Introduction
The lens is an avascular tissue located in front of
the vitreous body in the eyes. Light passes through the lens and
reaches the retina to form a clear image and therefore the
transparency of the lens is important. The lens contains two types
of cells, which are lens epithelial cells and lens fiber cells; the
organelles in the epithelial cells degrade and differentiate into
fiber cells (1). A cataract is one
of the most common eye diseases and the leading cause of blindness
worldwide (2). Age-related
cataracts is the most common type of this disease and it can
significantly lower quality of life (3). Perturbation of the lens redox status
has been considered as the major cause of age-related cataracts
(4). At present, surgical removal
of the lens is the primary therapy for this disease. However, there
is a lack of effective medical treatment; therefore, several
researchers have tried to explain its underlying mechanism in order
to delay or eliminate the occurrence of the disease and to identify
a therapeutic target (5,6). Hydrogen peroxide
(H2O2) elicits reactive oxygen species (ROS)
production and ROS increases oxidative stress and crystal protein
mutation in lens epithelial cells; therefore,
H2O2 is widely used in building a cataract
cell model to simulate the development of an aging lens and the
formation of age-related cataract (7).
Autophagy is a major intracellular degradation
system, which occurs in almost all cells (8). The dysfunctional cytoplasmic
materials, such as mitochondria and endoplasmic reticulum, are
sequestered by double-membrane autophagosomes and then delivered
into the lysosomes for degradation and recycling (9). Normally, autophagy is divided into
the following three types: Macroautophagy, microautophagy and
chaperone-mediated autophagy; the first type is the most widely
investigated (10). It has been
demonstrated that autophagy, especially macroautophagy, may play an
important role in the development of cataracts in the lens
(11). p62, an autophagy
substrate, is downregulated when autophagy is activated (12). By contrast, during autophagy,
microtubule-associated protein light chain 3 (LC3)B changes from
the soluble form (LC3BI) to the lipid-modified form (LC3BII).
Beclin is a critical component of the class III
phosphatidylinositol 3-kinase complex, which can regulate the
autophagy process (13).
Therefore, beclin, LC3BII/I and p62 are used as biomarkers to
demonstrate the occurrence of autophagy.
Thus far, >30 autophagy-related genes have been
reported. Among them, autophagy-related gene 4 (ATG4)
encodes a cysteine protease that contains four homologs
(ATG4A/ATG4B/ATG4C/ATG4D) (14). ATG4a can cleave
autophagy-related protein 8 (Atg8) near the C terminus,
allowing Atg8 to conjugate phosphatidylethanolamine (PE) via
the exposed glycine. This Atg8-PE system plays an important
role in autophagosome formation (15,16).
Although several studies have demonstrated that
ATG4 is pivotal in the development of some cancers, such as
colorectal cancer, cervical cancer and breast cancer, and it is a
promising therapeutic target to treat tumors (17–19),
to the best of the authors' knowledge, no research has connected it
to lens degradation and cataract formation. As autophagy is a
crucial process in lens degradation, the present study explored the
biological function of ATG4a in lens epithelial cells.
Materials and methods
Cell culture
The lens epithelial cell line HLE-B3 was purchased
from American Type Culture Collection. It was cultured in minimum
essential medium (MEM; HyClone; Cytiva) supplemented with 10% fetal
bovine serum (FBS; AusGeneX Pty, Ltd.) and 1%
penicillin-streptomycin solution (HyClone; Cytiva) in a humidified
environment of 5% CO2 at 37°C.
H2O2 (3%; Beijing Solarbio Science &
Technology Co., Ltd.) was diluted with MEM to a concentration of
~200 µmol/l. HLE-B3 cells were treated with 200 µmol/l
H2O2 and termed the
H2O2 group. Untreated HLE-B3 cells were
cultured as the control group.
Lentiviral transduction
To overexpress ATG4a, ATG4a sequence was
cloned into a lentiviral vector, pLenti-GIII- CMV-GFP-2A-Puro
vector (cat. no. LV082668; Applied Biological Materials, Inc.). An
empty vector was used as a negative control (NC). The sequencing
primers of the ATG4a lentiviral vector were as follows: CMV
sequencing primer, 5′-CGCAAATGGGCGGTAGGCGTG-3′; SV40 reverse
sequencing primer, 5′-TAGTCAGCCATGGGGCGGAGA-3′.
Cells were transferred to 6-well plates at a cell
density of 50–60% and cultured in MEM with 10% FBS for 24 h. Then,
the medium was replaced with Opti-MEM (Thermo Fisher Scientific,
Inc.) reduced-serum medium, to which 4.0 µg plasmid DNA and 10 µl
LipoGene™ 2000 Star Transfection Reagent (US Everbright, Inc.) with
0.5 ml Opti-MEM were added; the mixture was incubated for 20 min at
room temperature. The mixture in each plate was mixed with another
2 ml Opti-MEM and incubated under 5% CO2 in a 37°C
incubator for 6 h; then, the medium was replaced with complete
medium and cultured for another 48 h. Cells transfected with the
ATG4a and empty vectors were termed the overexpression
(OE)-ATG4a and NC groups, respectively. Total proteins were
harvested 36 h after transfection, and other experiments were
performed 24 h after transfection.
Transmission electron microscopy
(TEM)
Standard TEM was performed for the ultrastructural
analysis of the H2O2, control,
OE-ATG4a and NC groups. Cells were pretreated and fixed with
electron microscope-fixing fluid (2.5% glutaric dialdehyde
solution; Wuhan Servicebio Technology Co., Ltd.) for 2 h at room
temperature and then stored at 4°C. The cells were then embedded in
1% agarose and fixed in 1% osmic acid for 2 h at room temperature,
followed by dehydration with ethanol (50, 70, 80, 90, 95, 100%) and
acetone (100%) at room temperature for 15 min each. Cells were then
treated with 50% (2–4 h), 66% (overnight) and 100% (5–8 h) epoxy
resin at room temperature, followed by 100% epoxy resin, and heated
at 37°C overnight and then 60°C for 48 h. The agarose-embedded
cells were cut into thin sections (60–80 nm) and stained with 2%
uranyl acetate and 2% lead citrate for 15 min at room temperature.
Then, the sections were observed at 80 kV by a Hitachi TEM system
(HT7700, Hitachi High-Technologies Corporation). Cells and
autophagosomes were defined as structures measuring 5.0 and 2.0 µm,
respectively. The results were analyzed by ImageJ version 1.46r
(National Institutes of Health) and GraphPad Prism 5 (GraphPad
Software, Inc.) software.
Western blotting
Protein expression levels of autophagy biomarkers
were determined in the H2O2, control,
OE-ATG4a and NC groups. The total protein was extracted
using the Total Protein Extraction kit for Animal Cultured Cells
and Tissues (Invent Biotechnologies, Inc.) on ice and quantified
using BCA (Beyotime Institute of Biotechnology). The extracted
protein samples were stored at −80°C.
Equal amounts of protein (40 µg) were
electrophoresed via SDS-PAGE on 12% gels (Beyotime Institute of
Biotechnology) and blotted onto polyvinylidene fluoride membranes
(EMD Millipore). The membranes were blocked with QuickBlock™
blocking buffer for western blotting (cat. no. P0252; Beyotime
Institute of Biotechnology) for 15 min at room temperature and then
incubated overnight at 4°C with the following primary antibodies:
Anti-ATG4A (cat. no. ab223374; 1:1,000; Abcam), anti-p62 (cat. no.
ab109012; 1:5,000; Abcam), anti-LC3 (cat. no. ab192890; 1:2,000;
Abcam), anti-beclin (cat. no. ab207612; 1:2,000; Abcam), anti-Akt
(cat. no. YT0178; 1:1,000; ImmunoWay Biotechnology Company),
anti-phosphorylated (p-)Akt (cat. no. YP0006; 1:1,000; ImmunoWay
Biotechnology Company), anti-adenosine 5′-monophosphate-activated
protein kinase (AMPK; cat. no. YT0216; 1:1,000; ImmunoWay
Biotechnology Company), anti-p-AMPK (cat. no. YP0575; 1:1,000;
ImmunoWay Biotechnology Company), anti-GAPDH (cat. no. ab181602;
1:10,000; Abcam). After washing three times for 10 min each, the
membranes were incubated with the secondary antibodies (cat. no.
S0001; 1:2,000; Affinity Biosciences) for 2 h at room temperature
and then washed again in TBS with 0.1% Tween-20 three times for 10
min each. The membranes were visualized using the Super ECL Plus
kit (US Everbright, Inc.) and developed using a chemiluminescence
system (FluorChem FC2 imaging system, ProteinSimple). Signal
intensities were visualized by AlphaView software version 3.4.0.0
(ProteinSimple). Data were analyzed with ImageJ version 1.46r
(National Institutes of Health) and GraphPad Prism 5 (GraphPad
Software, Inc.) software. All experiments were performed in
triplicate.
EdU incorporation assay
Cell proliferation rates were analyzed using the EdU
incorporation assay in the OE-ATG4a and NC groups. HLE-B3
cells at a density of 80% were cultured in a 96-well plate, each
group had three replicates. The analysis was performed using the
Cell-Light EdU Apollo 488 In Vitro kit (cat. no. C10310-3;
Guangzhou RiboBio Co., Ltd.) following the manufacturer's protocol.
The percentage of EdU positive cells between the OE-ATG4a
and NC groups was compared using ImageJ version 1.46r (National
Institutes of Health) and SPSS 23.0 (IBM Corp.) software.
Flow cytometry analysis
Cell apoptosis (early and late apoptosis) in the
OE-ATG4a and NC groups was analyzed using a flow cytometer.
HLE-B3 cells were cultured in a 6-well plate and each group had
three replicates. The cells were harvested using trypsin without
EDTA and washed with PBS twice. The cells were resuspended in 1X
binding buffer at a concentration of >1×106 cells/ml.
Thereafter, 100 µl of the solution was transferred into a 5-ml
culture tube, to which 5 µl FITC Annexin V Apoptosis Detection kit
I (BD Biosciences) and 5 µl propidium iodide (PI) were added. The
mixture was gently vortexed for 15 min at room temperature in the
dark. Subsequently, 400 µl 1X binding buffer was added to each tube
and analyzed using BD LSRFortessa and FACSDiva version 6.2 (BD
Biosciences) within 1 h. The results were analyzed by ImageJ
version 1.46r (National Institutes of Health) and GraphPad Prism 5
(GraphPad Software, Inc.) software.
In situ cell death detection
Cell death was detected using the In Situ
Cell Death Detection kit (Roche Diagnostics) in the OE-ATG4a
and NC groups. HLE-B3 cells at a density of 80% were cultured in a
96-well plate at 37°C, each group had three replicates. In brief,
the cells were washed with PBS three times and fixed with 4%
paraformaldehyde in PBS at room temperature for 1 h. The cells were
then incubated in permeabilization solution for 2 min on ice. The
TUNEL reaction mixture was prepared and added into each well (50
µl/well); the cells were incubated at 37°C in the dark for 1 h.
DAPI (1:1,000) was used to stain the cell nucleus at room
temperature for 5 min in the dark and mounting medium was applied
to cells (cat. no. S2110; Beijing Solarbio Science & Technology
Co., Ltd). Images were captured by an Olympus IX71 fluorescence
microscope (Olympus Corporation) at magnification, ×200. The
percentage of dead cells between the OE-ATG4a and NC groups
was compared using ImageJ version 1.46r (National Institutes of
Health) and SPSS 23.0 (IBM Corp.).
Statistical analysis
All data were analyzed using SPSS version 23 (IBM
Corp.) and GraphPad Prism 5 (GraphPad Software, Inc.). The values
are presented as mean ± SD of three independent experiments. The
data were analyzed using the independent-samples t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
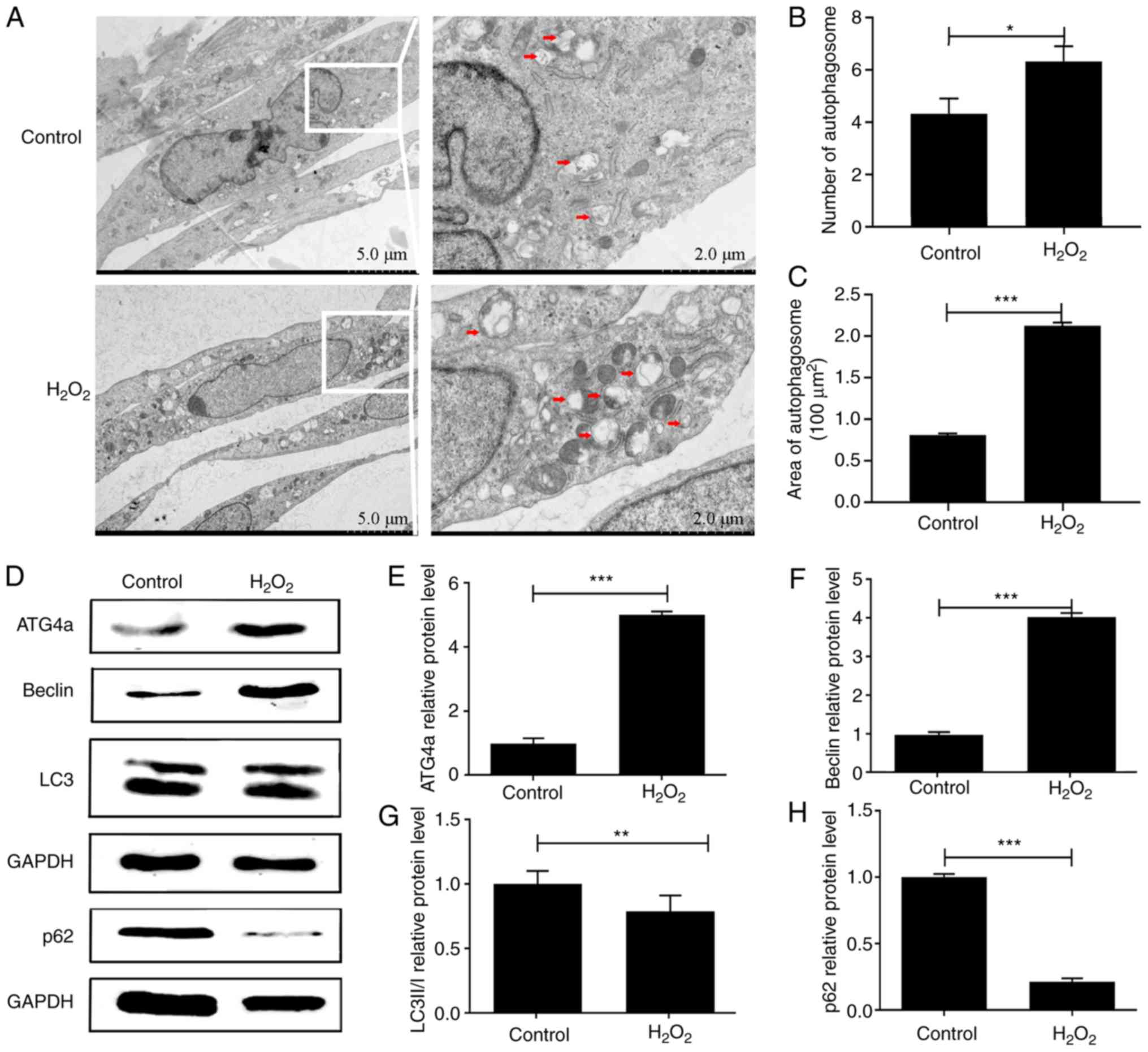
H2O2 can induce
autophagy in lens epithelial cells
Autophagy plays an important role in lens
degradation and cataract formation (11). To investigate whether
H2O2 can induce autophagy in lens epithelial
cells, TEM and western blotting were performed in both
H2O2 and control groups. TEM showed that,
compared with the control group, the number (P<0.05) and size
(P<0.001) of autophagosomes in the H2O2
group were significantly upregulated, indicating that the rate of
autophagy was increased (Fig.
1A-C). The results of western blotting showed that the protein
expression of ATG4a was significantly upregulated in the
H2O2 group compared with the control group
(P<0.001; Fig. 1D and E),
indicating that this gene might play an important role in autophagy
in lens epithelial cells. The protein expression of beclin was
significantly upregulated in the H2O2 group
compared with the control group (P<0.001; Fig. 1D and F). By contrast, the protein
expression of p62 was significantly downregulated (P<0.001;
Fig. 1D and H). Although the
expression of LC3BII/I (P<0.01; Fig. 1D and G) did not indicate the
upregulation of autophagy, the results of both TEM and western
blotting demonstrated the existence of autophagy in
H2O2-induced lens epithelial cells.
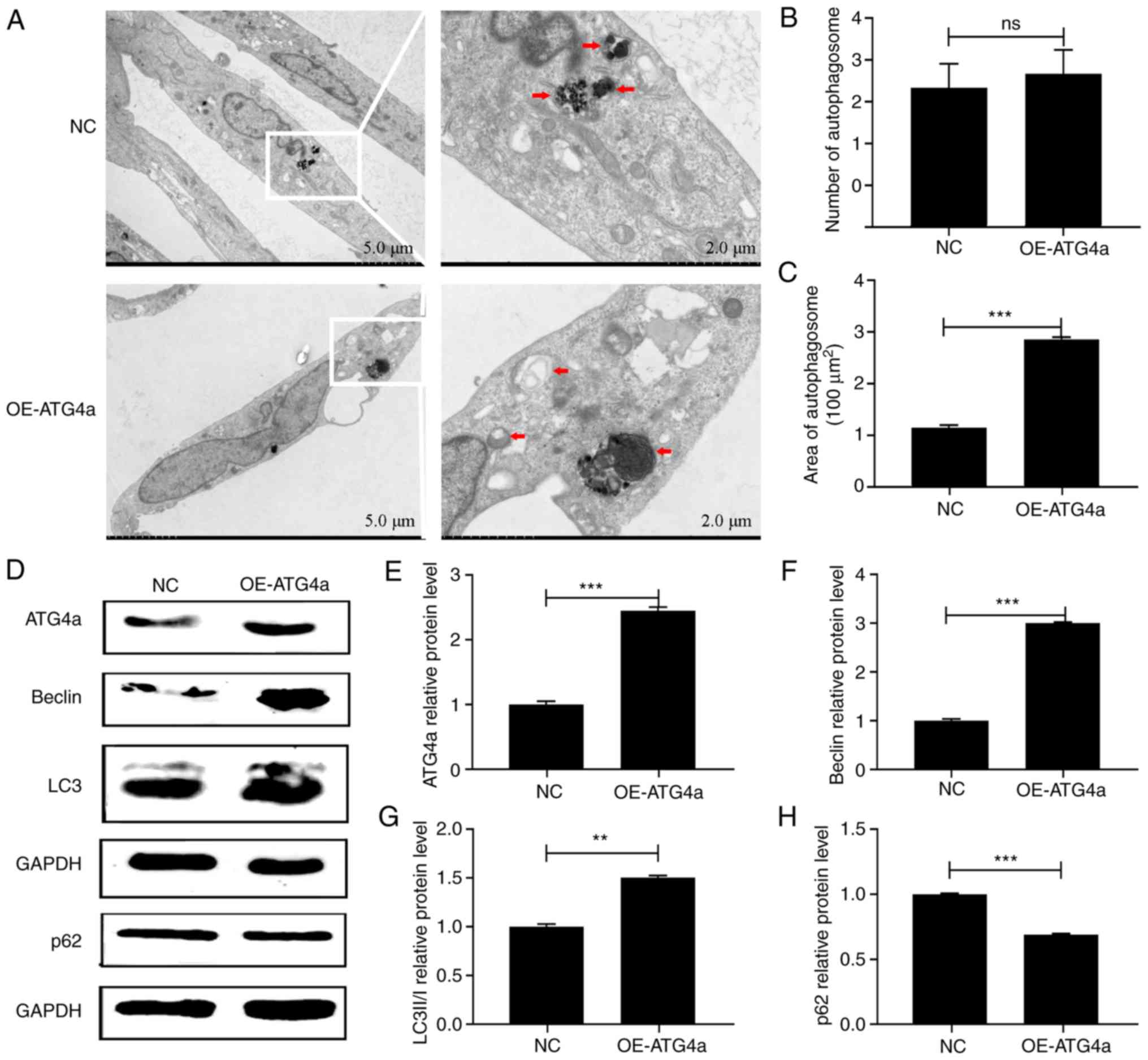
Overexpression of ATG4a activates
autophagy in lens epithelial cell
To confirm that ATG4a can specifically
regulate autophagy, TEM and western blotting were performed in the
OE-ATG4a and NC groups. TEM showed that in the
OE-ATG4a group (Fig. 2A)
the amount of autophagosomes was not significantly different from
that in the control group (P>0.05; Fig. 2B), but their size was significantly
increased (P<0.001; Fig. 2C).
Western blotting confirmed that the protein expression of beclin
(P<0.001) and LC3BII/I (P<0.01) was significantly upregulated
in the OE-ATG4a group compared with the NC group (Fig. 2D, F and G). By contrast, the
protein expression of p62 was significantly downregulated
(P<0.001; Fig. 2D and H),
verifying that ATG4a can activate autophagy in lens
epithelial cells.
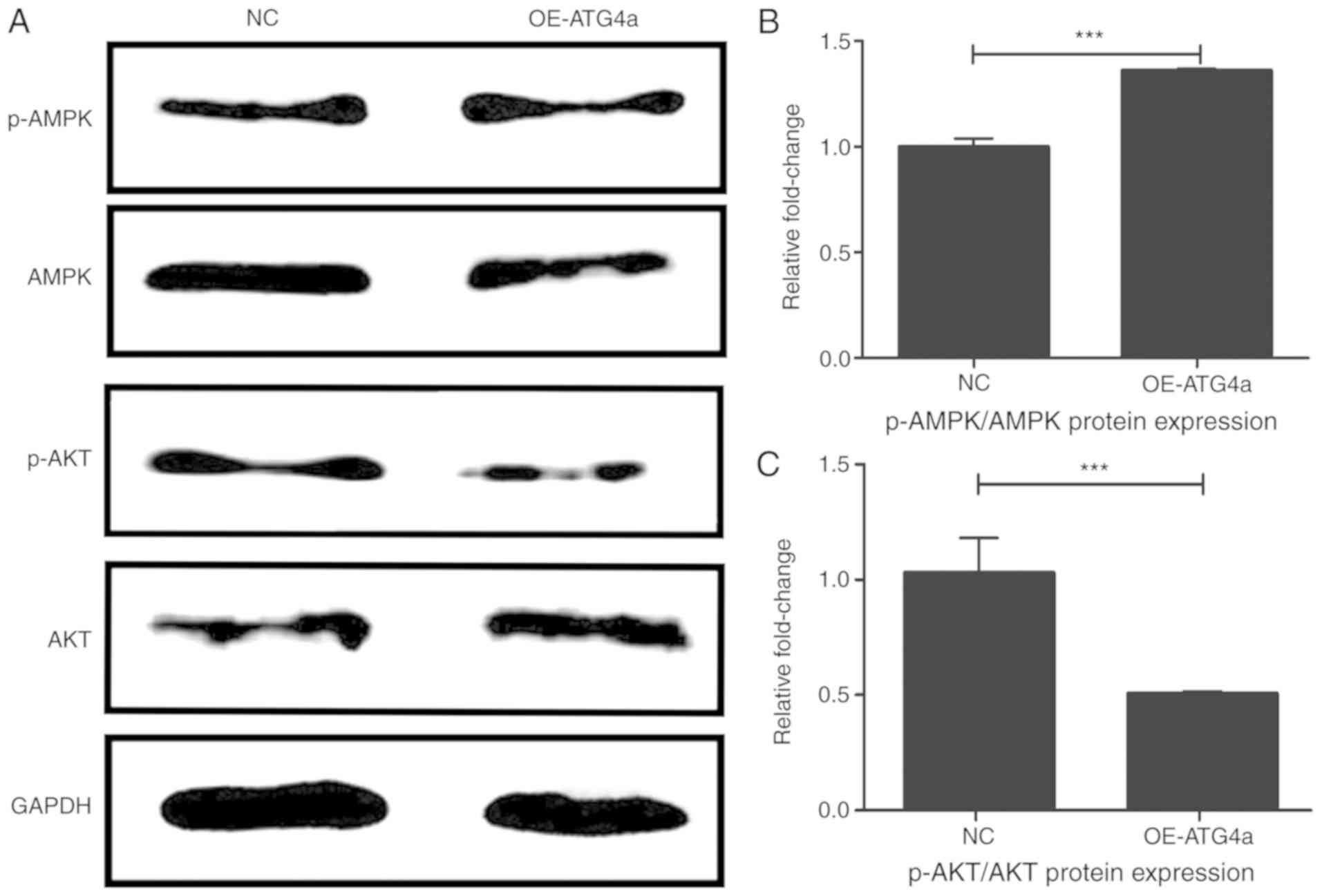
ATG4a influences the AMPK and Akt
pathways in lens epithelial cells
To investigate the autophagy pathways in which
ATG4a is involved, western blotting was performed in the
OE-ATG4a and NC groups (Fig.
3A). Compared with the NC group, the ratio of protein
expression of p-AMPK and AMPK was significantly elevated in the
OE-ATG4a group (P<0.001; Fig. 3B), indicating that the
overexpression of ATG4a significantly activated the
phosphorylation of AMPK. By contrast, the trend was reversed for
the ratio of p-Akt and Akt (P<0.001; Fig. 3C), indicating that the
overexpression of ATG4a can inhibit the activation of p-Akt.
These results suggested that ATG4a may play a role in
autophagy by promoting the AMPK pathway and inhibiting the Akt
pathway.
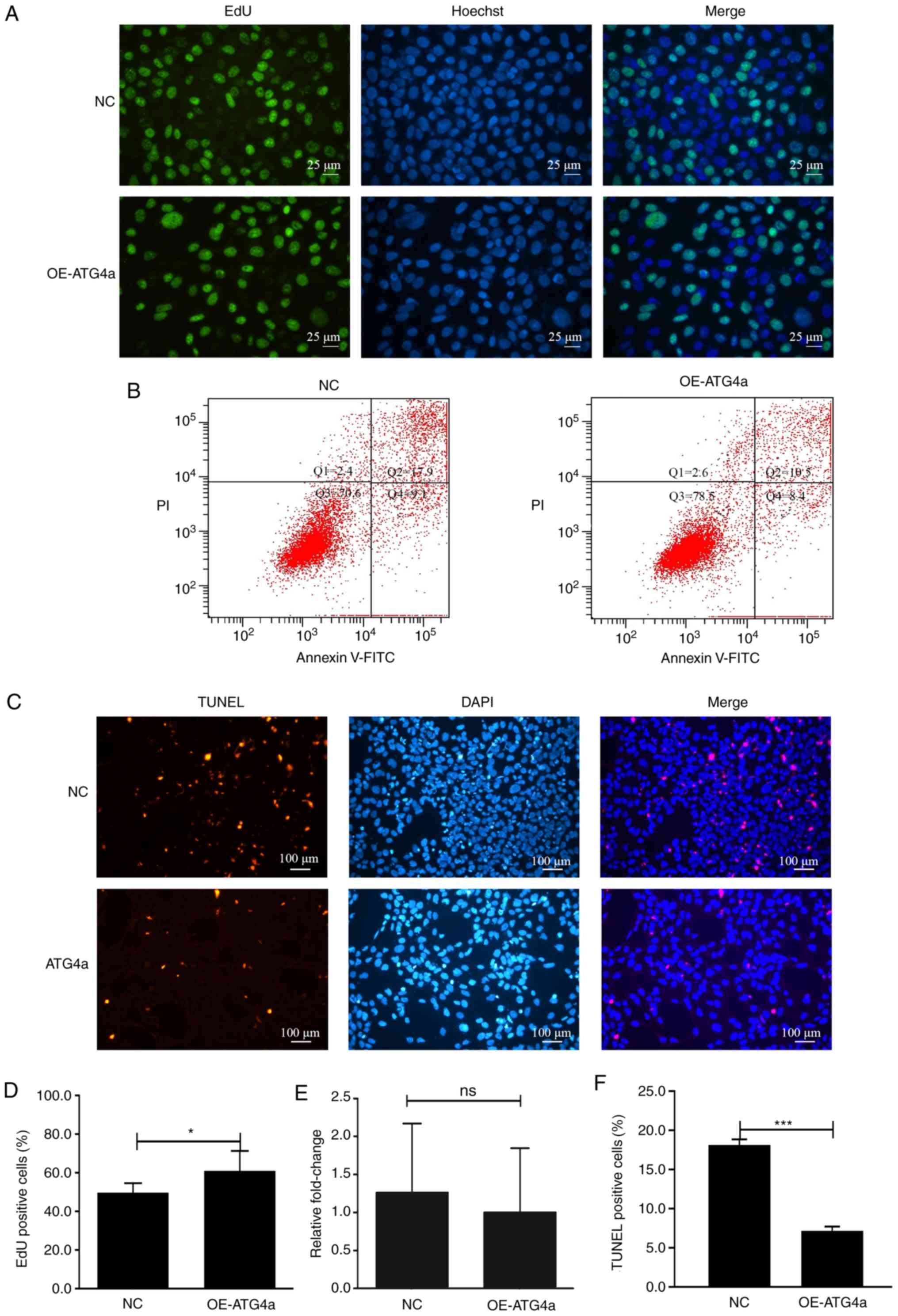
ATG4a promotes proliferation and
inhibits apoptosis
To investigate the relationship between ATG4a
and cell proliferation and apoptosis, the EdU incorporation assay,
FITC/PI double staining tested by flow cytometry and in situ
cell death detection assay were performed in the OE-ATG4a
and NC groups. The EdU incorporation assay showed that ATG4a
could significantly promote cell proliferation (P<0.05; Fig. 4A and D). Although the flow
cytometry analysis results did not show a significant difference
(P>0.05), it was observed that in the OE-ATG4a group, the
apoptosis rate of lens epithelial cells was lower compared with
that in the NC group, indicating that ATG4a may inhibit
apoptosis (Fig. 4B and E). To
further confirm this finding, a cell death detection assay was
performed in the OE-ATG4a and NC groups. The results showed
that, in the OE-ATG4a group, the percentage of TUNEL
positive cells was lower compared with that in the NC group
(P<0.001) and the difference was significant, indicating that
ATG4a can inhibit apoptosis (Fig. 4C and F).
Discussion
The present study for the first time, to the best of
the authors' knowledge, demonstrated that ATG4a-mediated
autophagy played an important role in the proliferation and
apoptosis of lens epithelial cells, and that this might occur via
the AMPK and Akt pathways.
H2O2-induced lens epithelial
cells have been widely used as a model for lens degradation and
cataract formation, according to previous studies, 200 µmol/l was
chosen as the concentration (20,21).
In the present study, this model was used to simulate the
degradation of the lens in the eyes. TEM showed that the number and
size of autophagosomes in the H2O2 group were
significantly higher compared with those in the control group. The
western blot analysis further validated the results; the protein
expression of beclin was significantly increased and the protein
expression of p62 was decreased after H2O2
treatment, suggesting that autophagy was significantly upregulated
in H2O2-treated lens epithelial cells.
Furthermore, western blotting showed that the protein expression of
ATG4a was upregulated in H2O2-treated lens
epithelial cells.
Briefly, ATG4a is an enzyme that can cleave LC3I to
produce LC3II; It plays an important role in the formation of
autophagic vesicle membranes (12). ATG4a is also the only protease
encoded by the autophagy-related genes (22). The upregulation of ATG4a in
H2O2-treated lens epithelial cells indicated
that ATG4a may play a key role in lens degradation.
Previous studies have demonstrated that ATG4a
can promote metastasis of tumor cells (23) and can be used as a target in
chemotherapy of cancer (24). To
explore the biological functions of ATG4a in lens epithelial
cells, a model of ATG4a overexpression was constructed. TEM
demonstrated that, compared with the NC group, the size of
autophagosomes increased in the OE-ATG4a group. Several
techniques including western blotting, immunofluorescence assay and
a dual-fluorescence mRFP-eGFP-LC3 system can evaluate the function
of effector proteins and autophagy flux (25,26).
In the present study, western blotting demonstrated that the
protein expression of beclin and LC3II/I was upregulated and that
of p62 was downregulated in the OE-ATG4a group. TEM is
considered one of the main and most important methods for the
detection of autophagy (27,28).
Furthermore, beclin, LC3II/I and p62 are routinely used as
biomarkers to measure the rate and occurrence of autophagy
(29–32). The results indicated that
ATG4a could activate autophagy in lens epithelial cells.
The Akt and AMPK signaling pathways are important
pathways in the autophagy process. Normally, Akt activates
mammalian target of rapamycin complex 1, which is a negative
regulator of autophagy, by inhibiting the tuberous sclerosis
complex 1/2 (TSC1/TSC2) protein complex and thus inhibiting
autophagy (33). In a previous
study, the ratio of p-Akt/Akt in H9C2 cells was significantly
reduced following exposure to H2O2 and
triggered autophagy, indicating that the Akt pathway at least
partly modulates autophagy (34).
AMPK activation leads to the phosphorylation and activation of
TSC1/2 and inhibition of mTOR; thus indicating that it is a
positive regulator of autophagy (35). In the present study, the ratio of
p-AMPK/AMPK was significantly increased and the ratio of p-Akt/Akt
was decreased in the OE-ATG4a group compared with the NC
group, indicating that these two pathways might be modulated by
ATG4a in lens epithelial cells, but the exact mechanism
needs further research. Nevertheless, the activation and inhibition
of these two autophagy pathways further demonstrated that
ATG4a can promote autophagy in lens epithelial cells.
Increasing evidence has demonstrated that cell
proliferation and apoptosis are affected by autophagy (8,36,37).
Studies have demonstrated that autophagy can promote cell growth
(38,39), but the relationship between
apoptosis and autophagy is complex and often appears contradictory
(36,40). Normally, autophagy can maintain
cell homeostasis under stressful conditions and prevent cell death
(8), but in some neurodegenerative
diseases, such as Alzheimer's disease, it can also be a pathogenic
factor (37). Therefore,
elucidating the exact functions of autophagy genes will improve our
knowledge on how to protect or induce cell death. The present study
demonstrated that ATG4a had a positive effect on cell
proliferation, using the EdU incorporation assay. As for apoptosis,
although the FITC/PI staining assay did not show a statistical
difference between the two groups, the in situ cell death
detection assay showed that the apoptosis rate in the
OE-ATG4a group was significantly downregulated compared with
the NC group, suggesting that ATG4A could inhibit apoptosis
in lens epithelial cells.
Taken together, the present study demonstrated that
ATG4a could induce autophagy in lens epithelial cells and
that this might activate the AMPK and inhibit Akt pathways.
Furthermore, ATG4a increased cell proliferation and
decreased apoptosis, indicating that ATG4a plays an
important role in lens degradation and cataract formation.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81870644).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY performed all experiments and wrote the
manuscript. JZ, YQ, FZ and LJ analyzed the experimental data. JZ
designed the present study and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATG4a
|
autophagy-related gene 4a
|
|
PE
|
phosphatidylethanolamine
|
|
TEM
|
transmission electron microscopy
|
References
|
1
|
Augusteyn RC: Growth of the lens: In vitro
observations. Clin Exp Optom. 91:226–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pascolini D and Mariotti SP: Global
estimates of visual impairment: 2010. Br J Ophthalmol. 96:614–618.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flaxman SR, Bourne RRA, Resnikoff S,
Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J,
Kempen JH, et al Vision Loss Expert Group of the Global Burden of
Disease Study, : Global causes of blindness and distance vision
impairment 1990–2020: A systematic review and meta-analysis. Lancet
Glob Health. 5:e1221–e1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao WJ and Yan YB: Increasing
susceptibility to oxidative stress by cataract-causing crystallin
mutations. Int J Biol Macromol. 108:665–673. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng H, Yang Z, Bai X, Yang M, Fang Y,
Zhang X, Guo Q and Ning H: Therapeutic potential of a dual mTORC1/2
inhibitor for the prevention of posterior capsule opacification: An
in vitro study. Int J Mol Med. 41:2099–2107. 2018.PubMed/NCBI
|
|
6
|
Crooke A, Huete-Toral F, Colligris B and
Pintor J: The role and therapeutic potential of melatonin in
age-related ocular diseases. J Pineal Res. 63:e124302017.
View Article : Google Scholar
|
|
7
|
Jin X, Jin H, Shi Y, Guo Y and Zhang H:
Long Non-Coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214
and activation of the Caspase-1 pathway. Cell Physiol Biochem.
42:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni Z, Gong Y, Dai X, Ding W, Wang B, Gong
H, Qin L, Cheng P, Li S, Lian J, et al: AU4S: A novel synthetic
peptide to measure the activity of ATG4 in living cells. Autophagy.
11:403–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang RC and Levine B: Autophagy in
cellular growth control. FEBS Lett. 584:1417–1426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morishita H and Mizushima N: Autophagy in
the lens. Exp Eye Res. 144:22–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv W, Sui L, Yan X, Xie H, Jiang L, Geng
C, Li Q, Yao X, Kong Y and Cao J: ROS-dependent Atg4 upregulation
mediated autophagy plays an important role in Cd-induced
proliferation and invasion in A549 cells. Chem Biol Interact.
279:136–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He R, Peng J, Yuan P, Xu F and Wei W:
Divergent roles of BECN1 in LC3 lipidation and autophagosomal
function. Autophagy. 11:740–747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Hou Y, Wang J, Chen X, Shao ZM and
Yin XM: Kinetics comparisons of mammalian Atg4 homologues indicate
selective preferences toward diverse Atg8 substrates. J Biol Chem.
286:7327–7338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakatogawa H, Ichimura Y and Ohsumi Y:
Atg8, a ubiquitin-like protein required for autophagosome
formation, mediates membrane tethering and hemifusion. Cell.
130:165–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
26:1749–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gil J, Ramsey D, Pawlowski P, Szmida E,
Leszczynski P, Bebenek M and Sasiadek MM: The influence of tumor
microenvironment on ATG4D gene expression in colorectal cancer
patients. Med Oncol. 35:159–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mao JJ, Wu LX, Wang W, Ye YY, Yang J, Chen
H, Yang QF, Zhang XY, Wang B and Chen WX: Nucleotide variation in
ATG4A and susceptibility to cervical cancer in Southwestern Chinese
women. Oncol Lett. 15:2992–3000. 2018.PubMed/NCBI
|
|
19
|
Antonelli M, Strappazzon F, Arisi I,
Brandi R, D'Onofrio M, Sambucci M, Manic G, Vitale I, Barilà D and
Stagni V: ATM kinase sustains breast cancer stem-like cells by
promoting ATG4C expression and autophagy. Oncotarget.
8:21692–21709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou W, Xu J, Wang C, Shi D and Yan Q:
miR-23b-3p regulates apoptosis and autophagy via suppressing SIRT1
in lens epithelial cells. J Cell Biochem. 120:19635–19646. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De-Qian K, Yue L, Li L and Guangying Z:
Downregulation of Smac attenuates
H2O2-induced apoptosis via endoplasmic
reticulum stress in human lens epithelial cells. Medicine
(Baltimore). 96:e74192017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández ÁF and López-Otín C: The
functional and pathologic relevance of autophagy proteases. J Clin
Invest. 125:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SW, Ping YF, Jiang YX, Luo X, Zhang
X, Bian XW and Yu PW: ATG4A promotes tumor metastasis by inducing
the epithelial-mesenchymal transition and stem-like properties in
gastric cells. Oncotarget. 7:39279–39292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu PF, Tsai KL, Hsu CJ, Tsai WL, Cheng
JS, Chang HW, Shiau CW, Goan YG, Tseng HH, Wu CH, et al: Drug
repurposing screening identifies tioconazole as an ATG4 inhibitor
that suppresses autophagy and sensitizes cancer cells to
chemotherapy. Theranostics. 8:830–845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH, Rao MV, Yang DS, Stavrides P, Im
E, Pensalfini A, Huo C, Sarkar P, Yoshimori T and Nixon RA:
Transgenic expression of a ratiometric autophagy probe specifically
in neurons enables the interrogation of brain autophagy in vivo.
Autophagy. 15:543–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun Y, Huang YH, Huang FY, Mei WL, Liu Q,
Wang CC, Lin YY, Huang C, Li YN, Dai HF, et al:
3′-epi-12β-hydroxyfroside, a new cardenolide, induces
cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in
lung cancer cells. Theranostics. 8:2044–2060. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Biazik J, Vihinen H, Anwar T, Jokitalo E
and Eskelinen EL: The versatile electron microscope: An
ultrastructural overview of autophagy. Methods. 75:44–53. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena AA, Adachi H, Adams CM, Adams PD, Adeli
K, et al: Guidelines for the use and interpretation of assays for
monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Runwal G, Stamatakou E, Siddiqi FH, Puri
C, Zhu Y and Rubinsztein DC: LC3-positive structures are prominent
in autophagy-deficient cells. Sci Rep. 9:101472019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bortnik S and Gorski SM: Clinical
applications of autophagy proteins in cancer: From potential
targets to biomarkers. Int J Mol Sci. 18:E14962017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan J, Yang X, Li J, Shu Z, Dai J, Liu X,
Li B, Jia S, Kou X, Yang Y, et al: Spermidine coupled with exercise
rescues skeletal muscle atrophy from D-gal-induced aging rats
through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a
signal pathway. Oncotarget. 8:17475–17490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Ji LY, Li L and Li JM: Oxidative
stress, autophagy and pyroptosis in the neovascularization of
oxygen-induced retinopathy in mice. Mol Med Rep. 19:927–934.
2019.PubMed/NCBI
|
|
33
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Jin X, Hu CF, Li R, Zhou Z and Shen
CX: Exosomes derived from mesenchymal stem cells rescue myocardial
ischaemia/reperfusion injury by inducing cardiomyocyte autophagy
via AMPK and Akt pathways. Cell Physiol Biochem. 43:52–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao M, Sun L, Yu XJ, Miao Y, Liu JJ, Wang
H, Ren J and Zang WJ: Acetylcholine mediates AMPK-dependent
autophagic cytoprotection in H9c2 cells during
hypoxia/reoxygenation injury. Cell Physiol Biochem. 32:601–613.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Booth LA, Tavallai S, Hamed HA,
Cruickshanks N and Dent P: The role of cell signalling in the
crosstalk between autophagy and apoptosis. Cell Signal. 26:549–555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katheder NS, Khezri R, O'Farrell F,
Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen
T, Juhász G, et al: Microenvironmental autophagy promotes tumour
growth. Nature. 541:417–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H and Zhang G: Activation of
CaMKKβ-AMPK-mTOR pathway is required for autophagy induction by
β,β-dimethylacrylshikonin against lung adenocarcinoma cells.
Biochem Biophys Res Commun. 517:477–483. 2019. View Article : Google Scholar : PubMed/NCBI
|