules without an open reading
frame (24,25). They are further classified into
antisense lncRNAs, intergenic non-coding RNAs (lincRNAs),
pseudogene lncRNAs, enhanced RNAs and intronic RNAs depending on
their location in relation to protein-coding genes. The class of an
lncRNA determines its functionality to a certain extent (26), with different lncRNAs being
involved in chromatin modification, transcription and
post-transcriptional regulation (27), making lncRNAs important regulators
of a number of physiological and pathological processes, including
OA (28). LncRNAs influence the
progression of OA by affecting the survival of chondrocytes and
synovial cells, and regulating the expression of factors associated
with arthritis, such as MMPs and type II collagen alpha 1 (29). For example, LncRNA HOX transcript
antisense RNA is significantly upregulated in the synovial fluid of
patients with TMJOA and was demonstrated to cause an IL-1β-induced
increase in the expression of MMP-1, MMP-3 and MMP-13 in the
primary chondrocytes of rabbits (14). Cartilage injury-related lncRNA
(lncRNA-CIR), highly expressed in the cartilage of patients with
OA, has been demonstrated to degrade cartilage matrix (30). In addition, human chondrocyte
inflammation-associated lincRNA (CILinc)01 and CILinc02 were
significantly downregulated in the chondrocytes of patients with
OA, and their knockdown promoted the IL-1β-induced expression of
IL-6 and IL-8 in the chondrocyte line TC28 (31). Preliminary experiments demonstrated
that the expression of AK094629 in the synovial tissue of patients
with OA was positively correlated with IL-1β, and that IL-1β
impedes the MSCs in the synovial fluid from differentiating to
cartilage by upregulating the secretion of IL-6 (9). Therefore, the present study
hypothesised that LncRNA AK094629 may be associated with the
development and progression of TMJOA.
Mitogen-activated protein kinase kinase kinase 4
(MAP3K4) is a 180-kDa protein that phosphorylates and activates
MAP2Ks, leading to the activation of MAPK pathways, including the
p38 pathway (32–34). A previous study demonstrated that
the p38 pathway contributed to IL-1β induced IL-6 expression
(35). According to the database
created by the University of California Santa Cruz (genome.ucsc.edu), AK094629 is the antisense lncRNA of
the nearby MAP3K4 gene. Therefore, it was hypothesised that MAP3K4
might be related to the expression of LncRNA AK094629 in SMSCs of
the TMJ.
The present study aimed to further explore the
association between lncRNA AK09469 and TMJOA in order to clarify
the regulatory mechanism between lncRNA AK094629 and the
IL-1β-induced upregulation of IL-6 in SMSCs.
Materials and methods
Ethics statement
The present study was approved by the Institutional
Ethics Board of the Hospital of Stomatology, Sun Yat-sen University
(Guangdong, China). Each patient who donated synovial specimens
signed an informed consent form before surgery.
Clinical samples and cell culture
Synovial specimens were obtained from ten surgically
treated patients with TMJOA. The samples were placed in 1.5 ml
RNase-free EP tubes containing TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA was then
extracted and analysed by reverse transcription-quantitative (RT-q)
PCR to determine the expression levels of IL-1β and AK094629.
Synovial tissues from the patients were washed three times with
phosphate-buffered saline (PBS, Gibco; Thermo Fisher Scientific,
Inc.) and cut into small pieces (<1 mm3).
Subsequently, 1 ml type I collagenase (Sigma-Aldrich; Merck KGaA)
was added to the tube, and digestion was performed in an incubator
at 37°C for 2.5 h. After digestion, the mixture was transferred to
a 15 ml tube and centrifuged at 350 × g for 5 min at room
temperature, the supernatant was discarded, and the precipitate was
resuspended in complete medium, composed of Dulbecco's Modified
Eagle Medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.) and 10%
foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
The suspension was seeded into petri dishes (5,000
cells/cm2) and cultured at 37°C in an incubator
containing 5% CO2. The medium was refreshed every 3
days. When the cells reached ~80% confluence, they were seeded into
new petri dishes after another round of digestion, centrifugation
and resuspension, according to the aforementioned protocol.as Cells
from passage 4–6 were selected for the experiments.
RT-qPCR
Total RNA from tissues and SMSCs was extracted using
TRIzol® reagent. The mRNA was then reverse-transcribed
into cDNA using the PrimeScript RT Master Mix (Perfect Real Time;
Takara Biotechnology Co., Ltd.) according to the manufacturer's
instructions. The following thermocycling conditions were used for
RT: 37°C for 15 min, 85°C for 5 sec and cooled to 4°C. qPCR was
performed using the SYBR® Green RT-qPCR kit (Roche
Diagnostics) and a Roche LightCycler 96 (Roche Diagnostics). The
relative mRNA expression levels of AK094629, MAP3K4 and IL-6 were
calculated using the 2−ΔΔCq method (36), followed by normalisation to the
GAPDH mRNA expression level. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 10 sec;
annealing at 58°C for 20 sec; and extension at 72°C for 30 sec,
with a total of 45 cycles. The sequences of the primers used are
listed in Table I. The
amplification efficiencies of GAPDH, AK094629, IL-6, and MAP3K4
primers were 1.03, 0.87, 1.14 and 1.10, respectively.
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Gene | Primer sequence
(3′→5′) | Amplification
efficiency (%) |
|---|
| GAPDH |
|
| F |
GACAGTCAGCCGCATCTTCT | 103 |
| R |
TTAAAAGCAGCCCTGGTGAC |
|
| AK094629 |
|
| F |
AGCGCTAAGAGTAAACGATGC | 97 |
| R |
GGGGAAGAAGAAATGCTAAAG |
|
| MAP3K4 |
|
| F |
CAATCGGACTGACTTCTGGATA | 105 |
| R |
TTGGGAACTTCGGACACTAAT |
|
| IL-6 |
|
| F |
ACTCACCTCTTCAGAACGAATTG | 104 |
| R |
CCATCTTTGGAAGGTTCAGGTTG |
|
Western blot analysis
Cells were collected and lysed using RIPA Lysis
Buffer (Beyotime Institute of Biotechnology) supplemented with
protease and phosphatase inhibitors (Beyotime Institute of
Biotechnology), which were added at a ratio of 1:99 to the RIPA
Lysis Buffer. The concentration of protein was measured using a
bicinchoninic assay (Beyotime Institute of Biotechnology), and the
protein sample was then split between two or three 200 µl EP tubes,
followed by the addition of loading buffer (sample to loading
buffer ratio, 4:1) and heating to 99°C for 10 min to denature the
proteins. Proteins were separated using 6 or 8% sodium dodecyl
sulphate polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology), transferred to polyvinylidene fluoride membranes
(EMD Millipore), and blocked with 5% non-fat milk (BD Biosciences)
for 1 h at room temperature. The blot was incubated with primary
antibodies, including rabbit anti-MAP3K4 (1:1,000; cat. no.
ab182165; Abcam), rabbit anti-IL-6 (1:1,000; cat. no. DF6087;
Affinity Biosciences), rabbit anti-p38 (1:1,000; cat. no. AF6456;
Affinity Biosciences), rabbit anti-phosphorylated (p)-p38 (1:1,000;
cat. no. AF4001; Affinity Biosciences) and rabbit anti-β-actin
(1:1,000; cat. no. AF7018; Affinity Biosciences) overnight at 4°C.
The blots were then washed with phosphate buffered saline
supplemented with 0.05% Tween 20 three times for 10 min at room
temperature and incubated with a horseradish peroxide-conjugated
IgG secondary antibody (1:2,000; cat. no. 7074P2; Cell Signaling
Technology) for 1 h at room temperature. The protein blots were
then visualised using an ECL Kit (EMD Millipore), and ImageJ
(version: 1.52t; National Institutes of Health) was used for
semi-quantitative analysis.
Cytometric bead array (CBA) assay
SMSCs were seeded into a 6-well plate and cultured
to ~80% confluence. After corresponding treatment, the supernatant
was collected. The concentration of IL-6 in the culture medium was
detected using the BD CBA Human IL-6 Protein Kit (BD Biosciences),
according to manufacturer's instructions. Subsequently, 50 µl of
culture medium from each treatment and assay diluent was added into
the respective assay tubes, with the tube containing the assay
diluent used as a negative control. Then, 50 µl of dilute IL-6
capture beads and 50 µl of dilute IL-6 phycoerythrin detection
reagent were added to each assay tube and incubated in the dark for
3 h at room temperature, and measurements were obtained using a
Cytoflex flow cytometer (Beckman Coulter, Inc.) and CytExpert
software (version 2.1; Beckman Coulter, Inc.).
Transfection of small interfering
(si)RNA or long non-coding (lnc)RNA smart silencer
The siRNA targeting MAP3K4 (Guangzhou RiboBio Co.,
Ltd.), siRNA negative control (siR NC; cat. no. siN0000001-1-5;
Guangzhou RiboBio Co., Ltd.), lncRNA smart silencer targeting
AK094629 (Guangzhou RiboBio Co., Ltd.) and lncRNA smart silencer NC
(cat. no. lnc3N0000001-1-5; Guangzhou RiboBio Co., Ltd.) were
diluted with 250 µl RNase-free water to a concentration of 10 mM
and preserved at −20°C. The NC used was a universal negative
control that did not target any known human, mouse or rat genes.
SMSCs were seeded into 6-well plates and transiently transfected
with siRNA or lncRNA smart silencer after culturing to ~80%
confluence. A total of 5 µl siRNA, siR NC, lncRNA smart silencer or
lncRNA smart silencer NC and 5 µl of Lipofectamine®
RNAiMax (Invitrogen; Thermo Fisher Scientific, Inc.) were pipetted
into different non-enzyme EP tubes and diluted with 120 µl of
opti-MEM (cat. no. 31985062, Gibco; Thermo Fisher Scientific,
Inc.), followed by incubation at room temperature for 15 min to
form the si-RNA/Lipofectamine® complexes. Then, 250 µl
of the complexes were added to each well of the 6-well plates and
cultured in an incubator at 37°C. After 48 h, the culture medium
was replaced and 10 ng/ml IL-1β was added to the IL-1β stimulated
groups, but not the control groups. Cells were incubated for 6 h at
37°C and then used for subequent experiments. The target sequences
of siRNA and lncRNA smart silencer used in the present study are
listed in Table II.
 | Table II.Target sequences of si-RNA and lncRNA
smart silencer. |
Table II.
Target sequences of si-RNA and lncRNA
smart silencer.
| RNA | Target sequence
(3′→5′) |
|---|
| siRNA |
|
| siMAP3K4-1 |
GCACTCTGTTTGTGGTTAA |
| siMAP3K4-2 |
GTGGAAGAAATACAGCTATA |
| siMAP3K4-3 |
GCAGCAGAATTCAGGCTTT |
| lncRNA smart
silencer |
|
| AK094629 |
GGACTGGAATGCTCCTACAG |
|
|
CAACAGACCAAGCTAACAGT |
|
|
GCTCAAAGTATGTTACTGCA |
|
|
ACTCCGGTCTCTTGACAGAA |
|
|
ACTTGGACTGGAATGCTGCA |
|
|
ATTTTCTGACCAGAAC |
Nucleoplasm separation
When the cells reached ~80% confluence, they were
digested and centrifuged at 350 × g for 5 min at room temperature,
the supernatant was then discarded, and 150 µl of cell
fractionation buffer (Invitrogen; Thermo Fisher Scientific, Inc.)
was added and mixed gently. The mixture was left on ice for 5 min,
centrifuged at 400 × g for 5 min at 4°C and the resulting
supernatant, containing the components of the cytoplasm, was
transferred to a new EP tube. Subsequently, 50 µl of cell
disruption buffer (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to the sediment, which contained the components of the
nucleus, and the mixture was then placed on ice for 5 min, followed
by addition of TRIzol® to extract the RNA.
Fluorescence in situ hybridisation
(FISH) assay
When the confluence of SMSCs in the confocal
laser-scanning dishes reached ~50%, the culture medium was
discarded, and the SMSCs were washed with PBS three times for 5 min
and fixed with 4% paraformaldehyde (PFA; Beyotime Institute of
Biotechnology) at room temperature for 10 min to cross-link the
protein and bind RNA to the protein. PFA was then removed, and the
sample was washed with PBS three times for 5 min and incubated with
70% ethanol at 4°C for 2 h. Next, 150 µl of hybridisation buffer
containing 20 nmol/l AK094629 probe (5′-TCTGTTGCGCTTACTGCTATAT-3′)
was added, and the sample was incubated at room temperature
overnight. After hybridisation, the solution was rinsed with 2X
saline-sodium citrate (SCC) for 10 min and 1X SCC two times for 5
min at room temperature. Subsequently, the cells were incubated
with anti-digoxigenin-fluorescein, fab fragments [1:200; Roche
Diagnostics (Shanghai) Co., Ltd.] at room temperature for 40 min
and washed with 2X SCC three times for 5 min. Then, 5 ng/ml DAPI
(Beyotime Institute of Biotechnology) was added, and the mixture
was incubated in the dark for 30 min. Finally, the samples were
observed under a fluorescence microscope (magnification, ×63). The
excitation and emission wavelengths of DAPI are 359 and 461 nm,
respectively, and the filter used to select these wavelengths was
410–579 nm. The excitation and emission wavelengths of FITC are 494
and 523 nm, and the filter used was 493–634 nm.
Statistical analysis
Differences between two groups were analysed using
Student's t-test. Analysis of variance (ANOVA) was performed to
determine significant differences between more than two groups, and
the least significant difference (LSD) or Bonferroni post hoc test
was used after ANOVA for additional comparisons. Correlation
between the expression of IL-1β and AK094629 was analysed using
Spearman's rank correlation test. P<0.05 was considered to
indicate a statistically significant difference.
Results
AK094629 in the synovial tissue of
patients with TMJOA positively correlates with IL-1β and is
upregulated by IL-1β in SMSCs
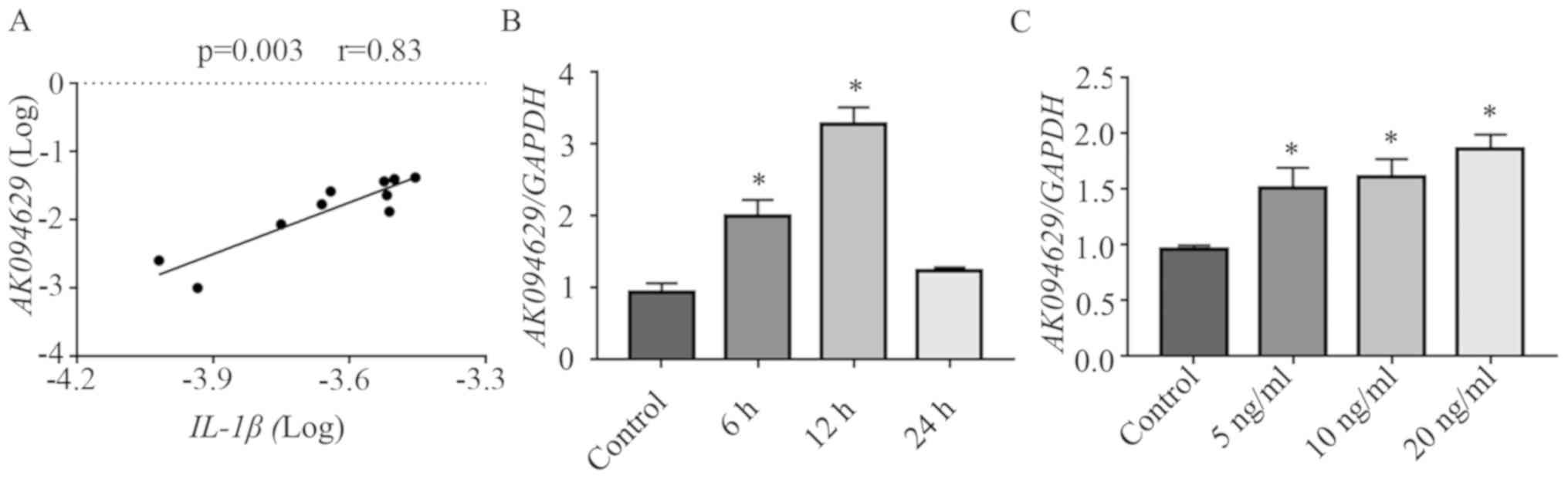
The expression of AK094629 and IL-1β in the synovial
tissues donated by ten patients with TMJOA were determined by
RT-qPCR. AK094629 positively correlated with IL-1β according to the
Spearman's rank correlation test (Fig.
1A). AK094629 was significantly upregulated in SMSCs stimulated
with 10 ng/ml IL-1β to for 6, 12 and 24 h compared with that in
unstimulated SMSCs (Fig. 1B). In
addition, SMSCs stimulated with 5, 10 and 20 ng/ml IL-1β for 12 h
demonstrated upregulated expression of AK094629 in a
concentration-dependent manner (Fig.
1C; Table SI).
AK094629 knockdown reverses
IL-1β-induced upregulation of IL-6
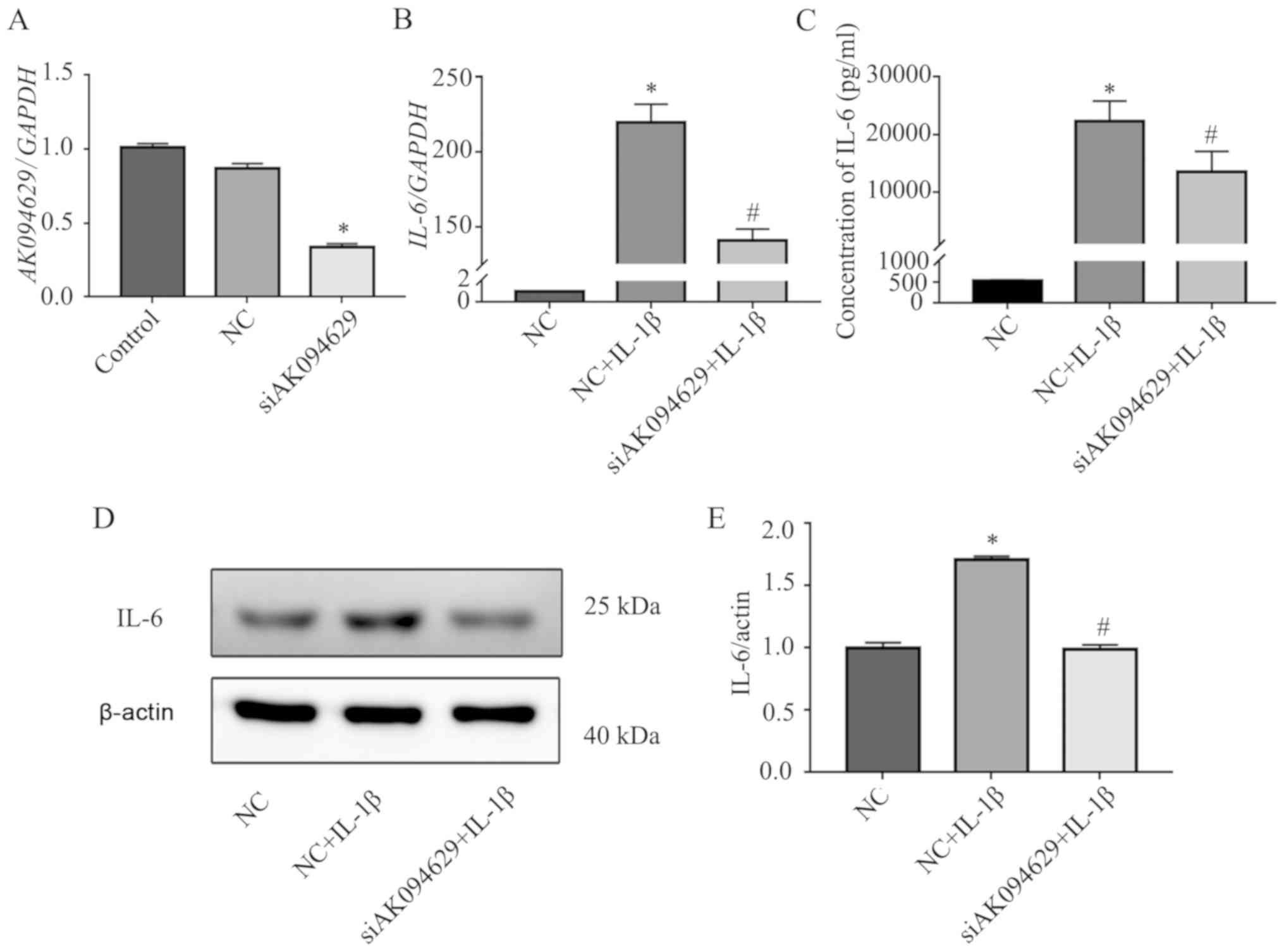
To determine the effects of AK094629 on
IL-1β-induced expression of IL-6 in SMSCs, the cells were
transfected with the AK094629 targeting lncRNA smart silencer. The
knockdown efficiency was ~60% as verified by RT-qPCR (Fig. 2A; Table SII). In addition, RT-qPCR, CBA
assay and western blotting analysis demonstrated that AK094629
knockdown attenuated the IL-1β induced upregulation of IL-6
(Fig. 2B-E; Tables SIII–%V).
Subcellular localisation of
AK094629
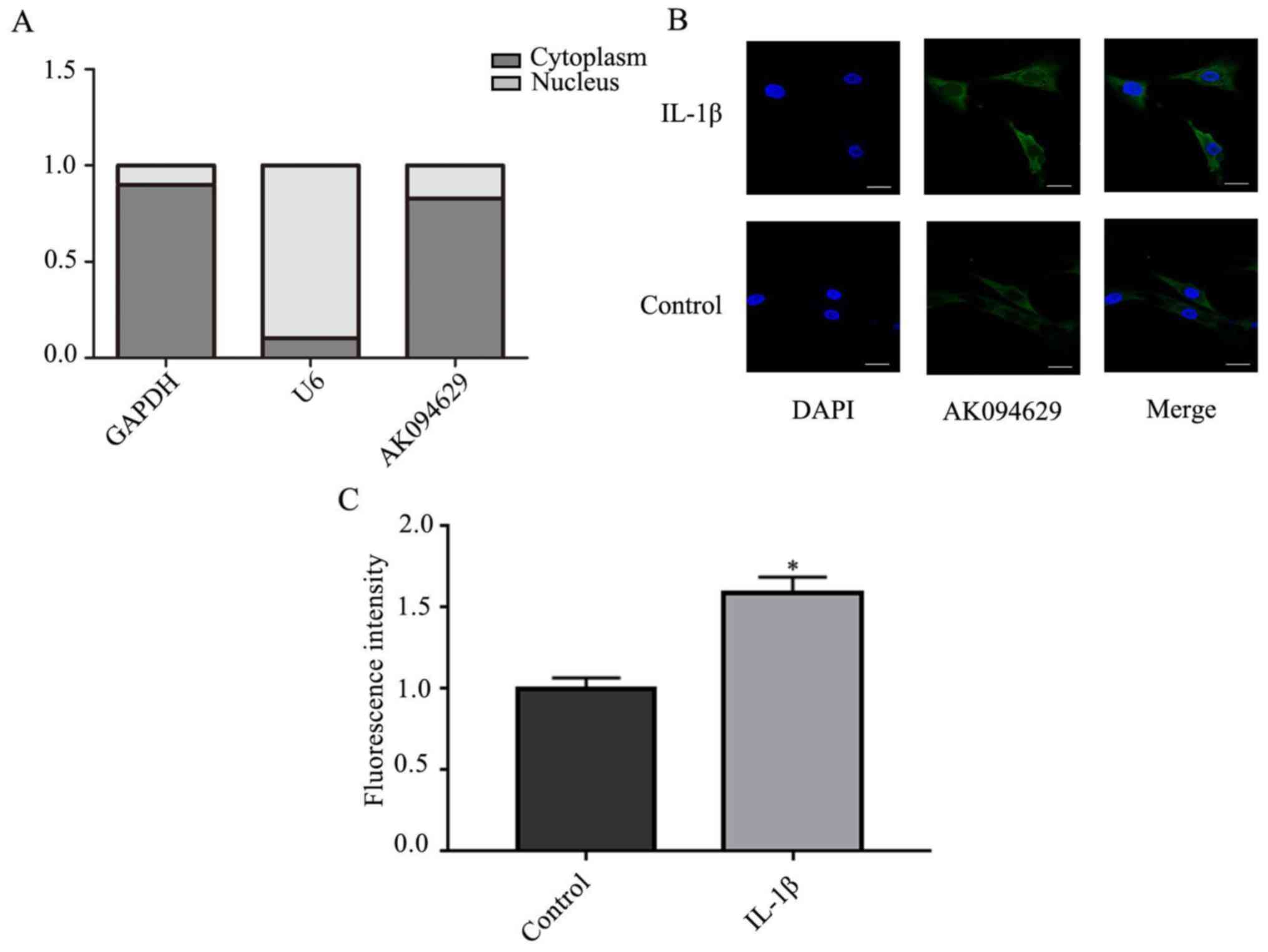
To determine the subcellular localisation of
AK094629, the expression of AK094629 in the cytoplasm and nucleus
was investigated. The results demonstrated that AK094629 was
predominantly located in the cytoplasm (Fig. 3A-C; Table SVI). The specificity of the
AK094629 probe in binding to AK094629 was verified using the FISH
assay (Fig. S1).
Knockdown of MAP3K4 attenuated the
IL-1β-induced upregulation of IL-6
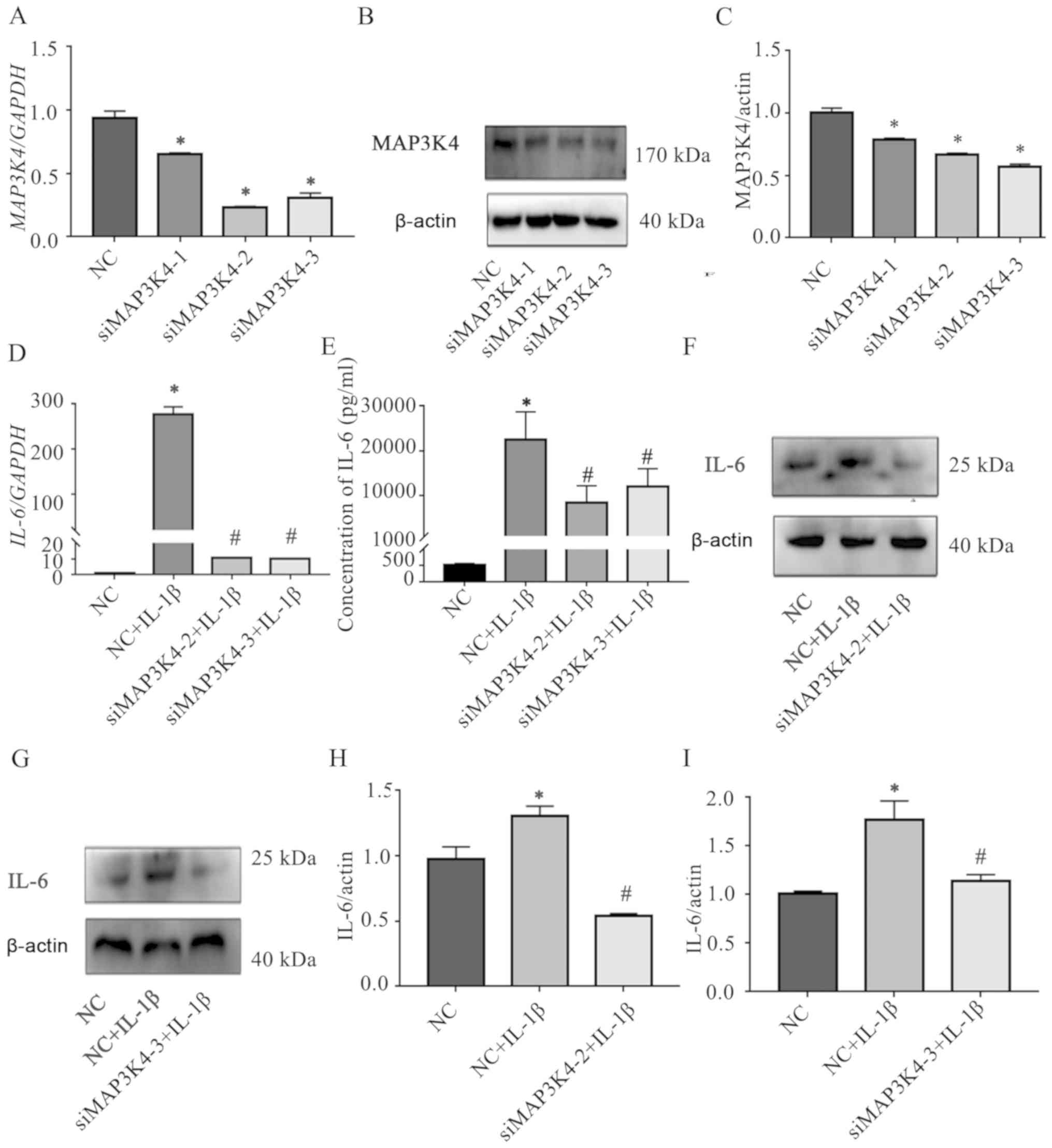
The role of MAP3K4 on the IL-1β-induced IL-6
expression in SMSCs was explored by transfecting three
MAP3K4-targeting siRNAs into the cells; the knockdown efficiencies
were verified by RT-qPCR and western blotting (Fig. 4A-C, Tables SVII–VIII), and two of the siRNAs with greater
knockdown efficiency were selected for subsequent experiments.
RT-qPCR, CBA and western blotting demonstrated that knockdown of
MAP3K4 inhibited the IL-1β-induced upregulation of IL-6 in SMSCs
(Fig. 4D-I, Tables SIX–XII). In addition, the expression levels
of MAP3K4 were significantly upregulated in SMSCs stimulated with
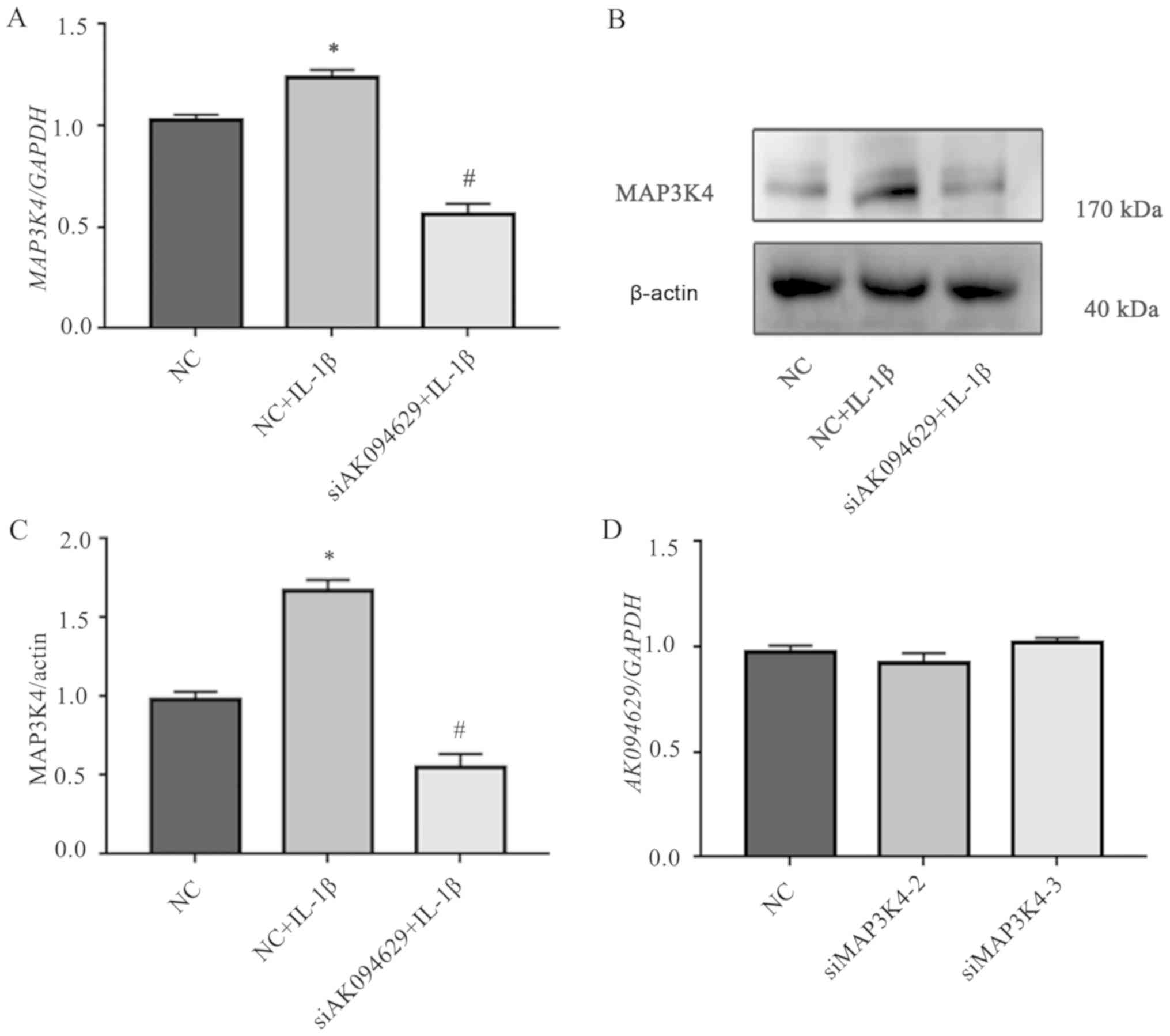
IL-1β compared with those in unstimulated cells (Fig. 5A-C; Tables SXIII–XVI).
AK094629 effects the expression of
MAP3K4, but MAP3K4 has no effect on the expression of AK094629
The association between AK094629 and MAP3K4 was
analysed by knocking down AK094629 and MAP3K4 with lncRNA smart
silencer and two siRNAs (siMAP3K4-2 and siMAP3K4-3), respectively.
Knockdown of AK094629 with the lncRNA smart silencer downregulated
the expression of MAP3K4 based on RT-qPCR and western blotting
results (Fig. 5A-C, Tables SV and XIII). However, no significant difference
was observed in the expression of AK094629 when MAP3K4 was knocked
down compared with the negative control (Fig. 5D; Table SXIV).
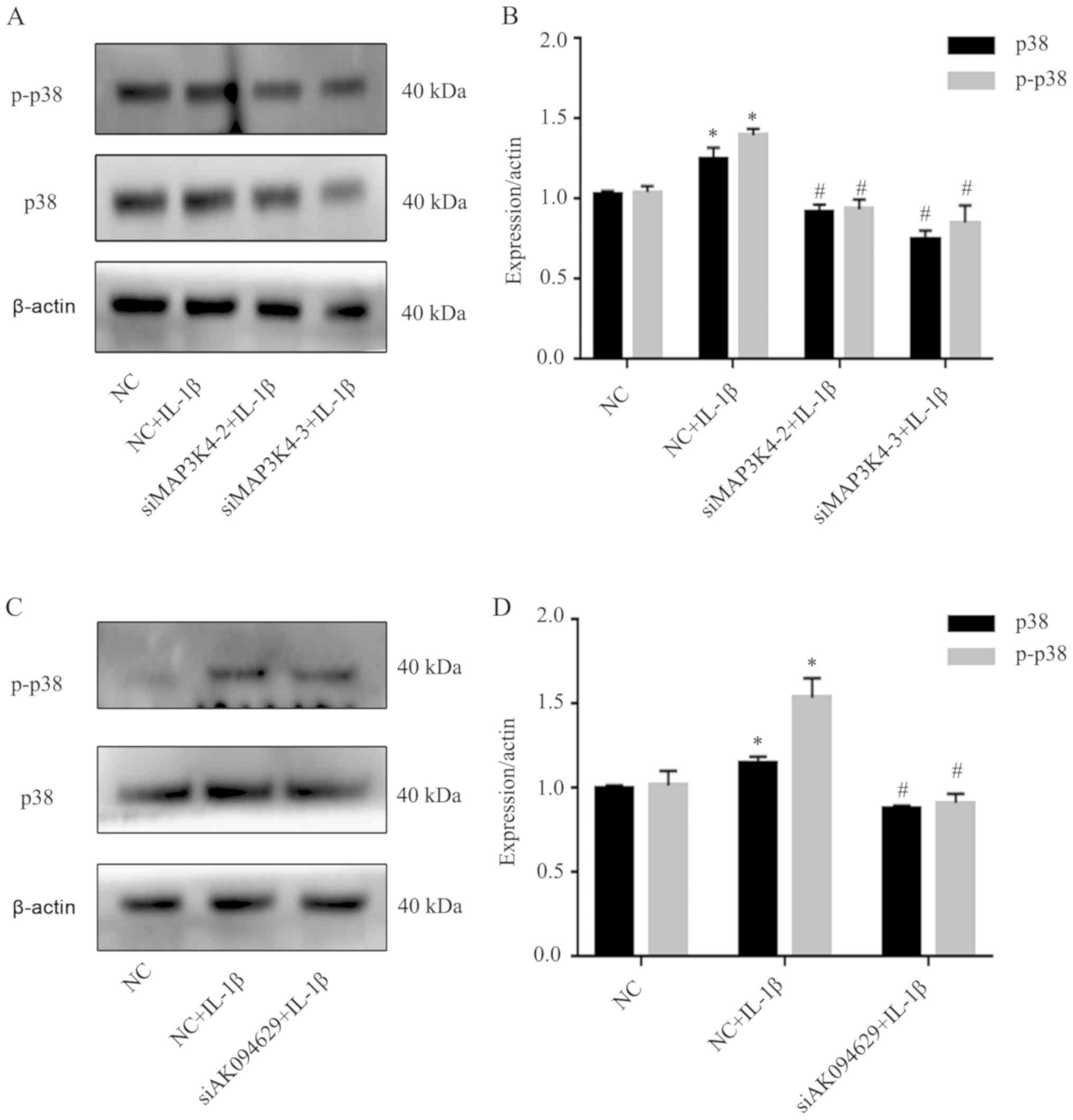
MAP3K4 or AK094629 knockdown inhibits
p38 expression
The effects on the expression levels of p38, a
molecule downstream of MAP3K4, following MAP3K4 and AK094629
knockdown were also explored. Western blotting demonstrated that
knockdown of MAP3K4 or AK094629 inhibited the expression of p38 and
p-p38 in SMSCs compared with the negative control group. (Fig. 6; Tables SXV and XVI).
Discussion
Although the causes of TMJOA are various and
complex, inflammation has been demonstrated to serve an important
role in its development (13).
IL-1β is one of the most important pro-inflammatory factors and a
mediator of cartilage degeneration and joint inflammation (37,38).
A previous study demonstrated that the expression of IL-1β was
significantly increased in the synovial fluid, synovial membrane
and cartilage of patients with OA (13). IL-1β can stimulate the production
of other inflammatory factors such as IL-6 (8,9),
which can subsequently promote the destruction of TMJ tissues by
stimulating osteoclast formation, bone resorption, chondrocyte MMP
production and inhibiting the differentiation of SMSCs to cartilage
(39,40). Therefore, blocking the effects
induced by IL-1β may be beneficial to the prognosis and outcome of
patients with TMJOA.
IL-6 is an exocrine protein, and for an exocrine
protein, the characterisation of extracellular expression levels is
more important than intracellular expression. In the present study,
this was performed using a CBA assay, which reflected the
expression level of IL-6 in the culture medium, thus displaying the
continuous accumulation of IL-6. However, the real-time
intracellular protein and gene expression levels provided more
detailed information about this process; thus, the intracellular
and extracellular expression levels of IL-6 in SMSCs were
characterised in the present study.
The abnormal expression of certain lncRNAs has been
reported to be associated with various pathological processes of
OA, including the degradation of ECM, inflammatory reaction,
apoptosis and angiogenesis (24,31).
The results of the present study demonstrated the expression of
AK094629 in the synovium of patients with TMJOA to be positively
correlated with IL-1β. This suggested that AK094629 may be involved
in the IL-1β-mediated pathophysiological processes and may be
linked to the occurrence and development of TMJOA.
Stem cell chemotaxis to the lesion and
differentiation to cartilage is an important repair mechanism of
articular cartilage damage (41).
A previous study demonstrated that IL-1β, which was significantly
upregulated in dysfunctional TMJs, impeded the mesenchymal stem
cells in the synovial fluid of the TMJ from differentiating to
cartilage through upregulating the secretion of IL-6 (9). In the present study, the expression
of AK094629 was also significantly upregulated in SMSCs of the TMJ
when stimulated with IL-1β. Knockdown of AK094629 reversed the
increase of IL-1β-induced IL-6 expression to some extent. These
results suggested that AK094629 contributes to the IL-1β-induced
upregulation of IL-6 in the SMSCs of the TMJ. Therefore, lncRNA
AK094629 may be a novel therapeutic target of TMJOA.
MAP3K4, also termed MEKK4, is a 180-kDa protein,
which is activated by growth factors, inflammatory factors and
environmental stress (42). MAP3K4
phosphorylates and activates MAP2Ks, leading to the activation of
MAPK pathways, including the pathways of p38 and JNK (32–34).
A previous study demonstrated that MAP3K4 serves an important role
in the development of skeletal muscles and the neural tube
(43), although its role in OA
remains unclear. The results of the present study demonstrated that
the expression of MAP3K4 was also significantly upregulated in
SMSCs after stimulation with IL-1β and that MAP3K4 was
downregulated at the gene and protein levels when AK094629 was
knocked down. In addition, no significant difference was observed
in the expression levels of AK094629 following the knockdown of
MAP3K4, whereas the IL-1β-induced upregulation of IL-6 was
inhibited in SMSCs. Therefore, MAP3K4 may be one of the downstream
targets of AK094629. A limitation of the present study was that
only siRNA for MAP3K4 knockdown was used; thus in order to explore
the relationship between AK094629 and MAP3K4, an MAP3K4 inhibitor
should have been used, and this result should also be confirmed
using clinical samples.
Based on the structural characteristics and cellular
localisation of lncRNAs, they are divided into several types and
influence gene expression through a number of mechanisms, such as
chromatin remodelling, competitive endogenous RNA, stability of RNA
and recruitment of scaffold proteins at the transcriptional and
post-transcriptional levels (44,45).
In the present study, the results of RT-qPCR and FISH demonstrated
that AK094629 was predominantly located in the cytoplasm. AK094629
is an antisense lncRNA of the nearby MAP3K4, and changes in their
levels were correlated; however, the mechanism through which lncRNA
AK094629 affects the expression of MAP3K4 is still unclear and
needs to be elucidated in future studies.
In summary, the present study demonstrated that in
the SMSCs of the TMJ, knockdown of the lncRNA AK094629 reversed the
IL-1β-induced upregulation of IL-6 by inhibiting MAP3K4 expression.
These findings indicated that lncRNA AK094629 may be a potential
novel therapeutic target in the treatment of TMJOA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The
Guangdong Medical Research Foundation (grant no. A2018385), The
Natural Science Foundation of Guangdong Province (grant no.
2018A030310329) and The National Natural Science Foundation of
China (grant no. 81800996).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZZ and YS conceived the experiments. JJ, YS and JS
conducted the experiments and drafted the manuscript. WL and LQ
analysed the data. KS and YH collected the synovial membrane tissue
specimens from the patients and participated in the cell culture
experiments. JZ and RY participated in the cell culture
experiments. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Institutional Ethics Board of the Hospital of Stomatology, Sun
Yat-sen University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
List T and Jensen RH: Temporomandibular
disorders: Old ideas and new concepts. Cephalalgia. 37:692–704.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poole AR: Osteoarthritis as a whole joint
disease. HSS J. 8:4–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res. 5:160442017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalladka M, Quek S, Heir G, Eliav E,
Mupparapu M and Viswanath A: Temporomandibular joint
osteoarthritis: Diagnosis and long-term conservative management: A
topic review. J Indian Prosthodont Soc. 14:6–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Souza RF, Lovato da Silva CH, Nasser M,
Fedorowicz Z and Al-Muharraqi MA: Interventions for the management
of temporomandibular joint osteoarthritis. Cochrane Database Syst
Rev. CD0072612012.PubMed/NCBI
|
|
6
|
Wang XD, Zhang JN, Gan YH and Zhou YH:
Current understanding of pathogenesis and treatment of TMJ
osteoarthritis. J Dent Res. 94:666–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui D, Li H, Xu X, Ye L, Zhou X, Zheng L
and Zhou Y: Mesenchymal stem cells for cartilage regeneration of
TMJ osteoarthritis. Stem Cells Int. 2017:59797412017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao W, Sun J, Liu W, Li W, Jia J, Ou F,
Su K, Zheng Y, Zhang Z and Sun Y: HDAC10 upregulation contributes
to interleukin 1β-mediated inflammatory activation of
synovium-derived mesenchymal stem cells in temporomandibular joint.
J Cell Physiol. 234:12646–12662. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu W, Sun Y, He Y, Zhang H, Zheng Y, Yao
Y and Zhang Z: IL-1β impedes the chondrogenic differentiation of
synovial fluid mesenchymal stem cells in the human
temporomandibular joint. Int J Mol Med. 39:317–326. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones BA and Pei M: Synovium-derived stem
cells: A tissue-specific stem cell for cartilage engineering and
regeneration. Tissue Eng Part B Rev. 18:301–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XD, Kou XX, Mao JJ, Gan YH and Zhou
YH: Sustained inflammation induces degeneration of the
temporomandibular joint. J Dent Res. 91:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ying W, Yuan F, He P and Ji P: Inhibition
of Notch1 protects against IL-1β-induced inflammation and cartilage
destruction in temporomandibular chondrocytes. Mol Med Rep.
15:4391–4397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Wang P, Jiang P, Lv Y, Dong C,
Dai X, Tan L and Wang Z: Upregulation of lncRNA HOTAIR contributes
to IL-1β-induced MMP overexpression and chondrocytes apoptosis in
temporomandibular joint osteoarthritis. Gene. 586:248–253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sirikaew N, Chomdej S, Tangyuenyong S,
Tangjitjaroen W, Somgird C, Thitaram C and Ongchai S:
Proinflammatory cytokines and lipopolysaccharides up regulate MMP-3
and MMP-13 production in Asian elephant (Elephas maximus)
chondrocytes: Attenuation by anti-arthritic agents. BMC Vet Res.
15:4192019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhai K, Duan H, Chen Y, Khan GJ, Cao WG,
Gao GZ, Shan LL and Wei ZJ: Apoptosis effects of imperatorin on
synoviocytes in rheumatoid arthritis through
mitochondrial/caspase-mediated pathways. Food Funct. 9:2070–2079.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pattappa G, Schewior R, Hofmeister I, Seja
J, Zellner J, Johnstone B, Docheva D and Angele P: Physioxia has a
beneficial effect on cartilage matrix production in interleukin-1
beta-inhibited mesenchymal stem cell chondrogenesis. Cells.
8:E9362019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gunson MJ, Arnett GW and Milam SB:
Pathophysiology and pharmacologic control of osseous mandibular
condylar resorption. J Oral Maxillofac Surg. 70:1918–1934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laavola M, Leppänen T, Hämäläinen M,
Vuolteenaho K, Moilanen T, Nieminen R and Moilanen E: IL-6 in
osteoarthritis: Effects of pine stilbenoids. Molecules.
24:E1092019. View Article : Google Scholar
|
|
21
|
Latourte A, Cherifi C, Maillet J, Ea HK,
Bouaziz W, Funck-Brentano T, Cohen-Solal M, Hay E and Richette P:
Systemic inhibition of IL-6/Stat3 signalling protects against
experimental osteoarthritis. Ann Rheum Dis. 76:748–755. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei H, Shen G, Deng X, Lou D, Sun B, Wu H,
Long L, Ding T and Zhao J: The role of IL-6 in bone marrow
(BM)-derived mesenchymal stem cells (MSCs) proliferation and
chondrogenesis. Cell Tissue Bank. 14:699–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Cao L, Li F, Ma C, Liu G and Wang Q:
Interleukin-6 from subchondral bone mesenchymal stem cells
contributes to the pathological phenotypes of experimental
osteoarthritis. Am J Transl Res. 10:1143–1154. 2018.PubMed/NCBI
|
|
24
|
Chen WK, Yu XH, Yang W, Wang C, He WS, Yan
YG, Zhang J and Wang WJ: lncRNAs: Novel players in intervertebral
disc degeneration and osteoarthritis. Cell Prolif. 50:2017.
View Article : Google Scholar
|
|
25
|
Khalil AM, Guttman M, Huarte M, Garber M,
Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van
Oudenaarden A, et al: Many human large intergenic noncoding RNAs
associate with chromatin-modifying complexes and affect gene
expression. Proc Natl Acad Sci USA. 106:11667–11672. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pearson MJ and Jones SW: Review: Long
noncoding RNAs in the regulation of inflammatory pathways in
rheumatoid arthritis and osteoarthritis. Arthritis Rheum.
68:2575–2583. 2016. View Article : Google Scholar
|
|
29
|
Cen X, Huang XQ, Sun WT, Liu Q and Liu J:
Long noncoding RNAs: A new regulatory code in osteoarthritis. Am J
Transl Res. 9:4747–4755. 2017.PubMed/NCBI
|
|
30
|
Giachelli CM, Speer MY, Li X, Rajachar RM
and Yang H: Regulation of vascular calcification: Roles of
phosphate and osteopontin. Circ Res. 96:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pearson MJ, Philp AM, Heward JA, Roux BT,
Walsh DA, Davis ET, Lindsay MA and Jones SW: Long intergenic
noncoding RNAs mediate the human chondrocyte inflammatory response
and are differentially expressed in osteoarthritis cartilage.
Arthritis Rheum. 68:845–856. 2016. View Article : Google Scholar
|
|
32
|
Aissouni Y, Zapart G, Iovanna JL, Dikic I
and Soubeyran P: CIN85 regulates the ability of MEKK4 to activate
the p38 MAP kinase pathway. Biochem Biophys Res Commun.
338:808–814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haque K, Pandey AK, Zheng HW, Riazuddin S,
Sha SH and Puligilla C: MEKK4 signaling regulates sensory cell
development and function in the mouse inner ear. J Neurosci.
36:1347–1361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abell AN and Johnson GL: MEKK4 is an
effector of the embryonic TRAF4 for JNK activation. J Biol Chem.
280:35793–35796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Sun Y, Zheng Y, Wang Z and Zheng Z:
p38 MAPK pathway promotes IL-6 and IL-8 secretion of synovial fluid
mesenchymal stem cells from temporomandibular joint inflamed by
IL-1β. J Pract Stomatol. 34:215–219. 2018.
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zwerina J, Redlich K, Polzer K, Joosten L,
Kronke G, Distler J, Hess A, Pundt N, Pap T, Hoffmann O, et al:
TNF-induced structural joint damage is mediated by IL-1. Proc Natl
Acad Sci USA. 104:11742–11747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tipton DA, Christian J and Blumer A:
Effects of cranberry components on IL-1β-stimulated production of
IL-6, IL-8 and VEGF by human TMJ synovial fibroblasts. Arch Oral
Biol. 68:88–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakashima T, Kobayashi Y, Yamasaki S,
Kawakami A, Eguchi K, Sasaki H and Sakai H: Protein expression and
functional difference of membrane-bound and soluble receptor
activator of NF-kappaB ligand: Modulation of the expression by
osteotropic factors and cytokines. Biochem Biophys Res Commun.
275:768–775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seol D, McCabe DJ, Choe H, Zheng H, Yu Y,
Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA and Martin JA:
Chondrogenic progenitor cells respond to cartilage injury.
Arthritis Rheum. 64:3626–3637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takekawa M, Posas F and Satio H: A Human
homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1,
mediates stress-induced activation of the p38 and JNK pathways.
EMBO J. 16:4973–4982. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abell AN, Rivera-Perez JA, Cuevas BD,
Uhlik MT, Sather S, Johnson NL, Minton SK, Lauder JM, Winter-Vann
AM, Nakamura K, et al: Ablation of MEKK4 kinase activity causes
neurulation and skeletal patterning defects in the mouse embryo.
Mol Cell Biol. 25:8948–8959. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huynh NP, Anderson BA, Guilak F and
McAlinden A: Emerging roles for long noncoding RNAs in skeletal
biology and disease. Connect Tissue Res. 58:116–141. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013. View Article : Google Scholar : PubMed/NCBI
|