Introduction
Temporomandibular disorder (TMD) is a joint disease
that causes pain and dysfunction of the temporomandibular joints
(TMJ) (1). It is a relatively
common disease and is currently estimated to afflict 5–12% of the
United States population (2).
Symptoms of TMD include decreased mandibular range of motion, pain
in the muscles of mastication, joint pain, associated joint noise
during function and a functional limitation or deviation of jaw
opening (3).
Inflammation, which can occur in the synovial
membrane or the capsule of the joint, is one of the reasons for the
pain in patients with TMD (4,5). A
variety of inflammatory cytokines have been observed in patients
with TMD, such as interleukin (IL)-1, IL-8 and monocyte chemotactic
protein 1 (MCP-1) (6). A number of
studies support the involvement of matrix metalloproteinases (MMPs)
in inflammatory processes by decomposition of the extracellular
matrix (ECM) and chemokines (7,8). A
close relationship has been observed between MMP-2 in the synovial
fluid and the degeneration of disc and articular cartilage in
patients with TMD (9). In
addition, neuropathic pain after nerve injury requires MMP-2 or
MMP-9 (10).
Peptidyl-prolyl isomerase 1 (Pin1) is a unique
peptidyl-prolyl cis/trans isomerase binding to and
isomerizing phosphorylated Ser/Thr-Pro motifs (11). Pin1 controls the function of
certain key regulators and contributes to a number of diseases
(12). It has been demonstrated
that Pin1 is an effective therapeutic target for Alzheimer's
disease, myocardial fibrosis, obesity and vascular dysfunction
(13–16). Furthermore, Pin1 leads to vascular
inflammation and atherosclerosis via the NF-κB signaling pathway
(17). Inhibition of Pin1
alleviates diabetes-induced endothelial dysfunction via NF-κB
signaling (16). However, the role
of PIN1 in IL-1β-stimulated temporomandibular chondrocytes is still
unclear.
Xanthan gum (XG) is a heteropolysaccharide
consisting of repeated pentasaccharide units, which are formed by
one glucuronic acid unit, two mannose units and two glucose units
(18). It has been reported that
XG exerts no cytotoxic effect on rabbit chondrocytes, but has
protective effects on these cells and relieves pain in a model of
osteoarthritis by modulating the production of MMPs and tissue
inhibitor of metalloproteinases 1 (TIMP-1) proteins (19–21).
Additionally, XG also suppresses matrix degradation by inhibiting
the expression of MMPs and promoting aggrecan and collagen II
content in the ECM by regulating the Wnt3α/β-catenin signaling
pathway (22). However, the role
and underlying mechanism of XG in TMD are still unclear.
The present aimed to determine the potential
function and mechanism of XG in IL-1β-stimulated temporomandibular
chondrocytes. The results indicated that XG may play a protective
role via the inhibition of the Pin1/NF-κB signaling pathway in TMD.
This study could provide a novel target for the treatment of
TMD.
Materials and methods
Isolation and culture of
chondrocytes
The animal care and procedures were approved by the
Ethics Committee of the Shandong University (Jinan, China).
Sprague-Dawley (SD) rats (n=10, male, 6-week-old, 140–160 g) were
purchased from Shandong University Animal Center. Rats were housed
in a pathogen-free animal care facility on a 12-h light/dark cycle
at 24°C and allowed full access to standard chow and water. The
health and behavior of rats were monitored once a day. The rats
were allowed to adapt for one week, and an intraperitoneal
injection of sodium pentobarbital (100 mg/kg) was administered to
euthanize the rats. Death was confirmed if there was no movement
and breathing. All experiments were performed in one day. The
temporomandibular cartilage was harvested and minced into small
pieces (<1 mm3). The pieces were primarily digested
with 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
for 30 min, and the supernatants were discarded. The sediment was
further digested with 0.2% collagenase II (Sigma-Aldrich; Merck
KGaA) for 4 h at 37°C. The extracted chondrocytes were passed
through a 70-µm pore size cell sieve and cultured overnight in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) at 5% CO2 and 37°C.
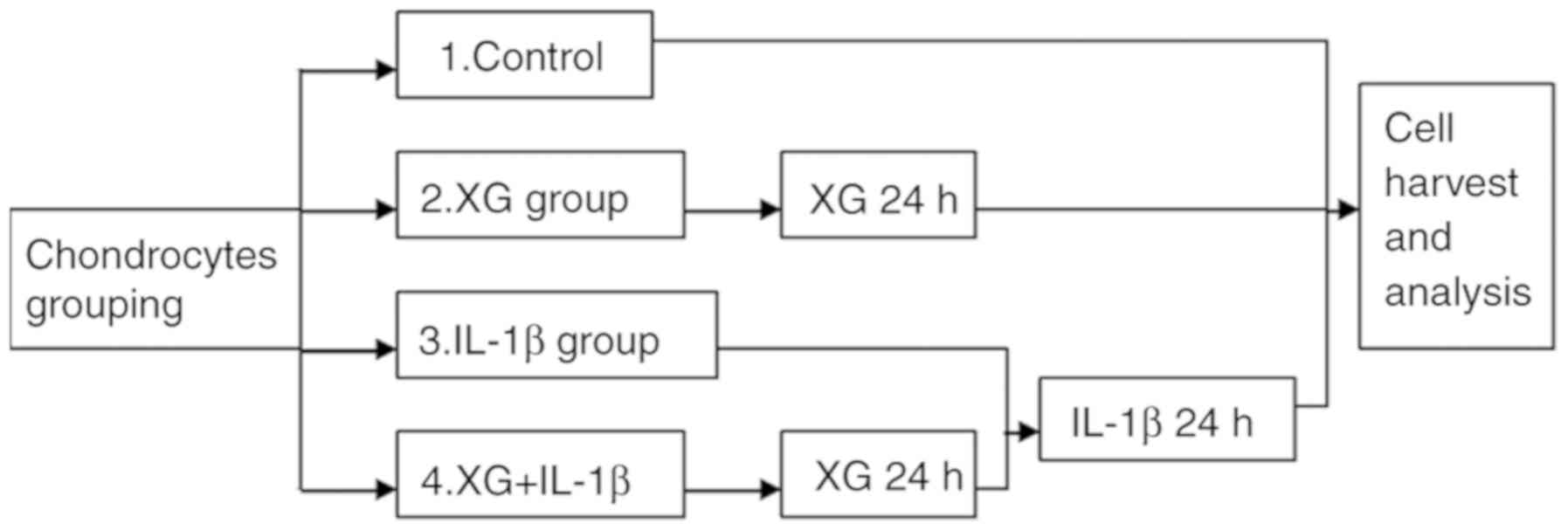
Cell grouping and stimulation
The primary temporomandibular chondrocytes were
randomly divided into four groups: Control, xanthan gum (XG), IL-1β
and XG+IL-1β. Cells in the IL-1β group were stimulated with 10
ng/ml IL-1β (Sigma-Aldrich; Merck KGaA) for 24 h at 24°C. Cells in
the XG+IL-1β group were cultured with 500 µg/ml XG for 24 h at 24°C
prior to IL-1β stimulation (Fig.
1).
ELISA
The secretion levels of inflammatory cytokines were
measured using ELISA kits for TNF-α (cat. no. RTA00) and IL-6 (cat.
no. R6000B; R&D Systems) as previously described (23). The plates were examined using an
absorption spectrometer (Thermo Fisher Scientific, Inc.) by
subtracting readings at 540 nm from the readings at 450 nm.
MTT assay
The primary chondrocytes were seeded into 96-well
plates. After stimulation, the MTT solution was added to each well
and then incubated for 2 h at 24°C. Cell viability was measured at
570 nm using a spectrophotometer (Thermo Fisher Scientific, Inc.).
The relative cell viability was calculated and compared with the
absorbance of the control group.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the primary
chondrocytes using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse-transcribed into cDNA using the
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) for 5 min at 25°C, 30 min at 42°C and 5 min at
95°C. Then, SYBR® Green Supermix (Bio-Rad Laboratories,
Inc.) was used to assess the transcript levels. The primer
sequences were as follows: MCP-1 forward,
5′-GAAGGAATGGGTCCAGACAT-3′ and reverse, 5′-ACGGGTCAACTTCACATTCA-3′;
inducible nitric oxide synthase (iNOS) forward,
5′-GTTCTCAGCCCAACAATACAAGA-3′ and reverse,
5′-GTGGACGGGTCGATGTCAC-3′; Pin1 forward,
5′-TCGGGAGAGAGGAGGACTTTG-3′ and reverse, 5′-GGAGGATGATGTGGATGCC-3′;
β-actin forward, 5′-TGTTGCCCTAGACTTCGAGCA-3′ and reverse,
5′-GGACCCAGGAAGGAAGGCT-3′. The thermocycling conditions included an
initial denaturation period of 1.5 min at 95°C, 40 cycles at 95°C
for 15 sec, and 60°C for 30 sec. β-actin was used as the reference
gene. The reaction was performed using the iCycler real-time PCR
system (Bio-Rad Laboratories, Inc.), and gene expression was
determined by the 2−ΔΔCq method (24).
Western blotting
Cells were harvested and lysed in RIPA buffer
(Beyotime Institute of Biotechnology). The bicinchoninic acid
method was used to determine the protein concentration. Proteins
(50 µg) were separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Amersham; GE Healthcare). The
membranes were blocked with 5% non-fat dry milk in TBS for 2 h at
room temperature and incubated overnight with anti-MCP-1 (1:500;
cat. no. ab25124; Abcam), anti-iNOS (1:500; cat. no. ab3523;
Abcam), anti-collagen I (1:500; cat. no. ab90395; Abcam),
anti-collagen III (1:500; cat. no. ab7778; Abcam), anti-MMP-2
(1:500; cat. no. ab92536; Abcam), anti-MMP-9 (1:500; cat.no.
ab38898; Abcam), anti-Pin1 (1:500; cat. no. ab192036; Abcam),
anti-NF-κB p65 (1:1,000; cat. no. 4764; Cell Signaling Technology,
Inc.), anti-phosphorylated NF-κB p65 (p-p65; 1:1,000; cat. no.
3039; Cell Signaling Technology, Inc.) and anti-β-actin (1:1,000;
cat. no. 4967; Cell Signaling Technology, Inc.) antibodies at 4°C.
After washing with TBS-0.1% Tween-20, the membranes were incubated
with the anti-rabbit horseradish peroxidase (HRP)
conjugated-secondary antibody (1:1,000; cat. no. 7074; Cell
Signaling Technology, Inc.) and anti-mouse HRP conjugated-secondary
antibody (1:1,000; cat. no. 7076; Cell Signaling Technology, Inc.)
at room temperature for 2 h. The protein bands were visualized by
ECL reagent (Pierce; Thermo Fisher Scientific, Inc.) and analyzed
by ImageJ software (v1.8.0, National Institutes of Health).
Gelatin zymography
Zymography was performed using a MMP gelatin
zymography kit (GenMed Scientific Inc.), as previously described
(25). Briefly, cellular proteins
were extracted by Native lysis buffer (cat. no. R0030; Beijing
Solarbio Science & Technology Co., Ltd.). Proteins (30 µg/lane)
were separated by 10% SDS-PAGE polymerized in the presence of 0.1%
gelatin. The gels were washed with the renaturing buffer for 2 h at
room temperature, incubated with the developing buffer for 20 h at
37°C, stained with Coomassie brilliant blue for 30 min at room
temperature and destained with the destaining buffer. Gel images
were captured using a Kodak Imager (Kodak) and MMP activity was
analyzed with the bright bands against the black background. The
bands in the gel were quantified using ImageJ software (v1.8.0,
National Institutes of Health).
Immunofluorescence
Chondrocytes were identified by staining collagen
II. Cells were fixed in 4% paraformaldehyde for 30 min at room
temperature (Beyotime Institute of Biotechnology) and permeabilized
by 0.1% Triton X-100 for 30 min at room temperature. Samples were
blocked with BSA for 30 min at room temperature, and then incubated
with PBS (control) or anti-collagen II primary antibodies (1:1,000;
cat. no. ab34712; Abcam) overnight at 4°C. Alexa 555-conjugated IgG
(1:500; cat. no. A-21428; Invitrogen; Thermo Fisher Scientific,
Inc.) was used as a secondary antibody. DAPI was used to stain
nuclei for 10 min at room temperature. Images were captured by
laser scanning confocal microscopy (LSM 710; Zeiss GmbH),
magnification, ×20. Data were analyzed using Image-Pro Plus 6.0
(Media Cybernetics, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS 15.0
(SPSS, Inc.). Data are expressed as the mean ± SD. Intergroup
comparisons were performed using Tukey's post hoc test following
one-way ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
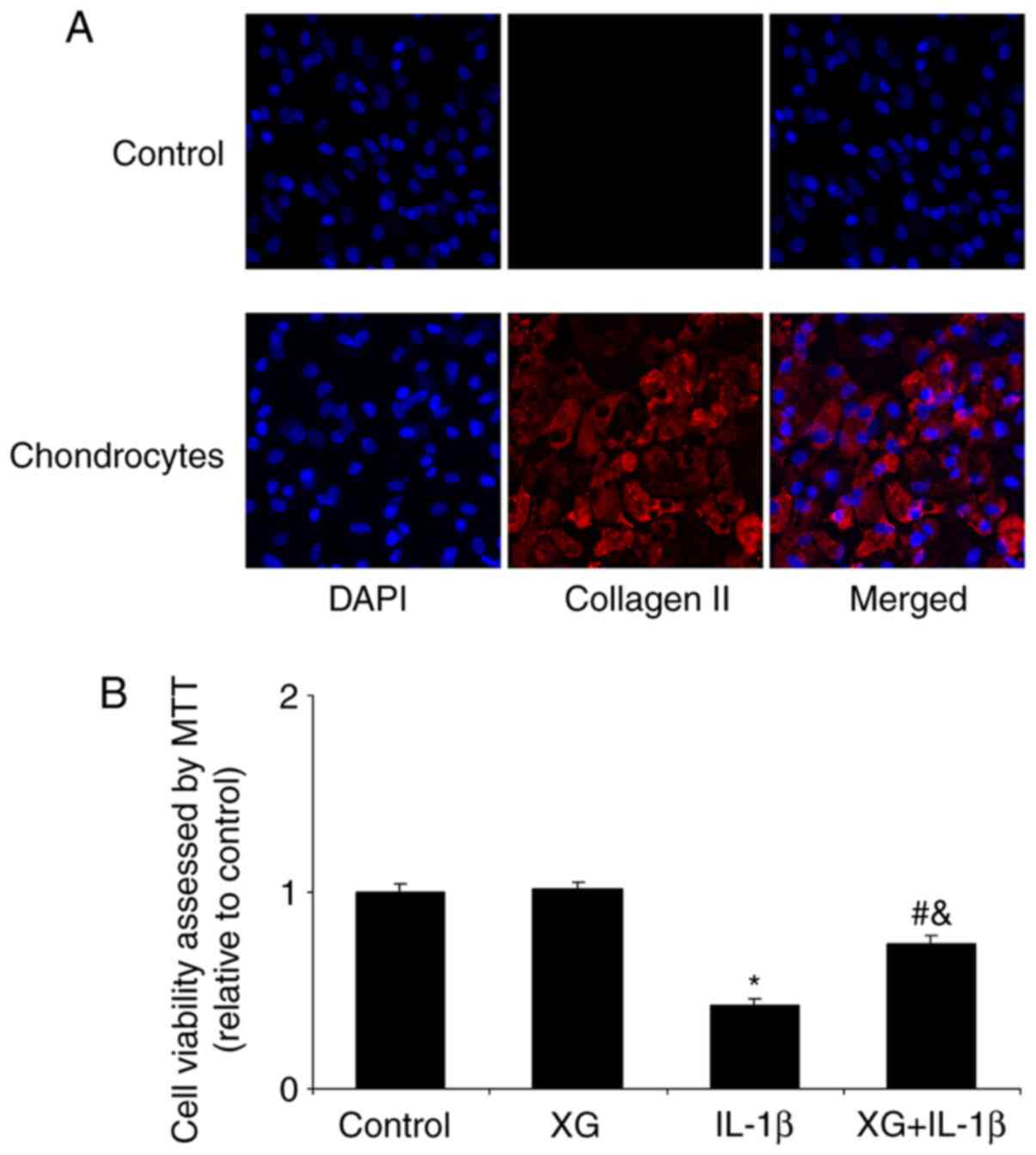
Identification of primary
chondrocytes
Chondrocytes were observed under a microscope
following extraction and an anti-collagen II antibody was used for
identification. As demonstrated in Fig. 2A by immunofluorescence, the
cytoplasm and the cell membrane were stained with red fluorescence
by the anti-collagen II antibody; no staining was observed in the
control group. Thus, chondrocytes were successfully extracted and
cultured.
XG increases the IL-1β-reduced cell
viability
Cell viability was analyzed by MTT assay. Compared
with the control, XG exhibited no significant effect on cell
viability, whereas IL-1β stimulation significantly reduced
chondrocyte viability (Fig. 2B).
However, pretreatment with XG partially reversed the IL-1β-reduced
cell viability, which suggested that XG exerted a protective effect
on chondrocytes in the presence of IL-1β.
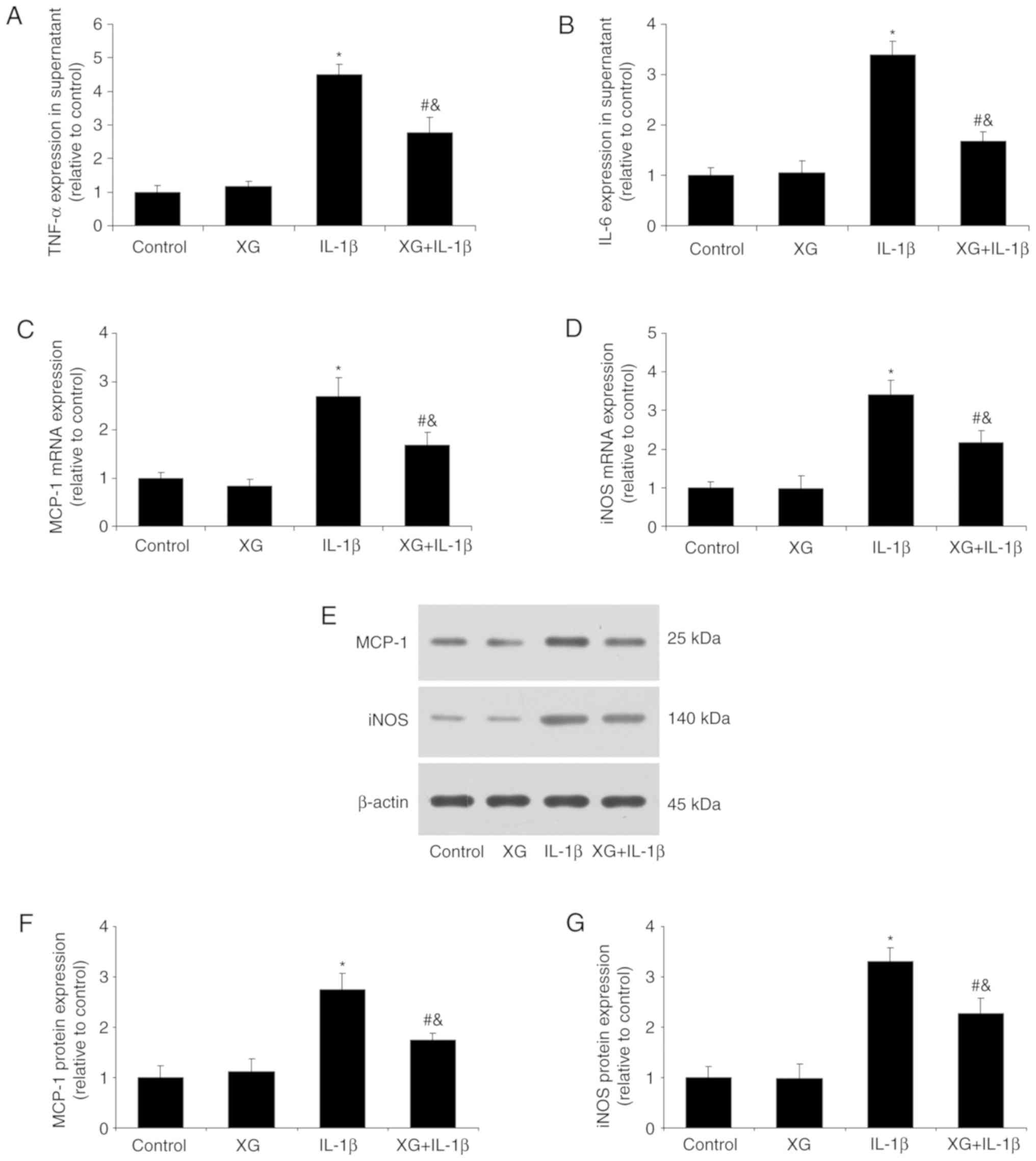
XG reduces the IL-1β-induced
inflammatory response
Following chondrocyte stimulation with IL-1β, the
secretion of TNF-α and IL-6 and the expression of MCP-1 and iNOS
were determined. No differences were observed between the control
and XG groups in inflammatory cytokine expression. However,
compared with the control, IL-1β stimulation increased the TNF-α
and IL-6 levels, whereas pretreatment with XG reduced them
(Fig. 3A and B). In addition, XG
significantly reduced the IL-1β-induced MCP-1 and iNOS mRNA
expression (Fig. 3C and D), as
well as MCP-1 and iNOS protein expression (Fig. 3E-G). These results suggested that
XG may suppress the IL-1β-induced inflammatory response in
chondrocytes.
XG increases collagen expression, but
reduces MMP expression and activity
The effects of XG on the expression of collagens and
MMPs, as well as MMP activity, were next investigated. Compared
with the control, IL-1β stimulation reduced the expression of
collagen, but increased MMP-2 and MMP-9 expression. However, XG
treatment had a protective effect, with increased collagen and
reduced MMPs expression (Fig.
4A-F). In addition, gelatin zymography revealed that IL-1β
increased the activity of MMP-2 and MMP-9, and XG pretreatment
reversed this effect (Fig. 4G-I).
These results suggested that XG may be responsible for maintaining
the balance between collagens and MMPs.
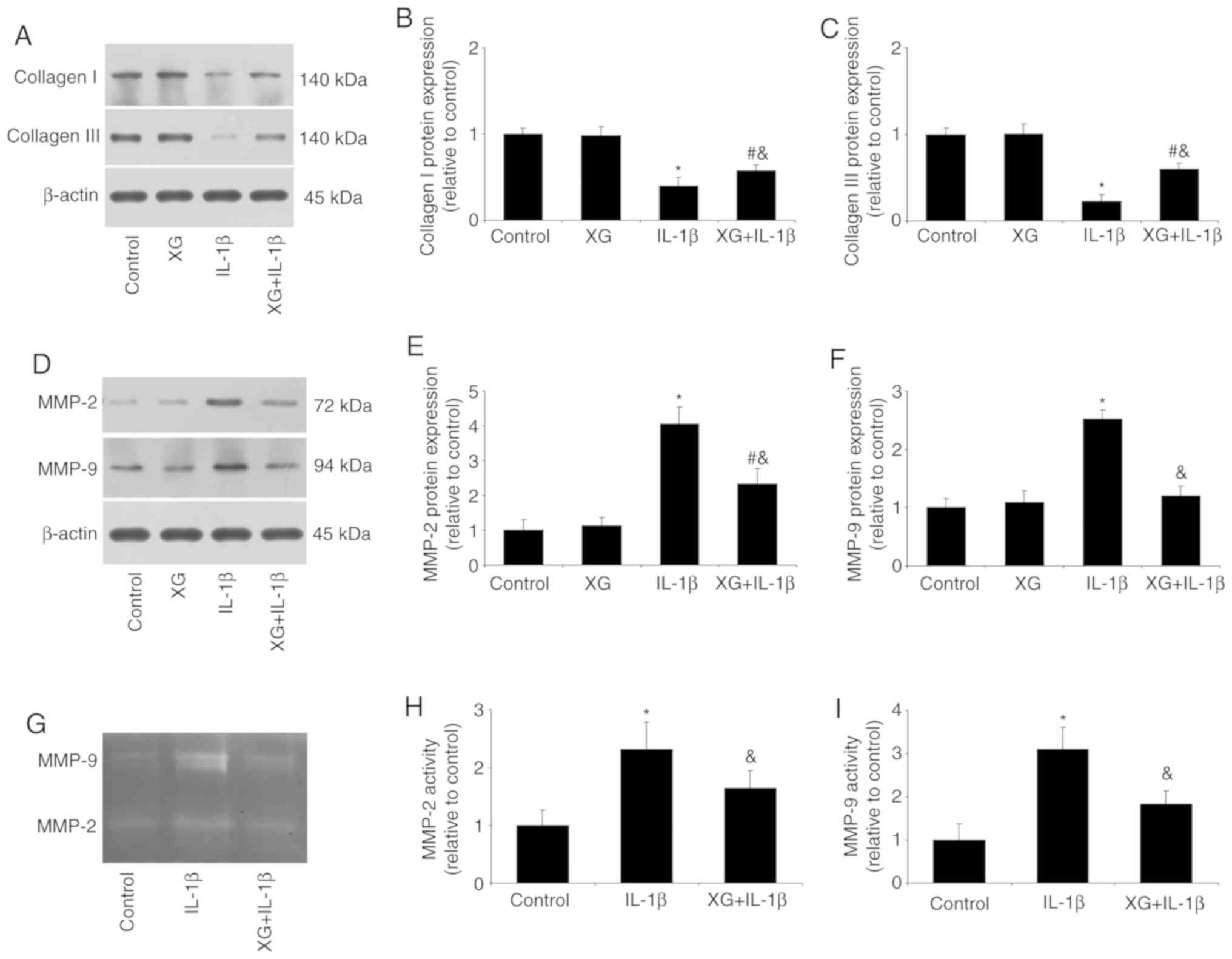 | Figure 4.XG increases the expression of
collagen, but reduces MMP expression and activity. Following
pretreatment of temporomandibular chondrocytes with XG and IL-1β
stimulation, collagen and MMP expression, as well as MMP activity
were analyzed. (A-C) IL-1β reduced collagen I and III protein
expression compared with the control group, whereas XG increased
collagen expression compared with IL-1β. (D-F) IL-1β increased
MMP-2 and MMP-9 protein expression compared with the control group,
whereas XG reduced MMP expression compared with IL-1β. (G-I)
Compared with their respective controls, IL-1β increased MMP-2 and
MMP-9 activity, whereas XG reduced MMP activity. *P<0.05 vs.
control; #P<0.05 vs. XG; &P<0.05
vs. IL-1β. XG, xanthan gum; MMP, matrix metalloproteinase; IL,
interleukin. |
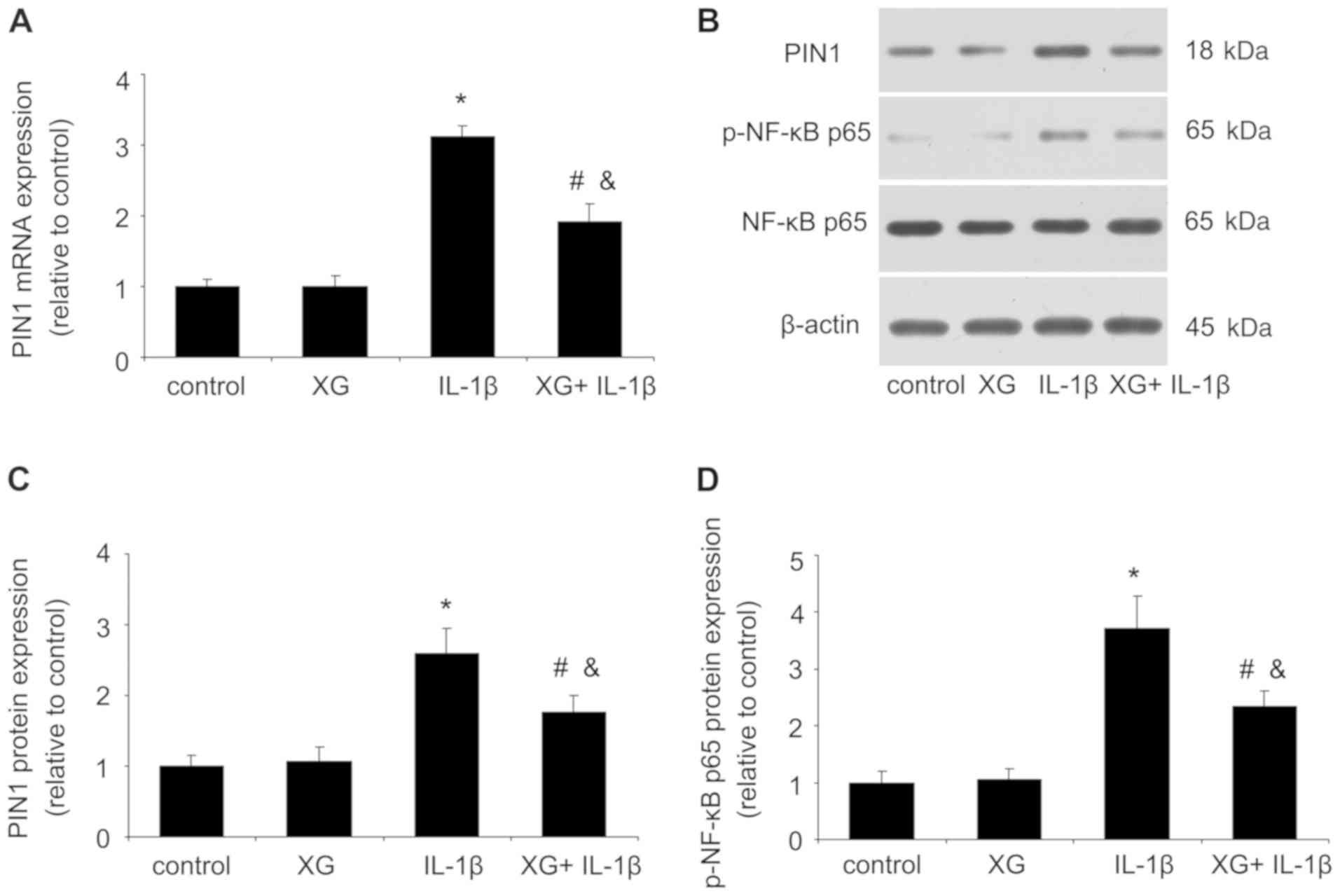
XG reduces the IL-1β-induced Pin1 and
p-p65 expression
After temporomandibular chondrocytes were stimulated
with IL-1β, the expression levels of Pin1 and p-p65 were measured
by RT-qPCR or western blotting. As presented in Fig. 5, no difference was observed between
the control and the XG groups. However, compared with the control
group, IL-1β significantly increased the mRNA and protein
expression of Pin1 and p-p65, whereas the XG+IL-1β group exhibited
reduced Pin1 and p-p65 expression compared with the IL-1β group.
These results suggested that XG may exert protective effects on
chondrocytes in presence of IL-1β through the Pin1/NF-κB signaling
pathway.
Discussion
TMD is a complicated disease characterized by pain
and dysfunction of TMJ (26).
Owing to a clearer understanding of its pathophysiological
properties, the therapy of TMD has been markedly developed in
recent years (27). XG is a
natural polysaccharide and an important industrial biopolymer. XG
is non-toxic, does not inhibit growth or cause skin or eye
irritation (28). XG has a number
of favorable properties, such as temperature stability,
pseudoplastic rheology and safety (28). A previous study demonstrated that
an intra-articular injection of XG alleviated pain and cartilage
damage in a rat model of osteoarthritis (20). In the present study, compared with
the control group, XG alone had no effect on cell viability,
whereas IL-1β stimulation reduced cell viability. However, XG
increased the IL-1β-reduced cell viability, suggesting a protective
role of XG in TMD.
Based on the latest medical knowledge, inflammatory
cytokines serve a crucial role in the pathogenesis of TMD (29). IL-1β is the most representative of
these inflammatory cytokines (30). IL-1β is a pro-inflammatory cytokine
that induces host defense processes, which can lead to joint
destruction (31). It has been
demonstrated that the level of IL-1β is significantly higher in
patients with TMD compared with that in asymptomatic volunteers
(32,33). In the present study, IL-1β was used
to stimulate primary temporomandibular chondrocytes. In
vitro, compared with the control group, IL-1β induced an
inflammatory response, including TNF-α and IL-6 expression, and
increased the mRNA and protein expression of MCP-1 and iNOS,
whereas XG treatment reduced the IL-1β-induced expression of these
inflammatory cytokines.
MMPs are a family of zinc-containing endopeptidases
that serve important roles in tissue remodeling and cartilage
degradation by degrading the ECM (7). Inflammation can induce the production
of MMPs and accelerate the degradation of the cartilage matrix
(34). The development of
inflammation increases the expression of MMP-2 and MMP-9 in the TMJ
of the rat (35). The present
study demonstrated that compared with the control group, IL-1β
stimulation reduced the expression of collagen, but increased the
expression of MMP-2 and MMP-9 compared with the control group,
whereas XG alleviated these effects, with increased collagen and
reduced MMP expression. In addition, XG reduced the IL-1β-induced
MMP activity. Therefore, XG may exert protection through
maintaining the balance between collagens and MMPs, which may
result in a balance between cartilage construction and
destruction.
Peptidylprolyl isomerases (PPIases) were discovered
in 1984 as enzymes that catalyze the cis/trans isomerization
of peptide bonds that precede the amino acid proline (36). As an important member of PPIases,
Pin1 regulates gene transcription, cell proliferation,
differentiation and apoptosis (37). Inhibition of Pin1 reduces airway
inflammation and pulmonary collagen deposition in asthma, lung
transplantation and liver fibrosis (38). Pin1 gene knockout attenuates
angiotensin II-induced abdominal aortic aneurysm by downregulation
of inflammation and MMPs in ApoE-knockout mice (39). In addition, Pin1 regulates IL-8
expression and the NF-κB signaling pathway in glioblastoma
(40). NF-κB is a key mediator in
the regulation of inflammatory genes (41). Various stimuli induce the NF-κB p65
nuclear translocation and subsequently upregulate the transcription
of various proinflammatory cytokines, including TNF-α, IL-1, IL-6
and IL-8 (42). In addition, the
phosphorylation of the p65 subunit regulates the activity of NF-κB
by binding to its target sites on DNA (43). It has been reported that
H2O2-induced oxidative stress increases Pin1
expression in PC12 cells (44).
Furthermore, IL-1β upregulates Mucin 5ac expression via
NF-κB-induced hypoxia-inducible factor-1α in asthma (45). IL-1β stimulation induces the
activation of NF-κB and mitogen-activated protein kinases (MAPKs),
as well as cytokines and chemokines (46). In the present study, IL-1β
increased the mRNA and protein expression of Pin1 and NF-κB p65
phosphorylation compared with the control, whereas XG treatment
reduced Pin1 and p-p65 expression. These results suggested that XG
may serve a protective role partly through the Pin1/NF-κB signaling
pathway. In addition, considering the role of MAPKs in
inflammation, IL-1β may induce MAPK phosphorylation by binding to
the IL-1β receptor, which needs further investigation.
There were some limitations to the present study.
Firstly, only in vitro experiments were performed. Secondly,
the role of apoptosis was not investigated. Thirdly, the nuclear
translocation of NF-κB p65 and PI3K/Akt cell signaling pathways
were not assessed. Thus, in our future experiments, in vivo
effects and apoptosis, as well as PI3K/Akt cell signaling will be
investigated.
In summary, the present study provided new insights
into the protective role and regulatory mechanisms of XG in TMD. XG
maintained cell viability and reduced IL-1β-induced expression of
inflammatory cytokines, as well as the imbalance between collagens
and MMPs. To the best of our knowledge, this study provided the
first evidence that XG may effectively treat TMD through Pin1/NF-κB
signaling pathway (Fig. 6).
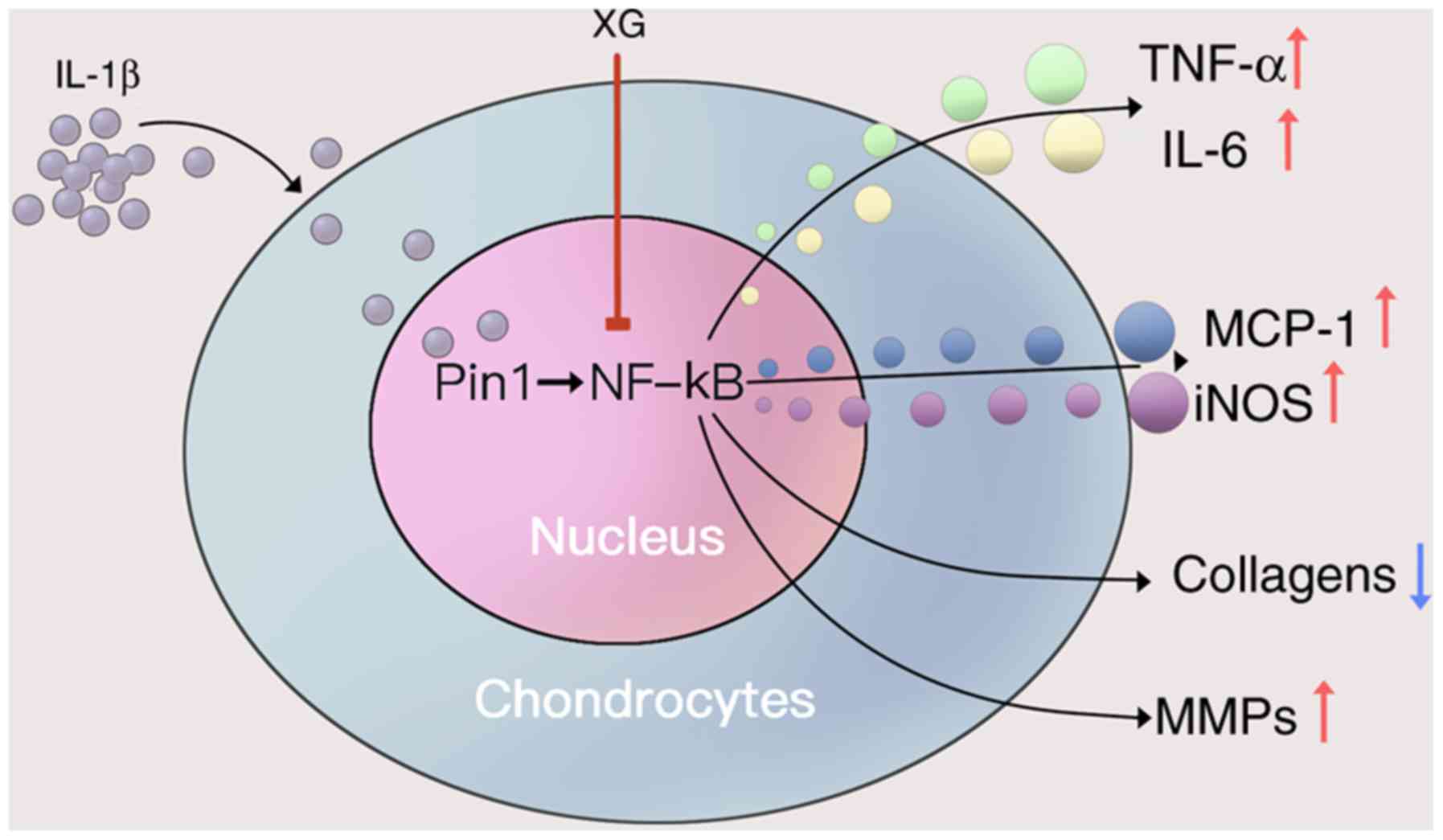 | Figure 6.Proposed protective mechanism of XG
on IL-1β-stimulated temporomandibular chondrocytes. IL-1β increased
the secretion of TNF-α and IL-6, as well as the expression of
MCP-1, iNOS and MMPs, but reduced the expression of collagens. XG
may reduce the IL-1β-induced effects by inhibiting the Pin1/NF-κB
signaling pathway. XG, xanthan gum; IL, interleukin; TNF-α, tumor
necrosis factor-α; MCP-1, monocyte chemoattractive protein-1; iNOS,
inducible nitric oxide synthase; MMPs, matrix metalloproteinases;
Pin1, peptidyl-prolyl isomerase 1; NF-κB, nuclear factor κB. |
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National
Nature Science Foundation of China (grant no. 61605060), and the
Key Research and Development Program of Shandong Province (grant
no. 2019GSF107052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WGY designed the experiments and wrote the
manuscript. FY and JLX performed the experiments. KYL, JLS and YGS
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal care and procedures were approved by the
Ethics Committee of the Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ying W, Yuan F, He P and Ji P: Inhibition
of Notch1 protects against IL-1β-induced inflammation and cartilage
destruction in temporomandibular chondrocytes. Mol Med Rep.
15:4391–4397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiffman E, Ohrbach R, Truelove E, Look
J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo
F, et al International RDC/TMD Consortium Network, International
association for Dental Research; Orofacial Pain Special Interest
Group, International Association for the Study of Pain, :
Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for
Clinical and Research Applications: recommendations of the
International RDC/TMD Consortium Network* and Orofacial Pain
Special Interest Group†. J Oral Facial Pain Headache. 28:6–27.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wadhwa S and Kapila S: TMJ disorders:
Future innovations in diagnostics and therapeutics. J Dent Educ.
72:930–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahlström L and Carlsson GE:
Temporomandibular disorders and oral health-related quality of
life. A systematic review. Acta Odontol Scand. 68:80–85. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamazaki Y, Ren K, Shimada M and Iwata K:
Modulation of paratrigeminal nociceptive neurons following
temporomandibular joint inflammation in rats. Exp Neurol.
214:209–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slade GD, Conrad MS, Diatchenko L, Rashid
NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W,
et al: Cytokine biomarkers and chronic pain: Association of genes,
transcription, and circulating proteins with temporomandibular
disorders and widespread palpation tenderness. Pain. 152:2802–2812.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji RR, Xu ZZ, Wang X and Lo EH: Matrix
metalloprotease regulation of neuropathic pain. Trends Pharmacol
Sci. 30:336–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizui T, Ishimaru J, Miyamoto K and Kurita
K: Matrix metalloproteinase-2 in synovial lavage fluid of patients
with disorders of the temporomandibular joint. Br J Oral Maxillofac
Surg. 39:310–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang
ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, et al: Distinct roles
of matrix metalloproteases in the early- and late-phase development
of neuropathic pain. Nat Med. 14:331–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tun-Kyi A, Finn G, Greenwood A, Nowak M,
Lee TH, Asara JM, Tsokos GC, Fitzgerald K, Israel E, Li X, et al:
Essential role for the prolyl isomerase Pin1 in Toll-like receptor
signaling and type I interferon-mediated immunity. Nat Immunol.
12:733–741. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu KP and Zhou XZ: The prolyl isomerase
PIN1: A pivotal new twist in phosphorylation signalling and
disease. Nat Rev Mol Cell Biol. 8:904–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pastorino L, Sun A, Lu PJ, Zhou XZ,
Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, et al: The prolyl
isomerase Pin1 regulates amyloid precursor protein processing and
amyloid-beta production. Nature. 440:528–534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Liang E, Song X, Du Z, Zhang Y and
Zhao Y: Inhibition of Pin1 alleviates myocardial fibrosis and
dysfunction in STZ-induced diabetic mice. Biochem Biophys Res
Commun. 479:109–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakatsu Y, Sakoda H, Kushiyama A, Zhang J,
Ono H, Fujishiro M, Kikuchi T, Fukushima T, Yoneda M, Ohno H, et
al: Peptidyl-prolyl cis/trans isomerase NIMA-interacting 1
associates with insulin receptor substrate-1 and enhances insulin
actions and adipogenesis. J Biol Chem. 286:20812–20822. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costantino S, Paneni F, Lüscher TF and
Cosentino F: Pin1 inhibitor Juglone prevents diabetic vascular
dysfunction. Int J Cardiol. 203:702–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Yu P, Jiang H, Yang X, Zhao J, Zou
Y and Ge J: The essential role of pin1 via nf-kappab signaling in
vascular inflammation and atherosclerosis in Apoe−/−
mice. Int J Mol Sci. 18:182017.
|
|
18
|
Zhang W, Wu J, Zhang F, Dou X, Ma A, Zhang
X, Shao H, Zhao S, Ling P, Liu F, et al: Lower range of molecular
weight of xanthan gum inhibits apoptosis of chondrocytes through
MAPK signaling pathways. Int J Biol Macromol. 130:79–87. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han G, Shao H, Zhu X, Wang G, Liu F, Wang
F, Ling P and Zhang T: The protective effect of xanthan gum on
interleukin-1β induced rabbit chondrocytes. Carbohydr Polym.
89:870–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao H, Han G, Ling P, Zhu X, Wang F, Zhao
L, Liu F, Liu X, Wang G, Ying Y, et al: Intra-articular injection
of xanthan gum reduces pain and cartilage damage in a rat
osteoarthritis model. Carbohydr Polym. 92:1850–1857. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao X, Chen Q, Dou X, Chen L, Wu J, Zhang
W, Shao H, Ling P, Liu F and Wang F: Lower range of molecular
weight of xanthan gum inhibits cartilage matrix destruction via
intrinsic bax-mitochondria cytochrome c-caspase pathway. Carbohydr
Polym. 198:354–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Han G, Ma M, Wei G, Shi X, Guo Z, Li
T, Meng H, Cao Y and Liu X: Xanthan gum ameliorates osteoarthritis
and mitigates cartilage degradation via regulation of the
wnt3a/β-catenin signaling pathway. Med Sci Monit. 25:7488–7498.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pastrana JL, Sha X, Virtue A, Mai J, Cueto
R, Lee IA, Wang H and Yang XF: Regulatory t cells and
atherosclerosis. J Clin Exp Cardiolog. 2012 (Suppl
12):22012.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang ES, Bai WW, Wang H, Zhang JN, Zhang
F, Ma Y, Jiang F, Yin M, Zhang MX, Chen XM, et al: PARP-1
(Poly[ADP-Ribose] Polymerase 1) Inhibition Protects From Ang II
(Angiotensin II)-Induced Abdominal Aortic Aneurysm in Mice.
Hypertension. 72:1189–1199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kou XX, Wu YW, Ding Y, Hao T, Bi RY, Gan
YH and Ma X: 17β-estradiol aggravates temporomandibular joint
inflammation through the NF-κB pathway in ovariectomized rats.
Arthritis Rheum. 63:1888–1897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Türp JC and Schindler HJ: Chronic
temporomandibular disorders. Schmerz. 18:109–117. 2004.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
García-Ochoa F, Santos VE, Casas JA and
Gómez E: Xanthan gum: Production, recovery, and properties.
Biotechnol Adv. 18:549–579. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wojdasiewicz P, Poniatowski LA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Evavold CL, Ruan J, Tan Y, Xia S, Wu H and
Kagan JC: The pore-forming protein gasdermin D regulates
interleukin-1 secretion from living macrophages. Immunity.
48:35–44.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Long X, Ke J, Meng QG, Lee WC,
Doocey JM and Zhu F: Regulation of HAS expression in human synovial
lining cells of TMJ by IL-1beta. Arch Oral Biol. 53:60–65. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kristensen KD, Alstergren P, Stoustrup P,
Küseler A, Herlin T and Pedersen TK: Cytokines in healthy
temporomandibular joint synovial fluid. J Oral Rehabil. 41:250–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Marks PH and Donaldson ML: Inflammatory
cytokine profiles associated with chondral damage in the anterior
cruciate ligament-deficient knee. Arthroscopy. 21:1342–1347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo
K, Kato T, Nishioka K and Yudoh K: Catabolic stress induces
features of chondrocyte senescence through overexpression of
caveolin 1: Possible involvement of caveolin 1-induced
down-regulation of articular chondrocytes in the pathogenesis of
osteoarthritis. Arthritis Rheum. 54:818–831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nascimento GC, Rizzi E, Gerlach RF and
Leite-Panissi CR: Expression of MMP-2 and MMP-9 in the rat
trigeminal ganglion during the development of temporomandibular
joint inflammation. Braz J Med Biol Res. 46:956–967. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fischer G, Bang H and Mech C:
Determination of enzymatic catalysis for the
cis-trans-isomerization of peptide binding in
proline-containing peptides. Biomed Biochim Acta. 43:1101–1111.
1984.(In German). PubMed/NCBI
|
|
37
|
Hanes SD: Prolyl isomerases in gene
transcription. Biochim Biophys Acta. 1850:2017–2034. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang JW, Hien TT, Lim SC, Jun DW, Choi HS,
Yoon JH, Cho IJ and Kang KW: Pin1 induction in the fibrotic liver
and its roles in TGF-β1 expression and Smad2/3 phosphorylation. J
Hepatol. 60:1235–1241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang ES, Cheng W, Yang RX, Bai WW, Liu X
and Zhao YX: Peptidyl-prolyl isomerase Pin1 deficiency attenuates
angiotensin II-induced abdominal aortic aneurysm formation in
ApoE−/− mice. J Mol Cell Cardiol. 114:334–344. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Atkinson GP, Nozell SE, Harrison DK,
Stonecypher MS, Chen D and Benveniste EN: The prolyl isomerase Pin1
regulates the NF-kappaB signaling pathway and interleukin-8
expression in glioblastoma. Oncogene. 28:3735–3745. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin WD, Wei SJ, Wang XP, Wang J, Wang WK,
Liu F, Gong L, Yan F, Zhang Y and Zhang M: Poly(ADP-ribose)
polymerase 1 inhibition protects against low shear stress induced
inflammation. Biochim Biophys Acta. 1833:59–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song N, Kim AJ, Kim HJ, Jee HJ, Kim M, Yoo
YH and Yun J: Melatonin suppresses doxorubicin-induced premature
senescence of A549 lung cancer cells by ameliorating mitochondrial
dysfunction. J Pineal Res. 53:335–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu S, Li H, Yu L, Wang N, Li X and Chen W:
IL-1β upregulates Muc5ac expression via NF-κB-induced HIF-1α in
asthma. Immunol Lett. 192:20–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu MM, Yang Q, Zhang J, Liu SM, Zhang Y,
Lin H, Huang ZF, Wang YY, Zhang XD, Zhong B, et al: TRIM38 inhibits
TNFα- and IL-1β-triggered NF-κB activation by mediating
lysosome-dependent degradation of TAB2/3. Proc Natl Acad Sci USA.
111:1509–1514. 2014. View Article : Google Scholar : PubMed/NCBI
|