Introduction
Non-small-cell lung cancer (NSCLC) is one of the
most fatal cancer types worldwide, accounting for ~80% of all
primary lung cancer types (1).
Statistically, 70–80% of patients with NSCLC will progress to an
advanced disease state once diagnosed (2). With regards to the current treatment
options, intensive chemotherapy combined with aggressive surgical
techniques have demonstrated significant successes; however, the
prognosis of patients with NSCLC remains disappointing due to the
chemoresistance, invasion and metastasis of the tumors (3,4).
Thus, there is an urgent requirement to develop novel
chemotherapies for the treatment of NSCLC.
Curcumin is a naturally active phenolic compound
extracted from the rhizome of the plant Curcuma longa, which
is used as a traditional Chinese medicine (5). Curcumin has become an increasingly
popular compound of interest, both in research and clinically,
among different types of cancer, including lung adenocarcinoma, a
type of NSCLC (6–8). It is reported to have anticancer
properties that are exerted by affecting multiple cellular
processes, such as cell proliferation, invasion, metastasis and
chemoresistance (9); however, the
mechanisms of action of curcumin in NSCLC remain largely
unknown.
MicroRNAs (miRNAs/miRs) are a class of endogenous
small non-coding RNAs of ~22 nucleotides in length that interact
with mRNAs, which leads to mRNA degradation or the inhibition of
translation (10). Functionally,
miRNAs are involved in diverse cellular functions, such as growth,
proliferation, apoptosis and migration (11). Accumulating evidence has
demonstrated that curcumin may represent a novel strategy for
cancer treatment by serving as an epigenetic regulator; initially,
curcumin was identified to exert epigenetic activity over miRNAs in
pancreatic cancer (12), and since
this, other studies have also reported an association between the
pharmacological effect of curcumin and miRNAs in lung cancer
(10). For example, Ye et
al (13) discovered that
miR-192-5p and miR-215 were involved in the proapoptotic effects of
curcumin, whereas Jin et al (14) also suggested that the
antiproliferative role of curcumin in human NSCLCs depended on the
increased expression levels of miR-192-5p and the inactivation of
the PI3K/AKT signaling pathway. However, apart from these studies,
to the best of our knowledge, there are very few studies into the
role of miR-192-5p in NSCLC progression, especially in processes
such as migration and invasion. In addition, the underlying
regulatory mechanism of curcumin in NSCLC remains to be fully
investigated.
The Wnt/β-catenin signaling pathway is one of the
main and most frequently dysregulated pathways in several types of
tumor, and it is suggested that altered cell proliferation,
invasive behaviors and cell resistance are all attributed to the
dysregulation of this signaling pathway (15,16).
Notably, several studies have reported that curcumin affects cell
proliferation through the Wnt/β-catenin signaling pathway in
multiple different types of cancer, including both colon cancer and
medulloblastoma (17,18). In fact, in lung cancer, this
signaling pathway has been demonstrated to participate in the
anticancer role of curcumin in tumor cell growth, invasion,
epithelial-mesenchymal transition and cancer stem cells traits
(15,16,19,20).
In addition, the involvement of miR-192-5p and the Wnt/β-catenin
signaling pathway is noted in several types of tumor (21–23),
excluding lung cancer; however, little is known regarding the
interaction between curcumin, miR-192-5p and the Wnt/β-catenin
signaling pathway in NSCLC.
In the present study, the expression levels of
miR-192-5p and c-Myc, an important oncogene, were investigated in
NSCLC cells following curcumin treatment. In addition, functional
and mechanistic assays were used to investigate the effect of
miR-192-5p dysregulation on curcumin-mediated cell proliferation,
invasion, migration and the Wnt/β-catenin signaling pathway in
NSCLC cells. In short, this study suggested a novel molecular
mechanism for curcumin in NSCLC.
Materials and methods
Cell culture and reagents
The human NSCLC cell lines, A427 and A549, and the
human embryonic kidney cell line 293T were purchased from the
American Type Culture Collection. The NSCLC cells were cultured in
RPMI-1640 medium (HyClone; GE Healthcare Life Sciences) and 293T
cells were maintained in DMEM (HyClone; GE Healthcare Life
Sciences). Both mediums were supplemented with 10% FBS (HyClone; GE
Healthcare Life Sciences) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) and cells were then
maintained in a humidified atmosphere of 5% CO2 at
37°C.
Curcumin treatment
Curcumin (100 mM in DMSO) was purchased from
Sigma-Aldrich (Merck KGaA). The working concentration of curcumin
was 10, 20 or 40 µM diluted in culture medium. Cells were cultured
in 6-well or 96-well plates (Corning, Inc.) and exposed to 10–40 µM
curcumin for 48 h at 37°C. The control group was treated with 0 µM
of curcumin diluted in 0.1% DMSO (24). The 20 µM curcumin concentration was
selected to treat A427 and A549 cells for further analysis.
Cell transfection
The hsa-miR-192-5p mimic
(5′-CUGACCUAUGAAUUGACAGCC-3′) and miR-negative control (NC) mimic
(5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from Shanghai
GenePharma Co., Ltd. The pcDNA3.1 (pcDNA; Invitrogen; Thermo Fisher
Scientific, Inc.) plasmid was used to construct the c-Myc
overexpression vector (pcDNA-c-Myc). Small interfering (si)RNA
against c-Myc (si-c-Myc; 5′-CCTGAGACAGATCAGCAACAA-3′) and its NC
(si-NC; 5′-TTCTCCGAACGTGTCACGT-3′), and the hsa-miR-192-5p
inhibitor (in-miR-192-5p; 5′-GGCUGUCAAUUCAUAGGUCAG-3′) and miR-NC
inhibitor (in-miR-NC; 5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased
from Shanghai GenePharma Co., Ltd. Cells at 70% confluency were
cultured in 6-well plates (Corning, Inc.) and the transfection of
plasmids (2 µg) and oligonucleotides (40 nM) was performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
were transfected for 48 h at 37°C prior to further studies.
MTT staining for cell viability
Cell viability was determined using an MTT reagent
(Sangon Biotech Co., Ltd.). Following curcumin treatment,
5×10−3 mg/ml MTT was added to each well and incubated
for 4 h at 37°C. The culture medium containing MTT was discarded
with micropipettor and formazan crystals were dissolved in 100 µl
dimethyl sulfoxide. The optical density (OD) was measured at 490 nm
using a SpectraMax M4 microplate reader (Molecular Devices, LLC).
Cell viability (%) was calculated using the following formula: OD
value of treated group/OD value of control group ×100. All
experiments were performed in quadruplicate.
Cell Counting Kit-8 (CCK-8) assay for
cell proliferation
The proliferative ability of the NSCLC cells was
determined using a CCK-8 assay (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Briefly,
1×104 A427 and A549 cells/well were plated into 96-well
plates (Corning, Inc.) for 24 h at 37°C. Subsequently, 20 µl CCK-8
solution (5 mg/ml) diluted in PBS was added to each well for
another 1 h and the absorbance was measured at 450 nm using a
SpectraMax M4 microplate reader (Molecular Devices, LLC). Results
were expressed as a percentage relative to the control group
(defined as 100%). Data are from ≥5 independent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using a PrimeScript™ RT reagent kit
(Takara Bio, Inc.) according to the manufacturer's instuctions. The
temperature conditions were as followed: 25°C for 10 min, 42°C for
15 min and 85°C for 5 min. qPCR was subsequently performed using a
SYBR Green PCR Master mix (Takara Bio, Inc.) and a TaqMan miRNA
assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol to amplify mRNAs and
miRNAs, respectively, on an ABI PRISM 7500 Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation was
95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and 60°C
for 30 sec, and the melting curve generation condition was 95°C for
15 sec, 60°C for 60 sec and 95°C for 15 sec. The following primer
pairs were used for the qPCR: c-Myc forward,
5′-GGCTCCTGGCAAAAGGTCA-3′ and reverse, 5′-CTGCGTAGTTGTGCTGATGT-3′;
miR-192-5p forward, 5′-GGACTTTCTTCATTCACACCG-3′ and reverse,
5′-GACCACTGAGGTTAGAGCCA-3′; GAPDH forward,
5′-CGAGCCACATCGCTCAGACA-3′ and reverse, 5′-GTGGTGAAGACGCCAGTGGA-3′;
and U6 forward, 5′-TCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. Expression levels were quantified
using the 2−ΔΔCq method (25) and miRNA was normalized to U6
(miRNA) and GAPDH (mRNA). The reactions were performed in
quadruplicate for each sample, with ≥3 independent runs.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Total protein
was quantified with Bradford method and 20 µg protein/lane was
separated via 10–12% SDS-PAGE, and then transferred onto PVDF
membranes. After blocking with 5% skimmed milk for 2 h at 25°C, the
membranes were incubated with the following primary antibodies (all
Abcam): Anti-c-Myc (1:1,000; cat. no. ab32072), anti-β-catenin
(1:4,000; cat. no. ab6302), anti-cyclin D1 (1:5,000; cat. no.
ab40754) and anti-β-actin (1:5,000; cat. no. ab8227) for overnight
at 4°C. After incubation with horseradish peroxidase-conjugated
secondary antibody anti-Rabbit (1:50,000; Abcam; cat. no. ab205718)
for 1 h at 25°C, the protein bands were visualized using an ECL
reagent (EMD Millipore). Protein expression was normalized to the
loading control β-actin on Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.).
Computational prediction
TargetScanHuman 7.1 database (http://www.targetscan.org/ENST00000377970.2) was
used to predict the potential miRNAs targeting Myc gene on the
3′untranslated region (c-Myc 3′UTR). The in silico data
provided a potential binding site between miR-192-5p and c-Myc
3′UTR on position 738–744.
Dual-luciferase reporter assay
The pMIR-REPORT Luciferase miRNA Expression Reporter
Vector and pMIR-REPORT β-galactosidase Reporter Control Vector,
were purchased from Invitrogen; Thermo Fisher Scientific, Inc. The
putative binding site of miR-192-5p in c-Myc 3′UTR was mutated. The
wild-type (WT) of c-Myc 3′UTR fragment (c-Myc-3′UTR-WT) and the
mutant type (MUT) of c-Myc 3′UTR fragment (c-Myc-3′UTR-MUT) were
cloned into the pMIR-REPORT miRNA Expression Reporter Vector,
respectively. 293T cells at 60% confluency were co-transfected with
100 ng pMIR-REPORT-c-Myc-WT/MUT vectors, pMIR-REPORT
β-galactosidase vectors and 50 nM miR-192-5p mimic/NC mimic using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following incubation for 48 h at 37°C, transfected 293T cells were
collected and the firefly luciferase and β-galactosidase activities
were detected using a Dual-Luciferase Reporter assay system
(Promega Corporation) according to the manufacturer's protocol.
Firefly luciferase activity was normalized to β-galactosidase
expression levels. Data are presented as the mean of ≥3 independent
transfections.
RNA immunoprecipitation (RIP)
Following the transfection of miR-192-5p mimic or
miR-NC, RIP was performed in cell extracts in RIPA lysis buffer
(Beyotime Institute of Biotechnology). A Magna RIP™ RNA-binding
protein immunoprecipitation kit (EMD Millipore) was used according
to the manufacturer's protocol to obtain the RIP that bound to
argonaute 2 (Ago2; Abcam; 1:20; cat. no. ab32381) or IgG (1:50;
Abcam; cat. no. ab2410) antibody. Then, RT-qPCR was performed to
detect the expression levels of c-Myc mRNA in RIP.
Transwell assays
For the migration and invasion assays, A427 and A549
cells (5×105) were suspended in 200 µl RPMI-1640 medium
without FBS and plated in the upper chambers of Transwell plates
(Corning, Inc.) without or with Matrigel (Corning, Inc.) for
migration and invasion, respectively. The Matrigel membrane was
established using Matrigel (1:8) after air drying for 16 h at 4°C.
RPMI-1640 medium supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) was plated in the lower chambers. Following
incubation at 37°C for 48 h, the migratory and invasive cells in
the lower chambers were stained with 0.2% crystal violet for 15 min
at 25°C. Absorbance was measured at a wavelength of 570 nm using a
microplate reader and the migratory/invasive ability was presented
as a percentage of the control group.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc.) and data are presented
as the mean ± SD. Statistical differences between groups were
determined using a one-way ANOVA with Tukey's post hoc analysis.
P<0.05 was considered to indicate a statistically significant
result.
Results
Curcumin treatment suppresses NSCLC
cell progression in vitro
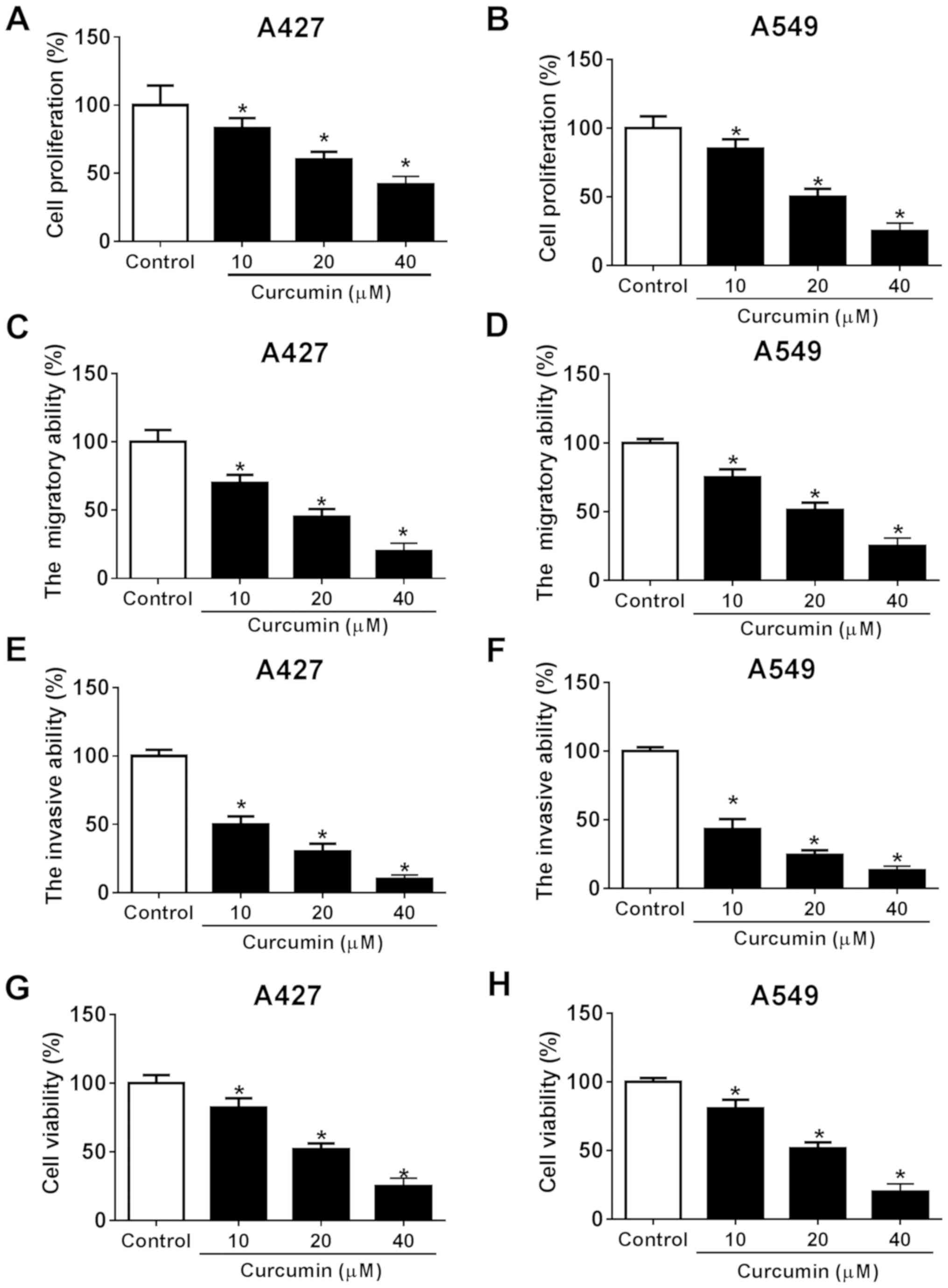
Following the exposure of NSCLC A427 and A549 cells
to different concentrations of curcumin (10, 20 and 40 µM), the
tumor-suppressive role of curcumin in NSCLC in vitro was
investigated. Cellular proliferation was significantly decreased in
both cell lines following 10, 20 and 40 µM curcumin treatment
compared with the control cells (Fig.
1A and B), which was consistent with the cell viability status
(Fig. 1G and H). In addition, the
migratory ability of NSCLC cells was determined; the migratory
ability of both cell lines was significantly attenuated by 10, 20
and 40 µM of curcumin compared with the control (Fig. 1C and D). Curcumin was also found to
significantly reduce the invasive ability of both cell lines at all
concentrations (10, 20 and 40 µM) compared with the control
(Fig. 1E and F). Of note, the
effects of curcumin on NSCLCs were all observed to occur in a
dose-dependent manner. These results suggested that curcumin
treatment may promote NSCLC cell cytotoxicity and suppress cell
migration and invasion, leading to the antitumor effect in NSCLC
in vitro.
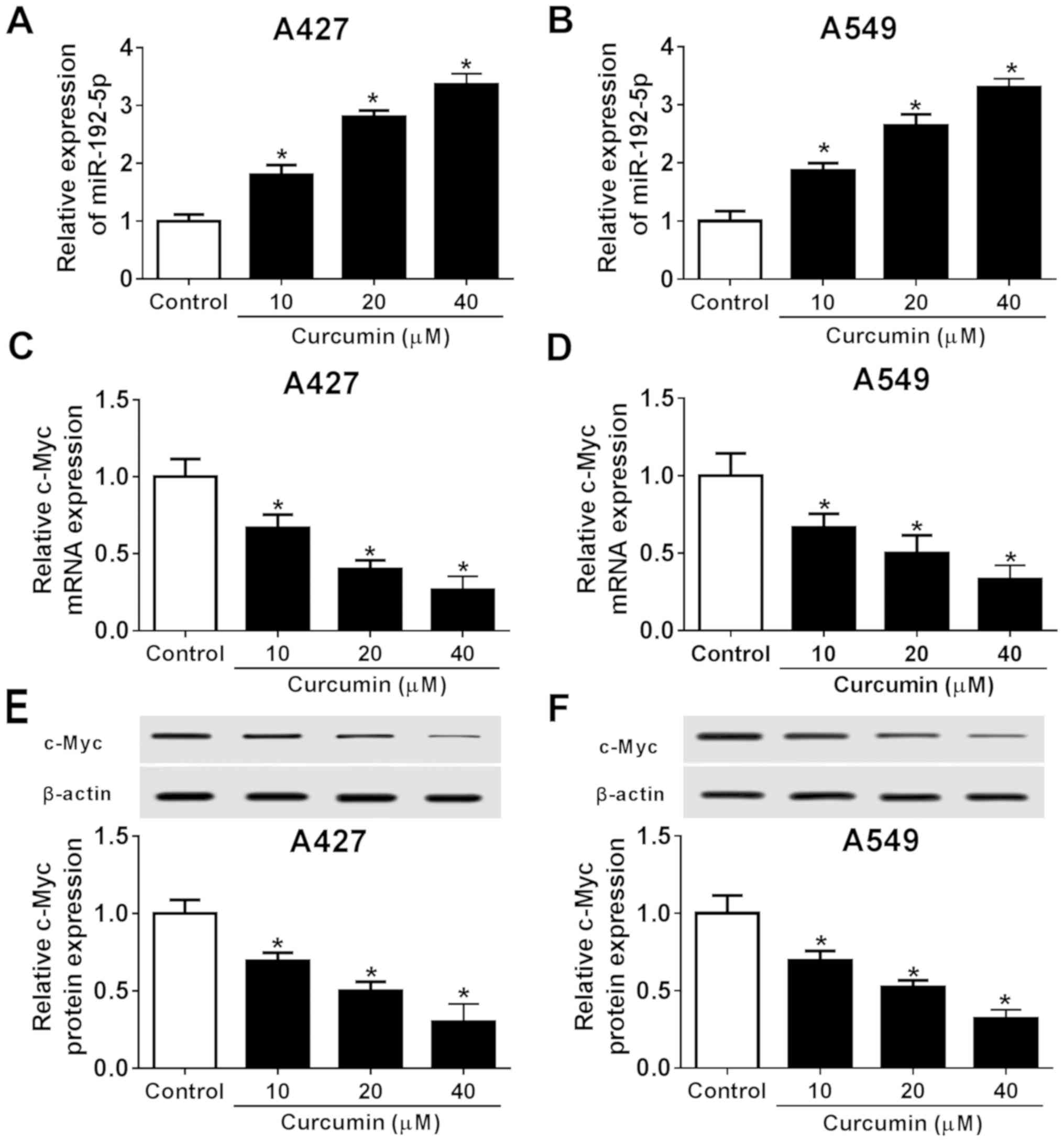
Expression levels of miR-192-5p and
c-Myc are altered following curcumin treatment in NSCLC
To determine the potential association between
miR-192-5p and c-Myc in NSCLC, RT-qPCR and western blotting were
used to quantify their expression levels in both A427 and A549
cells exposed to different concentrations of curcumin (10, 20 and
40 µM). Curcumin treatment led to a significant
concentration-dependent increase in miR-192-5p expression levels
compared with the control (Fig. 2A and
B), whereas the expression levels of c-Myc at both the mRNA
(Fig. 2C and D) and protein level
(Fig. 2E and F) were significantly
decreased in a dose-dependent manner in both cell lines compared
with the control. These findings suggested that miR-192-5p
expression was increased and c-Myc expression was decreased in
NSCLC cells following curcumin exposure, which may contribute to
curcumin-induced NSCLC cell cytotoxicity and suppression of cell
migration and invasion. Subsequently, the role of miR-192-5p and
c-Myc in the role of curcumin in NSCLC cells were further
investigated, and 20 µM of curcumin was used (Figs. 1 and 2).
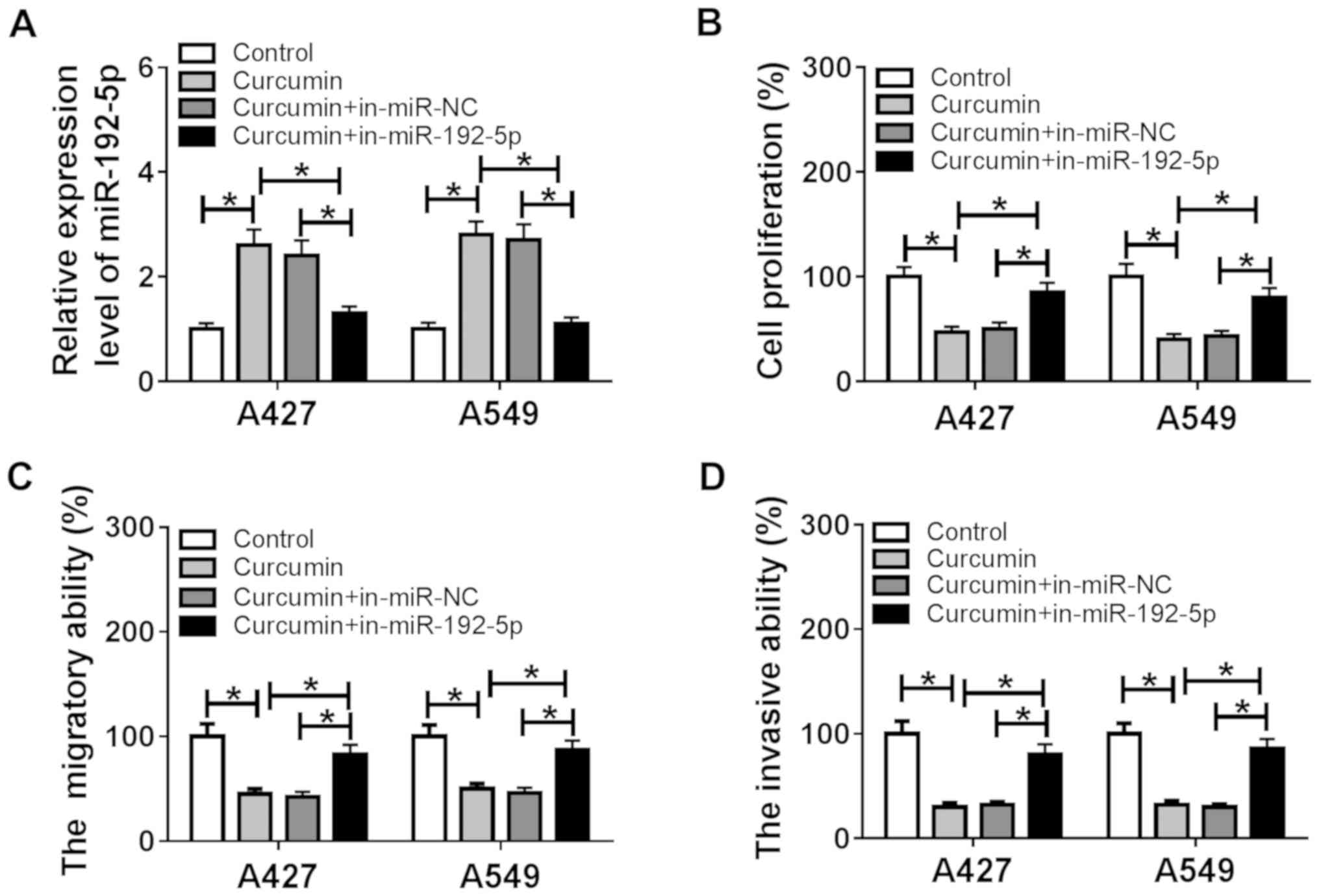
miR-192-5p knockdown reverses the
inhibitory effects of curcumin on NSCLC cell proliferation,
migration and invasion
The dysregulation of miR-192-5p expression following
curcumin treatment was confirmed in the human NSCLC cell lines,
A427 and A549. Thus, the functions of miR-192-5p following its
knockdown by transfection in curcumin-induced anticancer activity
were further investigated. Following the transient transfection of
the in-miR-192-5p into A427 and A549 cells, a significant decrease
was observed in miR-192-5p expression levels in A427 and A549 cells
compared with the in-miR-NC-transfected cells, as indicated by
RT-qPCR (Fig. S1A). The increased
miR-192-5p expression levels induced by curcumin were impaired
following the exogenous administration of in-miR-192-5p (Fig. 3A). In addition, following 20 µM
curcumin treatment, in-miR-192-5p-transfected cells demonstrated
significantly increased proliferation rates in A427 and A549 cells
compared with the curcumin + in-miR-NC group (Fig. 3B). The curcumin induced a decrease
in cell migratory and invasive abilities in A427 and A549 cells,
which was significantly reversed by miR-192-5p inhibition (Fig. 3C and D). These results indicated
that the beneficial effects of curcumin were partially abolished by
miR-192-5p knockdown, suggesting that miR-192-5p upregulation may
be behind the underlying anticancer role of curcumin in NSCLC.
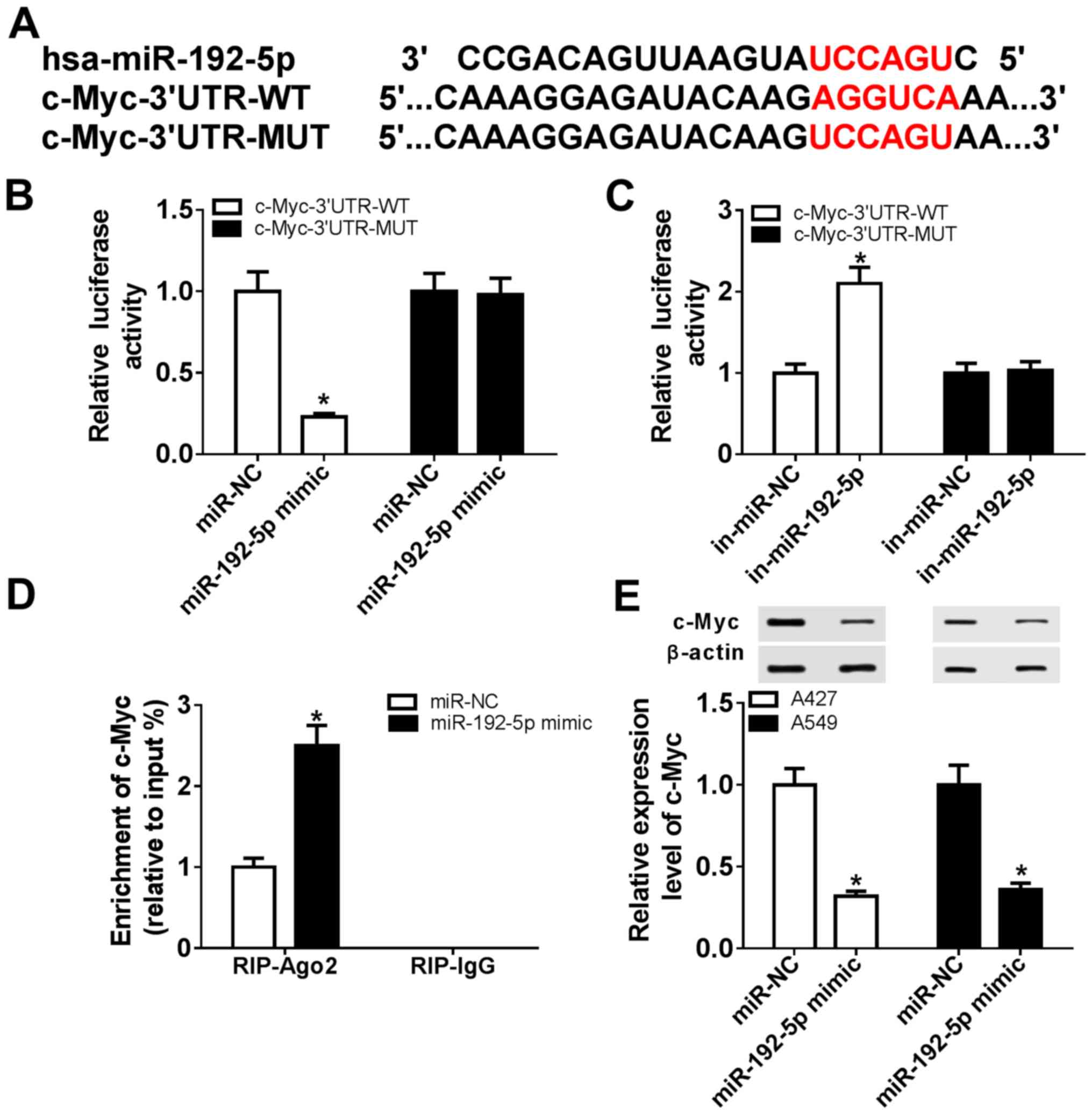
c-Myc is a direct target of
miR-192-5p
Accumulating evidence has reported that c-Myc
undertakes important roles in the numerous biological functions of
miRNAs in NSCLC (26–28); however, the regulatory effect of
miR-192-5p on c-Myc remains largely unknown. In this study, a
potential binding site between miR-192-5p and c-Myc was identified
using computational prediction on the TargetScanHuman database
(Fig. 4A). Then, c-Myc-WT and
c-Myc-MUT 3′UTRs were cloned into pMIR vectors, and a
dual-luciferase reporter assay was used to demonstrate that the
relative luciferase activity of c-Myc-3′UTR-WT in 293T cells was
significantly reduced following co-transfection with miR-192-5p
mimic compared with the miR-NC group (Fig. 4B), whereas the relative luciferase
activity was significantly increased following co-transfection with
in-miR-192-5p compared with the in-miR-NC group (Fig. 4C). The miR-192-5p mimic
transfection was also validated; a significant increase in
miR-192-5p expression levels was observed in A427 and A549 cells in
the miR-192-5p mimic-transfected cells compared with the miR-NC
group, as indicated by RT-qPCR (Fig.
S1B). However, there were no significant differences in
c-Myc-3′UTR-MUT-transfected cells between the NCs- and
inhibitor/mimic-transfected cells (Fig. 4B and C). A RIP assay further
validated the existence of a binding site between miR-192-5p and
c-Myc (Fig. 4D), and western
blotting demonstrated that c-Myc expression was significantly
inhibited by the miR-192-5p mimic in A427 and A549 cells compared
with the miR-NC group (Fig. 4E).
These results suggested that miR-192-5p may negatively regulate
c-Myc expression through target binding.
Curcumin exerts its anticancer role
through the miR-192-5p/c- Myc axis in NSCLC in vitro
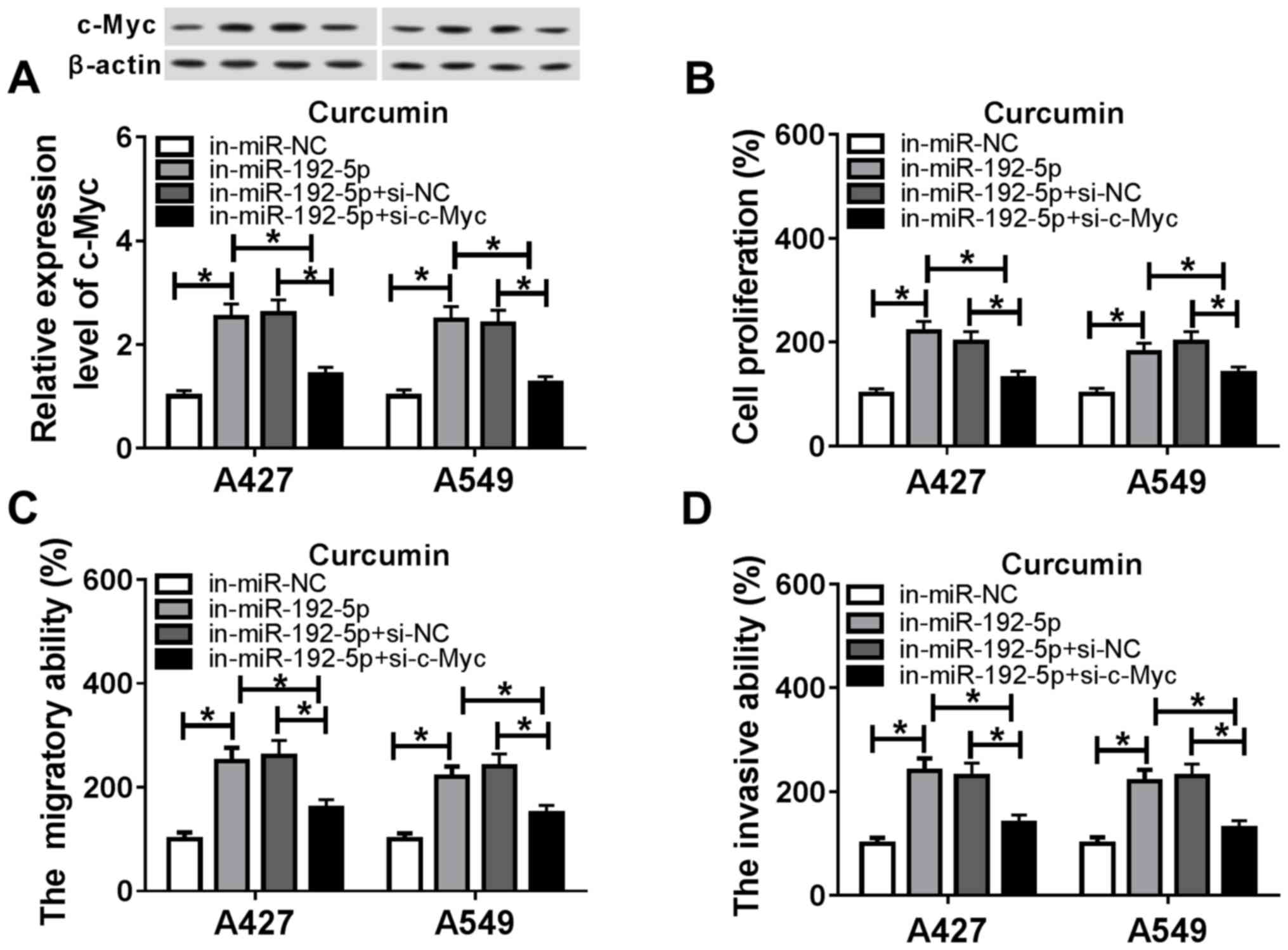
Rescue experiments were used to clarify the role of
c-Myc in mediating the biological action of miR-192-5p in A427 and
A549 cells treated with curcumin. The transfection of in-miR-192-5p
in both cell lines under curcumin treatment significantly increased
the expression levels of c-Myc compared with the in-miR-NC group
(Fig. 5A), whereas the
co-transfection of in-miR-192-5p and si-c-Myc resulted in a
significant decrease in c-Myc expression levels compared with the
in-miR-192-5p + si-NC group. In addition, in cells transfected with
in-miR-192-5p, cell proliferation was significantly increased
compared with the in-miR-NC group (Fig. 5B); however, in combination with
si-c-Myc, this increase was subsequently blocked (Fig. 5B). The downregulation of c-Myc
expression in in-miR-192-5p-transfected cells also partly abolished
the promotive effects of in-miR-192-5p transfection on cell
migration and invasion (Fig. 5C and
D). The transfection efficiency of si-c-Myc was assessed using
RT-qPCR and western blotting (Fig.
S1C and D). Moreover, pcDNA-c-Myc resulted in the
overexpression of c-Myc (Fig. S1E and
F), and this transfection also counteracted the suppressive
effect of miR-192-5p overexpression on c-Myc expression and the
abilities of cell proliferation, migration and invasion in
curcumin-treated cells (Fig.
S2A-D). These results suggested that the miR-192-5p/c-Myc axis
may mediate the anticancer effects of curcumin in NSCLC.
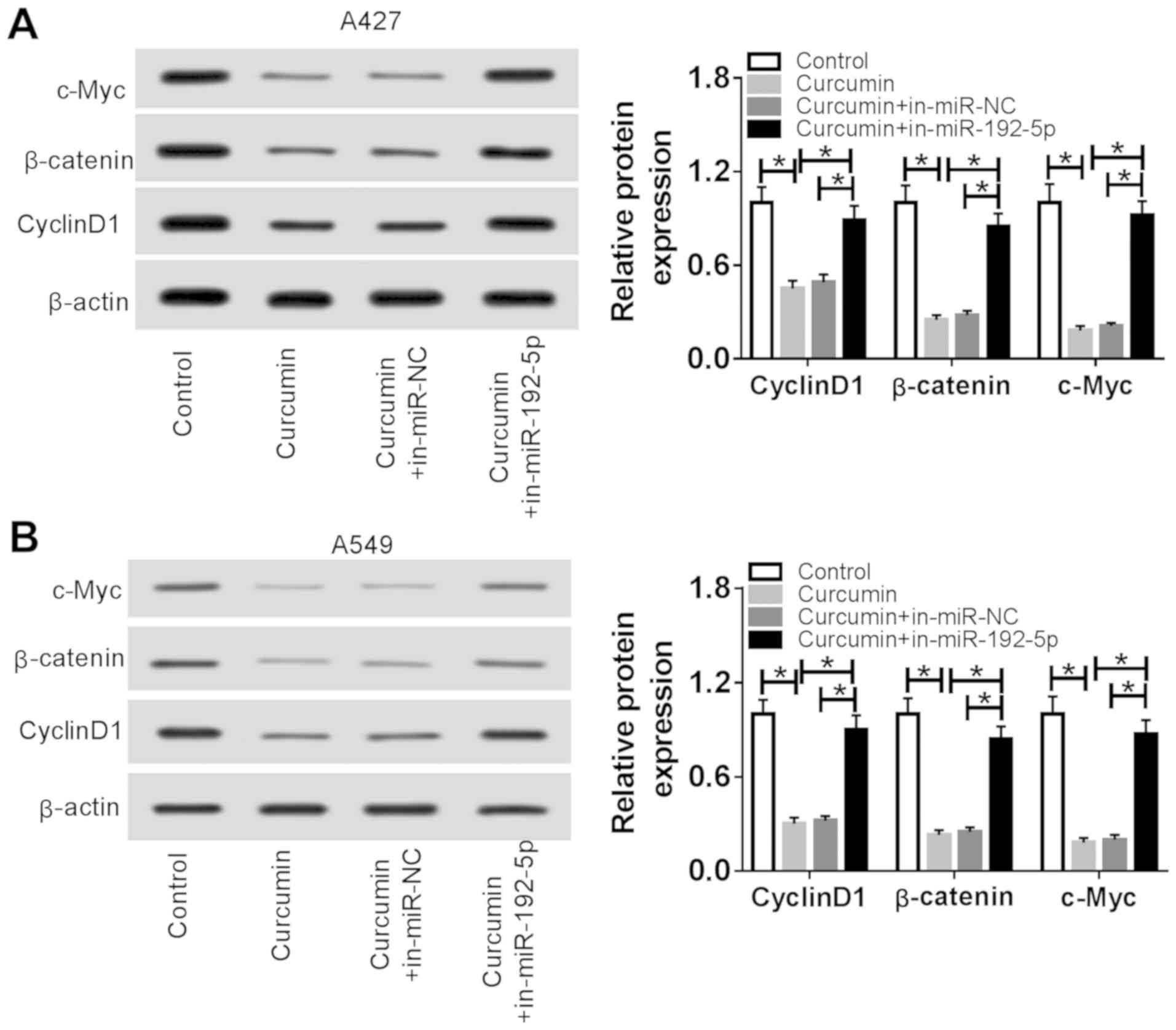
Curcumin inactivates the Wnt/β-catenin
signaling pathway by upregulating miR-192-5p expression in NSCLC
cells in vitro
To determine whether curcumin affected the
Wnt/β-catenin signaling pathway through miR-192-5p, the expression
of Wnt/β-catenin signaling pathway-related proteins was detected in
A427 and A549 cells transfected with in-miR-192-5p followed by
treatment with 20 µM curcumin for 48 h using western blotting. The
expression levels of β-catenin, cyclin D1 and c-Myc were
significantly reduced by curcumin treatment alone compared with the
control group (Fig. 6A and B);
however, these decreased expression levels were subsequently
significantly reversed in cells treated with curcumin and
transfected with in-miR-192-5p compared with the curcumin +
in-miR-NC group. These findings suggested that the curcumin-induced
inactivation of the Wnt/β-catenin signaling pathway in NSCLC may
depend on the high expression levels of miR-192-5p.
Discussion
Curcumin, isolated from the rhizomes of the plant
Curcuma longa, has been reported to exhibit various
anti-inflammatory, antiangiogenic, antiproliferative and
antioxidant properties (29,30).
It has been suggested that curcumin may induce apoptosis in
malignant cells, and therefore, it demonstrates great potential as
a cancer treatment (31). In
addition, several studies have observed that curcumin exerts growth
suppressive effects in both small cell lung cancer and NSCLC cells
(32,33); however, the effect of curcumin in
NSCLC, in addition to its associated mechanism of action, remain
poorly understood. The present study confirmed the cytotoxicity of
curcumin in human NSCLC cell lines A427 and A549, and curcumin was
found to inhibit cell proliferation, migration and invasion in a
dose-dependent manner. Moreover, the expression levels of
miR-192-5p and c-Myc were discovered to be increased and decreased,
respectively, following curcumin exposure, which suggested that
miR-192-5p upregulation may be an important event for the
curcumin-mediated antiproliferative, antimigratory and
anti-invasive effects.
The close interaction between miRNAs and the
anti-lung cancer role of curcumin has been previously reported. For
example, Zhang et al (34,35)
demonstrated that curcumin promoted apoptosis in
cisplatin-resistant A549 cells through miRNA signaling pathways,
including the downregulation of miR-186 expression. Zhan et
al (36) constructed a miRNA
gene network that is attributed to the antimetastatic role of
curcumin in A549 cells, such as let-7a-3p, miR-1262 and miR-330-5p.
Similarly, Jiao et al (37)
identified that curcumin induces metastasis inhibition depending on
a miRNA/transcription factor/target gene network, including the
miR-34a-5p/miR-34c-5p/miR-302b-3p/lymphoid enhancer-binding factor
1/cyclin D1/Wnt1/c-Myc axis. Moreover, curcumin has also been found
to be effective in increasing paclitaxel sensitivity in cancer stem
cells, which may occur by interacting with miRNAs (38,39).
In the present study, the increased expression levels of miR-192-5p
initiated the antiproliferative, antimigratory and anti-invasive
roles of curcumin in A549 and A427 cells by targeting c-Myc
expression. Taken together, these results suggested that curcumin
could be an epigenetic agent that could provide a new therapeutic
strategy for cancer treatment, and the miRNAs/genes network,
including the miR-192-5p/c-Myc axis, may serve a vital role in the
anticancer activity of curcumin in lung cancer.
Previous studies have shown that the increased
expression levels of miR-192-5p contribute to curcumin-induced
antitumor effects in NSCLC (13,14).
To date, the tumor-suppressive activities of curcumin have mainly
focused on its ability to promote cell apoptosis, and enhance
chemo- and radiosensitization, in addition to its ability to
inhibit cell proliferation and migration in lung cancer cells, and
modulate miRNAs (11,40). For example, antagonizing miR-192-5p
was found to attenuate curcumin-stimulated apoptosis promotion in
H460, A427 and A549 cells through the p53/miR-192-5p/X-linked
inhibitor of apoptosis protein axis (13), of which miR-192-5p was identified
as one of the most responsive miRNAs following curcumin treatment.
Another study reported that the ectopic expression of miR-192-5p
enhanced the effects of curcumin on the inhibition of cell
viability and the promotion of apoptosis in A549 cells through the
PI3K/AKT signaling pathway (14).
Nonetheless, to the best of our knowledge, numerous roles of
miR-192-5p exist in NSCLC progression, which have not been
investigated in detail, such as migration and invasion. The present
study suggested that the upregulation of miR-192-5p contributed to
the curcumin-induced antiproliferative effects in both A549 and
A427 cells. Furthermore, the migratory and invasive abilities of
the cells were also significantly inhibited by curcumin treatment,
whereas miR-192-5p inhibition could partially reverse the
curcumin-induced antimigration and anti-invasion effects through
inactivation of the Wnt/β-catenin signaling pathway by decreasing
the expression levels of β-catenin, cyclin D1 and c-Myc.
As a well-documented oncogene, c-Myc has been known
to be extensively involved in cell cycle progression and apoptosis
(41). The dysregulation of this
gene has been associated with multiple types of cancer, including
NSCLC (42): For example, it was
reported that c-Myc was a major target for miR-145, which
negatively mediated eukaryotic translation initiation factor 4E, a
downstream target of c-Myc, in NSCLC (25); epidermal growth factor receptor was
discovered to promote lung tumorigenesis through activating c-Myc,
which in turn stimulated miR-7 expression (43); and increased expression levels of
c-Myc were demonstrated to reverse the inhibitory effect of
miR-449c on NSCLC tumor growth in vivo (44). In addition, cell proliferation and
invasion in A549 cells were also altered by the miR-376a/c-Myc axis
in a previous study (45).
Therefore, to investigate whether c-Myc was a direct target gene of
miR-192-5p, a dual-luciferase reporter assay and RIP were
performed; it was observed that c-Myc was negatively regulated by
miR-192-5p, and that the overexpression of c-Myc relieved the
inhibition of miR-192-5p on A427 and A549 cell proliferation,
migration and invasion. However, the anticancer effect of
miR-192-5p induction through c-Myc knockdown was only investigated
in the human NSCLC cell lines, A427 and A549, in the present study,
and to the best of our knowledge, there is currently no published
xenograft experiments that have focused on the increased expression
levels of miR-192-5p as the underlying mechanism of the
curcumin-mediated tumor-suppressive role (11,13,14).
The Wnt pathway, especially the Wnt/β-catenin
dependent pathway is the main target for therapeutic interventions
in cancer types, including lung cancer (46,47).
Activating the Wnt/β-catenin leads to the accumulation of β-catenin
and further promotes oncogenes, including c-Myc and Cyclin D1
(48). Curcumin can inhibit the
Wnt/β-catenin pathway in lung cancer stem cells and lung cancer
cells (15,16). Moreover, miR-192-5p is also
associated with the Wnt/β-catenin pathway in malignant tumors
(49,50). However, the relationship between
curcumin, miR-192-5p and the Wnt/β-catenin pathway have not been
previously investigated. Thus, the present results suggested that
miR-192-5p upregulation mediated the antitumor role of curcumin in
NSCLC by activating the Wnt/β-catenin pathway and targeting
c-Myc.
In conclusion, the present study demonstrated that
curcumin exhibited antiproliferative and antimigratory and
anti-invasive properties in NSCLC in vitro, which were
largely mediated through the increased expression levels of
miR-192-5p by targeting c-Myc and inhibiting the Wnt/β-catenin
signaling pathway. These findings suggested a novel mechanism for
the anticancer function of curcumin and suggested that the
restoration of miR-192-5p could be used as a novel therapeutic
approach for NSCLC treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP and CZ conceptualized and designed the
experiments, analyzed the data, and prepared the original draft of
the manuscript. YS and ZL performed the experiments, collected and
validated the data, and revised it critically for important
intellectual content prior to reviewing and editing. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gerber DE, Laccetti AL, Xuan L, Halm EA
and Pruitt SL: Impact of prior cancer on eligibility for lung
cancer clinical trials. J Natl Cancer Inst. 106(pii):
dju3022014.PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lochowski M, Lochowska B, Rebowski M,
Brzezinski D, Cieslik-Wolski B and Kozak J: Five-year survival
analysis and prognostic factors in patients operated on for
non-small cell lung cancer with N2 disease. J Thorac Dis.
10:3180–3186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stinchcombe TE and Socinski MA: Current
treatments for advanced stage non-small cell lung cancer. Proc Am
Thorac Soc. 6:233–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correction, . New perspectives of curcumin
in cancer prevention. Cancer Prev Res (Phila). 10:3712017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibrahim S, Tagami T, Kishi T and Ozeki T:
Curcumin marinosomes as promising nano-drug delivery system for
lung cancer. Int J Pharm. 540:40–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maran A, Yaszemski MJ, Kohut A and Voronov
A: Curcumin and osteosarcoma: Can invertible polymeric micelles
help? Materials (Basel). 9(pii): E5202016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan MA, Akhtar N, Sharma V and Pathak K:
Product development studies on sonocrystallized curcumin for the
treatment of gastric cancer. Pharmaceutics. 7:43–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye MX, Li Y, Yin H and Zhang J: Curcumin:
Updated molecular mechanisms and intervention targets in human lung
cancer. Int J Mol Sci. 13:3959–3978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du X, Zhang J, Wang J, Lin X and Ding F:
Role of miRNA in lung cancer-potential biomarkers and therapies.
Curr Pharm Des. 23:5997–6010. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lelli D, Pedone C, Majeed M and Sahebkar
A: Curcumin and lung cancer: The role of microRNAs. Curr Pharm Des.
23:3440–3444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simental-Mendia LE and Sahebkar A:
Modulation of microRNAs by curcumin in pancreatic cancer. Clin
Nutr. 35:15852016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye M and Zhang J and Zhang J, Miao Q, Yao
L and Zhang J: Curcumin promotes apoptosis by activating the
p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer.
Cancer Lett. 357:196–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin H, Qiao F, Wang Y, Xu Y and Shang Y:
Curcumin inhibits cell proliferation and induces apoptosis of human
non-small cell lung cancer cells through the upregulation of
miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol
Rep. 34:2782–2789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Wei C and Xi Z: Curcumin suppresses
proliferation and invasion in non-small cell lung cancer by
modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell
Dev Biol Anim. 50:840–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu JY, Yang X, Chen Y, Jiang Y, Wang SJ,
Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al: Curcumin suppresses
lung cancer stem cells via inhibiting wnt/β-catenin and sonic
hedgehog pathways. Phytother Res. 31:680–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He M, Li Y, Zhang L, Li L, Shen Y, Lin L,
Zheng W, Chen L, Bian X, Ng HK and Tang L: Curcumin suppresses cell
proliferation through inhibition of the Wnt/β-catenin signaling
pathway in medulloblastoma. Oncol Rep. 32:173–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dou H, Shen R, Tao J, Huang L, Shi H, Chen
H, Wang Y and Wang T: Curcumin suppresses the colon cancer
proliferation by inhibiting wnt/β-catenin pathways via miR-130a.
Front Pharmacol. 8:8772017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JY, Wang X, Wang XJ, Zheng BZ, Wang
Y, Wang X and Liang B: Curcumin inhibits the growth via
Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur Rev
Med Pharmacol Sci. 22:7492–7499. 2018.PubMed/NCBI
|
|
20
|
Xu JH, Yang HP, Zhou XD, Wang HJ, Gong L
and Tang CL: Role of Wnt inhibitory factor-1 in inhibition of
bisdemethoxycurcumin mediated epithelial-to-mesenchymal transition
in highly metastatic lung cancer 95D cells. Chin Med J (Engl).
128:1376–1383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Peng Y, Huang Y, Yang M, Yan R,
Zhao Y, Cheng Y, Liu X, Deng S, Feng X, et al: SMG-1 inhibition by
miR-192/-215 causes epithelial-mesenchymal transition in gastric
carcinogenesis via activation of Wnt signaling. Cancer Med.
7:146–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Chen Z, Huang J, Chen F, Ye W,
Ding G and Wang X: Bioinformatics identification of dysregulated
microRNAs in triple negative breast cancer based on microRNA
expression profiling. Oncol Lett. 15:3017–3023. 2018.PubMed/NCBI
|
|
23
|
Zhang H, Zhang C, Feng R, Zhang H, Gao M
and Ye L: Investigating the microRNA-mRNA regulatory network in
acute myeloid leukemia. Oncol Lett. 14:3981–3988. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Chen Y, Ge Y, Hu Y, Li M and Jin
Y: Inhalation treatment of primary lung cancer using liposomal
curcumin dry powder inhalers. Acta Pharm Sin B. 8:440–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang HW, Crawford M, Fabbri M, Nuovo G,
Garofalo M, Nana-Sinkam SP and Friedman A: A mathematical model for
microRNA in lung cancer. PLoS One. 8:e536632013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui J, Mo J, Luo M, Yu Q, Zhou S, Li T,
Zhang Y and Luo W: C-Myc-activated long non-coding RNA H19
downregulates miR-107 and promotes cell cycle progression of
non-small cell lung cancer. Int J Clin Exp Pathol. 8:12400–12409.
2015.PubMed/NCBI
|
|
28
|
Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng
A and Hu J: MiRNA-145 inhibits non-small cell lung cancer cell
proliferation by targeting c-Myc. J Exp Clin Cancer Res.
29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park W, Amin AR, Chen ZG and Shin DM: New
perspectives of curcumin in cancer prevention. Cancer Prev Res
(Phila). 6:387–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Datta R, Halder SK and Zhang B: Role of
TGF-β signaling in curcumin-mediated inhibition of tumorigenicity
of human lung cancer cells. J Cancer Res Clin Oncol. 139:563–572.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X
and Yin H: Curcumin promotes apoptosis in A549/DDP
multidrug-resistant human lung adenocarcinoma cells through an
miRNA signaling pathway. Biochem Biophys Res Commun. 399:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Du Y, Wu C, Ren X, Ti X, Shi J,
Zhao F and Yin H: Curcumin promotes apoptosis in human lung
adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep.
24:1217–1223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhan JW, Jiao DM, Wang Y, Song J, Wu JH,
Wu LJ, Chen QY and Ma SL: Integrated microRNA and gene expression
profiling reveals the crucial miRNAs in curcumin anti-lung cancer
cell invasion. Thorac Cancer. 8:461–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiao DM, Yan L, Wang LS, Hu HZ, Tang XL,
Chen J, Wang J, Li Y and Chen QY: Exploration of inhibitory
mechanisms of curcumin in lung cancer metastasis using a
miRNA-transcription factor-target gene network. PLoS One.
12:e01724702017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu Y, Wang J, Liu L, Yu L, Zhao N, Zhou X
and Lu X: Curcumin increases the sensitivity of
Paclitaxel-resistant NSCLC cells to Paclitaxel through
microRNA-30c-mediated MTA1 reduction. Tumour Biol.
39:10104283176983532017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suresh R, Ali S, Ahmad A, Philip PA and
Sarkar FH: The role of cancer stem cells in recurrent and
drug-resistant lung cancer. Adv Exp Med Biol. 890:57–74. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Bai W and Zhang W: MiR-21
suppresses the anticancer activities of curcumin by targeting PTEN
gene in human non-small cell lung cancer A549 cells. Clin Transl
Oncol. 16:708–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen S, Gu T, Lu Z, Qiu L, Xiao G, Zhu X,
Li F, Yu H, Li G and Liu H: Roles of MYC-targeting long non-coding
RNA MINCR in cell cycle regulation and apoptosis in non-small cell
lung Cancer. Respir Res. 20:2022019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Jia Y, Zhao S, Zhang X, Wang X,
Han X, Wang Y, Ma M, Shi J and Liu L: BIN1 reverses PD-L1-mediated
immune escape by inactivating the c-MYC and EGFR/MAPK signaling
pathways in non-small cell lung cancer. Oncogene. 36:6235–6243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chou YT, Lin HH, Lien YC, Wang YH, Hong
CF, Kao YR, Lin SC, Chang YC, Lin SY, Chen SJ, et al: EGFR promotes
lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc
pathway that targets the Ets2 transcriptional repressor ERF. Cancer
Res. 70:8822–8831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miao LJ, Huang SF, Sun ZT, Gao ZY, Zhang
RX, Liu Y and Wang J: MiR-449c targets c-Myc and inhibits NSCLC
cell progression. FEBS Lett. 587:1359–1365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Cong W, Wu G, Ju X, Li Z, Duan X,
Wang X and Gao H: MiR-376a suppresses the proliferation and
invasion of non-small-cell lung cancer by targeting c-Myc. Cell
Biol Int. 42:25–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park M, Kim M, Hwang D, Park M, Kim WK,
Kim SK, Shin J, Park ES, Kang CM, Paik YK and Kim H:
Characterization of gene expression and activated signaling
pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol.
27:580–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang DW, Lee SW, Hwang WC, Lee BH, Choi
YS, Suh YA, Choi KY and Min DS: Phospholipase D1 Acts through
Akt/TopBP1 and RB1 to regulate the E2F1-dependent apoptotic program
in cancer cells. Cancer Res. 77:142–152. 2017. View Article : Google Scholar : PubMed/NCBI
|