Introduction
The hippocampus serves major roles in learning and
memory and has been indicated to be the most susceptible brain
region during normal aging (1,2).
During the aging process, numerous neuropathological and
neurobiological alterations occur in the hippocampus, such as
induction of the neuroinflammatory process, alterations in synaptic
plasticity, a reduction in the ability of protein repair and a
decrease in neuronal activity (3–6).
Chemokines serve diverse roles in tissue homeostasis
and immune reactions (7). It has
been reported that various chemokines and chemokine receptors were
widely expressed in neurons and glial cells of the central nervous
system (CNS), and they were indicated to function as critical
regulators in intercellular communication in the CNS under normal
or pathological conditions including stroke, Alzheimer's disease
and multiple sclerosis (8–11). Among chemokines, macrophage
inflammatory protein-3α (MIP-3α), which is also known as C-C motif
chemokine 20, has been indicated to interact specifically with its
unique receptor, C-C chemokine receptor (CCR) type 6 (CCR6)
(12). A number of studies have
reported that CCRs, including CCR6, were expressed in hippocampal
neurons, and they were considered to serve as protective factors
that are associated with neuronal survival (13–18).
Previous studies have focused on the role and the
alteration in the expression of MIP-3α and CCR6 in response to
pathological neuroinflammatory conditions, such as autoimmune
encephalomyelitis, Alzheimer's disease, epilepsy and cerebral
ischemia (15,18–20).
However, age-related alterations in the basal expression of MIP-3α
and CCR6 in the brain under normal condition have not yet been
fully elucidated. Mongolian gerbils are considered to be a suitable
model for studies on aging, as it has been indicated that
age-dependent alterations in behavioral and biological processes in
gerbils are similar to those in humans (21,22).
Therefore, the aim of the current study was to investigate
age-dependent alterations in the protein expression of MIP-3α and
CCR6 in the hippocampus during the normal aging process in
gerbils.
Materials and methods
Animals
In the present study, male Mongolian gerbils
(Meriones unguiculatus) (n=36) at the age of postnatal month
(PM) 1 (25–30 g) as the young, PM 6 (65–75 g) as the adult, PM 12
(75–85 g) as the middle-aged and PM 24 (85–95 g) as the aged group,
were used. The gerbils (n=9 animals/group) were obtained from the
Experimental Animal Center of Kangwon National University in the
Republic of Korea. The gerbils were housed under conventional
housing conditions at an ambient temperature of 23±3°C, a relative
humidity of 55±5% and a 12-h light/dark cycle. Free access to food
and water was permitted. All experimental procedures including
animal handling and use were reviewed and approved by the
Institutional Animal Care and Use Committee of Kangwon National
University (approval no. KW-200113-2).
Immunohistochemistry
For tissue preparation, all gerbils used in the
present study were profoundly anaesthetized using an
intraperitoneal injection of 60 mg/kg sodium pentobarbital, and
cardiac perfusion was conducted. Confirmation of death was
evaluated with vital signs including heart beats, pupillary
response, and respiratory pattern (lack of cardiac activity for 5
min through cardiac palpation, unresponsiveness to light with
dilated pupils using light shone into the eyes of the animal and
lack of spontaneously breathing pattern with shallow and irregular
breathing pattern). The gerbil brains were rinsed via transcardial
perfusion of 0.1M PBS (pH 7.4) and fixed via perfusion of 4%
paraformaldehyde in 0.1M PBS 20 min at room temperature. The brains
were removed and post-fixed with the same fixative for 6 h at room
temperature. The brain tissues including hippocampi were frontally
sectioned to 30 µm thickness using a cryostat at −30°C.
To examine age-related alterations in neurons,
microglia, MIP-3α and CCR6 in the hippocampus, immunohistochemical
staining was performed according to a previous study (18). In brief, the sections were treated
with 0.3% hydrogen peroxide (H2O2) in PBS for
30 min and 10% normal goat serum (cat. no. S-1000, Vector
Laboratories INC.) in 0.05 M PBS for 30 min. and incubated with
mouse anti-neuronal nuclei (cat. no. MAB377, NeuN; 1:800; EMD
Millipore) as a marker of neurons, rabbit anti-ionized
calcium-binding adapter molecule 1 (cat. no. 019-19741, Iba1;
1:200; FUJIFILM Wako Pure Chemical Corporation) as a marker for
microglia, rabbit anti-MIP-3α (cat. no. ab9829, 1:50; Abcam) or
rabbit anti-CCR6 (ab78429, 1:200; Abcam) for 10 h at 4°C.
Subsequently, the tissues were incubated with biotinylated horse
anti-mouse (cat. no. BA-2001) or goat anti-rabbit (cat. no.
BA-1000) IgG (1:200; Vector Laboratories Inc.) for 2 h at room
temperature, and then to streptavidin peroxidase complex (cat. no.
PK-7200, 1:200; Vector Laboratories Inc.) for 45 min at room
temperature. Finally, the signal was visualized with
3,3′-diaminobenzidine.
For quantitative analysis of NeuN, Iba1, MIP-3α and
CCR6 immunoreactivity, six sections per animal with a 120-µm
thickness interval were selected. Digital images of NeuN, Iba1,
MIP-3α and CCR6 immunoreactive structures were captured at 20×
magnification using an a light microscope (BX53, Olympus
Corporation) equipped with a digital camera (Axiocam; Carl Zeiss
AG), which was connected to a monitor. Firstly, the numbers of
NeuN-immunoreactive neurons were counted in the target regions in
the hippocampus, according to the method that was reported in a
previous study (23). Briefly,
NeuN-immunoreactive neurons were captured in a 250×250 µm region at
the center of the stratum pyramidale of the hippocampus proper
[cornu ammonis (CA)1-3 fields] and the granule cell layer of the
dentate gyrus. The number of NeuN-immunoreactive neurons in each
sample was quantified using Optimas v6.5 software (CyberMetrics
Corporation). Secondly, according to a previously published method
(18), the alterations in Iba-1
immunoreactivity were evaluated in the CA1-3 fields and the dentate
gyrus. In addition, alterations in MIP-3α and CCR6 immunoreactivity
were evaluated in hippocampal cells as relative immunoreactivity
(RI) using ImageJ v1.59 software (National Institutes of Health).
Briefly, the images of Iba1, MIP-3α and CCR6 were captured in the
target cells or layers of the immunoreactive structures, calibrated
to an array of 512×512 pixels and measured using a 0–255 grayscale
system (white to dark signal corresponded to 255 to 0). After the
background was subtracted, the staining intensity was calculated
and expressed as RI. The ratio of RI in each immunoreactive
structure was expressed as a percentage, with the PM 1 group
designated as 100%.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. The differences in the mean RI among the groups were
analyzed three times using one-way ANOVA followed by Bonferroni's
multiple comparisons test using SPSS v17.0 software (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Alterations in NeuN immunoreactive
neurons
In the PM 1, 6, 12 and 24 groups, NeuN
immunoreactivity was primarily localized in neurons of the stratum
pyramidale, which are called pyramidal neurons, in the hippocampal
CA1-3 fields (Fig. 1A-D). The
distribution pattern of NeuN immunoreactive neurons in the CA1-3
fields was similar in all groups. As in the hippocampus proper, the
distribution pattern of NeuN immunoreactive neurons in the dentate
gyrus did not differ between the groups (Fig. 1E-H). Furthermore, the numbers of
NeuN immunoreactive neurons in both the CA1-3 fields and the
dentate gyrus were similar in all groups (Fig. 1I).
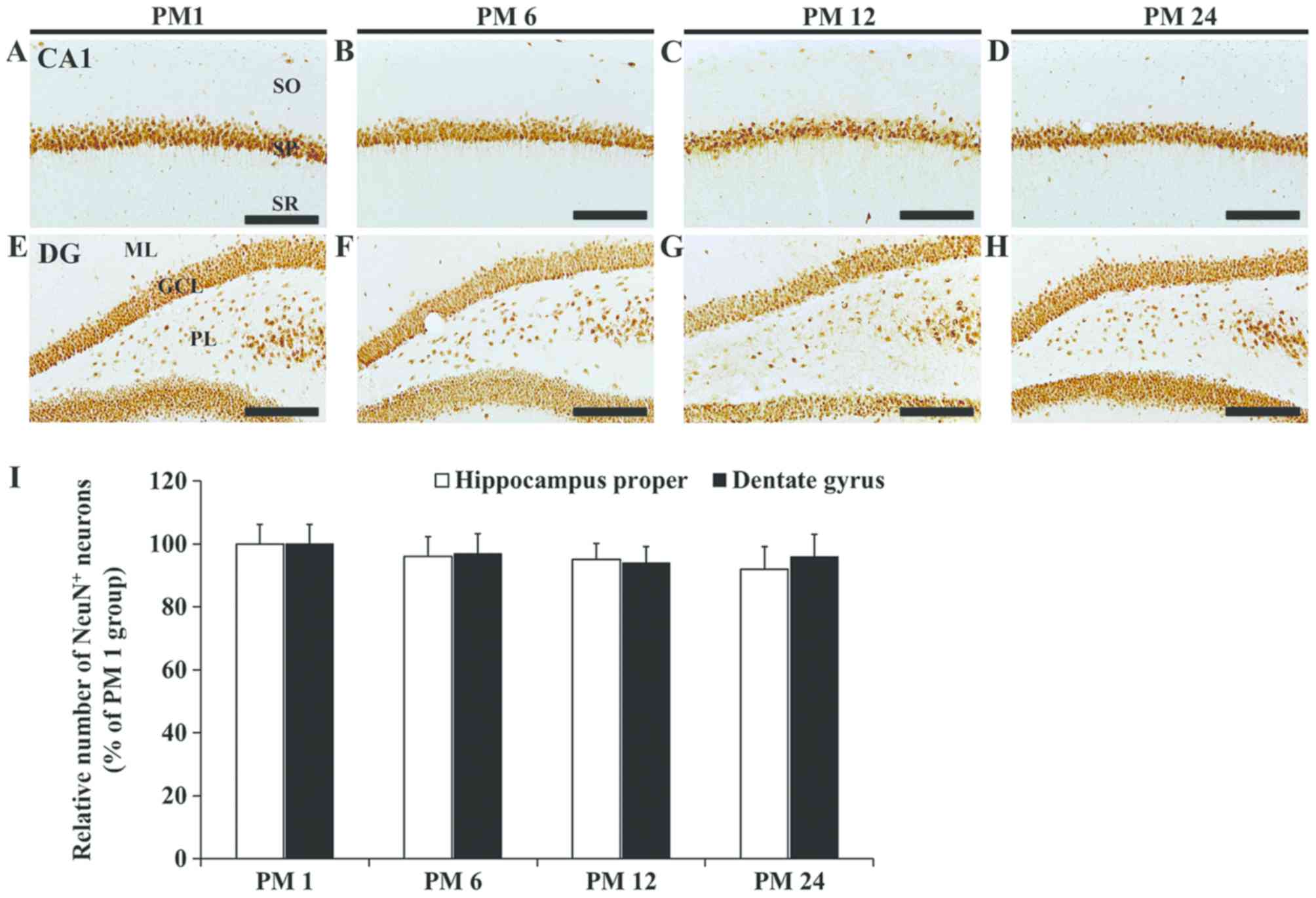 | Figure 1.NeuN immunohistochemistry. NeuN
immunoreactivity in the CA1 field in the (A) PM 1, (B) PM 6, (C) PM
12 and (D) PM 24 group, and in the DG in the (E) PM 1, (F) PM 6,
(G) PM 12 and (H) PM 24 group. Scale bar=100 µm. (I) Relative
number of NeuN immunoreactive neurons in the SP and GCL of the PM
1, 6, 12 and 24 groups (n=9 per group). The data are presented as
the mean ± standard error of the mean. GCL, granule cell layer; ML,
molecular layer; PL, polymorphic layer; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; DG, dentate gyrus; CA,
cornu ammonis; PM, postnatal month; NeuN anti-neuronal nuclei. |
Alterations in Iba1
immunoreactivity
In the PM 1 group, Iba1 immunoreactive microglia
were detected in the stratum oriens and radiatum of the hippocampus
proper (Fig. 2A). Iba1
immunoreactivity in the PM 6 group was decreased by 27.5% compared
with the PM 1 group (Fig. 2B and
I). In the PM 12 group, Iba1 immunoreactivity was similar to
that in the PM 1 group (Fig. 2C and
I). However, in the PM 24 group, an increase of 25.9% in Iba1
immunoreactivity was observed compared with the PM 12 group
(Fig. 2D and I). In this this
group, certain Iba1 immunoreactive microglia were identified as
activated microglia (enlarged cell bodies and thickened
processes).
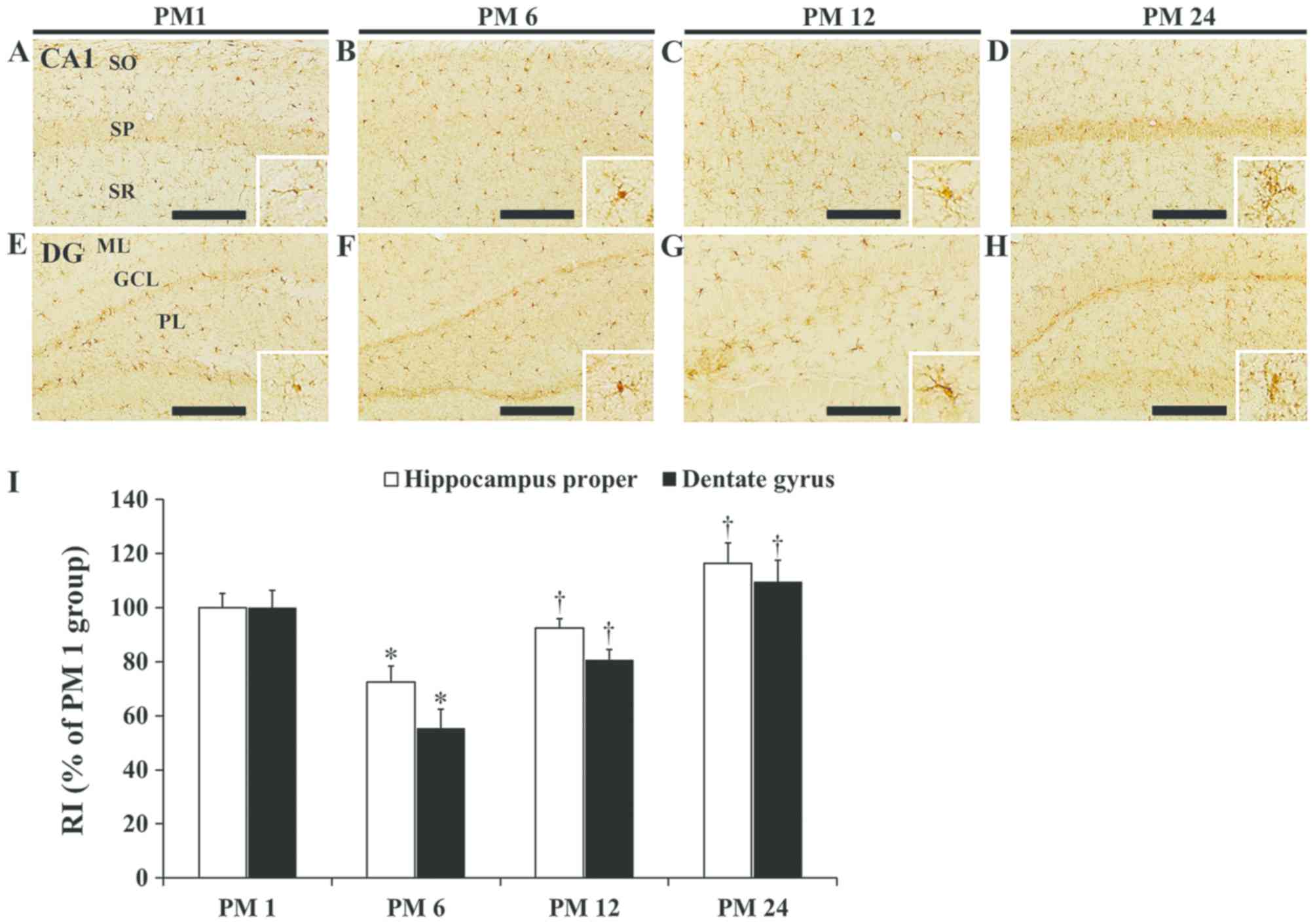 | Figure 2.Iba-1 immunohistochemistry. Iba1
immunoreactivity in the CA1 field in the (A) PM 1, (B) PM 6, (C) PM
12 and (D) PM 24 group, and in the DG in the (E) PM 1, (F) PM 6,
(G) PM 12 and (H) PM 24 group. Microglia in high magnification
(×100) are presented in the smaller panels. Scale bar, 100 µm
(A-H). (I) RI as % of Iba1 immunoreactivity in the CA1 field and
the DG of the PM 1, 6, 12 and 24 groups (n=9 per group). The data
are presented as the mean ± standard error of the mean. *P<0.05
vs. PM 1; †P<0.05 vs. PM 6 and PM 12. GCL, granule
cell layer; ML, molecular layer; PL, polymorphic layer; SO, stratum
oriens; SP, stratum pyramidale; SR, stratum radiatum; RI, relative
immunoreactivity; DG, dentate gyrus; CA, cornu ammonis; PM,
postnatal month; Iba1, ionized calcium-binding adapter molecule
1. |
In the PM 1 group, Iba1 immunoreactive microglia
were primarily localized in the molecular and polymorphic layers of
the dentate gyrus (Fig. 2E). Iba1
immunoreactivity in the PM 6 and 12 groups was decreased to 55.3
and 80.7%, respectively, compared with the PM 1 group (Fig. 2F, G and I). In the PM 24 group, an
increase of 35.9% in Iba1 immunoreactivity was observed compared
with the PM 12 group (Fig. 2H and
I).
Alterations in MIP-3α
immunoreactivity
In the PM1 group, MIP-3α immunoreactivity in the
hippocampus proper was detected in pyramidal cells of the stratum
pyramidale of the CA1-3 fields (Fig.
3A and E). In the PM 6 and 12 groups, MIP-3α immunoreactivity
in pyramidal cells was decreased to 62.2 and 72.1%, respectively,
compared with the PM 1 group (Fig. 3B,
C, F, G and M). A considerable increase in MIP-3α
immunoreactivity was observed in pyramidal cells of the PM 24
group, as RI was increased to 215.9% compared with the PM 1 group
(Fig. 3D, H and M).
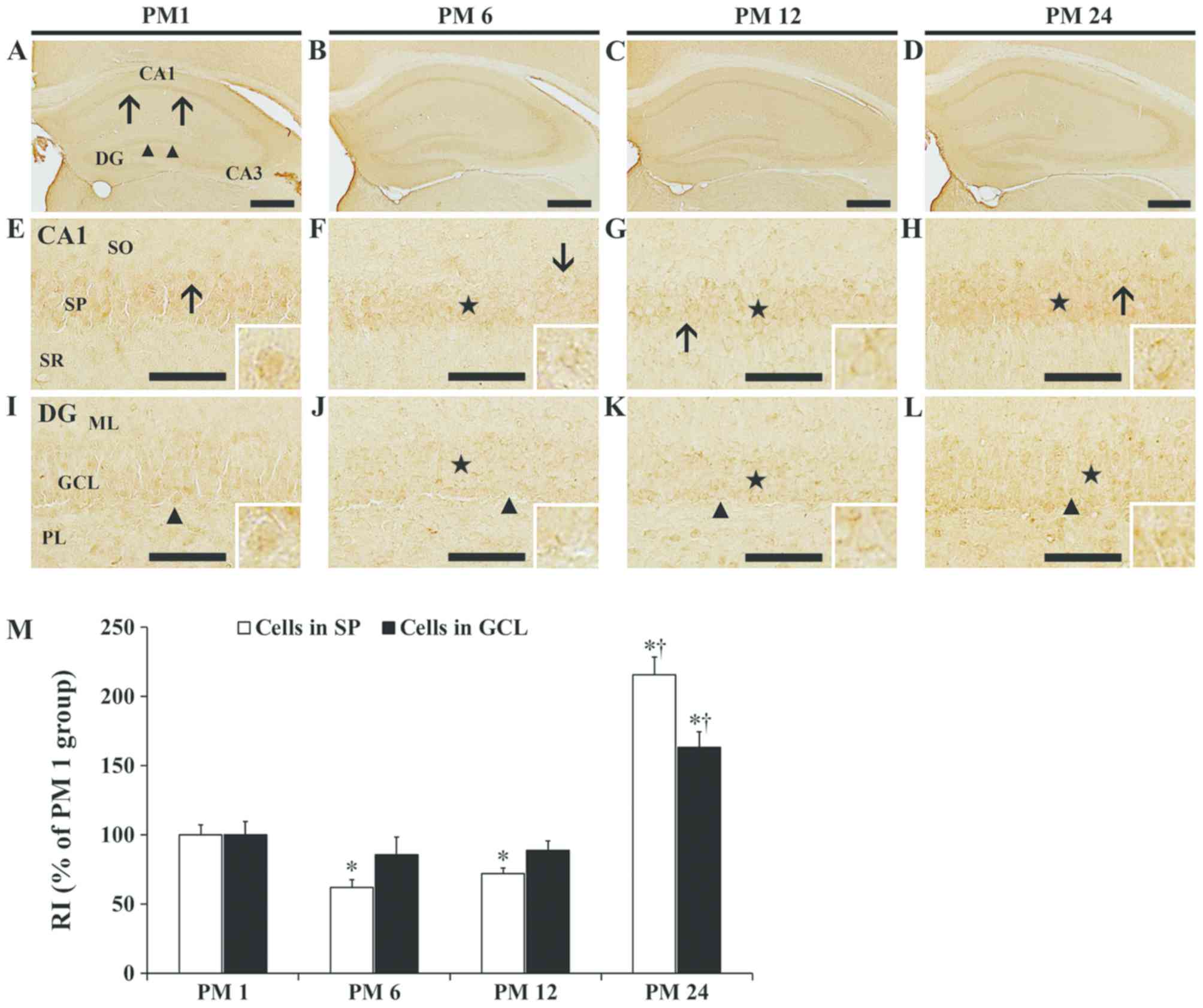 | Figure 3.MIP-3α immunohistochemistry. MIP-3α
immunoreactivity in the hippocampus in the (A) PM 1, (B) PM 6, (C)
PM 12 and (D) PM 24 group, in the CA1 field in the (E) PM 1, (F) PM
6, (G) PM 12 and (H) PM 24 group, and in the DG in the (I) PM 1,
(J) PM 6, (K) PM 12 and (L) PM 24 group. Scale bar, 400 µm (A-C)
and 50 µm (D-I). (M) RI as % of MIP-3α immunoreactivity in the SP
and GCL of the PM 1, 6, 12 and 24 groups (n=9 per group). The data
are presented as the mean ± standard error of the mean. *P<0.05
vs. PM 1; †P<0.05 vs. PM 12. GCL, granule cell layer;
ML, molecular layer; PL, polymorphic layer; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; RI, relative
immunoreactivity; DG, dentate gyrus; CA, cornu ammonis; PM,
postnatal month; MIP-3α, macrophage inflammatory protein-3α. |
In the PM 1 group, MIP-3α in the dentate gyrus was
detected primarily in granule cells of the granule cell layer
(Fig. 3A and I). Similar to the
hippocampus proper, MIP-3α immunoreactivity in the dentate gyrus
was decreased in the PM 6 and 12 groups to 85.9 and 88.9%,
respectively, compared with the PM 1 group (Fig. 3B, C and J-M). In the PM 24 group,
MIP-3α immunoreactivity was increased to 163.3% compared with the
PM 1 group (Fig. 3D, L and M).
Alterations in CCR6
immunoreactivity
In the PM 1 group, a strong CCR6 immunoreactivity
was primarily detected in the stratum pyramidale of the hippocampus
proper (Fig. 4A and E). In the PM
6 and 12 groups, CCR6 immunoreactivity in the stratum pyramidale
was faint (Fig. 4B, C, F, G and
M). In the PM 24 group, CCR6 immunoreactivity in the stratum
pyramidale was increased compared with the PM 6 and 12 groups,
exhibiting an RI of 37.0% of that in the PM 1 group (Fig. 4D, H and M).
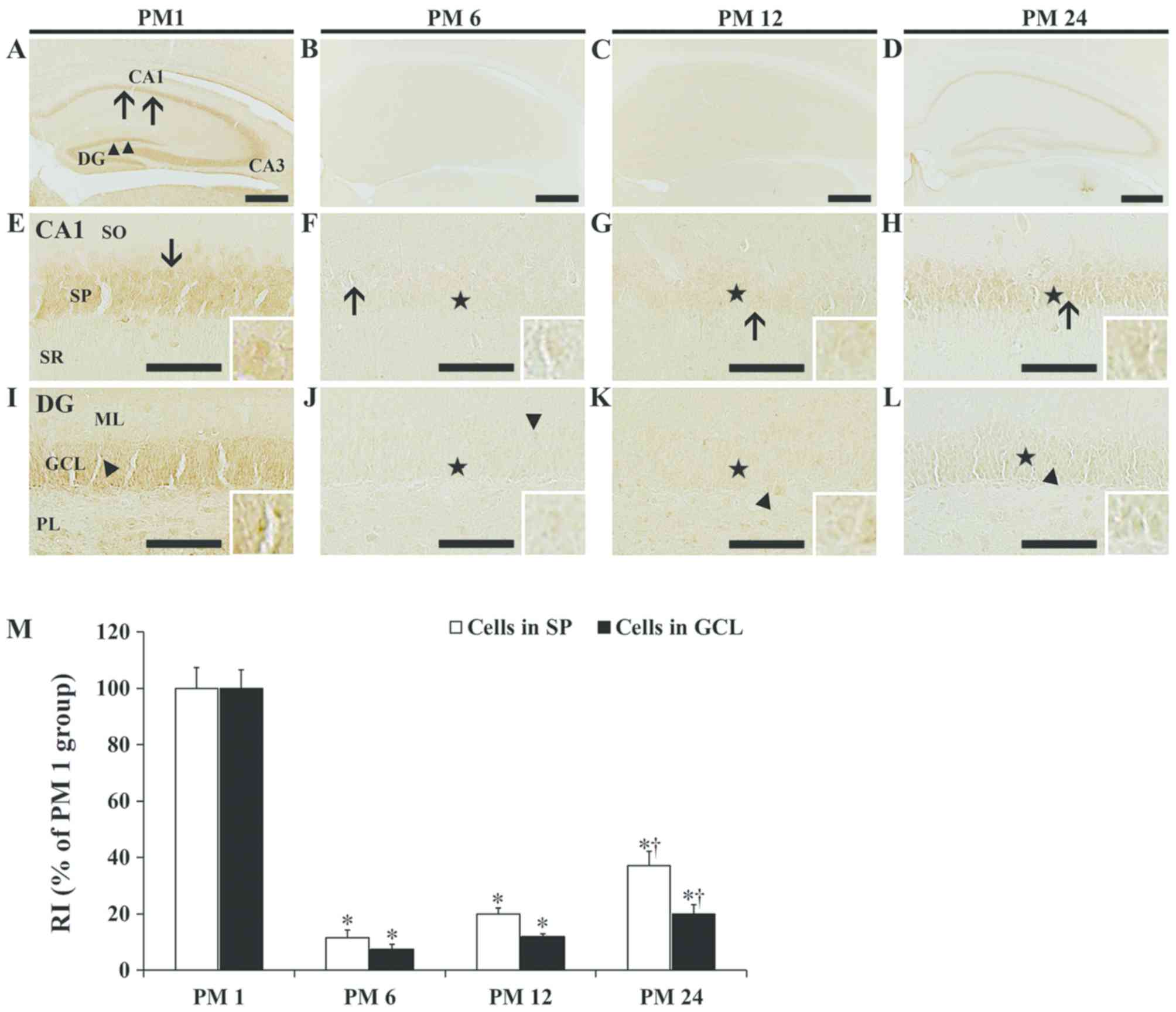 | Figure 4.CCR6 immunohistochemistry. CCR6
immunoreactivity in the hippocampus in the (A) PM 1, (B) PM 6, (C)
PM 12 and (D) PM 24 group, in the CA1 field in the (E) PM 1, (F) PM
6, (G) PM 12 and (H) PM 24 group, and in the DG in the (I) PM 1,
(J) PM 6, (K) PM 12 and (L) PM 24 group. Scale bars, 400 µm (A-C)
and 50 µm (D-I). (M) RI as % of CCR6 immunoreactivity in the SP and
GCL of the PM 1, 6, 12 and 24 groups (n=9 per group). The data are
presented as the mean ± standard error of the mean. *P<0.05 vs.
PM 1; †P<0.05 vs. PM 12. GCL, granule cell layer; ML,
molecular layer; PL, polymorphic layer; SO, stratum oriens; SP,
stratum pyramidale; SR, stratum radiatum; RI, relative
immunoreactivity; DG, dentate gyrus; CA, cornu ammonis; PM,
postnatal month; CCR6, C-C chemokine receptor type 6. |
In the PM 1 group, a strong CCR6 immunoreactivity
was also detected in the granule cell layer of the dentate gyrus
(Fig. 4A and I). As in the case of
the hippocampus proper, CCR6 immunoreactivity in the PM 6 and 12
groups was faint (Fig. 4B, C, J, K and
M). In the PM 24 group, CCR6 immunoreactivity in the granule
cell layer was increased compared with the PM 6 and 12 groups, with
an RI of 19.9% of that in the PM 1 group (Fig. 4D, L and M).
Discussion
In the current study, the distribution pattern of
NeuN immunoreactive neuronal cells in the hippocampus proper and
dentate gyrus did not differ among the PM 1, 6, 12 and 24 groups,
and the numbers of the neurons were indicated to be similar in all
groups. This is in accordance with previous studies, which have
reported that the neuronal number in the hippocampus proper and
dentate gyrus in aged rodents was preserved (23–27).
This finding indicates that the number of neurons in the rodent
hippocampus does not alter during aging.
It has been reported that Iba1 immunoreactivity,
which is a marker of microglia, was higher in the gerbil
hippocampus in the young (PM 1) compared with the adult (PM 6)
group (28). In the current study,
Iba1 immunoreactivity in the hippocampus was decreased in the PM 6
and increased in the PM 24 group, compared with the PM 1 group.
This finding indicates that neuroinflammation may be increased in
the hippocampus during aging. Previous studies have revealed that
microglia serve critical roles in neuroinflammation in the brain
during normal aging, as they have been indicated to increase in
numbers and size to acquire an active phenotype in the aged brains
(28–31). Concomitantly with microglial
activation, the expression of pro-inflammatory cytokines, such as
interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α), has
also been indicated to alter in the hippocampus during the aging
process. Balschun et al (32), reported that the basal mRNA
expression of IL-1β was decreased in the hippocampus of middle-aged
(12- to 16-month-old) compared with young (3-month-old) rats. On
the contrary, Gavilan et al (3) reported that no significant
differences were observed in the mRNA expression of IL-1β and TNF-α
in the hippocampus between young and middle-aged rats. In addition,
it has been indicated that the protein expression of
cyclooxygenase-2 in the hippocampus of the adult gerbil was
decreased compared with the young group (33). By contrast, the expression of
pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, have
been revealed to be increased in aged compared with young and
middle-aged hippocampi in the normal aging process in rodents
(3,31,34).
In addition, it has been reported that altered levels of cytokines
and chemokines in aged brains were associated with an age-related
cognitive decline (34–37). The age-related cognitive decline or
impairment may be associated with the neuroinflammation that occurs
in aged brains, and has been indicated to result in reductions in
synaptic activity, pre-synaptic molecules, post-synaptic densities
and dendritic and axonal arborization (3,38–40).
It has been reported that both MIP-1α and MIP-1β
were increased in the hippocampus of 30-month-old mice compared
with the 4-month-old mice (41),
suggesting that an age-dependent increase in chemokines may
contribute to the decline of brain function during normal aging
(41). In addition, it has been
indicated that C-C motif chemokine 2/monocyte chemoattractant
protein-1 expression was increased in the aged mouse hippocampus
(42). Furthermore, Subramanian
et al (20) reported that
CCR6 mRNA expression was increased in the brain of older
symptomatic and pre-symptomatic 3×Tg Alzheimer's disease mice and
suggested that variations in the CCR6 level may be associated with
alterations in cognition and learning behavior. In the current
study, it was revealed that MIP-3α and CCR6 immunoreactivities in
the hippocampal cells of the PM 6 and 12 groups were decreased
compared with the PM 1 group, while they were subsequently
increased in the PM 24 group. This result is consistent with the
findings by Liu et al (15), who suggested that CCR6 in pyramidal
and granule cells may participate in regulating the activities of
hippocampal principal neurons. In addition, it has been reported
that MIP-3α was increased in aged hippocampal principal cells
concomitantly with inflammation, and CCR6 was increased in the
neurons as a protective factor that is associated with neuronal
survival an animal model of status epilepticus and ischemic stroke
(15,18). Therefore, it may be hypothesized
that an increased protein expression of MIP-3α and CCR6 in the aged
hippocampus may be associated with a decline in brain functions,
including cognition and learning, during the normal aging
process.
However, certain limitations exist to the present
study. In the current study, age-dependent alterations in the
protein expression of MIP-3α and CCR6 in the hippocampal subregions
were investigated during the normal aging process. Notably, the
mechanisms via which MIP-3α and CCR6 expression was regulated
during the aging process, were not examined. Potential upstream
regulators, which may affect MIP-3α and CCR6 expression in the
hippocampus at various aging stages, should be elucidated in future
studies. In addition, the association between age-dependent
alterations in neuroinflammation and the altered expression of
MIP-3α and CCR6 in aged animals should be addressed in future
studies.
In conclusion, the current study revealed that
MIP-3α and CCR6 immunoreactivities in the gerbil hippocampus were
altered during the normal aging process, and indicated that both
MIP-3α and CCR6 were decreased in the adult compared with the young
hippocampus, and increased again in the aged compared with the
adult hippocampus.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Basic Science
Research Program of the National Research Foundation (NRF) of Korea
(grant nos. NRF-2017R1D1A1B03029311, NRF- 2017R1D1A1B03030161 and
NRF- 2018R1D1A1B07049453).
Availability of data and materials
All data generated or analyzed during the current
study are included in the published article.
Authors' contributions
CHL, JHA and JHP conceived the projects. JHA, JHP,
MHW and CHL were responsible for the experimental design, data
collection and manuscript writing. TKL, GEY, MCS and JHC performed
the experiments, data analysis and critical comments on the present
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures including animal
handling and use were reviewed and approved by the Institutional
Animal Care and Use Committee of Kangwon National University
(approval no. KW-180124-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bettio LEB, Rajendran L and Gil-Mohapel J:
The effects of aging in the hippocampus and cognitive decline.
Neurosci Biobehav Rev. 79:66–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geinisman Y, Detoledo-Morrell L, Morrell F
and Heller RE: Hippocampal markers of age-related memory
dysfunction: Behavioral, electrophysiological and morphological
perspectives. Prog Neurobiol. 45:223–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gavilan MP, Revilla E, Pintado C, Castaño
A, Vizuete ML, Moreno-González I, Baglietto-Vargas D, Sánchez-Varo
R, Vitorica J, Gutiérrez A and Ruano D: Molecular and cellular
characterization of the age-related neuroinflammatory processes
occurring in normal rat hippocampus: Potential relation with the
loss of somatostatin GABAergic neurons. J Neurochem. 103:984–996.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee CH, Park JH, Choi JH, Yoo KY, Ryu PD
and Won MH: Heat shock protein 90 and its cochaperone, p23, are
markedly increased in the aged gerbil hippocampus. Exp Gerontol.
46:768–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lister JP and Barnes CA: Neurobiological
changes in the hippocampus during normative aging. Arch Neurol.
66:829–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ojo B, Rezaie P, Gabbott PL, Davies H,
Colyer F, Cowley TR, Lynch M and Stewart MG: Age-related changes in
the hippocampus (loss of synaptophysin and glial-synaptic
interaction) are modified by systemic treatment with an
NCAM-derived peptide, FGL. Brain Behav Immun. 26:778–788. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bajetto A, Bonavia R, Barbero S and
Schettini G: Characterization of chemokines and their receptors in
the central nervous system: Physiopathological implications. J
Neurochem. 82:1311–1329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cartier L, Hartley O, Dubois-Dauphin M and
Krause KH: Chemokine receptors in the central nervous system: Role
in brain inflammation and neurodegenerative diseases. Brain Res
Brain Res Rev. 48:16–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glabinski AR and Ransohoff RM: Chemokines
and chemokine receptors in CNS pathology. J Neurovirol. 5:3–12.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schilling M, Strecker JK, Ringelstein EB,
Schabitz WR and Kiefer R: The role of CC chemokine receptor 2 on
microglia activation and blood-borne cell recruitment after
transient focal cerebral ischemia in mice. Brain Res. 1289:79–84.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perez-Canadillas JM, Zaballos A, Gutierrez
J, Varona R, Roncal F, Albar JP, Márquez G and Bruix M: NMR
solution structure of murine CCL20/MIP-3alpha, a chemokine that
specifically chemoattracts immature dendritic cells and lymphocytes
through its highly specific interaction with the beta-chemokine
receptor CCR6. J Biol Chem. 276:28372–28379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horuk R, Martin AW, Wang Z, Schweitzer L,
Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, et
al: Expression of chemokine receptors by subsets of neurons in the
central nervous system. J Immunol. 158:2882–2890. 1997.PubMed/NCBI
|
|
14
|
Lee JC, Ahn JH, Kim IH, Park JH, Yan BC,
Cho GS, Ohk TH, Park CW, Cho JH, Kim YM, et al: Transient
ischemia-induced change of CCR7 immunoreactivity in neurons and its
new expression in astrocytes in the gerbil hippocampus. J Neurol
Sci. 336:203–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JX, Cao X, Liu Y and Tang FR: Altered
expression of neuronal CCR6 during pilocarpine induced status
epilepticus in mice. Epilepsy Res. 126:45–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meucci O, Fatatis A, Simen AA, Bushell TJ,
Gray PW and Miller RJ: Chemokines regulate hippocampal neuronal
signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA.
95:14500–14505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meucci O, Fatatis A, Simen AA and Miller
RJ: Expression of CX3CR1 chemokine receptors on neurons and their
role in neuronal survival. Proc Natl Acad Sci USA. 97:8075–8080.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Noh Y, Kim SS, Ahn JH, Ohk TG,
Cho JH, Lee TK, Kim H, Song M, Lee JC, et al: Time-course changes
and new expressions of MIP-3α and its receptor, CCR6, in the gerbil
hippocampal CA1 area following transient global cerebral ischemia.
Neurochem Res. 43:2102–2110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serafini B, Columba-Cabezas S, Di Rosa F
and Aloisi F: Intracerebral recruitment and maturation of dendritic
cells in the onset and progression of experimental autoimmune
encephalomyelitis. Am J Pathol. 157:1991–2002. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subramanian S, Ayala P, Wadsworth TL,
Harris CL, Vandenbark AA, Quinn JF and Offner H: CCR6: A biomarker
for Alzheimer's-like disease in a triple transgenic mouse model. J
Alzheimers Dis. 22:619–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheal ML: The gerbil: A unique model for
research on aging. Exp Aging Res. 12:3–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vincent AL, Rodrick GE and Sodeman WA: The
Mongolian gerbil in aging research. Exp Aging Res. 6:249–260. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahn JH, Choi JH, Park JH, Kim IH, Cho JH,
Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al: Long-term
exercise improves memory deficits via restoration of myelin and
microvessel damage, and enhancement of neurogenesis in the aged
gerbil hippocampus after ischemic stroke. Neurorehabil Neural
Repair. 30:894–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn JH, Lee TK, Park JH, Cho JH, Kim IH,
Lee JC, Hong S, Jeon YH, Kang Il J, Lee YJ, et al: Age-dependent
differences in myelin basic protein expression in the hippocampus
of young, adult and aged gerbils. Lab Anim Res. 33:237–243. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rapp PR and Gallagher M: Preserved neuron
number in the hippocampus of aged rats with spatial learning
deficits. Proc Natl Acad Sci USA. 93:9926–9930. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
West MJ: Regionally specific loss of
neurons in the aging human hippocampus. Neurobiol Aging.
14:287–293. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasmussen T, Schliemann T, Sørensen JC,
Zimmer J and West MJ: Memory impaired aged rats: No loss of
principal hippocampal and subicular neurons. Neurobiol Aging.
17:143–147. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi JH, Lee CH, Hwang IK, Won MH, Seong
JK, Yoon YS, Lee HS and Lee IS: Age-related changes in ionized
calcium- binding adapter molecule 1 immunoreactivity and protein
level in the gerbil hippocampal CA1 region. J Vet Med Sci.
69:1131–1136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cox FF, Carney D, Miller AM and Lynch MA:
CD200 fusion protein decreases microglial activation in the
hippocampus of aged rats. Brain Behav Immun. 26:789–796. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lynch MA: Age-related neuroinflammatory
changes negatively impact on neuronal function. Front Aging
Neurosci. 1:62010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Matsuwaki T, Yamanouchi K and
Nishihara M: Involvement of progranulin in modulating
neuroinflammatory responses but not neurogenesis in the hippocampus
of aged mice. Exp Gerontol. 95:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balschun D, Randolf A, Pitossi F,
Schneider H, Del Rey A and Besedovsky HO: Hippocampal interleukin-1
beta gene expression during long-term potentiation decays with age.
Ann N Y Acad Sci. 992:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kang Il J and Won MH: Cyclooxygenase-2 immunoreactivity and
protein level in the gerbil hippocampus during normal aging.
Neurochem Res. 35:99–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Griffin R, Nally R, Nolan Y, McCartney Y,
Linden J and Lynch MA: The age-related attenuation in long-term
potentiation is associated with microglial activation. J Neurochem.
99:1263–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scheinert RB, Asokan A, Rani A, Kumar A,
Foster TC and Ormerod BK: Some hormone, cytokine and chemokine
levels that change across lifespan vary by cognitive status in male
Fischer 344 rats. Brain Behav Immun. 49:216–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Speisman RB, Kumar A, Rani A, Foster TC
and Ormerod BK: Daily exercise improves memory, stimulates
hippocampal neurogenesis and modulates immune and neuroimmune
cytokines in aging rats. Brain Behav Immun. 28:25–43. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Villeda SA, Plambeck KE, Middeldorp J,
Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik
D, et al: Young blood reverses age-related impairments in cognitive
function and synaptic plasticity in mice. Nat Med. 20:659–663.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chung HY, Cesari M, Anton S, Marzetti E,
Giovannini S, Seo AY, Carter C, Yu BP and Leeuwenburgh C: Molecular
inflammation: Underpinnings of aging and age-related diseases.
Ageing Res Rev. 8:18–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tha KK, Okuma Y, Miyazaki H, Murayama T,
Uehara T, Hatakeyama R, Hayashi Y and Nomura Y: Changes in
expressions of proinflammatory cytokines IL-1beta, TNF-alpha and
IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain
Res. 885:25–31. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yankner BA, Lu T and Loerch P: The aging
brain. Annu Rev Pathol. 3:41–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Felzien LK, McDonald JT, Gleason SM,
Berman NE and Klein RM: Increased chemokine gene expression during
aging in the murine brain. Brain Res. 890:137–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terao A, Apte-Deshpande A, Dousman L,
Morairty S, Eynon BP, Kilduff TS and Freund YR: Immune response
gene expression increases in the aging murine hippocampus. J
Neuroimmunol. 132:99–112. 2002. View Article : Google Scholar : PubMed/NCBI
|


















