Introduction
Neuromyelitis optica (NMO), also known as Devic's
syndrome, is a severe demyelinating disease of the central nervous
system that causes recurrent seizures. NMO mainly affects the optic
nerves and the spinal cord, but it can also affect the brain, to a
lesser extent (1). NMO is
difficult to distinguish clinically from multiple sclerosis (MS) as
both disorders have similar characteristics in the early stages of
disease. NMO was once considered a subtype of MS, and was even
termed optica-spinal MS in Japan. However, a previous study has
suggested that NMO is an independent clinical entity that is
distinct from MS (2). NMO is
caused by an autoimmune antibody (NMO-IgG) targeting aquaporin-4
(AQP4), a plasma membrane water transport channel expressed in the
glia (3–5). Binding of NMO-IgG to AQP4 on
astrocytes can cause complement-mediated and cell-mediated injury
to astrocytes, triggering a series of inflammatory cascades that
result in the release of cytokines, activation of microglia and
accumulation of leukocytes. Together, these responses result in
neuronal injury and cause the clinical symptoms of NMO (6–8).
NMO remains to be an incurable disease. The goal of
treating acute NMO events is to improve relapse symptoms and
restore neurological functions, while the aim of long-term
immunosuppression is to prevent further attacks (9–11).
For long-term immunosuppression, patients usually receive either B
cell-targeted therapies, such as intravenous rituximab or oral
azathioprine, and/or prednisone (12–20).
Other therapeutic options include mycophenolate mofetil (21), methotrexate (22) or mitoxantrone. Mitoxantrone can
cause adverse effects, such as cardiotoxicity or leukemia, and is
thus generally not considered for first-line treatment (11,23–28).
A novel strategy for NMO treatment is blocking the binding of
NMO-IgG to AQP4. Based on this strategy, a high-affinity,
non-pathogenic monoclonal antibody (aquaporumab) was developed and
exhibited positive effects in clinical trials (29). Aquaporumab can relieve NMO symptoms
by competing with NMO-IgG to bind to AQP4 (29). However, monoclonal antibody drugs
can also have disadvantages, for example, they may activate the
complement system and cause pathological changes similar to those
observed in NMO, or they may induce the production of autoimmune
antibodies, thus reducing their protective effects (10).
In this context, inhibitors that block binding
between NMO-IgG and AQP4 may represent potential drug candidates
for treating NMO. A previously established reporter cell-based
high-throughput screening system identified a small molecule
inhibitor (isotetrandrine, obtained from an extract of the herb
Mahonia japonica) that can block binding between NMO-IgG and
AQP4 in a dose-dependent manner (30). In the present study, the natural
compounds from another herb (Petroselinum crispum) that
exhibited similar inhibitory effects in the screening system were
purified. Another compound from this herb, geraldol, inhibited
binding of NMO-IgG to AQP4.
Materials and methods
Cells, animals and antibodies
Chinese hamster lung fibroblast cells V79 (American
Type Culture Collection; cat. no. CCL-93) were cultured in DMEM
medium (D7777; Sigma-Aldrich; Merck KGaA). Fischer rat thyroid
epithelial cells FRTL which exogenously expresses M23-AQP4 were
supplied by Liaoning Medical College and established from a plasmid
transfection (30). FRTL were
cultured in F-12-modified Coon's medium (Sigma-Aldrich; Merck
KGaA). Both culture media were supplemented with 10% (v/v) FBS
(Sigma-Aldrich; Merck KGaA) and 2 mM glutamine. Cells were cultured
at 37°C under a humidified atmosphere containing 5%
CO2.
In total, 30 female (age, 6-week-old; weight ~18 g)
and 20 male (age, 8-week-old; weight ~23 g) AQP4−/− mice
(Dalian Medical University) were used in the present study, as
previously described (31). Mice
were housed in a 20–26°C specific pathogen-free animal facility on
a 12 h light/dark cycle and 15 times per hour air exchange, with
constant access to food and water. Mice were mated until birth,
then neonatal mice were used for primary astrocytes isolation.
Protocols for mouse experiments were approved by the Laboratory
Animal Ethics Committee of School of Life Science, Jilin University
(Changchun, China; approval no. 2017-nsfc049).
NMO-IgG was purified from the sera of patients with
NMO and concentrated using a Melon Gel IgG Purification kit (Thermo
Fisher Scientific, Inc.) and Amicon Ultra Centrifugal Filter Units
(EMD Millipore) according to the manufacturer's protocol. NMO serum
and control (non-NMO) serum were obtained from clinical patients
whose sera were NMO-IgG positive or negative, respectively
(32). This study was approved by
Ethics Committee of The China-Japan Union Hospital, Jilin
University (approval no. 2017022238). Written informed consent was
obtained from all patients.
Primary screening procedures
The procedure for primary screening of various herb
fractions inhibiting the binding of NMO-IgG to AQP4 was performed
as previously described (30).
Briefly, V79-AQP4 cells were plated in black-walled and
clear-bottomed 96-well tissue culture plates (Costar; Corning,
Inc.) and maintained in complete DMEM at 37°C for ~48 h until they
reached 90% confluence. Overall, 80 wells were used to test natural
fractions or natural compounds which were isolated and maintained
in the lab of the present study; the first row of each plate was
used as a negative control (V79-AQP4, no test compound) and the
last row of each plate was used as a positive control (V79-null, no
test compound). Each well was washed three times with PBS, fixed
with 4% paraformaldehyde (PFA) at room temperature for 10 min and
blocked with 3% BSA (Merck KGaA) at room temperature for 30 min
(100 µl/well). Test natural fractions were added to each well (0.5
µl 20 mM solution in DMSO) and incubated for 15 min at room
temperature. After washing twice with PBS, NMO-IgG (0.2 µg/ml) and
horseradish peroxidase (HRP)-labeled goat anti-human IgG secondary
antibody (1:1,000, Santa Cruz Biotechnology, Inc.; cat. no.
sc-2907) were added to each well and incubated at 37°C for 1 h.
After washing three times with PBS, enhanced chemiluminescent
substrate (ECL-Plus; GE Healthcare) was added to measure HRP
activity. Chemiluminescence was measured after 5 min using a plate
reader (TECAN Infinite 200; Tecan Group Ltd.).
Isolation and identification of target
natural compounds
P. crispum was purchased from a commercial
herb supplier (Hongjian Herb Store). The isolation procedures were
performed as previously described (33). The aerial parts of P.
crispum were crushed and extracted using different solvents
(petroleum, ethyl acetate, chloroform, n-butanol; all were 100%;
purchased from Beijing Chemical Works) at room temperature to
achieve maximum solubility for the target peak (bioactive
fraction). The extracts (50 g) were further purified by high-speed
countercurrent chromatography (HSCCC; TBE-300; Tauto Biotechnique
Co., Ltd.), which was coupled with a UV detector (cat. no.
TBD-23UV; Tauto Biotech Co., Ltd.) with the following settings;
Rotation rate, 800 rpm; solvent, 70% methanol; flow rate, 10
ml/min; three multilayer-coiled polytetrafluoroethylene columns
(internal diameter, 3.0 mm; total volume, 300 ml) connected in
series at a temperature of 25°C. Each peak fraction was manually
collected and concentrated using a rotatory evaporator under
reduced pressure, yielding 1–4 g concentrate for each fraction.
After bioactivity screening, four continuous peak fractions derived
from ethyl acetate extraction were selected to isolate single
compound. Then, 20 µg of each concentrate was dissolved in
acetonitrile for subsequent analytical reverse-phase high
performance liquid chromatography (HPLC; Shimadzu LC-2010AHT;
Shimadzu Corporation) with a UV detector. Geraldol obtained from
Sigma-Aldrich (Merck KGaA) was used as an internal reference
compound. A 4.6×25 mm C18 column (particle size, 5 µm; Beckman
Ultrasphere ODS) was used for peak separation at 20°C. The mobile
phase consisted of 52% methanol and 48% water containing 2% acetic
acid. The injection volume was 10 µl and the flow rate was 1.0
ml/min. The effluent was examined at 360 nm. Peak identification
was performed using liquid chromatography/mass spectrometry and
nuclear magnetic resonance (NMR). Mass spectrometry was performed
using QuattroMicro (Waters) equipment with scans from 50 to 1,000
m/z. The positive electrospray ionization (ESI) mode was used, with
N2 as the curtain gas, nebulizer gas and collision gas
(11, 9 and 7 psi as the optimal values). The ESI needle voltage was
set to 4,500 V and the turbo-gas temperature was set at 425°C.
1H and 13C NMR spectra were recorded using a
Bruker Avance 500 spectrometer (Bruker Optics-Beijing) operating at
500 MHz for 1H and 100 MHz for 13C
spectra.
Primary astrocyte cultures
Primary astrocytes were isolated from the cortices
of 20 1-day-old wild-type and AQP4−/− mice as previously
described (34). Briefly, the
cerebral hemispheres of the mice were separated, and the meninges,
hippocampus, basal ganglia and olfactory bulb were removed.
Cortical tissue was then isolated using forceps under a microscope,
and digested in 0.25% trypsin-EDTA in DMEM at 37°C for 15 min. The
digested cells were centrifuged at 300 × g for 10 min at room
temperature, and cultured on a poly-L-ornithine hydrobromide-coated
(P3655; Sigma-Aldrich; Merck KGaA) 96-well plate or glass
coverslips in DMEM supplemented with 10% FBS (Sigma-Aldrich; Merck
KGaA) at 37°C under a humidified atmosphere containing 5%
CO2. The medium was changed every other day. To purify
astrocytes, the culture plates were shaken in a rotator at 180 rpm
overnight and then at 220 rpm for 4 h when confluency reached ~30%.
To prevent proliferation of other cell types, cell mixtures were
treated with 10 µM cytosine arabinoside for 48 h. The medium was
replaced with DMEM containing 3% FBS and 0.15 mM dibutyryl cAMP to
induce differentiation when confluency reached ~50%. Once astrocyte
confluency had reached >90%, the complement-dependent
cytotoxicity (CDC) assay was performed.
CDC assay
For the CDC assay, cells were incubated with P.
crispum fractions, isolated geraldol or DMSO for 15 min,
followed by treatment of NMO-IgG (2.5 µg/ml) and 5% human
complement (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C. Control
human serum collected from patients without NMO (1:200) was added
as a negative control. CellTiter-Glo® Luminescent Cell
Viability Assay (Promega Corporation) was used to measure cell
viability and its decrease according to CDC. Briefly, after
incubation at 37°C for 48 h, 100 µl of CellTiter-Glo®
reagent was added to each well and mixed by gentle vortexing,
swirling or inverting to obtain a homogeneous solution.
Luminescence was recorded after 5 min using a microplate reader
(Fluostar Optima; BMG Lab Tech GmbH). All experiments were repeated
twice with four replicates.
Immunofluorescence staining
V79 cells expressing M23-AQP4 were cultured on round
glass coverslips in 24-well plate (Corning, Inc.) for 24 h. After
washing three times with PBS, the cells were blocked with 3% BSA
(Merck KGaA) at room temperature for 1 h. Geraldol or DMSO was
added in 3% BSA and incubated at room temperature for 15 min,
followed by the addition of NMO-IgG (20 µg/ml) or control serum
from patients without NMO (1:200) at room temperature for 1 h.
V79-AQP4 cells were washed with PBS three times and incubated with
Alexa Fluor 555-conjugated goat anti-human IgG secondary antibody
(1:400; Invitrogen; Thermo Fisher Scientific, Inc; cat. no.
A-21433). For AQP4 immunofluorescence staining, V79-AQP4 cells were
fixed in 4% PFA at room temperature for 10 min and permeabilized
with 0.5% Triton X-100 at room temperature for 10 min. After
blocking with 3% BSA at room temperature for 1 h, the cells were
incubated with rabbit anti-AQP4 antibody (1:200; Santa Cruz
Biotechnology; cat. no. sc-32739) at room temperature for 1 h.
Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200; Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. A32731) was used as the
secondary antibody. Fluorescence images were obtained and observed
using fluorescence microscopy (IX71; Olympus Corporation).
Cell viability assay
Cell viability was measured in 96-well plates with a
colorimetric assay using the Cell Proliferation reagent (WST-1;
Roche; cat. no. 11644807001) according to the manufacturer's
protocol. Cells at 70% confluency were treated with serial
dilutions of geraldol (2, 4, 8, 16, 32, 64, 128 or 256 µM) for 24
h. WST-1 reagent (10 µl/well) was added to cultured cells (100
µl/well) and incubated at 37°C for 1 h. Absorbance was measured at
440 nm. All experiments were repeated twice with 4 replicates.
Detection of apoptosis
Apoptosis was detected using a Cell Death Detection
ELISAPLUS kit (Roche) according to the manufacturer's
protocol. This photometric enzyme immunoassay is used for
quantitative in vitro detection of cytoplasmic
histone-associated DNA fragments (mono- and oligo-nucleosomes)
following programmed cell death. Cells at 70% confluency were
treated serial dilutions of geraldol (2, 4, 8, 16, 32, 64, 128 or
256 µM) for 24 h. Cell lysates were then placed in a
streptavidin-coated microplate and incubated with a mixture of
biotinylated anti-histone and anti-DNA peroxidase antibodies that
were included in the kit at room temperature for 2 h. The amount of
peroxidase retained in the immunocomplex was photometrically
determined after reaction with 100 µl
2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)- diammonium
salt substrate at room temperature for 15 min. Absorbance was
measured at 405 nm. All experiments were repeated twice with four
replicates.
Osmotic water permeability assay
Osmotic water permeability of the plasma membrane
was assessed using a slightly modified version of a previously
described calcein fluorescence quenching method (31). Briefly, cells were cultured on
glass coverslips pre-coated for 24 h, incubated with 5 µM
calcein-AM (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for
15 min, and then transferred to a perfusion chamber designed for
rapid solution exchange. The time course of cytoplasmic calcein
fluorescence in response to cell shrinkage induced by exchange of
perfusate between PBS (300 mOsmol) and hypertonic PBS (500 mOsmol
containing 200 mM D-mannitol) was measured. A reciprocal
exponential time constant (τ=1/s) was used as an indicator of cell
shrinkage, where τ is the time from the initiation of the osmotic
switch to the point where cytoplasmic calcein fluorescence
quenching reaches its maximum. All experiments were repeated twice
with four replicates.
Statistical analysis
Statistical analysis was performed using the
unpaired two-tailed Student's t-test in SPSS (version 17.0; SPSS,
Inc.). Multiple comparisons between groups were performed using a
one-way ANOVA followed by Sidak multiple comparisons test with
GraphPad Prism software (version 7; GraphPad Software, Inc.). Data
are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and structural
characterization of geraldol
Fractions from the herb P. crispum were
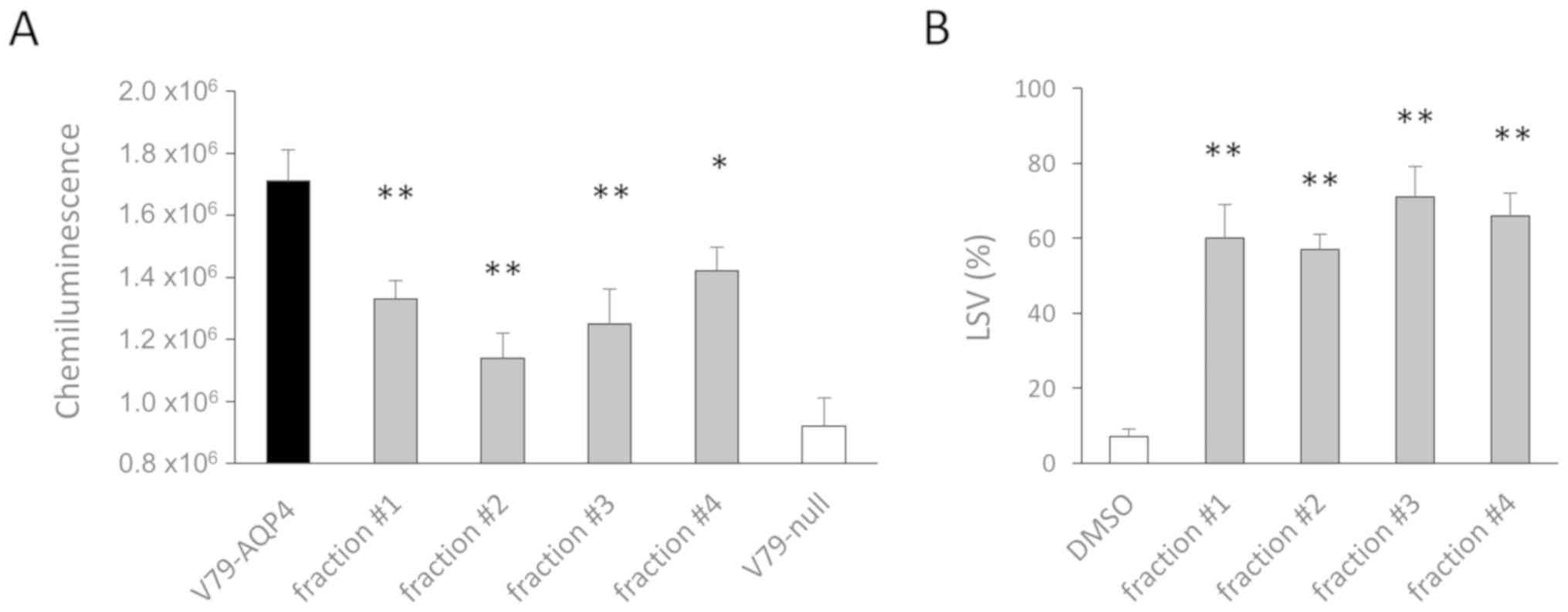
isolated by solvent extraction and subject to a screening as
reported in a previous study (30). During the primary screening, four
continuous fractions isolated from ethyl acetate extraction were
identified to have inhibitory effects on NMO-IgG binding to AQP4
(Fig. 1A). These fractions also
exhibited inhibitory effects on NMO-IgG-dependent complement
cytotoxicity in secondary screens (Fig. 1B).
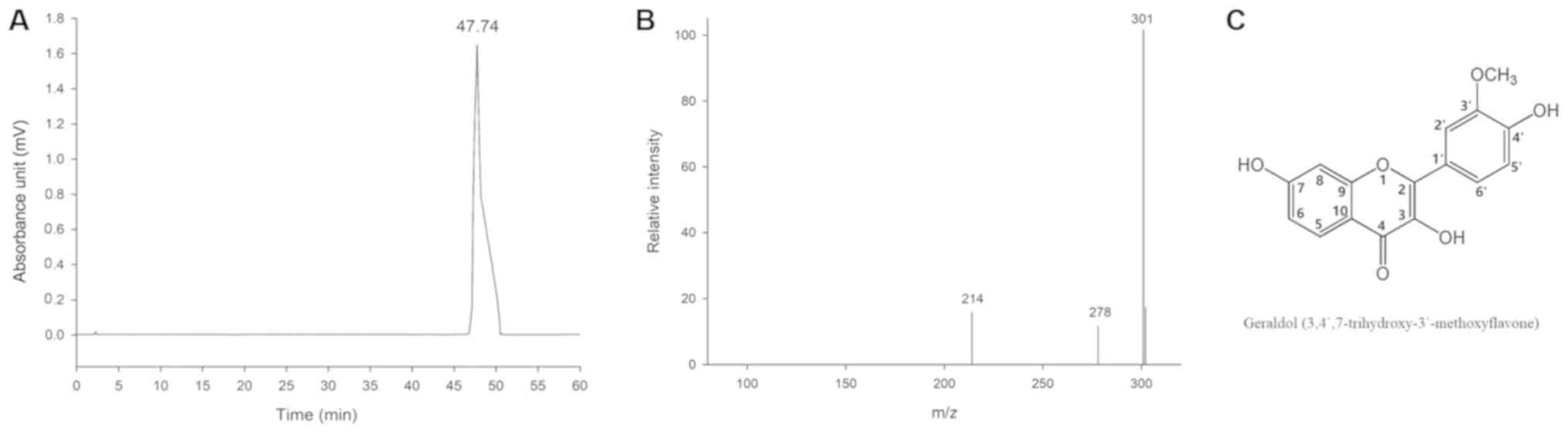
This bioactivity-guided phytochemical study led to
the isolation of the flavonoid geraldol from all the four
fractions. The purity of the compound was assessed using analytical
HPLC. As presented in Fig. 2, only
a single peak with a retention time of 47.74 min was observed. The
purity of the peak was determined to be 99.5%. These results
indicated that >99% pure geraldol could be obtained successfully
using HSCCC separation under optimized conditions. The
HSCCC-purified fraction was structurally characterized using
ESI-mass spectrometry (ESI-MS) and NMR as follows:
Molecular formula:
C16H12O6. 1H NMR (500
MHz, CDCl3): δ (ppm) 7.93 (1H, d, J=8.8 Hz,
H-5), 6.91 (1H, dd, J=2.2 Hz, 8.8 Hz, H-6), 6.97
(1H, d, J=2.2 Hz, H-8), 7.77 (1H, d, J=2.1
Hz, H-2′), 6.94 (1H, d, J=8.5 Hz, H-5′), 7.70
(1H, dd, J=2.1 Hz, 8.5 Hz, H-6′), 9.66 (1H,
s, OH-3), 10.74 (1H, s, OH-7), 9.08 (1H, s,
OH-4′) and 3.83 (1H, s, OCH3−3′).
13C NMR (100 MHz, CD3OD): δ (ppm) 144.9
(C-2), 137.3 (C-3), 172.0 (C-4), 126.4 (C-5), 114.7 (C-6), 162.3
(C-7), 102.1 (C-8), 156.3 (C-9), 114.2 (C-10), 122.6 (C-1′), 111.7
(C-2′), 147.4 (C-3′), 148.4 (C-4′), 115.5 (C-5′), 121.4 (C-6′) and
55.6 (OCH3−3′).
Geraldol blocks NMO-IgG binding to
AQP4
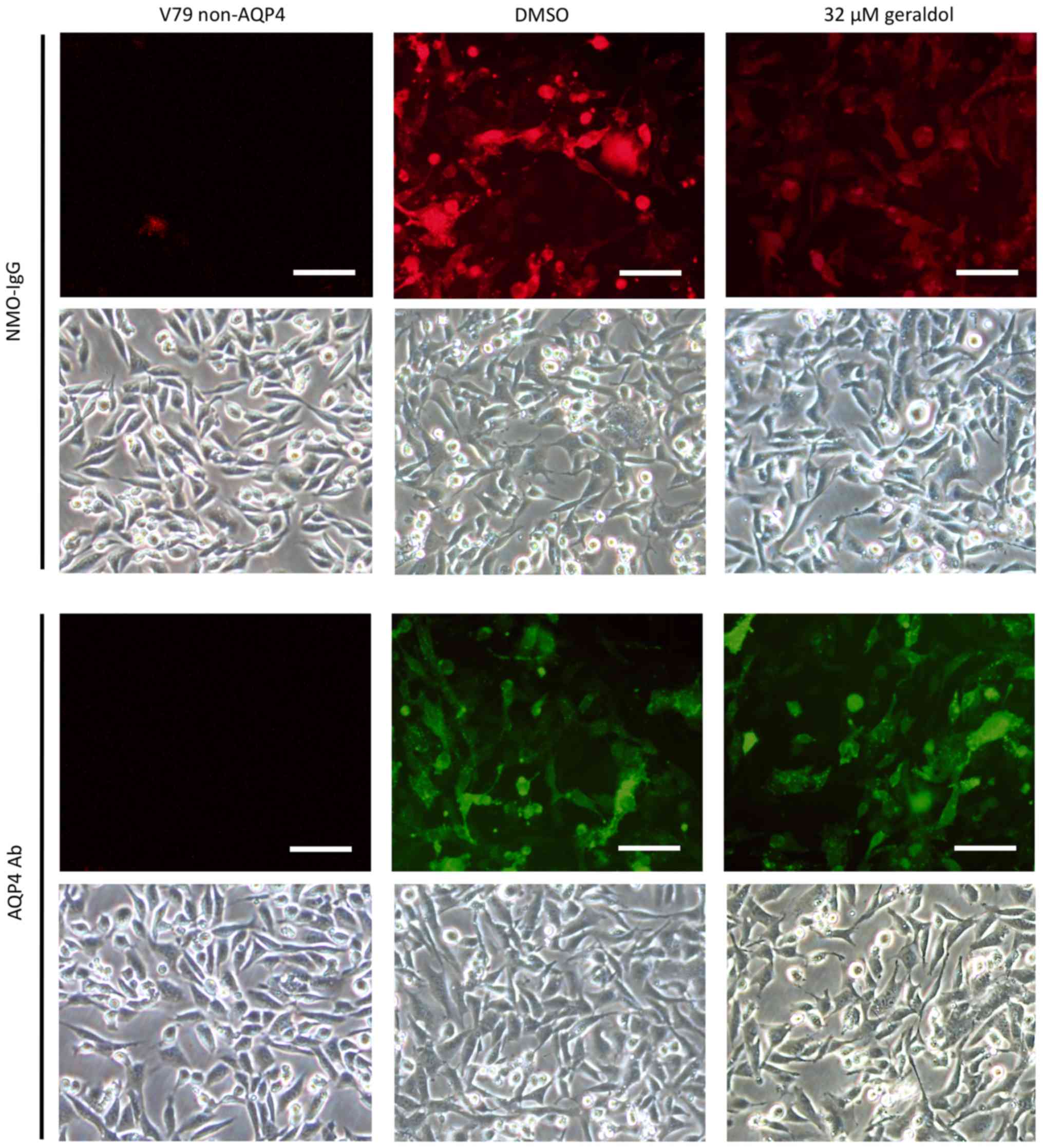
The inhibitory effect of geraldol on NMO-IgG binding
to AQP4 was analyzed using immunofluorescence assays. After the
V79-AQP4 cells were incubated with NMO-IgG, fixed and
permeabilized, binding of Alexa Fluor 555-conjugated anti-human
secondary antibody was measured in the presence or absence of
geraldol. As presented in Fig. 3,
red fluorescence was decreased when cells were incubated with
geraldol. Meanwhile, an anti-C terminal AQP4 antibody and Alexa
Fluor 488-labeled secondary antibody were used to test the
expression of AQP4. The green fluorescence produced by staining
with anti-C terminal AQP4 antibody was unaltered by geraldol
treatment. There was an approximately equal total number of cells
in the different groups (indicated by light microscopy).
Geraldol decreases CDC in both
NMO-IgG/complement- treated FRTL-AQP4 cells and primary
astrocytes
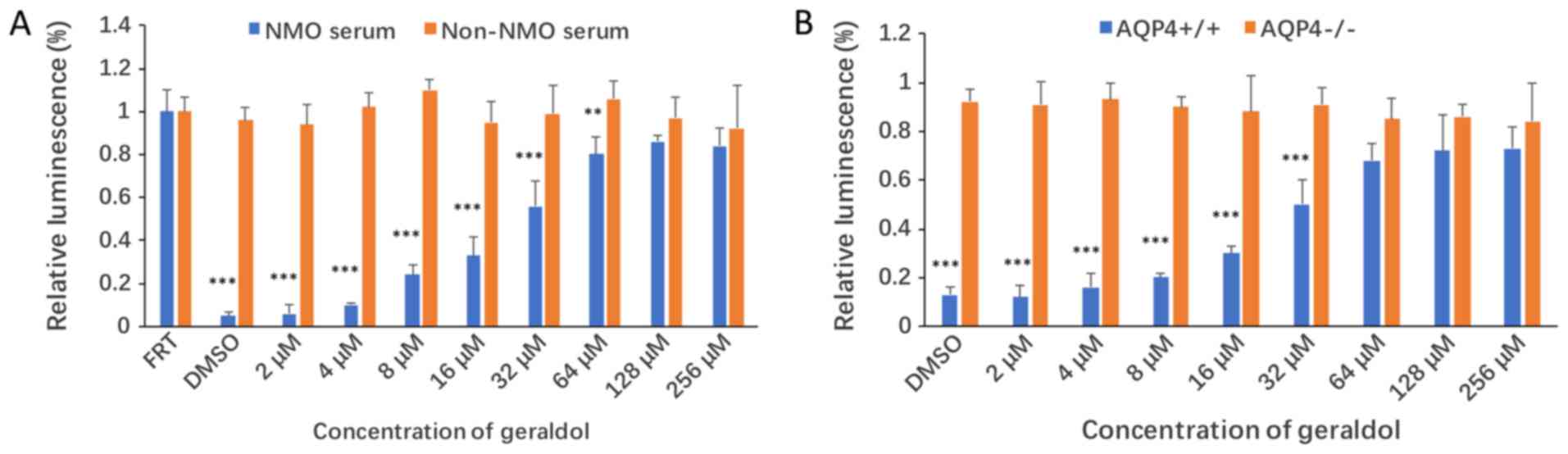
Next, the inhibitory effects of geraldol on CDC were
evaluated. FRTL cells stably expressing human AQP4 were incubated
with NMO-IgG for 60 min in the presence of human complement, along
with either geraldol or DMSO. The cytotoxicity of geraldol was also
measured using this system. As presented in Fig. 4A, geraldol increased the viability
of NMO-IgG/complement-treated FRTL-AQP4 cells in a
concentration-dependent manner. The half maximal inhibitory
concentration (IC50) of geraldol was 24.6 µM. A group of
FRTL cells without AQP4 expression were used as the negative
control. In order to confirm the effects of geraldol purified from
a natural source, a commercial geraldol compound was also purchased
and its inhibitory effects were tested in the same CDC assay with
similar results (data not shown).
The inhibitory effect of geraldol on CDC was also
assessed in cultured primary astrocytes from wild-type and
AQP4−/− mice (Fig. 4B).
Geraldol increased the viability of cultured
NMO-IgG/complement-treated primary astrocytes from wild-type mice
in a concentration-dependent manner. The IC50 of
geraldol was 30.3 µM. No inhibitory effect was observed in cultured
primary astrocytes from AQP4−/− mice.
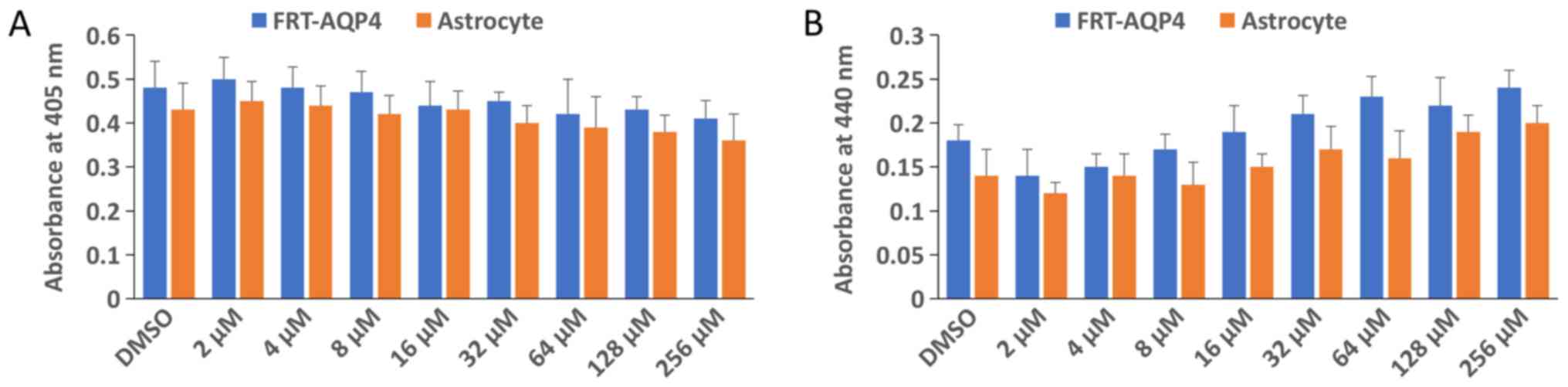
Geraldol does not affect the viability
of FRTL-AQP4 cells and primary astrocytes
To determine whether geraldol affects cell
viability, its effects on cell proliferation were analyzed using
the WST-1 assay and cell apoptosis using the cell death assay. As
presented in Fig. 5A and B, no
significant differences in cell proliferation or apoptosis were
observed within the geraldol dose range used in the present
study.
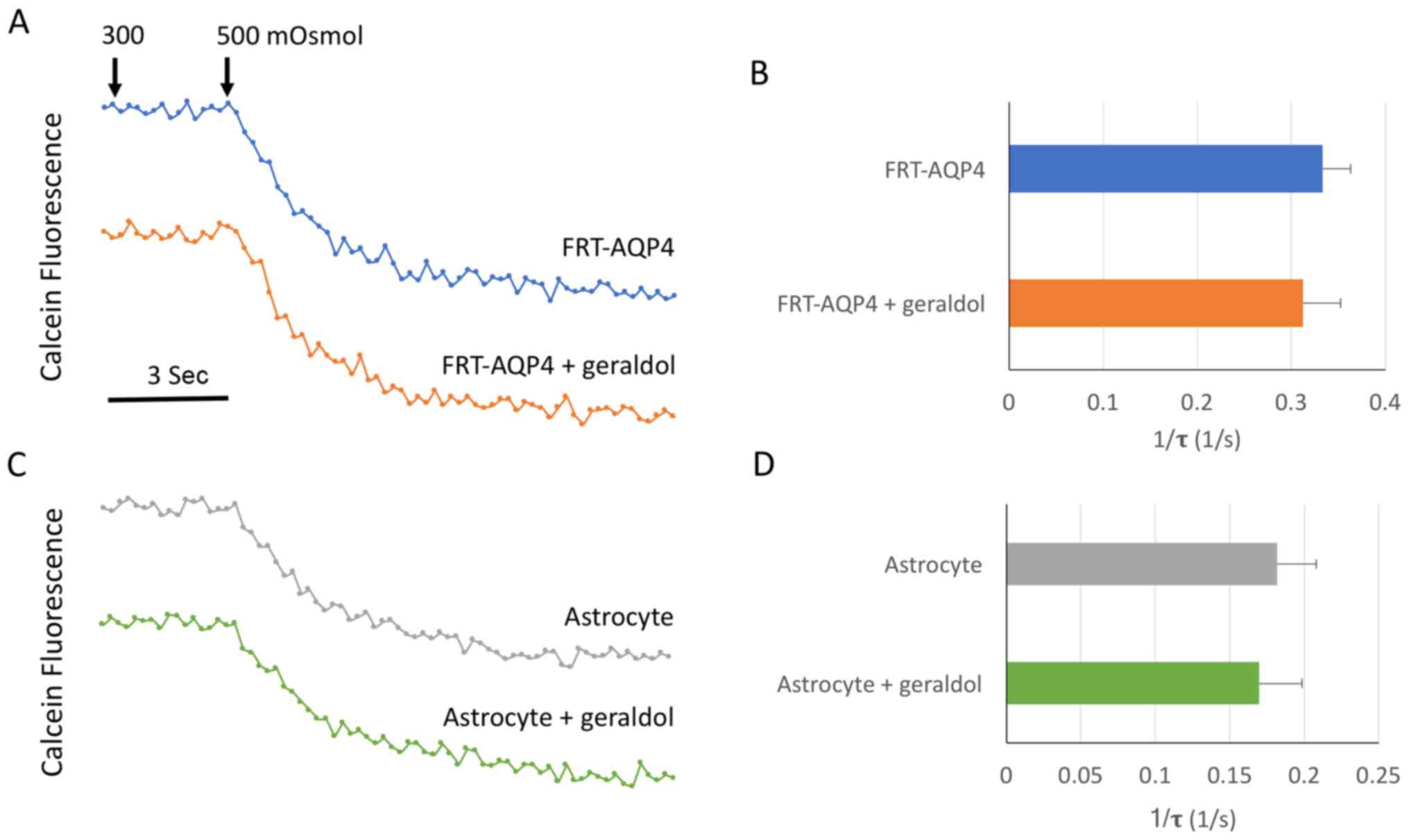
Geraldol does not affect the water
permeability of FRTL-AQP4 cells and primary astrocytes
Whether geraldol influences the osmotic water
permeability of AQP4 was further investigated in the present study.
Using a calcein-AM fluorescence quenching-based method as
previously described (31),
geraldol did not significantly influence osmotic water permeability
in either FRTL-AQP4 cells or astrocytes (Fig. 6).
Discussion
Serum NMO-IgG targets astrocytic AQP4, which is the
most highly expressed aquaporin in astrocytes (6,35–37).
NMO-IgG/AQP4 antibodies are present in up to 80% of patients with
NMO (6,38–40).
Although drugs and therapies are available for NMO, including
methylprednisolone, immunosuppression and plasma exchange, relapse
may still occur, making NMO an incurable disease (9–11).
Inhibitors of NMO-IgG binding to AQP4 are potential
therapeutic candidates for NMO. An ideal inhibitor should
effectively block binding of NMO-IgG to AQP4 without harming other
cells. In a previous study, inhibitors of NMO-IgG binding to AQP4
were identified (30). The
activity of isotetrandrine, an alkaloid compound purified from
fractions of Mahonia japonica, was demonstrated using a CDC
experiment. It was also demonstrated that isotetrandrine had low
cytotoxicity and did not affect the water transport function of
AQP4 (30). In the present study,
the inhibitory effect of a flavonoid compound (geraldol, purified
from a fraction of P. crispum) on NMO-IgG binding to AQP4
was examined.
P. crispum, commonly known as parsley, is a
culinary herb that was originally found in the Mediterranean
region, but now grown worldwide. Its main constituents include
coumarins, furanocoumarins (bergapten and imperatori), ascorbic
acid, carotenoids, flavonoids, apiole, various terpenoic compounds,
phenyl propanoids, phthalides and tocopherol (41,42).
P. crispum has been used in a number of different medicinal
applications, acting as an antimicrobial, antianemic,
anticoagulant, antihyperlipidemic, antihypertensive, diuretic,
hypoglycemic, hypouricemic, antioxidative and estrogenic agent
(43). Geraldol was purified from
P. crispum fractions via HPLC and it was demonstrated that
this molecule inhibited binding of NMO-IgG to AQP4.
Geraldol is a flavonoid. Flavonoid compounds have
broad biological properties, including antioxidative, free-radical
scavenging, as well as anti-inflammatory/infective effects
(44). These compounds usually
have low cytotoxicity, and thus, may be safe for potential
therapeutic use. Geraldol has been reported to show mild inhibitive
effects against the interaction of human papillomavirus-16-E6 with
caspase-8 (45). In addition,
geraldol is a 3′-methoxylated metabolite of the flavonoid fisetin,
which has distinct antioxidant, anti-inflammatory and
anti-angiogenic properties (46).
Geraldol blocked NMO-IgG binding to AQP4 in
immunofluorescence assays and reduced cytotoxicity in
NMO-IgG/complement-treated FRTL-AQP4 cells and primary astrocytes.
Furthermore, it did not affect the viability of FRTL-AQP4 cells and
primary astrocytes, or water permeability in either cell system.
These data suggest that geraldol is an effective inhibitor of
NMO-IgG binding to AQP4, and could be a candidate molecule for use
in NMO therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by he National
Natural Science Fund (grant no. 81771304), the National Natural
Science Youth Fund (grant no. 81601234 and 81601073), the Health
Special Fund of Jilin Province (grant no. SCZSY201616), Science
Technology Innovation Development Fund of Jilin (grant no.
201750246), Traditional Chinese Medicine Science and Technology
Fund of Jilin Province (grant no. 2019140) and Jilin Province
Health Research Talent Special Project Fund (grant
no.2019SCZ037).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and MS designed the experiments and analyzed the
experimental results. JW and SW carried out the experiments and
wrote the manuscript. HX, WL, DW, LZ YL, JC and FL also performed
the experiments, and helped write and review the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Protocols for mouse experiments were approved by The
Laboratory Animal Ethics Committee of School of Life Science, Jilin
University (Changchun, China; approval no. 2017-nsfc049). The use
of patient blood samples was approved by Ethics Committee of The
China-Japan Union Hospital, Jilin University (approval no.
2017022238). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cree BA, Goodin DS and Hauser SL:
Neuromyelitis optica. Semin Neurol. 22:105–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Seze J, Lebrun C, Stojkovic T, Ferriby
D, Chatel M and Vermersch P: Is Devic's neuromyelitis optica a
separate disease? A comparative study with multiple sclerosis. Mult
Scler. 9:521–525. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hinson SR, Pittock SJ, Lucchinetti CF,
Roemer SF, Fryer JP, Kryzer TJ and Lennon VA: Pathogenic potential
of IgG binding to water channel extracellular domain in
neuromyelitis optica. Neurology. 69:2221–2231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marignier R, Nicolle A, Watrin C, Touret
M, Cavagna S, Varrin-Doyer M, Cavillon G, Rogemond V, Confavreux C,
Honnorat J and Giraudon P: Oligodendrocytes are damaged by
neuromyelitis optica immunoglobulin G via astrocyte injury. Brain.
133:2578–2591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parratt JD and Prineas JW: Neuromyelitis
optica: A demyelinating disease characterized by acute destruction
an regeneration of perivascular astrocytes. Mult Scler.
16:1156–1172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paul F, Jarius S, Aktas O, Bluthner M,
Bauer O, Appelhans H, Franciotta D, Bergamaschi R, Littleton E,
Palace J, et al: Antibody to aquaporin 4 in the diagnosis of
neuromyelitis optica. PLoS Med. 4:e1332007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuoka T, Matsushita T, Kawano Y,
Osoegawa M, Ochi H, Ishizu T, Minohara M, Kikuchi H, Mihara F,
Ohyagi Y and Kira J: Heterogeneity of aquaporin-4 autoimmunity and
spinal cord lesions in multiple sclerosis in Japanese. Brain.
130:1206–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nicchia GP, Mastrototaro M, Rossi A,
Pisani F, Tortorella C, Ruggieri M, Lia A, Trojano M, Frigeri A and
Svelto M: Aquaporin-4 orthogonal arrays of particles are the target
for neuromyelitis optica autoantibodies. Glia. 57:1363–1373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trebst C, Berthele A, Jarius S, Trebst C,
Berthele A, Jarius S, Kümpfel T, Schippling S, Wildemann B and
Wilke C; Neuromyelitis optica Studiengruppe (NEMOS), : Diagnosis
and treatment of neuromyelitis optica. Consensus recommendations of
the neuromyelitis optica study group. Nervenarzt. 82:768–777.
2011.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wildemann B, Jarius S and Paul F:
Neuromyelitis optica. Nervenarzt. 84:436–441. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimbrough DJ, Fujihara K, Jacob A,
Lana-Peixoto MA, Leite MI, Levy M, Marignier R, Nakashima I, Palace
J, de Seze J, et al: Treatment of neuromyelitis optica: Review and
recommendations. Mult Scler Relat Disord. 1:180–187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarius S, Aboul-Enein F, Waters P, Kuenz
B, Hauser A, Berger T, Lang W, Reindl M, Vincent A and
Kristoferitsch W: Antibody to aquaporin-4 in the long-term course
of neuromyelitis optica. Brain. 131:3072–3080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bedi GS, Brown AD, Delgado SR, Usmani N,
Lam BL and Sheremata WA: Impact of rituximab on relapse rate and
disability in neuromyelitis optica. Mult Scler. 17:1225–1230. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SH, Kim W, Li XF, Jung IJ and Kim HJ:
Repeated treatment with rituximab based on the assessment of
peripheral circulating memory B cells in patients with relapsing
neuromyelitis optica over 2 years. Arch Neurol. 68:1412–1420. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenberg BM, Graves D, Remington G,
Hardeman P, Mann M, Karandikar N, Stuve O, Monson N and Frohman E:
Rituximab dosing and monitoring strategies in neuromyelitis optica
patients: Creating strategies for therapeutic success. Mult Scler.
18:1022–1026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pellkofer H, Suessmair C, Schulze A,
Hohlfeld R and Kuempfel T: Course of neuromyelitis optica during
inadvertent pregnancy in a patient treated with rituximab. Mult
Scler. 15:1006–1008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costanzi C, Matiello M, Lucchinetti CF,
Weinshenker BG, Pittock SJ, Mandrekar J, Thapa P and McKeon A:
Azathioprine: Tolerability, efficacy, and predictors of benefit in
neuromyelitis optica. Neurology. 77:659–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe S, Misu T, Miyazawa I, Nakashima
I, Shiga Y, Fujihara K and Itoyama Y: Low-dose corticosteroids
reduce relapses in neuromyelitis optica: A retrospective analysis.
Mult Scler. 13:968–974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bichuetti DB, Lobato de Oliveira EM,
Oliveira DM, Amorin de Souza N and Gabbai AA: Neuromyelitis optica
treatment: Analysis of 36 patients. Mult Scler. 67:1131–1136.
2010.
|
|
20
|
Mandler RN, Ahmed W and Dencoff JE:
Devic's neuromyelitis optica: A prospective study of seven patients
treated with prednisone and azathioprine. Neurology. 51:1219–1220.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacob A, Matiello M, Weinshenker BG,
Wingerchuk DM, Lucchinetti C, Shuster E, Carter J, Keegan BM,
Kantarci OH and Pittock SJ: Treatment of neuromyelitis optica with
mycophenolate mofetil: Retrospective analysis of 24 patients. Arch
Neurol. 66:1128–1133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitley J, Elsone L, George J, Waters P,
Woodhall M, Vincent A, Jacob A, Leite MI and Palace J: Methotrexate
is an alternative to azathioprine in neuromyelitis optica spectrum
disorders with aquaporin-4 antibodies. J Neurol Neurosurg
Psychiatry. 84:918–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Kim W, Park MS, Sohn EH, Li XF and
Kim HJ: Efficacy and safety of mitoxantrone in patients with highly
relapsing neuromyelitis optica. Arch Neurol. 68:473–479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weinstock-Guttman B, Ramanathan M, Lincoff
N, Napoli SQ, Sharma J, Feichter J and Bakshi R: Study of
mitoxantrone for the treatment of recurrent neuromyelitis optica
(Devic disease). Arch Neurol. 63:957–963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cabre P, Olindo S, Marignier R, Jeannin S,
Merle H and Smadja D; Aegis of French National Observatory of
Multiple Sclerosis, : Efficacy of mitoxantrone in neuromyelitis
optica spectrum: Clinical and neuroradiological study. J Neurol
Neurosurg Psychiatry. 84:511–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dörr J, Bitsch A, Schmailzl KJ, Chan A,
von Ahsen N, Hummel M, Varon R, Lill CM, Vogel HP, Zipp F and Paul
F: Severe cardiac failure in a patient with multiple sclerosis
following low-dose mitoxantrone treatment. Neurology. 73:991–993.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stroet A, Hemmelmann C, Starck M, Zettl U,
Dörr J, Friedemann P, Flachenecker P, Fleischer V, Zipp F, Nückel
H, et al: Incidence of therapy-related acute leukaemia in
mitoxantrone-treated multiple sclerosis patients in Germany. Ther
Adv Neurol Disord. 5:75–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stroet A, Gold R and Chan A: Acute myeloid
leukemia in Italian patients with multiple sclerosis treated with
mitoxantrone. Neurology. 78:933; author reply 933–934. 2012.
View Article : Google Scholar
|
|
29
|
Tradtrantip L, Zhang H, Saadoun S, Phuan
PW, Lam C, Papadopoulos MC, Bennett JL and Verkman AS: Anti-
aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis
optica. Ann Neurol. 71:314–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun M, Wang J, Zhou Y, Wang Z, Jiang Y and
Li M: Isotetrandrine reduces astrocyte cytotoxicity in
neuromyelitis optica by blocking the binding of NMO-IgG to
aquaporin 4. Neuroimmunomodulation. 23:98–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Solenov E, Watanabe H, Manley GT and
Verkman AS: Sevenfold-reduced osmotic water permeability in primary
astrocyte cultures from AQP-4-deficient mice, measured by a
fluorescence quenching method. Am J Physiol Cell Physiol.
286:C426–C432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Su W, Wang J, Pisani F, Frigeri A
and Ma T: Detection of anti-aquaporin-4 autoantibodies in the sera
of Chinese neuromyelitis optica patients. Neural Regen Res.
8:708–713. 2013.PubMed/NCBI
|
|
33
|
Khan M, Yu B, Rasul A, Al Shawi A, Yi F,
Yang H and Ma T: Jaceosidin induces apoptosis in U87 glioblastoma
cells through G2/M phase arrest. Evid Based Complement Alternat
Med. 2012:7030342012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Swanson RA, Liu J, Miller JW, Rothstein
JD, Farrell K, Stein BA and Longuemare MC: Neuronal regulation of
glutamate transporter subtype expression in astrocytes. J Neurosci.
17:932–940. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lennon VA, Kryzer TJ, Pittock SJ, Verkman
AS and Hinson SR: IgG marker of optic-spinal multiple sclerosis
binds to the aquaporin-4 water channel. J Exp Med. 202:473–477.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lennon VA, Wingerchuk DM, Kryzer TJ,
Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I and Weinshenker
BG: A serum autoantibody marker of neuromyelitis optica:
Distinction from multiple sclerosis. Lancet. 364:2106–2112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jarius S, Franciotta D, Bergamaschi R,
Wright H, Littleton E, Palace J, Hohlfeld R and Vincent A: NMO-IgG
in the diagnosis of neuromyelitis optica. Neurology. 68:1076–1077.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jarius S, Probst C, Borowski K, Franciotta
D, Wildemann B, Stoecker W and Wandinger KP: Standardized method
for the detection of antibodies to aquaporin-4 based on a highly
sensitive immunofluorescence assay employing recombinant target
antigen. J Neurol Sci. 291:52–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jarius S, Franciotta D, Paul F,
Bergamaschi R, Rommer PS, Ruprecht K, Ringelstein M, Aktas O,
Kristoferitsch W and Wildemann B: Testing for antibodies to human
aquaporin-4 by ELISA: Sensitivity, specificity, and direct
comparison with immunohistochemistry. J Neurol Sci. 320:32–37.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Waters P, Jarius S, Littleton E, Leite MI,
Jacob S, Gray B, Geraldes R, Vale T, Jacob A, Palace J, et al:
Aquaporin-4 antibodies in neuromyelitis optica and longitudinally
extensive transverse myelitis. Arch Neurol. 65:913–919. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Snoussi M, Dehmani A, Noumi E, Flamini G
and Papetti A: Chemical composition and antibiofilm activity of
Petroselinum crispum and Ocimum basilicum essential
oils against Vibrio spp. strains. Microb Pathog. 90:13–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chaudhary SK, Ceska O, Têtu C, Warrington
PJ, Ashwood- Smith MJ and Poulton GA: Oxypeucedanin, a major
furocoumarin in parsley, Petroselinum crispum. Planta Med.
52:462–464. 1986. View Article : Google Scholar
|
|
43
|
Mahmood S, Hussain S and Malik F: Critique
of medicinal conspicuousness of parsley (Petroselinum
crispum): A culinary herb of mediterranean region. Pak J Pharm
Sci. 27:193–202. 2014.PubMed/NCBI
|
|
44
|
Seelinger G, Merfort I and Schempp CM:
Anti-oxidant, anti-inflammatory and anti-allergic activities of
luteolin. Planta Med. 74:1667–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yuan CH, Filippova M, Tungteakkhun SS,
Duerksen-Hughes PJ and Krstenansky JL: Small molecule inhibitors of
the HPV16-E6 interaction with caspase 8. Bioorg Med Chem Lett.
22:2125–2129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jo JH, Jo JJ, Lee JM and Lee S:
Identification of absolute conversion to geraldol from fisetin and
pharmacokinetics in mouse. J Chromatogr B Analyt Technol Biomed
Life Sci. 1038:95–100. 2016. View Article : Google Scholar : PubMed/NCBI
|