Introduction
Gliomas have been recognized as one of the most
common invasive malignancies of the central nervous system, with
high recurrence rates, high mortality and low rates of cure
(1,2). Although the diagnosis and treatment
of glioma has made great progress in recent years, the overall
survival of glioma patients remains low (3,4).
Currently, there is no effective treatment for gliomas; therefore,
novel treatments are required. Elucidating the possible molecular
mechanisms of action is also an important aspect for the
development of treatments for gliomas.
Tubeimoside-1 (TBMS1) is a triterpenoid saponin the
sugar chains of which are connected by 3-hydroxy-3-methylglutaric
acid to form a unique macrocyclic structure. TBMS1 is extracted
from the tubers of a traditional Chinese medicinal plant,
Bolbostemma paniculatum (Maxim.): Franquet
(Cucurbitaceae), as recorded in the Supplement to the
Compendium of Materia Medica (5).
It is conventionally used as a natural medicine to treat a variety
of diseases by producing anti-inflammatory and immunosuppressive
activities (6,7). In addition, TBMS1 has been revealed
to have antitumor effects and is considered a candidate for
treating various types of cancer (8). Notably, TBMS1 was revealed to exert a
direct cytotoxic effect on glioma cell lines and induce apoptosis
(9). However, the exact role TBMS1
plays in glioma cells remains to be elucidated.
Serine/threonine kinase Akt kinase regulates diverse
cellular processes, including cell survival, proliferation,
angiogenesis and migration (10).
Akt is a main downstream effector of PI3K whose dysregulation
causes aberrant Akt activity. Therefore, targeting this pathway may
have an effect on cancer treatment (11). In addition, dysfunction of the
PI3K/Akt pathway has been revealed to cause human glioma cell
apoptosis (12). TBMS1 has been
also reported to act as a potent apoptosis inducer by modulating
PI3K/Akt signaling (13). PI3K/Akt
has not been reported to be modified in TBMS1-treated glioma
cancer, to the best of the authors' knowledge.
In the present study, the mechanisms of action
underlying TBMS1-induced cytotoxicity, apoptosis and cell cycle
arrest in human glioma cells were investigated. It was also
demonstrated that the PI3K/Akt-mediated signaling pathway was
involved in the anti-glioma effects of TBMS1 in human glioma
cells.
Materials and methods
TBMS1
TBMS1 with HPLC ≥98% was obtained from the National
Institute for the Control of Pharmaceutical and Biological
Products. TBMS1 powder was processed into a 1-mg/ml stock solution
with DMEM (Gibco; Thermo Fisher Scientific, Inc.) and stored at
−20°C.
Cell culture
The human glioma U251 cell line was obtained from
the Type Culture Collection of the Chinese Academy of Sciences.
Normal human astrocyte HA cell line was obtained from Lonza Group
Ltd. All cell lines were incubated in DMEM supplemented with 10%
FBS with antibiotics 100 U/ml penicillin and 100 µg/ml streptomycin
(all from Gibco; Thermo Fisher Scientific, Inc.) in a humidified
incubator containing 5% CO2 at 37°C.
Cell proliferation assay
The cell viability was detected using MTT assays
(Sigma-Aldrich; Merck KGaA). U251 and HA cell lines were seeded
(5×103 cells/well) for 24 h. The cells were treated with
TBMS1 (0–50 µg/ml) at 37°C for 24, 48 or 72 h or TBMS1 (30 µg/ml)
in the presence or absence of PI3K/Akt inhibitor LY294002 (20 µM;
cat. no. L9908; Sigma-Aldrich; Merck KGaA) for 24 h. Then, 20 µl
MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
cell sample for 4 h at 37°C. The cells were then dissolved using
100 µl DMSO, shaken for 10 min on a mini shaker and analyzed using
a multi-well plate reader at 570 nm on a microplate reader (Thermo
Fisher Scientific, Inc.). The proliferation rate (%) was calculated
using the following formula: Proliferation rate =
ODadministrated/ODcontrol × 100%.
BrdU incorporation assay
DNA synthesis was assessed via the incorporation of
BrdU into newly synthesized strands. U251 cells (5×103
cells/well) were seeded in 96-well plates, cultured for 24 h and
treated with 0, 20, 30 or 40 µg/ml TBMS1 at 37°C for 24 h. BrdU
labelling was commenced through the addition of 10 µl/ml labelling
solution, at a final concentration of 10 µM, to the medium. After
the cells were incubated at 37°C for 6 h, labelling was stopped and
the uptake of BrdU was measured using a Cell Proliferation ELISA
kit (Roche Diagnostics) according to the manufacturer's
protocol.
Cell cycle assay
U251 cells (5×103 cells/well) were seeded
in six-well plates and treated with 0, 20, 30 or 40 µg/ml TBMS1 at
37°C for 24 h. Cells were collected, washed twice with cold PBS,
fixed with 70% cold alcohol at −20°C overnight and then stained
with 10 µl propidium iodide (PI; 1 mg/ml) in the presence of 1%
RNase A for 30 min at 37°C, for cell cycle detection. Analysis was
performed using Cell Quest and Mod Fit programs (v5.1, BD
Biosciences) as described previously (14). The proportions of cells in the
G0/G1, S and G2/M phases were presented as DNA histograms.
Hoechst 33342 staining assay
U251 glioma cells (5×103 cells/well) were
seeded in 6-well culture plates and then treated with TBMS1 (0, 20,
30 or 40 µg/ml) at 37°C for 24 h. The cells were washed twice with
cold PBS and then fixed with cold methanol and acetic acid (3/1,
v/v) at 4°C for 30 min. After washing with PBS, a solution of
Hoechst 33342 staining dye was added to the cells at 37°C for 30
min in the dark. After a final wash in PBS, a single, randomly
selected field of view of the cells was visualized under a
fluorescence microscope (magnification, ×400; Nikon
Corporation).
Annexin V/PI flow cytometric
assay
The effect of TBMS1 on the apoptosis of U251 cells
was analyzed using flow cytometry. Cells (5×103
cells/well) were seeded in 6-well plates and treated with 0, 20, 30
or 40 µg/ml TBMS1 at 37°C for 24 h and then washed with cold PBS
and resuspended in incubation buffer. The rate of apoptosis was
examined by staining the samples with Annexin V-fluorescein
5-isothiocyanate (FITC) and PI for 20 min in the dark at room
temperature using an Annexin V-FITC staining kit according to the
manufacturer's protocol (BD Biosciences). Subsequently, flow
cytometric analyses were performed using a FACS-Canto flow
cytometer (Beckman Coulter, Inc.) with Cell Quest software (v5.1,
BD Biosciences).
Western blot assay
U251 cells were exposed to TBMS1 (0, 20, 30 or 40
µg/ml) or TBMS1 (30 µg/ml) in the presence or absence of PI3K/Akt
inhibitor LY294002 (20 µM) at 37°C for 24 h. The proteins were
extracted using a cocktail of protein lysate solution supplemented
with protease inhibitors (Beyotime Institute of Biotechnology). The
cell lysate was centrifuged at 700 × g for 5 min at 4°C and the
supernatant fraction was collected for western blotting. Cell
lysates were collected, and the total protein was assessed using
the BCA protein assay kit (Tiangen Biotech Co., Ltd.). A total of
20 µg total protein samples were separated on 12% SDS-PAGE,
transferred onto PVDF membranes (EMD Millipore) and blocked using
Tris-buffered saline containing 5% non-fat milk for 30 min at room
temperature. These membranes were incubated with the corresponding
primary antibodies overnight at 4°C. After being washed three
times, the membranes were incubated with a secondary antibody for 1
h at room temperature. ECL (EMD Millipore) was used to detect
protein bands. Band densities were quantified using Image J 1.45s
software (National Institutes of Health). The antibodies [Akt (cat.
no. sc377457; 1:1,000), phosphorylated (p-)Ser 473-Akt (cat. no.
sc52940; 1:1,000), p-Thr 308-Akt (cat. no. sc271966; 1:1,000), p21
(cat. no. sc817; 1:200), cyclin B1 (cat. no. sc245; 1:1,000), Bad
(cat. no. sc8044; 1:1,000), Bax (cat. no. sc7480; 1:1,000), Bcl-2
(cat. no. sc509; 1:500), caspase-3 (cat. no. sc7272; 1:1,000),
caspase-9 (cat. no. sc56076; 1:1,000) and GAPDH (cat. no. sc365062;
1:1,000), mouse IgG-horseradish peroxidase (HRP; cat. no. sc2748;
1:2,000) and rat IgG-HRP (cat. no. sc2750; 1:2,000)] were purchased
from Santa Cruz Biotechnology, Inc. The antibody poly-ADP ribose
polymerase (PARP; product no. 9542; 1:1,000) and apoptosis-inducing
factor (AIF; cat. no. 4642; 1:1,000) were purchased from Cell
Signaling Technology, Inc. The antibody cyclin-dependent kinase 1
(CDK1; cat. no. PA5-14438; 1:1,000) was purchased from Thermo
Fisher Scientific, Inc.
Statistical analysis
All data represent at least 3 independent
experiments and are expressed as the mean ± standard deviation.
Statistical analysis with multiple comparisons were performed using
one-way ANOVAs followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
TBMS1 suppresses the growth of glioma
cells through the PI3K/Akt signaling pathway
To investigate the effect of TBMS1 on glioma cells,
MTT was used to analyze changes in cell viability. As revealed in
Fig. 1A, the viability of U251
cells decreased in a time- and concentration-dependent manner.
However, the effect of TBMS1 on HA cells was less pronounced than
in U251 cells. The IC50 concentrations of TBMS1-treated
U251 cells at 24, 48 and 72 h were 31.55±1.60, 28.38±1.21 and
25.30±1.26, respectively. When U251 cells were treated with TBMS1
for 24 h, the average viability of the cells at concentrations of
20, 30 and 40 µg/ml was reduced to 74.62%, 51.40% and 26.32%,
respectively, compared with the control group (0 µg/ml). Therefore,
concentrations of 20, 30 and 40 µg/m at 24 h were selected for
further experiments to research the mechanism of action of TBMS1 in
glioma cells.
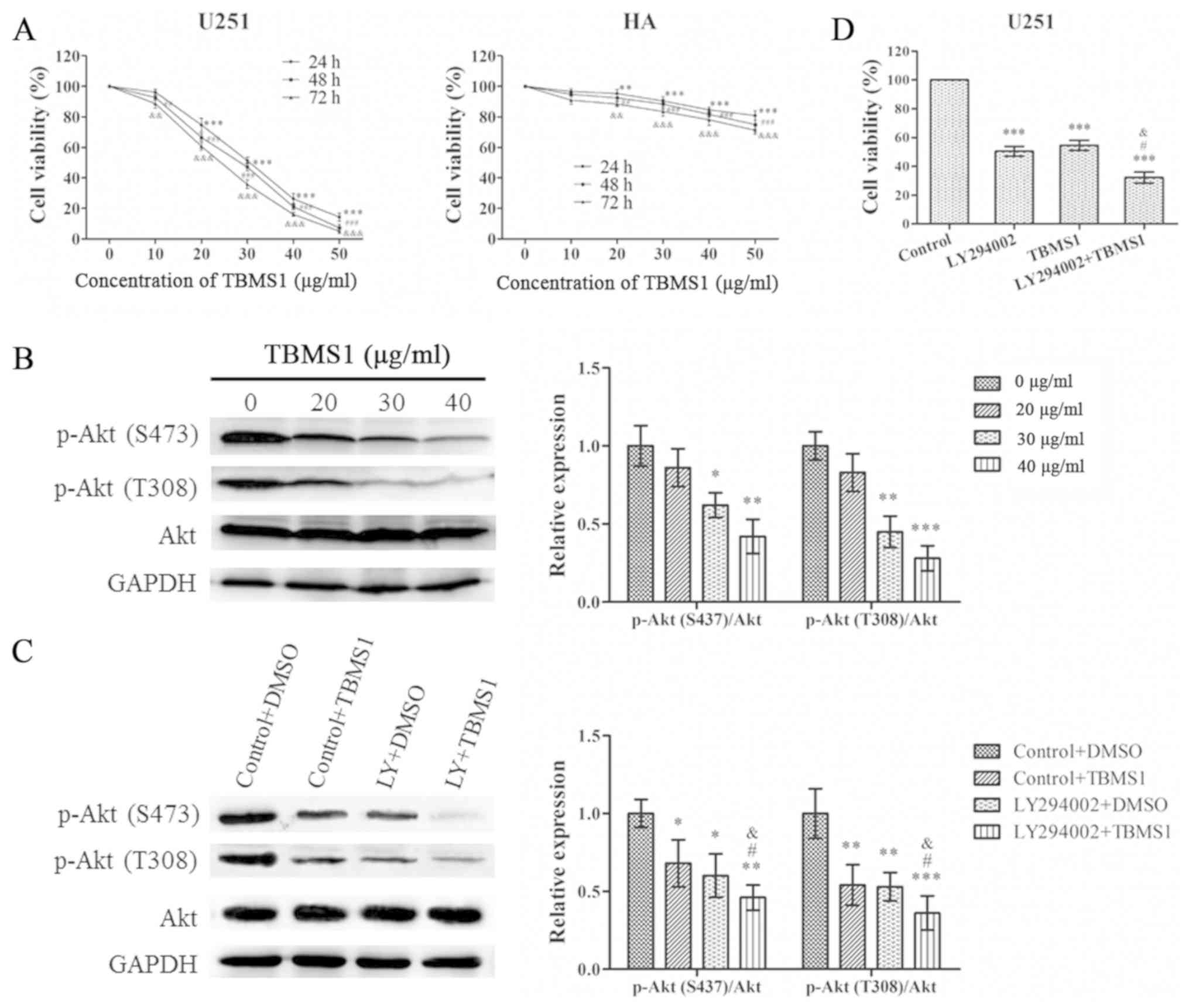 | Figure 1.TBMS1 suppresses the growth of glioma
cells by inhibiting the PI3K/Akt signaling pathway. U251 and HA
cells were incubated with 0–50 µg/ml of TBMS1 for 24, 48 or 72 h.
(A) Cell viability was determined by MTT. **P<0.01 and
***P<0.001 vs. TBMS1 (at 24h, 0 µg/ml); ##P<0.01
and ###P<0.001 vs. TBMS1 (at 48 h, 0 µg/ml);
&&P<0.01 and
&&&P<0.001 vs. TBMS1 (at 72h, 0 µg/ml).
(B) The expression levels and quantitative values of p-Akt (S473),
p-Akt (T308) and total Akt in U251 cells were monitored by western
blot analysis. U251 cells were treated with TBMS1 (30 µg/ml) in the
presence or absence of PI3K/Akt inhibitor LY294002 for 24 h.
*P<0.05, **P<0.01 and ***P<0.001 vs. TBMS1 (0 µg/ml). (C)
The expression levels and quantitative values of p-Akt (S473),
p-Akt (T308) and total Akt in U251 cells were monitored by western
blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs.
Control+DMSO; #P<0.05 vs. LY294002+DMSO;
&P<0.05 vs. Control+TBMS1. (D) Cell viability was
determined by MTT. ***P<0.001 vs. Control; #P<0.05
vs. LY294002; &P<0.05 vs. TBMS1. TBMS1,
tubeimoside-1; p-, phosphorylated. |
In order to evaluate the effect of TBMS1 on the
PI3K/Akt pathway in U251 cells, Akt phosphorylation and total Akt
expression in U251 cells were also examined. As revealed in
Fig. 1B, TBMS1 inhibited the
phosphorylation of Akt in a concentration-dependent manner, while
expression of total Akt was unchanged. To confirm the results,
LY294002 was used to treat U251 cells alone or in combination with
TBMS1 (30 µg/ml). As revealed in Fig.
1C, the phosphorylation of Akt at Thr308 and Ser473
significantly decreased in cells treated with TBMS1 alone, LY294002
alone and TBMS1 combined with LY294002 compared with the control
group, which was treated only with DMSO. As revealed in Fig. 1D, compared with the control group
(0 µg/ml), cell viability was significantly decreased in cells
exposed to TBMS1 alone, LY294002 alone or the combination
treatment. These results indicated that TBMS1 had the potential to
inhibit human glioma cell growth through the PI3K/Akt signaling
pathway.
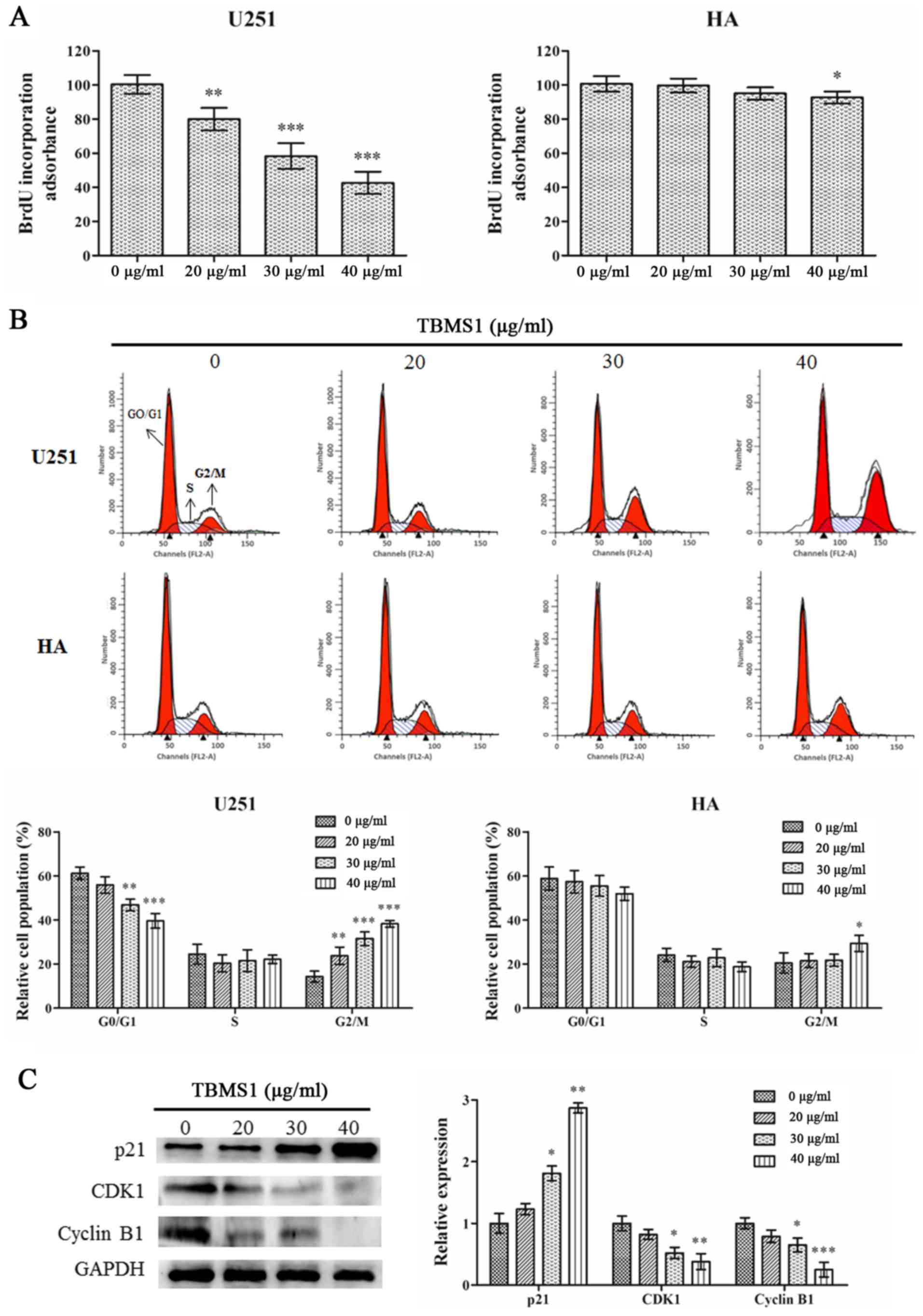
TBMS1 arrests the G2/M phase of glioma
cells by regulating the p21/CDK1/cyclin B1 signaling pathway
To explore the mechanisms of action underlying the
inhibition of cell viability in the TBMS1 treatment group, cell
cycle changes in U251 cells and HA cells were investigated. BrdU
incorporation was used to examine the effect of TBMS1 on DNA
synthesis. As revealed in Fig. 2A,
TBMS1 suppressed DNA synthesis in U251 cells in a dose-dependent
manner (P<0.01) while having a reduced effect in HA cells, with
a significant response only observed at the highest dose (40 µg/ml;
P<0.05).
Cell cycle progression was assessed using flow
cytometry. Flow cytometric analysis demonstrated that with TBMS1
treatment at 20, 30 or 40 µg/ml, the percentage of U251 cells in
the G2/M phase increased from 14.30% to 23.76%, 31.56% and 38.24%,
respectively (Fig. 2B). The
percentage of HA cells slightly increased only at the highest dose
of TBMS1 (40 µg/ml), indicating that TBMS1 induced cell cycle
arrest in the G2/M phase in glioma U251 cells.
To investigate the molecular basis of the effect of
TBMS1 on cell cycle arrest, western blotting was used to study the
expression levels of regulatory proteins. As a checkpoint of G2/M
phase, the expression levels of CDK1 and cyclin B1 were studied.
Fig. 2C demonstrated that the
protein expression levels of CDK1 and cyclin B1 were significantly
decreased in the TBMS1-treated group, in a dose-dependent manner.
As a major regulator of the CDK1/cyclin B1 signaling pathway, p21
protein expression was significantly increased. These data further
demonstrated that TBMS1 could induce G2/M arrest in glioma cells by
regulating the p21/CDK1/cyclin B1 signaling pathway.
TBMS1 induces apoptosis of glioma
cells by blocking the Bcl-2 signaling pathway
The effects on apoptosis of treatment with TBMS1
were investigated using Hoechst 33342 staining and Annexin
V-FITC/PI staining. As revealed in Fig. 3A, the Hoechst 33342 staining assay
demonstrated that following treatment with TBMS1 for 24 h,
chromatin was agglutinated, nuclear fragmentation was observed and
apoptotic bodies were formed in U251 cells, but there was no
significant change in HA cells. Flow cytometric results
demonstrated that in the treated U251 cells, the cells experienced
typical morphological changes associated with apoptosis. The
results of flow cytometric analysis demonstrated that rates of
apoptosis were 16.67, 29.65 and 39.07% in the cells treated with
20, 30 or 40 µg/ml of TBMS1 respectively for 24 h as compared to
9.51% in the control cells (Fig.
3B). No effect was observed in the HA cells. These results
indicated that TBMS1 could induce apoptosis in glioma cells.
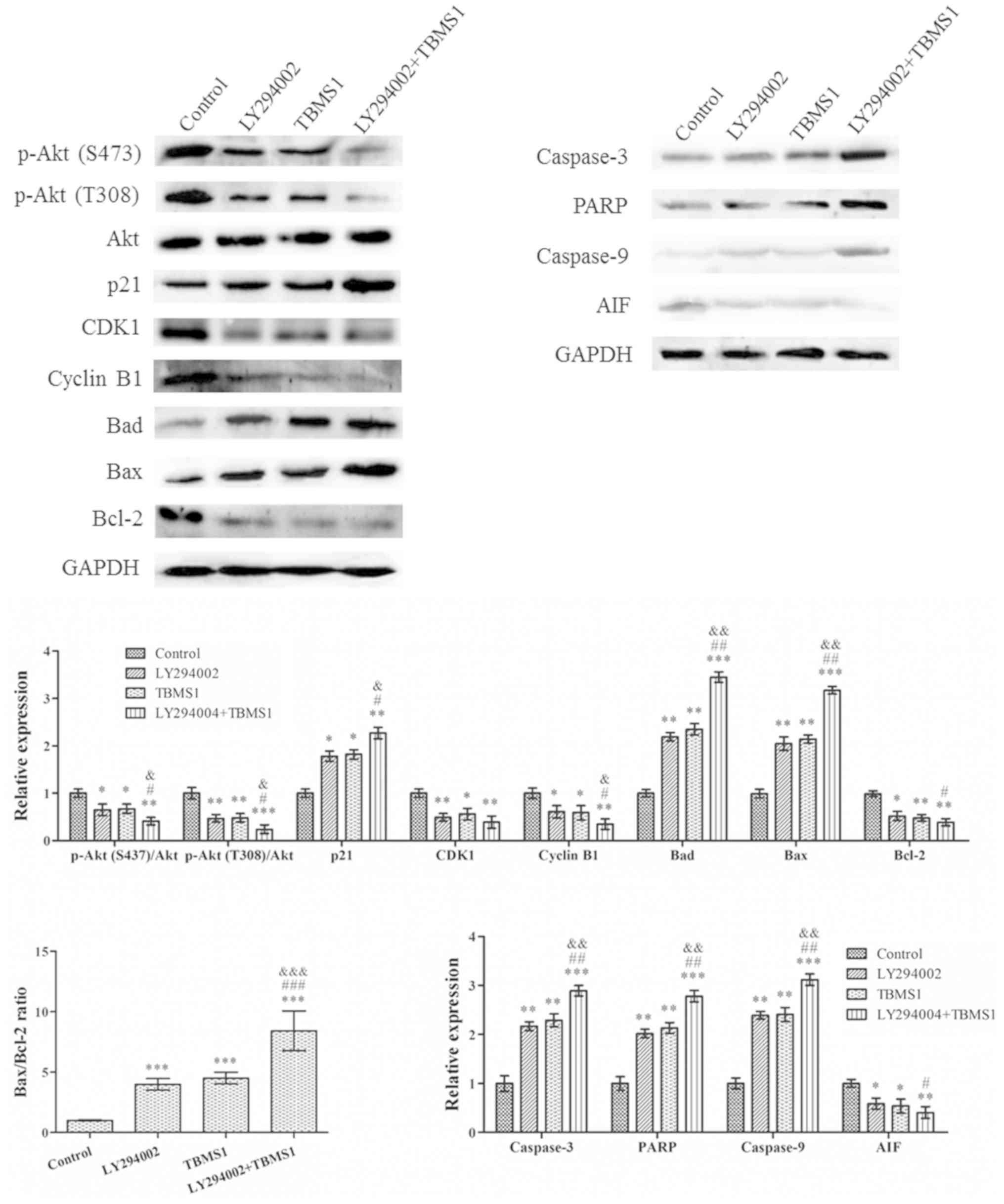
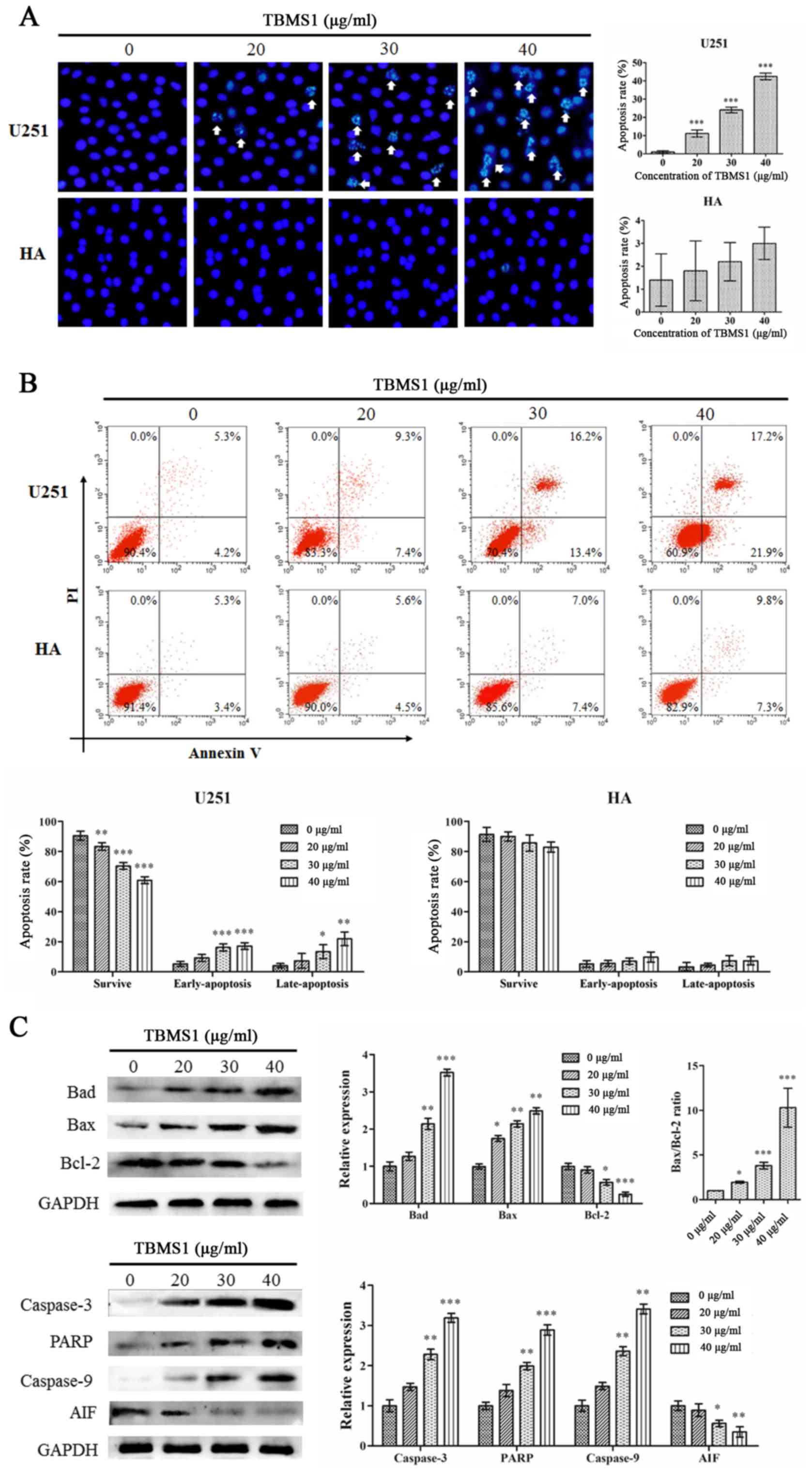 | Figure 3.TBMS1 induces the apoptosis of glioma
cells by blocking Bcl-2. U251 and HA cells were incubated with 20,
30 or 40 µg/ml of TBMS1 for 24 h. The cells were stained using (A)
Hoechst 33342 staining and (B) Annexin V-FITC/PI double staining. A
statistical analysis was performed for apoptosis. (C) The
expression levels and quantitative values of Bad, Bax, PARP,
caspase-3, caspase-9, AIF and Bcl-2 were monitored via western blot
analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. TBMS1 (0
µg/ml). TBMS1, tubeimoside-1; FITC, fluorescein 5-isothiocyanate;
PI, propidium iodide; PARP, poly-ADP ribose polymerase; AIF,
apoptosis-inducing factor. |
To confirm the results, expression changes of the
apoptosis regulators Bad, Bax, caspase-3, PARP, caspase-9, AIF and
Bcl-2 were analyzed by immunoblotting. As revealed in Fig. 3C, with increasing doses of TBMS1,
the protein expression levels of Bad, Bax, caspase-3, PARP and
caspase-9 were increased while the expression levels of AIF and
Bcl-2 proteins were decreased. In addition, TBMS1 increased the
Bax/Bcl-2 ratio. These results demonstrated that TBMS1 increased
cell death by suppressing the Bcl-2 signaling pathway.
TBMS1 attenuates the progression of
glioma cells through the PI3K/Akt-mediated signaling pathways
The PI3K/Akt signaling pathway is a potential
survival signaling pathway in a number of systems and its
inactivation can inhibit growth and induce apoptosis. In order to
detect whether gliomas are inhibited by TBMS1 inducing the
inactivation of the PI3K/Akt signaling pathway, U251 cells were
treated with TBMS1 (30 µg/ml) in the presence or absence of 20 µM
LY294002. Western blotting was then performed to explore
alterations in the protein expression levels. The results indicated
that the use of TBMS1 alone, LY294002 alone or a combination
treatment for 24 h, amplified the effect of Akt dephosphorylation
in U251 cells (Fig. 4), while
total Akt protein levels remained constant in all of the
treatments. In addition, the protein expression levels of p-Akt was
significantly reduced in cells treated with TBMS1 and LY294002
together, compared with that of TBMS1 or LY294002 alone. Compared
with TBMS1 (30 µg/ml) treatment, the CDK-interacting protein, p21,
expression levels demonstrated the greatest increase whereas CDK1
and cyclin B1 levels were reduced the most in cells that were
treated with TBMS1 and LY294002 combined. Following combined
treatment, the Bad, Bax, caspase-3, PARP and caspase-9 levels
increased, while the AIF and Bcl-2 levels decreased. In addition,
the Bax/Bcl-2 ratio also demonstrated the largest increase
following combination therapy, compared with single therapies
alone.
Discussion
As a common intracranial tumor, gliomas account for
~40% of intracranial tumors (15).
Despite recent advances in the surgical and medical treatment of
glioma, the prognosis of patients with malignant glioma is still
poor (16). Previous research has
revealed that a traditional Chinese herb, TBMS1, inhibits
proliferation and promotes apoptosis in U251 glioma cancer cells
(9). This apoptotic response is
associated with the regulation of the expression of the Bcl-2 gene
family and the mitochondrial pathway of apoptosis by inducing
intracellular reactive oxygen species (ROS) (9). Based on previous research, the
present study further explored changes in the PI3K/Akt pathway
proteins that regulate apoptosis and changes in related pathways
(9). The development and
progression of glioma is usually associated with molecular changes
in the PI3K/Akt signaling pathway (17), which is responsible for a variety
of biological processes, including cell metabolism, survival, cell
cycle progression, regulation of apoptosis, protein synthesis and
genomic instability (18). It is
an important intracellular pathway which is frequently aberrantly
activated in various types of human cancer (19). Therefore, targeting the PI3K/Akt
pathway can have implications for the treatment of numerous cancer
types (20). Akt is a downstream
protein of PI3K, a lipid kinase consisting of a catalytic subunit
(21). The Akt protein contains
two phosphorylation sites, Thr308 and Ser473, whose activation
requires phosphorylation by the phosphoinositide-dependent kinase 1
and rapamycin-insensitive complex (21). Thr308, located in the core
activation region of the catalytic protein kinase, and Ser473,
located in the C-terminal hydrophobic motif, can fully activate Akt
(22). Akt phosphorylation is
widely considered as a marker for the activation of the PI3K/Akt
signaling pathway (23). Aberrant
activation of the PI3K/Akt signaling pathway has been reported in
gliomas (23). The present study
demonstrated that TBMS1 inhibited the viability of U251 cells and
the phosphorylation of Akt. LY294002 demonstrated the same effect.
In addition, the combination of TBMS1 and LY294002 enhanced these
inhibitions. These results demonstrated that TBMS1 can inhibit
human glioma cell activity through Akt phosphorylation.
Progression through the cell cycle in normal cells
is regulated through various checkpoints during cell division and
impaired regulation of checkpoints may lead to uncontrolled cell
proliferation (24). Cell cycle
dysregulation is the basis for characterizing abnormal cell
proliferation in cancer (25). It
has been reported that maintaining proper cell cycle progression is
an effective therapeutic strategy to prevent tumor growth (25). In the present study, BrdU
incorporation and flow cytometry demonstrated that TBMS1 could
inhibit DNA synthesis and lead to the accumulation of U251 cells in
the G2/M phase in a concentration-dependent manner. The cell cycle
control system is based on two major protein families, the cyclins
and CDKs (26). Typically, the
expression levels of cyclins are dynamic, while CDKs act as
catalytic subunits and stabilize the expression levels of the
cyclins (26). During the
transition from G2 to M phase, cyclin B1 activates CDK1, resulting
in an increase in activated CDK1 (26). In glioma cells, the constitutive
activation of the PI3K/Akt signaling cascade and a decrease in p21
protein expression levels are common, leading to flawed cell cycle
progression (27). The present
study indicated that TBMS1 decreased cyclin B1, CDK1 and Akt
phosphorylation levels, and increased the levels of the CKD1
inhibitor p21. Therefore, the PI3K/Akt/p21/CDK1/cyclinB1 signaling
pathways were involved in TBMS1-induced glioma cell cycle G2/M
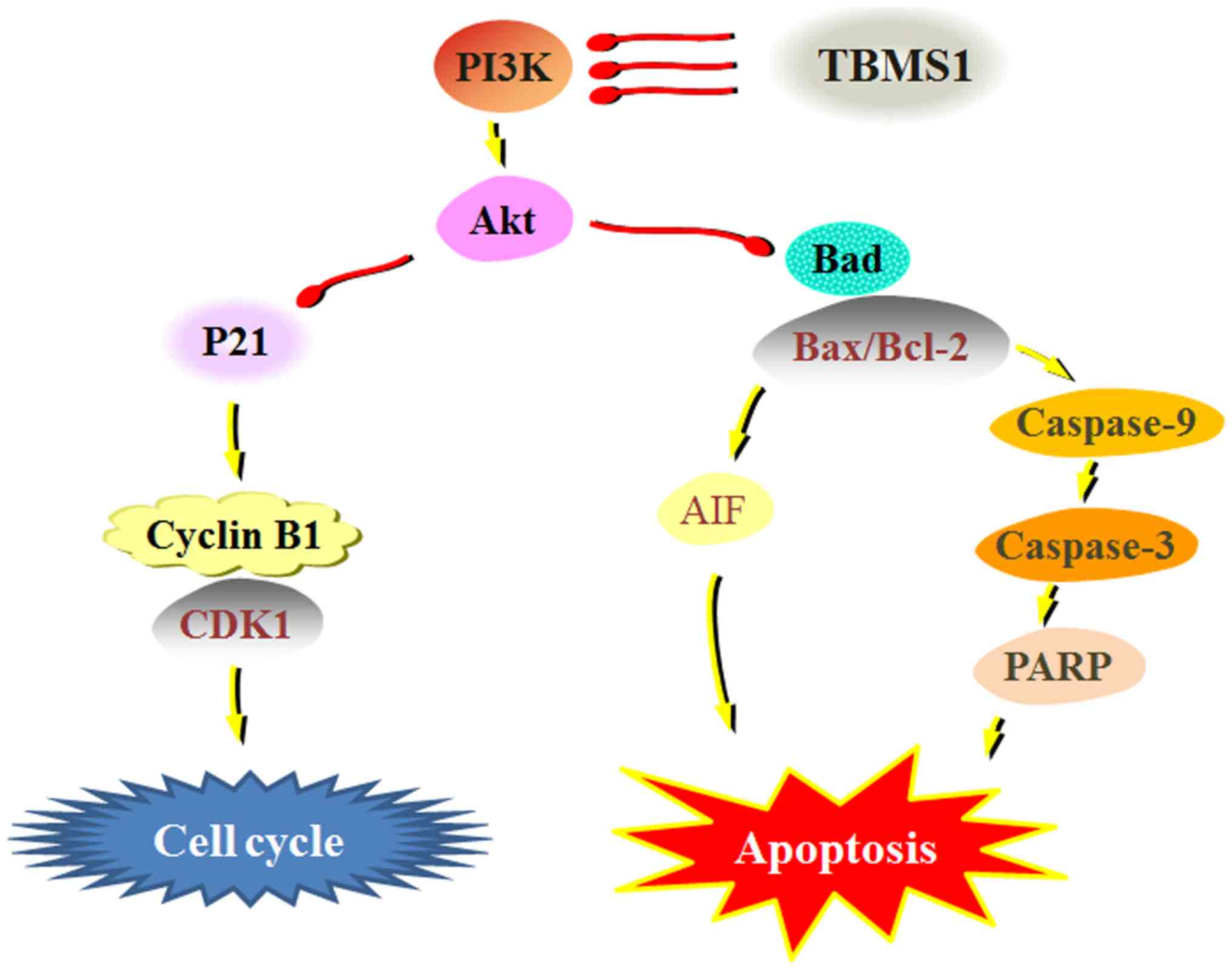
arrest (Fig. 5).
Apoptosis defects play an important role in the
pathogenesis of tumors: The cytotoxic effects of a number of
antitumor drugs are usually accompanied by the induction of
apoptosis (28). The present study
demonstrated that TBMS1 induced apoptosis, as revealed by the
typical morphologic features such as clear chromatin condensation,
nuclear fragmentation and apoptotic bodies in the treated U251
cells. Cell apoptosis signaling pathways are classified into the
death receptor (extrinsic) pathway and the mitochondrial
(intrinsic) pathway (29). The
Bcl-2 family, including anti-apoptotic components such as Bcl-2 and
Bcl-xL, and pro-apoptotic components such as Bax, Bak and Bad, are
involved in the regulation of apoptosis through the mitochondrial
pathway (30). The promotion of
Bad expression and the increase in the Bax/Bcl-2 ratio are
associated with apoptosis in a variety of human cancer types
(31). The ratio of Bax/Bcl-2 is
involved in the release of AIF and endonuclease G from
mitochondria, also referred to as the caspase-independent pathway
(32). Mitochondria-dependent
signaling occurs through the cleavage of caspase-9, which
subsequently activates downstream caspase-3, leading to the
cleavage of various key cellular substrates (including PARP),
thereby inducing apoptosis (33).
Previous studies have demonstrated that TBMS1 exerts anti-growth
and induces apoptotic activity against cancer cells by activating
caspases and Bcl-2 family proteins (34,35).
The present study demonstrated that after treatment with TBMS1,
p-Akt, AIF, Bad, caspase-3, caspase-8 and caspase-9 expression
levels were significantly decreased and that the Bax/Bcl-2 ratio
was increased, indicating that TBMS1 promoted cell death.
Inhibition of Akt phosphorylation results in downregulation of
Akt-regulated anti-apoptotic proteins, thereby promoting the death
of apoptotic cells (36).
Inhibition of interference with Akt phosphorylation has been
revealed to be sufficient to promote mitochondrial membrane
depolarization and apoptosis in numerous types of cancer cells
(17). To further explore the
relationship between apoptotic regulator proteins and Akt
phosphorylation, glioma cell lines were treated with TBMS1,
LY294002 or a combination treatment of the two drugs. The results
demonstrated that the PI3K/Akt inhibitor, LY294002, reduced Akt
phosphorylation and AIF expression, and also increased Bad,
caspase-3, caspase-8 and caspase-9 expression levels as well as the
Bax/Bcl-2 ratio. TBMS1 and LY294002 enhanced these responses. These
data revealed that the mechanism of action by which TBMS1 triggered
mitochondria-mediated apoptosis may be through the PI3K/Akt
signaling pathway.
Collectively, TBMS1 exhibited an inhibitory effect
on cellular growth, promoted apoptosis and induced cell cycle
arrest by suppressing the PI3K/Akt-mediated signaling pathways in
glioma cells. This provides the rationale for in vivo
studies on the utilization of TBMS1 as a potential cancer
therapeutic compound.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LJC and ZS contributed to the conception and design
of the study. HTX and ZXC performed experimental procedures. YM and
MMW conducted data analysis. LJC and ZS were involved in revising
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Diamandis P and Aldape K: World Health
Organization 2016 Classification of Central Nervous System Tumors.
Neurol Clin. 36:439–447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi J, Dong B, Cao J, Mao Y, Guan W, Peng
Y and Wang S: Long non-coding RNA in glioma: Signaling pathways.
Oncotarget. 8:27582–27592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olar A and Aldape KD: Using the molecular
classification of glioblastoma to inform personalized treatment. J
Pathol. 232:165–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu L, Ma R, Wang Y and Nishino H: Potent
anti-tumor activity and low toxicity of tubeimoside 1 isolated from
Bolbostemma paniculatum. Planta Med. 60:204–208. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XH, Sun NX, Guo RX, Xing JL and Liu
XN: Efficacy research of tubeimoside against the experimental
herpes simplex keratitis. Rec Adv Ophthalmol. 22:373–376. 2002.
|
|
7
|
He D, Huang B, Fu S, Li Y, Ran X, Liu Y,
Chen G, Liu J, Liu D and Tubeimoside I: Tubeimoside I protects
dopaminergic neurons against inflammation-mediated damage in
lipopolysaccharide (LPS)-evoked model of Parkinson's disease in
rats. Int J Mol Sci. 19:E22422018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Islam MS, Wang C, Zheng J, Paudyal N, Zhu
Y and Sun H: The potential role of tubeimosides in cancer
prevention and treatment. Eur J Med Chem. 162:109–121. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
10
|
Wu T, Cui H, Xu Y, Du Q, Zhao E, Cao J,
Nie L, Fu G and Ren A: The effect of tubeimoside-1 on the
proliferation, metastasis and apoptosis of oral squamous cell
carcinoma in vitro. OncoTargets Ther. 11:3989–4000. 2018.
View Article : Google Scholar
|
|
11
|
Jiang SL, Guan YD, Chen XS, Ge P, Wang XL,
Lao YZ, Xiao SS, Zhang Y, Yang JM, Xu XJ, et al: Tubeimoside-1, a
triterpenoid saponin, induces cytoprotective autophagy in human
breast cancer cells in vitro via Akt-mediated pathway. Acta
Pharmacol Sin. 40:919–928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baryawno N, Sveinbjörnsson B, Eksborg S,
Chen CS, Kogner P and Johnsen JI: Small-molecule inhibitors of
phosphatidylinositol 3-kinase/Akt signaling inhibit
Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma
growth. Cancer Res. 70:266–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi H, Bi H, Sun X, Dong H, Jiang Y, Mu H,
Li W, Liu G, Gao R and Su J: Tubeimoside-1 inhibits the
proliferation and metastasis by promoting miR-126-5p expression in
non-small cell lung cancer cells. Oncol Lett. 16:3126–3134.
2018.PubMed/NCBI
|
|
14
|
Srivastav AK, Dubey D, Chopra D, Singh J,
Negi S, Mujtaba SF, Dwivedi A and Ray RS: Oxidative stress-mediated
photoactivation of carbazole inhibits human skin cell physiology. J
Cell Biochem. 121:1273–1282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grier JT and Batchelor T: Low-grade
gliomas in adults. Oncologist. 11:681–693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bush NA, Chang SM and Berger MS: Current
and future strategies for treatment of glioma. Neurosurg Rev.
40:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Gao W, Zhang L, Zhang D, Zhao Z
and Bao Y: Deoxypodophyllotoxin inhibits cell viability and
invasion by blocking the PI3K/Akt signaling pathway in human
glioblastoma cells. Oncol Rep. 41:2453–2463. 2019.PubMed/NCBI
|
|
18
|
Ebrahimi S, Hosseini M, Shahidsales S,
Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM and Avan A:
Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic
Strategy for the Treatment of Pancreatic Cancer. Curr Med Chem.
24:1321–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Q, Jiang W and Hou P: Emerging role
of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer
Biol pii. S1044-579X(18)30136-6. 2019. View Article : Google Scholar
|
|
20
|
Fleischer A, Ghadiri A, Dessauge F,
Duhamel M, Rebollo MP, Alvarez-Franco F and Rebollo A: Modulating
apoptosis as a target for effective therapy. Mol Immunol.
43:1065–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Wu C, Chen N, Gu H, Yen A, Cao L,
Wang E and Wang L: PI3K/Akt/mTOR signaling pathway and targeted
therapy for glioblastoma. Oncotarget. 7:33440–33450. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parsons DW, Jones S, Zhang X, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et
al: An integrated genomic analysis of human glioblastoma
multiforme. Science. 321:1807–1812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wiman KG and Zhivotovsky B: Understanding
cell cycle and cell death regulation provides novel weapons against
human diseases. J Intern Med. 281:483–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arellano M and Moreno S: Regulation of
CDK/cyclin complexes during the cell cycle. Int J Biochem Cell
Biol. 29:559–573. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang R, Yi L, Dong Z, Ouyang Q, Zhou J,
Pang Y, Wu Y, Xu L and Cui H: Tigecycline inhibits glioma growth by
regulating miRNA-199b-5p-HES1-AKT pathway. Mol Cancer Ther.
15:421–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robertson JD and Orrenius S: Molecular
mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev
Toxicol. 30:609–627. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Creagh EM: Caspase crosstalk: Integration
of apoptotic and innate immune signalling pathways. Trends Immunol.
35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kvansakul M and Hinds MG: Structural
biology of the Bcl-2 family and its mimicry by viral proteins. Cell
Death Dis. 4:e9092013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soriano ME and Scorrano L: The interplay
between BCL-2 family proteins and mitochondrial morphology in the
regulation of apoptosis. Adv Exp Med Biol. 687:97–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forbes-Hernández TY, Giampieri F,
Gasparrini M, Mazzoni L, Quiles JL, Alvarez-Suarez JM and Battino
M: The effects of bioactive compounds from plant foods on
mitochondrial function: A focus on apoptotic mechanisms. Food Chem
Toxicol. 68:154–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Palmer CS, Osellame LD, Stojanovski D and
Ryan MT: The regulation of mitochondrial morphology: Intricate
mechanisms and dynamic machinery. Cell Signal. 23:1534–1545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Xu X and He P: Tubeimoside-1
inhibits proliferation and induces apoptosis by increasing the Bax
to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549
cells. Mol Med Rep. 4:25–29. 2011.PubMed/NCBI
|
|
35
|
Chen WJ, Yu C, Yang Z, He JL, Yin J, Liu
HZ, Liu HT and Wang YX: Tubeimoside-1 induces G2/M phase arrest and
apoptosis in SKOV-3 cells through increase of intracellular
Ca2+ and caspase-dependent signaling pathways. Int J
Oncol. 40:535–543. 2012.PubMed/NCBI
|
|
36
|
Chao Y, Wang Y, Liu X, Ma P, Shi Y, Gao J,
Shi Q, Hu J, Yu R and Zhou X: Mst1 regulates glioma cell
proliferation via the AKT/mTOR signaling pathway. J Neurooncol.
121:279–288. 2015. View Article : Google Scholar : PubMed/NCBI
|