Introduction
Idiopathic scoliosis (IS) is defined as scoliosis
with an unknown cause that presents as a spinal 3-dimensional
deformity during growth and development, and has an increasing
prevalence (current incidence rate, >2%) and poses a worldwide
threat to human health (1–3). The most common time for the
development of IS to occur is during the fastest-growing state of
juveniles (age, 10–16 years), which is the most active period of
bone development and osteoblast proliferation (4–7).
Moreover, effective inhibition of osteoblast proliferation and
induction of apoptosis contribute to the prevention and treatment
of IS (8). Therefore, medication
that can induce osteoblast apoptosis should be investigated as a
potential therapeutic strategy for IS. Previous studies have
reported that melatonin exerts an influence on the progression of
IS (9–14). Furthermore, our previous studies
revealed that high concentrations of melatonin significantly
inhibit osteoblast viability (15,16),
thus indicating a possible clinical application of melatonin in
preventing and controlling the progression of IS (17); however, the mechanism is yet to be
elucidated.
The endoplasmic reticulum (ER) is a critical
subcellular organelle responsible for regulation of Ca2+
homoeostasis and transmembrane protein synthesis and folding
(18,19). External stimulation induces the
disruption of ER physiological function, which triggers ER stress
(ERS) (20,21). Moreover, ERS can reduce cell damage
and restore cell function by activating the unfolded protein
response (UPR) (22,23). However, excessive or prolonged ERS
can induce cell apoptosis (24–26),
and it has been reported that melatonin can initiate ERS-induced
apoptosis in human hepatoma cells (27). In addition, excessive ERS caused by
a high concentration of melatonin is a direct factor for inducing
apoptosis (28). It is also
speculated that these effects be due to the high concentration of
melatonin caused by Ca2+ overload and the induction of
osteoblastic ERS, but this is yet to be elucidated.
The ER is highly sensitive to stress; it maintains
its structure and function by regulating membrane proteins, and
selectively transports membrane proteins to deliver biological
effects (29). However, the type
of ER target protein that melatonin interacts with to export
relevant membrane proteins, transfer biological information to the
cell membrane, open Ca2+ channels on the cell membrane
surface and induce ERS is not yet fully understood. Golgi membranes
of HeLa cells using small interfering RNA screen technology and,
when exposed to external stimuli, will rapidly redistribute on the
plasma membrane, causing Ca2+ influx in the cell
membrane (30). Furthermore, it
has been reported that Septin4, a subtype of the Septin
superprotein family, is a necessary component of cytoskeletal
proteins and may affect vesicle trafficking, apoptosis and numerous
important physiological processes (31–34).
The present study hypothesized that septin4 may also be a target
protein of melatonin-induced osteoblasts, activate ERS and induce
apoptosis. However, the mechanism via which melatonin induces
osteoblast apoptosis to regulate IS is yet to be elucidated.
Therefore, the aim of the present study was to further investigate
the mechanism underlying the effect of melatonin on osteoblast
development and its possible target of osteoblasts, which may
provide a novel insight and direction for treatment of IS.
Materials and methods
Cell culture and treatment
The clonal human fetal osteoblastic (hFOB 1.19) cell
line (35) was donated by Dr
Malayannan Subramaniam (Department of Biochemistry and Molecular
Biology, Mayo Clinic; Rochester, USA) (36). Cells were cultured in a humidified
incubator with 5% CO2 atmosphere at 37°C and in a 1:1
mixture of DMEM and F-12 medium (HyClone; GE Healthcare Life
Sciences) with 10% FBS (HyClone; GE Healthcare Life Sciences) and a
100 µg/ml streptomycin and 100 U/ml penicillin solution (HyClone;
GE Healthcare Life Sciences). The cell culture medium was changed
every other day. The osteoblastic cells were sub-cultured using
0.25% trypsin with EDTA (HyClone; GE Healthcare Life Sciences) to
replace the cells. Osteoblasts were cultured to passages 8–11 and
plated at 1×104 cells/cm2 for 24 h before
treatment. Melatonin (2, 4 and 6 mM), obtained from Sigma-Aldrich
(Merck KGaA), was dissolved in medium containing 0.2% DMSO and 10%
FBS to treat the cells for 24 h in 5% CO2 at 37°C.
Plasmids, cell transfections
The full-length human Septin4 plasmid (Shanghai
Genechem Co., Ltd.) was cloned to Flag-tagged destination vectors
(Shanghai Genechem Co., Ltd.) according to the requirements of
immunoblotting. Cell transfections were performed using
Lipofectamine® 2000 (37°C for 48 h; 90% cell density)
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The control group comprised cells
transfected with control plasmid. The time interval between
transfection and subsequent experimentation was 24 h.
Cell viability assay
Cell viability was measured using an MTT assay
(Sigma-Aldrich; Merck KGaA). hFOB 1.19 cells were seeded in 96-well
plates at a density of 6×103 cells/well and allowed to
adhere for 24 h at 37°C before being treated. Then, cells were
transfected with the control plasmid or Flag-Septin4 plasmid. The
time interval between transfection and subsequent experimentation
was 24 h. Subsequently, hFOB 1.19 cells were divided into four
groups randomly: i) Transfected with control plasmid; ii)
transfected with control plasmid and exposed to melatonin (2, 4 and
6 mM); iii) transfected with Flag-Septin4 plasmid; and iv)
transfected with Flag-Septin4 plasmid and exposed to melatonin (2,
4 and 6 mM). The cells were transfected for 48 h and treated with
melatonin for 48 h at 37°C. Medium was replaced with 90 µl fresh
culture medium, and 10 µl MTT (5 mg/ml) solution was added to each
well. After incubation at 37°C for 4 h, the supernatant was removed
and 110 µl DMSO was added to each well. The mixture was agitated on
a plate for 10 min before the optical density of each well was
measured at 490 nm using an Absorbance Reader. Data from five
independent experiments were analyzed.
Annexin V-FITC/propidium iodide (PI)
double-staining for cell apoptosis analysis
Apoptotic cells were detected using an Annexin
V-FITC/PI kit (Dojindo Molecular Technologies, Inc.) and quantified
using a BD LSRFortesa (BD Biosciences); data were analyzed using BD
FACSDiva Software (version 6.2; BD Biosciences). hFOB 1.19 cells
were seeded in 6-well plates at a density of 1×105
cells/well, and then the four different groups of cells were
transfected for 48 h and treated with 4 mM melatonin for 48 h at
37°C. The treated cells were digested with 0.25% trypsin (without
EDTA), centrifuged at 300 × g for 3 min at 4°C and then washed with
cold PBS twice. After the supernatant had been discarded, cells
were resuspended in 500 µl binding buffer, and Annexin-V-FITC (5
µl) and PI (5 µl) were added and mixed. The suspensions were
incubated in dark for 20 min at room temperature prior to flow
cytometric analysis. Finally, cells were analyzed using a BD
FACScan flow cytometer equipped with ModFit LT v3.0 (Becton,
Dickinson and Company) within 1 h. Cells were classified as
follows: Viable (Annexin V-FITC-/PI-), early apoptotic (Annexin
V-FITC+/PI-), late apoptotic (Annexin V-FITC+/PI+) or necrotic
(Annexin V-FITC-/PI+). The experiment was repeated three times.
Western blotting
Monoclonal mouse anti-Septin4 (1:4,000; cat. no.
ab166788; Abcam), polyclonal rabbit anti-glucose-regulated protein
(GRP)94 (1:1,000; cat. no. ab2791; Abcam), GRP78 (1:1,000; cat. no.
ab21685; Abcam), polyclone rabbit anti-caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), monoclonal mouse anti-Flag
(1:1,000; cat. no. 8146; Cell Signaling Technology, Inc.) and
monoclonal mouse anti-β-actin (1:10,000; cat. no. 4970; ProteinTech
Group, Inc.) antibodies were obtained commercially. In addition,
goat anti-mouse and anti-rabbit secondary antibodies (both 1:500;
cat. nos. 7076 and 4414, respectively) were purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.
Following treatment with 4 mM melatonin for 48 h at
37°C, proteins were extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology) for 30 min at 4°C and centrifuged at
2,500 × g for 20 min at 4°C. The supernatant fluid containing total
cell lysate was harvested. The concentrations of protein were
determined using the bicinchoninic acid protein assay (Beyotime
Institute of Biotechnology). Each sample (60 µg protein/lane) was
separated via 10% SDS-PAGE and transferred to 0.22 µm PVDF
membranes at 80 V for 90 min at 4°C. After blocking with 5% BSA
(Beyotime Institute of Biotechnology) in TBST (10% Tween 20) at
room temperature for 1 h and agitation for 1 h at 4°C, PVDF
membranes were incubated overnight with corresponding antibodies
(GRP78, GRP94, Septin4, caspase-3, Flag and β-actin) at 4°C.
Subsequently, after washing four times with TBST for 15 min each
time, membranes were visualized using goat anti-mouse or goat
anti-rabbit IgG antibodies conjugated with horseradish peroxidase
at 1:10,000 for 2 h at room temperature. Membranes were washed four
times with TBST for 15 min, and an EC3 Imaging system (UVP LLC) was
used to scan the specific bands. All of the bands were normalized
to β-actin content of the same sample and ImageJ software (version
1.51; National Institutes of Health) was used to measure the
protein expression.
Phalloidin staining
Phalloidin (AAT Bioquest, Inc.) was used to stain
fibrillar (F)-actin. In total, 1X Phalloidin conjugate working
solution was prepared according to the manufacturer's instructions.
hFOB 1.19 cells were seeded in 24-well plates at a density of
1.2×104 cells/well and allowed to adhere for 24 h before
being treated at 37°C. Then, the various groups of cells were
transfected for 48 h and treated with 4 mM melatonin for 48 h at
37°C. Subsequently, treated cells were washed three times with PBS
solution and fixed using 4% paraformaldehyde for 30 min at room
temperature. After rinsing the cells three times in PBS, 0.1%
Triton X-100 in PBS was added to fixed cells for 15 min at room
temperature to increase permeability, and then cells were washed
three times with PBS. Subsequently, cells were incubated with
phalloidin-conjugate working solution (500 µl/well) at room
temperature in the dark for 30 min to stain the cells. After
rinsing cells gently with PBS three times to remove excess
phalloidin, 10% DAPI (Beijing Solarbio Science & Technology
Co., Ltd.) was used at room temperature for 30 min in the dark for
nucleus staining. The cells were washed again with PBS solution
three times, and then were observed and imaged via fluorescence
microscopy (magnification, ×20) (Olympus Corporation).
Statistical analysis
Quantitative data from ≥3 independent experiment
were analyzed using GraphPad Prism 5 software (GraphPad Software,
Inc.). Differences in data between two groups were analyzed using
an independent samples t-test, and differences between multiple
groups were assessed using one-way ANOVA with Tukey's test. Data
are presented as the mean ± SD, and P<0.05 was considered to
indicate a statistically significant difference.
Results
High concentration of melatonin
promotes ERS and ERS-induced apoptosis, and decreases the viability
of hFOB 1.19 cells
To determine the effect of different concentrations
of melatonin on the viability of osteoblasts, an MTT assay was
performed. Firstly, 0, 2, 4 and 6 mM melatonin were used to treat
osteoblasts for 48 h, and it was revealed that the viability of
osteoblasts decreased in association with increased melatonin
concentration (Fig. 1A). In
addition, compared with the control group, cells treated with
higher concentrations of melatonin exhibited significantly enhanced
apoptosis (Fig. 1B). Moreover, the
expression levels of ERS-related genes (GRP78 and GRP94) and
apoptosis-related genes (cleaved caspase-3) were significantly
increased in a dose-dependent manner following a rise in melatonin
concentration (Fig. 1C).
Therefore, the results indicated that a high concentration of
melatonin promoted ERS, which induced apoptosis and decreased the
viability of osteoblasts.
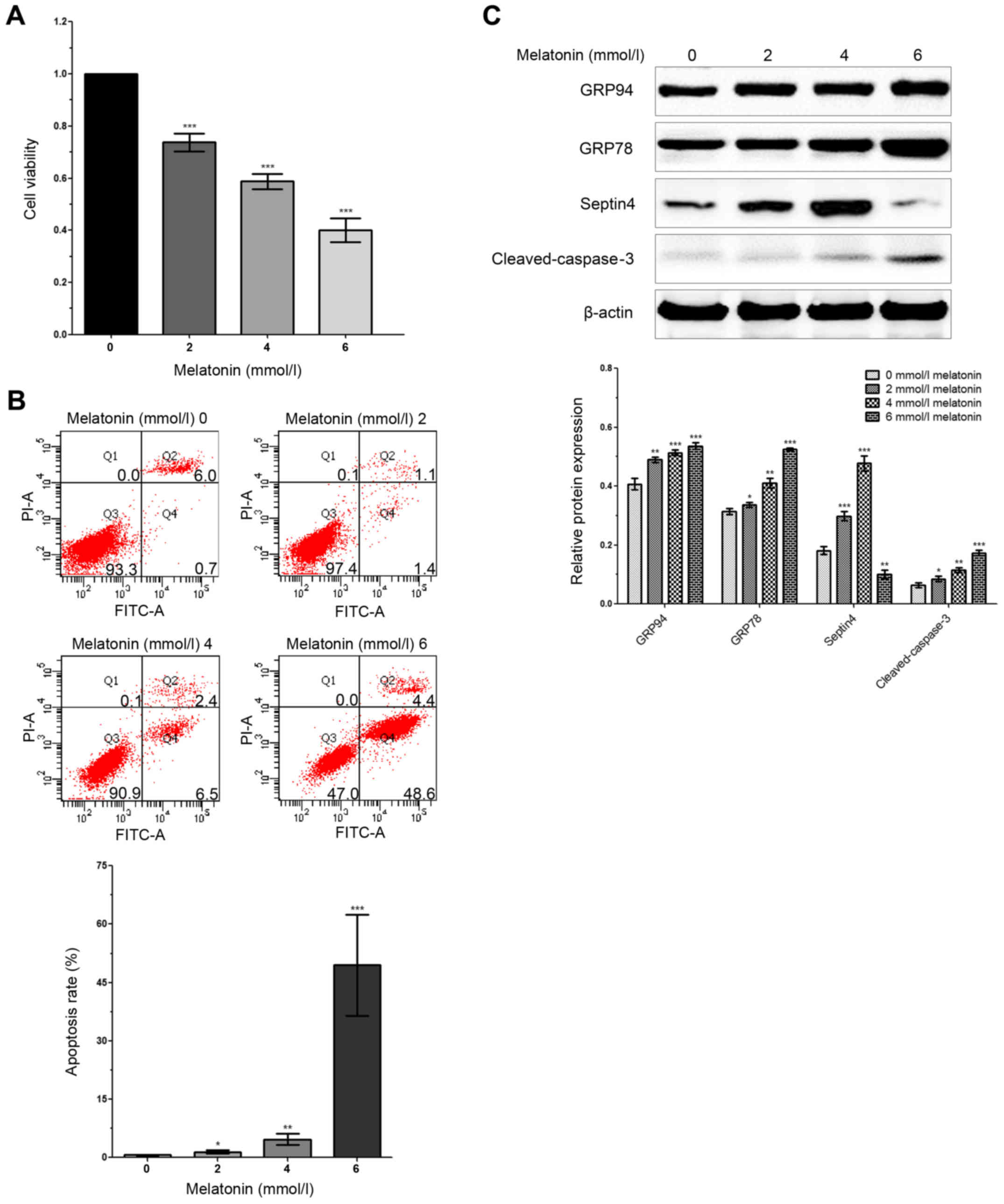 | Figure 1.Expression of Septin4 is increased in
hFOB 1.19 cells treated with a high concentration of melatonin. (A)
hFOB 1.19 cells were treated with 0, 2, 4 and 6 mmol/l of melatonin
for 48 h. Cell viability was assessed via the MTT assay. (B) Flow
cytometry was performed to analyze apoptosis in hFOB 1.19 cells,
which were treated with 0, 2, 4 and 6 mmol/l of melatonin. (C)
Western blotting was performed to detect the expression levels of
GRP78, GRP94, cleaved caspase-3 and Septin4 after treatment with 0,
2, 4 and 6 mmol/l of melatonin for 48 h. *P<0.05, **P<0.01,
***P<0.001 vs. 0 mmol/l group. GRP, glucose regulated protein;
PI propidium iodide. |
Septin4 expression affects ERS and
apoptosis and decreases the viability of hFOB 1.19 cells treated
with a high concentration of melatonin
The present study also assessed the importance of
Septin4 in bone metabolic diseases. It was demonstrated that,
compared with the control group, the expression of Septin4 in
osteoblasts treated with a high concentration of melatonin was
significantly increased. Moreover, the expression of Septin4 was
significantly increased in a dose-dependent manner in association
with melatonin concentration (Fig.
1C). Thus, the results suggested that Septin4 may be associated
with melatonin-induced osteoblast apoptosis via ERS (Fig. 1).
Overexpression of Septin4 promotes
apoptosis in hFOB 1.19 cells induced by treatment with a high
concentration of melatonin
To further examine the effect of Septin4 on high
concentration of melatonin-induced osteoblasts apoptosis, cells
were transfected with control plasmid or Flag-Septin4 plasmid for
48 h and treated with 0, 2, 4 and 6 mM melatonin for 48 h. It was
identified that overexpression of Septin4 further inhibited
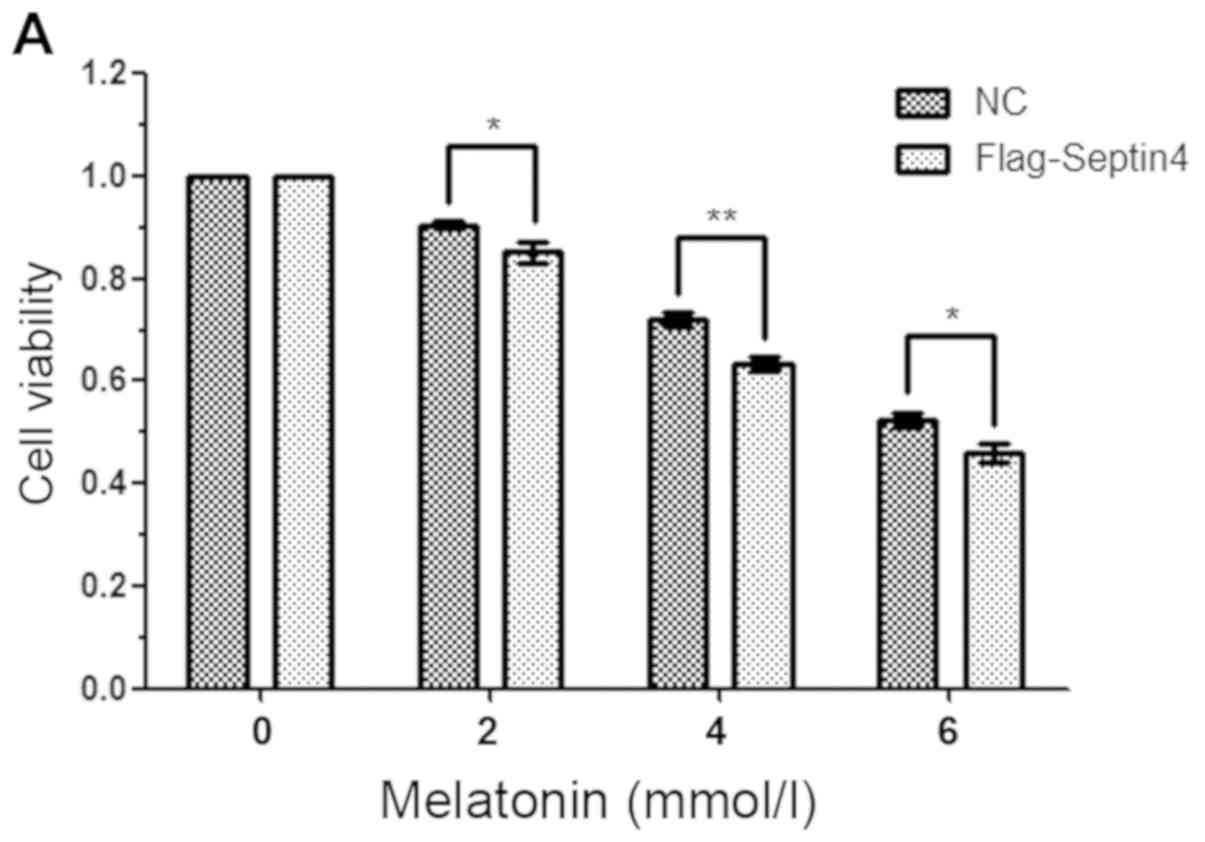
osteoblast viability compared with the control plasmid (Fig. 2A). Furthermore, the results
indicated that overexpression of Septin4 resulted in a
significantly higher rate of apoptosis (NC with melatonin 7.63% vs.
Flag-Septin4 with melatonin 18.43%; P<0.001; Fig. 2B); however, this effect was not
observed in the absence of melatonin. Collectively, the results
demonstrated that Septin4 significantly decreased cell viability
and promoted apoptosis, following induction using a high
concentration of melatonin (Fig.
2).
Overexpression of Septin4 increases
ERS in hFOB 1.19 cells ERS and apoptosis pathway-associated genes
expression levels induced by treatment with high concentration of
melatonin
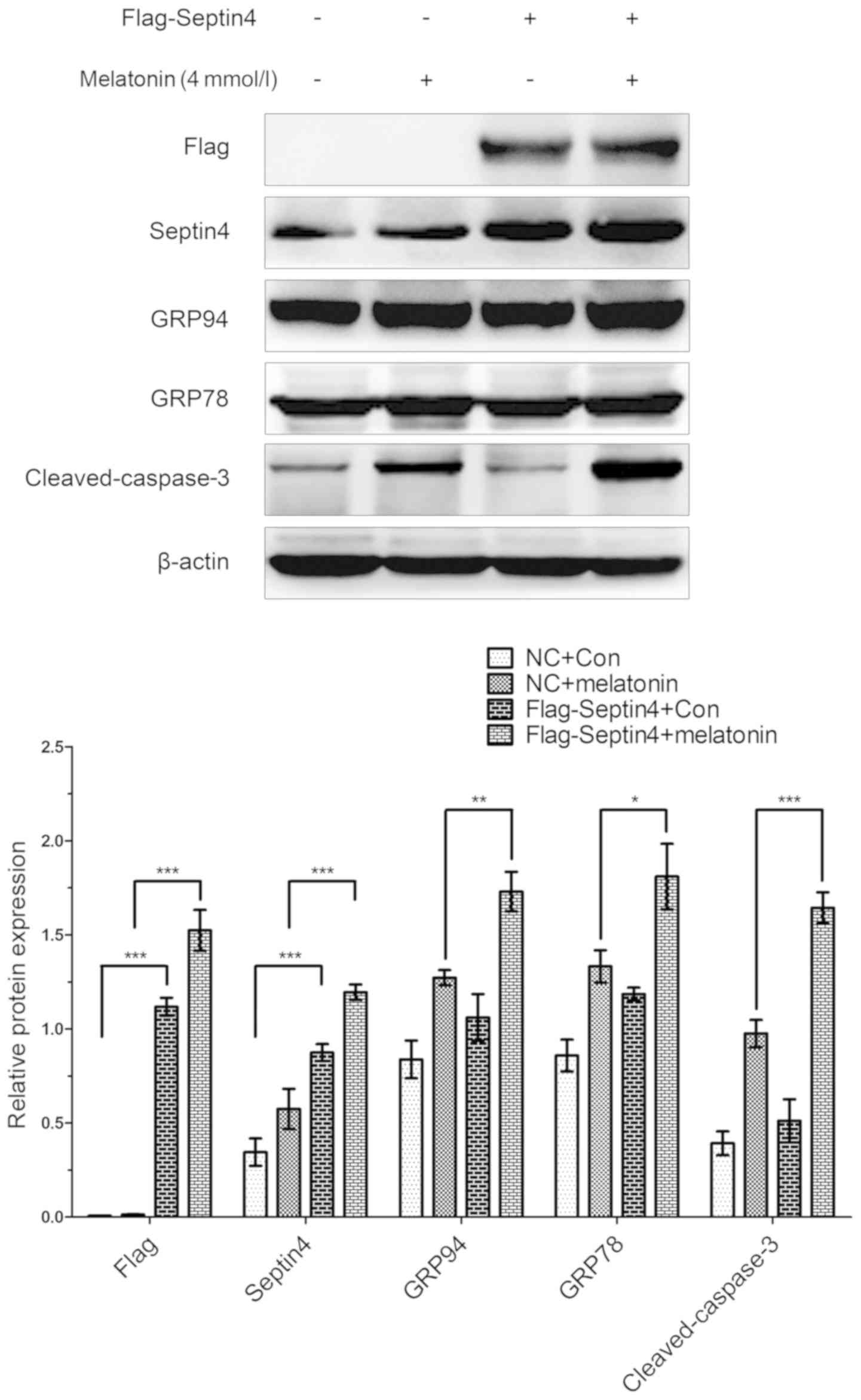
In addition, the effect of overexpressing Septin4 on
the expression levels of ERS- and apoptosis-associated pathway
genes were assessed, as well as the effects induced by a high
concentration of melatonin in osteoblasts. Cells were transfected
with either the Flag-Septin4 plasmid or control plasmid for 48 h
and treated with 0 or 4 mM melatonin for 48 h. It was identified
that both the expression levels of ERS-associated genes (GRP78 and
GRP94) and apoptosis-associated genes (cleaved caspase-3) were
increased in the cells transfected with the Flag-Septin4 plasmid
and treated with melatonin, compared with cells transfected with
the control plasmid and administered melatonin (Fig. 3). However, in the absence of
melatonin treatment, the expression levels of these genes were not
significantly different between the Flag-Septin4 plasmid and the
control plasmid. Therefore, the results indicated that Septin4
mediates ERS and osteoblast apoptosis, and its effect was dependent
on treatment with melatonin (Fig.
3).
Overexpression of Septin4 increases
cytoskeleton destruction induced by treatment with a high
concentration of melatonin in hFOB 1.19 cells
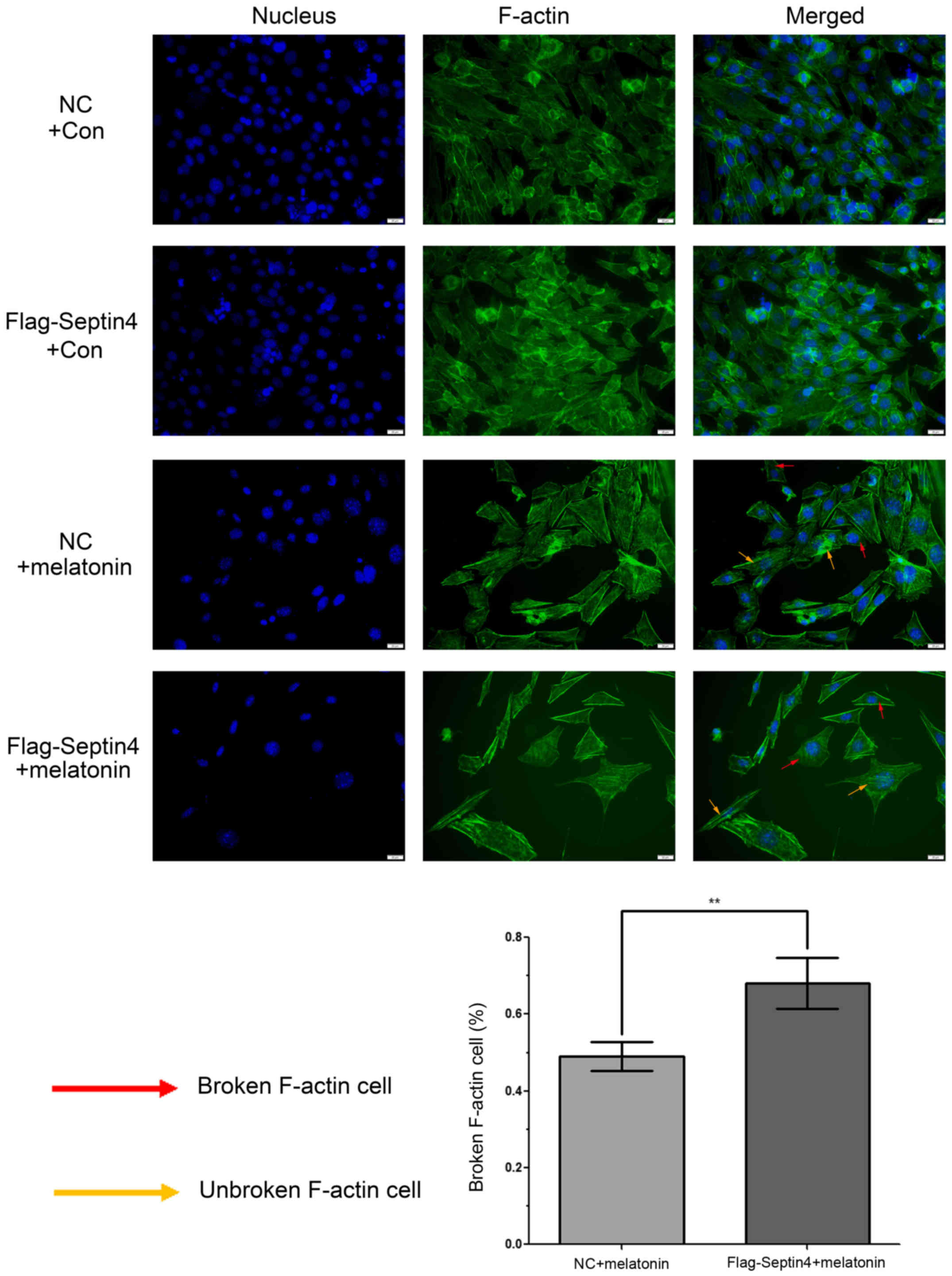
To determine whether a high concentration of
melatonin can induce cytoskeleton destruction, phalloidin was used
to stain F-actin and DAPI was used for nuclei staining. Then,
fluorescence microscopy was performed to analyze the staining
profiles and it was revealed that F-actin was well aligned along
the axis of hFOB cells in control groups (Fig. 4). However, when hFOB cells were
treated with 4 mM melatonin for 48 h, actin microfilament staining
was blurry or non-existent. Moreover, the size of the cells
increased, and the cell count decreased in the melatonin treatment
groups. In addition, statistical analysis demonstrated that this
event had a 1.39-fold increase (P<0.01) in cells with the
overexpression Flag-Septin4 plasmid compared with those that
expressed the control plasmid. Collectively, the results
demonstrated that overexpression of Septin4 increased cytoskeleton
destruction, which was induced by treatment with a high
concentration of melatonin in hFOB 1.19 cells. (Fig. 4).
Discussion
It has been reported that the level of melatonin is
associated with IS (37), and the
clinical application of melatonin can also be used to treat
patients with partial IS, which may be associated with
melatonin-induced proliferation and apoptosis in osteoblasts
(38). However, the optimal
effective concentration and mechanism of action are yet to be
elucidated. The present results are in line with those from
previous studies, which have reported that low concentrations of
melatonin (1 nM-100 µM) promote osteoblast proliferation, while
high concentrations of melatonin (>1 mM) significantly inhibit
osteoblast viability (15,16,39).
In order to provide more accurate results, various high
concentrations of melatonin (2, 4 and 6 mM) were selected in the
present study, and 48 h was selected as the most suitable
observation time to examine melatonin function in osteoblasts,
which was based on our previous studies (40,41).
In the present study, it was demonstrated that a decrease in
viability of osteoblasts was associated with an increase in
melatonin concentration. Furthermore, it was revealed that
melatonin induced osteoblast apoptosis via regulation of Septin4,
resulting in the promotion of ERS-mediated apoptosis mechanisms
involving the GRP94/caspase-3 pathways. This indicates a possible
clinical application for melatonin in the prevention and reversal
of IS progression.
GRP94 is a member of the HSP90 family and is
primarily localized in the ER. GRP94 serves as a molecular
chaperone involved in the folding, transport and secretion of
proteins, and regulates apoptosis and antigen presentation
(42). Furthermore, glucose
regulated protein 78 (GRP78) is a core regulator of UPR (43). GRP78 expression is upregulated in
ERS, which inhibits protein synthesis and decreases cellular stress
in the early stage of ERS. However, prolonged ERS activates the
CHOP and caspase-12 pathways, which induce cell apoptosis and
result in upregulation of the apoptosis marker protein caspase-3
(44). The present results suggest
that a high concentration of melatonin induced osteoblast ERS,
which was positively associated with melatonin concentration.
Furthermore, both of the expression levels of pathway-related genes
of ERS (GRP78 and GRP94) and apoptosis (cleaved caspase-3) were
significantly increased in a dose-dependent manner of melatonin
concentration, which indicates that ERS may serve an important role
in cell apoptosis induced by high concentrations of melatonin.
However, the target protein by which melatonin induces ERS and cell
apoptosis has not been elucidated.
It has been revealed that Septin4, an ER membrane
protein and a receptor associated with ERS, inhibits hepatocellular
carcinoma via the regulation of hepatoma cell apoptosis (32). Moreover, previous studies have
reported that Septin4 aggravates cell apoptosis induced by
transforming growth factor-β, etoposide and staurosporine, and
Septin4 affects the regulation of myofibroblast transformation and
hepatic fibrosis induced by chemokine ligands 4 (32,45).
Thus, these findings reveal the important role of Septin4 in
apoptosis. Furthermore, the present results indicate that
increasing melatonin concentration are associated with an increase
in the expression levels of Septin4 and ERS-related proteins (GRP78
and GRP94). It was also identified that the expression of the
apoptosis-associated protein caspase-3 was positively associated
with Septin4 expression, which suggests that Septin4 may regulate
bone metabolism via apoptotic pathways. Subsequently, Septin4 was
overexpressed and it was demonstrated that melatonin-induced
osteoblast injury was further exacerbated, which was indicated by
the further decrease in cell viability, apoptotic exacerbations and
a significant increase in GRP78, GRP94 and caspase-3 expression
levels.
In addition, Septin4 is an important component of
the cytoskeleton; thus, phalloidin was used to stain F-actin and
DAPI in the nucleus. The present results demonstrated that
cytoskeleton destruction, cell morphology changes and the reduction
in the number of cells were increased after osteoblasts had
overexpressed Septin4 and were treated with melatonin; however,
Septin4 did not serve this role independently of melatonin
treatment. It was originally speculated that the expression of
Septin4 should be increased with 6 mM melatonin,; however, after
the repeated experiments, the expression of Septin4 was decreased;
this effect may due to the excessive concentration of melatonin,
which resulted in enhanced apoptosis and destruction of the cell
structure and Septin4 is a cytoskeletal protein, and could not be
identified. However, these effects should be investigated in future
research. A limitation of the present study was that a
Z-VAD-FMK/pan-caspase inhibitor was not used to prevent
melatonin-induced Septin4 expression levels. Therefore, further
studies are required to investigate the association between Septin4
and apoptosis.
In conclusion, the present results indicated that
high concentrations of melatonin may result in ERS and induce
osteoblast apoptosis, and that Septin4 is a membrane protein
associated with ER Ca2+ ion regulation and a target for
high-concentration melatonin to act on osteoblasts. The present
results may provide a novel direction for the treatment of abnormal
proliferation of osteoblasts and indicated that suppressing Septin4
may be a novel target for the prevention of IS.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Liaoning Province (grant no. 2019-BS-294) and
National Natural Science Foundation of China (grant no.
81472044).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LT, SZha and ZT contributed to the study design,
data collection and analysis, as well as the writing of the
manuscript. YZ conceived, designed and coordinated the study. KW,
SZho and WD contributed to the experimental methods. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IS
|
idiopathic scoliosis
|
|
ERS
|
endoplasmic reticulum stress
|
|
GRP78
|
glucose regulated protein 78
|
|
GRP94
|
glucose regulated protein 94
|
References
|
1
|
Roach JW: Adolescent idiopathic scoliosis.
Orthop Clin North Am. 30:353–365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinstein SL, Dolan LA, Cheng JC,
Danielsson A and Morcuende JA: Adolescent idiopathic scoliosis.
Lancet. 371:1527–1537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Z, Tang NL, Xu L, Qin X, Mao S, Song
Y, Liu L, Li F, Liu P, Yi L, et al: Genome-wide association study
identifies new susceptibility loci for adolescent idiopathic
scoliosis in Chinese girls. Nat Commun. 6:83552015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou T, Chen C, Xu C, Zhou H, Gao B, Su D,
Liao Z, Li Y, Yang S and Su P: Mutant MAPK7-induced idiopathic
scoliosis is linked to impaired osteogenesis. Cell Physiol Biochem.
48:880–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park WW, Suh KT, Kim JI, Kim SJ and Lee
JS: Decreased osteogenic differentiation of mesenchymal stem cells
and reduced bone mineral density in patients with adolescent
idiopathic scoliosis. Eur Spine J. 18:1920–1926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Chen H, Leung RKK, Choy KW, Lam
TP, Ng BKW, Qiu Y, Feng JQ, Cheng JCY and Lee WYW: Aberrant
miR-145-5p/β-catenin signal impairs osteocyte function in
adolescent idiopathic scoliosis. FASEB J. Jun 15–2018.(Epub ahead
of print). View Article : Google Scholar
|
|
7
|
Wang WJ, Sun C, Liu Z, Sun X, Zhu F, Zhu
ZZ and Qiu Y: Transcription factor Runx2 in the low bone mineral
density of girls with adolescent idiopathic scoliosis. Orthop Surg.
6:8–14. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu S, Tao ZB, Tao L and Zhu Y: Melatonin
induces mitochondrial apoptosis in osteoblasts by regulating the
STIM1/cytosolic calcium elevation/ERK pathway. Life Sci.
248:1174552020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aota Y, Terayama H, Saito T and Itoh M:
Pinealectomy in a broiler chicken model impairs endochondral
ossification and induces rapid cancellous bone loss. Spine J.
13:1607–1616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Letellier K, Azeddine B, Parent S, Labelle
H, Rompré PH, Moreau A and Moldovan F: Estrogen cross-talk with the
melatonin signaling pathway in human osteoblasts derived from
adolescent idiopathic scoliosis patients. J Pineal Res. 45:383–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man GC, Wong JH, Wang WW, Sun GQ, Yeung
BH, Ng TB, Lee SK, Ng BK, Qiu Y and Cheng JC: Abnormal melatonin
receptor 1B expression in osteoblasts from girls with adolescent
idiopathic scoliosis. J Pineal Res. 50:395–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Man GC, Wang WW, Yeung BH, Lee SK, Ng BK,
Hung WY, Wong JH, Ng TB, Qiu Y and Cheng JC: Abnormal proliferation
and differentiation of osteoblasts from girls with adolescent
idiopathic scoliosis to melatonin. J Pineal Res. 49:69–77.
2010.PubMed/NCBI
|
|
13
|
Yim AP, Yeung HY, Sun G, Lee KM, Ng TB,
Lam TP, Ng BK, Qiu Y, Moreau A and Cheng JC: Abnormal skeletal
growth in adolescent idiopathic scoliosis is associated with
abnormal quantitative expression of melatonin receptor, MT2. Int J
Mol Sci. 14:6345–6358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono H, Machida M, Saito M, Nishiwaki Y,
Kato H, Hosogane N, Chiba K, Miyamoto T, Matsumoto M and Toyama Y:
Mechanism of osteoporosis in adolescent idiopathic scoliosis:
Experimental scoliosis in pinealectomized chickens. J Pineal Res.
51:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Melatonin delays cell proliferation by inducing G1 and G2 /M phase
arrest in a human osteoblastic cell line hFOB 1.19. J Pineal Res.
50:222–231. 2011.PubMed/NCBI
|
|
16
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Prevention of ERK activation involves melatonin-induced G(1) and
G(2) /M phase arrest in the human osteoblastic cell line hFOB 1.19.
J Pineal Res. 53:60–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong XC, Zhu Y, Ge R, Liu LF and Yuan W:
Effect of melatonin on the extracellular-regulated kinase signal
pathway activation and human osteoblastic cell line hFOB 1.19
proliferation. Int J Mol Sci. 16:10337–10353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellgaard L, Molinari M and Helenius A:
Setting the standards: Quality control in the secretory pathway.
Science. 286:1882–1888. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gething MJ and Sambrook J: Protein folding
in the cell. Nature. 355:33–45. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goodall JC, Wu C, Zhang Y, McNeill L,
Ellis L, Saudek V and Gaston JS: Endoplasmic reticulum
stress-induced transcription factor, CHOP, is crucial for dendritic
cell IL-23 expression. Proc Natl Acad Sci USA. 107:17698–17703.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim TJ, Joo C, Seong J, Vafabakhsh R,
Botvinick EL, Berns MW, Palmer AE, Wang N, Ha T, Jakobsson E, et
al: Distinct mechanisms regulating mechanical force-induced
Ca2+ signals at the plasma membrane and the ER in human
MSCs. Elife. 4:e048762015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang C, Wang Y, Zhang H and Han F: The
role of endoplasmic reticulum stress in neurodegenerative disease.
Apoptosis. 22:1–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ivanova EA and Orekhov AN: The role of
endoplasmic reticulum stress and unfolded protein response in
atherosclerosis. Int J Mol Sci. 17(pii): E1932016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li ZR, Yang L, Zhen J, Zhao Y and Lu ZN:
Nobiletin protects PC12 cells from ERS-induced apoptosis in OGD/R
injury via activation of the PI3K/AKT pathway. Exp Ther Med.
16:1470–1476. 2018.PubMed/NCBI
|
|
25
|
Xiao B, Liu C, Liu BT, Zhang X, Liu RR and
Zhang XW: TTF1-NPs induce ERS-mediated apoptosis and inhibit human
hepatoma cell growth in vitro and in vivo. Oncol Res. 23:311–320.
2016. View Article : Google Scholar
|
|
26
|
Li B, He X, Zhuang M, Niu B, Wu C, Mu H,
Tang F, Cui Y, Liu W, Zhao B, et al: Melatonin ameliorates
busulfan-induced spermatogonial stem cell oxidative apoptosis in
mouse testes. Antioxid Redox Signal. 28:385–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F
and Wei W: Melatonin sensitizes human hepatoma cells to endoplasmic
reticulum stress-induced apoptosis. J Pineal Res. 52:322–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Yin H, Tao Y, Zhong S, Yu H, Li J,
Bai Z and Ou Y: Antitumor effects and mechanisms of
pyropheophorbide-α methyl ester-mediated photodynamic therapy on
the human osteosarcoma cell line MG-63. Int J Mol Med. 45:971–982.
2020.PubMed/NCBI
|
|
29
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma S, Quintana A, Findlay GM, Mettlen
M, Baust B, Jain M, Nilsson R, Rao A and Hogan PG: An siRNA screen
for NFAT activation identifies septins as coordinators of
store-operated Ca2+ entry. Nature. 499:238–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He X, Bao J, Chen J, Sun X, Wang J, Zhu D,
Song K, Peng W, Xu T and Duan Y: Adenovirus-mediated
over-expression of Septin4 ameliorates hepatic fibrosis in mouse
livers infected with Schistosoma japonicum. Parasitol Int.
64:487–492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu D, Wang J, Sun X, Chen J, Duan Y, Pan
J, Xu T, Qin Y, He X and Huang C: Septin4_i1 regulates apoptosis in
hepatic stellate cells through peroxisome proliferator-activated
receptor-γ/Akt/B-cell lymphoma 2 pathway. J Histochem Cytochem.
63:163–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen S, Liu M, Wu Y, Saiyin H, Liu G and
Yu L: Involvement of SEPT4_i1 in hepatocellular carcinoma: SEPT4_i1
regulates susceptibility to apoptosis in hepatocellular carcinoma
cells. Mol Biol Rep. 39:4519–4526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kinoshita M: Diversity of septin
scaffolds. Curr Opin Cell Biol. 18:54–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harris SA, Enger RJ, Riggs BL and
Spelsberg TC: Development and characterization of a conditionally
immortalized human fetal osteoblastic cell line. J Bone Miner Res.
10:178–186. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Subramaniam M, Jalal SM, Rickard DJ,
Harris SA, Bolander ME and Spelsberg TC: Further characterization
of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: Bone
formation in vivo and karyotype analysis using multicolor
fluorescent in situ hybridization. J Cell Biochem. 87:9–15. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Liu Z, Man CW, Guo J, Han X, Hu Z,
Ng TB, Zhao Z, Li J, Wang W, et al: The effect of exogenous
melatonin on reducing scoliotic curvature and improving bone
quality in melatonin-deficient C57BL/6J mice. Sci Rep. 9:62022019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Machida M, Dubousset J, Yamada T and
Kimura J: Serum melatonin levels in adolescent idiopathic scoliosis
prediction and prevention for curve progression-a prospective
study. J Pineal Res. 46:344–348. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han Y, Kim YM, Kim HS and Lee KY:
Melatonin promotes osteoblast differentiation by regulating Osterix
protein stability and expression. Sci Rep. 7:57162017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tao L and Zhu Y: Melatonin regulates
CRE-dependent gene transcription underlying osteoblast
proliferation by activating Src and PKA in parallel. Am J Transl
Res. 10:86–100. 2018.PubMed/NCBI
|
|
41
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
Periostin has a protective role in melatonin-induced cell apoptosis
by inhibiting the eIF2α-ATF4 pathway in human osteoblasts. Int J
Mol Med. 41:1003–1012. 2018.PubMed/NCBI
|
|
42
|
Melnick J, Dul JL and Argon Y: Sequential
interaction of the chaperones BiP and GRP94 with immunoglobulin
chains in the endoplasmic reticulum. Nature. 370:373–375. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burikhanov R, Zhao Y, Goswami A, Qiu S,
Schwarze SR and Rangnekar VM: The tumor suppressor Par-4 activates
an extrinsic pathway for apoptosis. Cell. 138:377–388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garrison JB, Correa RG, Gerlic M, Yip KW,
Krieg A, Tamble CM, Shi R, Welsh K, Duggineni S, Huang Z, et al:
ARTS and Siah collaborate in a pathway for XIAP degradation. Mol
Cell. 41:107–116. 2011. View Article : Google Scholar : PubMed/NCBI
|