Introduction
Testosterone is an important steroid hormone. A
recent study has indicated that the harmonized reference range for
total testosterone in healthy, non-obese young males aged 19–39
years was 264–916 ng/dl (1). The
physiological serum testosterone concentrations are <50 ng/dl in
adult females and <10 ng/dl in infants and children of both
sexes (2). The quantification of
testosterone levels serves an important role in the diagnosis and
treatment of various diseases, such as hypogonadism, polycystic
ovary and hirsutism (1,3,4).
Immunoassays have been developed to quantify
testosterone levels, including radioimmunoassay, chemiluminescence
analysis, electrochemiluminescence analysis, and light-initiated
chemiluminescent assay (5,6). A considerable inaccuracy of
testosterone assays has been reported, especially regarding
testosterone quantification in females, children and male patients
with hypogonadism (7–9).
Liquid chromatography tandem mass spectrometry
(LC-MS/MS) combines the separation ability of liquid chromatography
with the detection ability of mass spectrometry, which enables
LC-MS/MS to exhibit unique advantages in the detection of small
molecules (10). Moreover, isotope
dilution mass spectrometry (ID-MS) has been recommended as a
reference measurement method by the Advisory Committee on Quantity
of Materials (11). However, to
the best of our knowledge, ID-MS has been rarely used in clinical
laboratories. Isotope dilution comprises the addition of a known
amount of isotope-labeled analyte to each calibrator, followed by
quality control and sample assessment (12). Although isotope-labeled analytes
exhibit similar recovery, chromatography and ionization
characteristics compared with unlabeled analytes, they can be
distinguished on the mass spectrum based on their different mass
numbers (12). Using stable
isotope-labeled analytes as internal standards (IS) may
counterbalance the analyte loss and matrix effect (ME) during
sample pretreatment and LC-MS/MS analysis. The variability in the
precision and accuracy of different mass spectrometry methods
(seven high performance liquid chromatography tandem mass
spectrometry assays, and one gas chromatography tandem mass
spectrometry assays) has been reported, and the results of the
comparison of eight mass spectrometry methods showed that the
within-run variability at 297 and 8.47 ng/dl ranged between
1.40–11.36% CV and 2.52–25.58% CV, respectively (13). Sample preparation includes several
stages, such as derivatization procedure, liquid-liquid extraction
(LLE) and solid phase extraction, which has been indicated to
result in the poor sensitivity and low extraction efficiency of the
different methods (14–16). Therefore, certain mass spectrometry
methods for testosterone determination cannot meet the clinical
requirements. In the present study, a method for quantitative
analysis of total testosterone in human serum was developed via
isotope dilution (ID) ultra-performance liquid chromatography
(UPLC) tandem mass spectrometry (ID-UPLC-MS/MS). The results of the
current study indicated that this method was suitable for detection
of testosterone in males and females, with high accuracy and
precision.
Materials and methods
Samples
Clinical serum samples were collected at the Gong'an
Hospital (Tianjin, China), between March 2019 and June 2019. The
serum samples (n=30) that were used to assess the applicability of
the present method were obtained from both outpatient and inpatient
individuals. Samples with sufficient serum (at least 2 ml), clear
appearance, no jaundice, no hemolysis and no blood lipids were
collected. The average age of 15 female patients was 38 years
(range, 20–58), while the average age of 15 male patients was 49
years (range, 18–85). A total of three levels of quality control
(QC) materials were prepared via combining human serum samples from
healthy donors. A total of 8 subjects were recruited from Tianjin
Gong'an Hospital, between April 2019 and June 2019. Upon study
entry, all participants underwent a detailed medical examination
and clinical history review. The donors were enrolled in the study
to meet the following requirements: Age 18–55 years; normal renal
and liver function; on no medications known to interfere with
androgen synthesis or action (e.g., cimetidine, spironolactone,
finasteride, testosterone, flutamide, lupron); not have
participated in a drug study within the last 3 months; available
for blood draws between 7:00 a.m. and 10 a.m. The total
testosterone of healthy donor samples was tested on the ARCHITECT
i2000 analyzer (Abbott Pharmaceutical Co. Ltd.), and the results of
testosterone concentration met the expected requirements. The
donors were 4 male and 4 female subjects. The average age of the
male donors was 31 years (range, 25–36 years), and the average age
of the female donors was 28 years (range, 24–37 years). The 8
donors exhibited a normal testosterone concentration. All serum
samples were stored in aliquots at −80°C until used. The study
protocol was approved by the Ethics Committee of Tianjin Medical
University (approval no. TMUHMEC2017008) and written informed
consent was obtained from each participant prior to study
entry.
Apparatus and reagents
Testosterone (≥99.6%) was purchased from National
Pharmaceutical Engineering Research Center and served as a
calibration standard. 16,16,17-d3-testosterone (T-D3) with an
isotopic purity of 99.37% was purchased from Sigma-Aldrich (Merck
KGaA) and was used as an IS. Methanol, acetonitrile, n-hexane and
ethyl acetate were purchased form Merck KGaA, and formic acid was
purchased from Waters Corporation. Ammonium acetate and sodium
carbonate were purchased from Shanghai Fuchen Chemicals Co., Ltd.
All solvents were high-performance liquid chromatography grade and
chemicals were reagent grade. The steroids used for interference
testing were obtained from Shanghai Yuanye Bio-Technology Co., Ltd.
and National Institute of Metrology of China. Electrospray
ionization UPLC-MS/MS analysis was performed using a
Xevo® TQ-XS Triple Quadrupole Mass Spectrometry
instrument (Waters Corporation) with an ACQUITY UPLC®
I-Class PLUS system (Waters Corporation).
Calibrator preparation
Testosterone was dissolved in anhydrous methanol to
prepare the primary stock solution (PSS) with a concentration of
1.00 mg/ml. A total of ten calibrator levels covering a range of
1.00–1,000.00 ng/dl were prepared with methanol from 1.00 mg/ml
PSS. T-D3 was dissolved in anhydrous methanol to prepare IS
solution with a concentration of 1,000.00 ng/dl.
Sample preparation
The samples for testosterone determination were
prepared via LLE as previously described, with certain
modifications (17). The serum
samples were processed together with QC samples, reagent blank and
ten levels of calibrators. Samples (100 µl) and IS solution (100
µl; 1,000 ng/dl) were mixed for 15 min at room temperature. Acid
buffer (100 µl; 0.5 mol/l ammonium acetate; pH 5.5) was added, and
then mixed for 2 h at room temperature. LLE was performed twice
with 500 µl ethyl acetate/n-hexane solution (3:2; v/v) to extract
the analytes. The combined organic extracts were evaporated to
dryness using a nitrogen blowing instrument and the sample extract
was re-dissolved in basic buffer (200 µl; 0.2 mol/l sodium
carbonate; pH 9.8). This solution was extracted twice using
n-hexane (500 µl each). The combined organic layers were dried and
reconstituted with methanol (100 µl) for UPLC-MS/MS analysis.
UPLC-MS/MS conditions
For the chromatographic assay, an ACQUITY UPLC™ BEH
C18 (2.1×100 mm; 1.7 µm) analytical column was heated to 40°C with
0.1% formic acid in water (buffer A) and acetonitrile (buffer B).
The injection volume was 5 µl and the samples were maintained at
5°C in the autosampler. The gradient elution procedure is presented
in Table SI.
For the mass spectrometry assay, the mass
spectrometry instrument was operated with electrospray ionization
in the positive ion mode with ion spray source temperature at
150°C. The temperature of desolvation gas was 400°C and the
nebuliser pressure of the gas was 7.0 bar (101.5 psi). The flow
rate of desolvation gas was 600 l/h, and the flow rate of counter
blow gas with conical hole was 150 l/h. The ion selection
parameters of testosterone and T-D3 are presented in Table I.
 | Table I.Ion selection parameters for T and
T-D3. |
Table I.
Ion selection parameters for T and
T-D3.
| Materials | Quantitative ion
pairs, m/z | Confirmation ion
pairs, m/z | Cone voltage, V | Collision voltage,
eV |
|---|
| T | 289.2/97.0 | 289.2/108.9 | 38 | 24 |
|
|
| 289.2/97.0 | 38 | 20 |
| T-D3 | 292.2/97.0 | 292.2/108.9 | 44 | 24 |
|
|
| 292.2/97.0 | 44 | 24 |
Data analysis
The UPLC-MS/MS raw data were processed using
MassLynx version 4.2 software (Waters Corporation). The level of
testosterone was analyzed using MedCalc version 18.2.1 (MedCalc
Software Ltd.). Origin version 9.0 (OriginLab) and GraphPad prism
version 6.0 (GraphPad Software, Inc.) were used for the analysis of
chromatograms and histograms. The peak area ratio of testosterone
quantifier ion to IS quantifier ion was used for quantification. To
establish the best fit for the calibration curve, the peak area
ratio of testosterone and IS was obtained from four groups of
calibration runs and plotted with the target concentration using a
linear model with no weighting, weight of 1/X, 1/X2 or
1/variance of Y. The average sum of squared residuals and the
average relative sum of squared residuals were estimated via
comparing the calculated concentration with the target
concentration. A linear calibration curve using a weight of 1/X was
selected, as this model exhibited the smallest average sum of
squared residuals among all linear models. The analyte
concentration in serum was calculated using the peak area ratio for
the sample and the regression parameters of the established 1/X
weighted calibration curve.
Method validation
The method verification procedure followed the
guidelines of Clinical and Laboratory Standards Institute (CLSI)
C62-A (18) and China National
Accreditation Committee GL037 for performance evaluation (19).
Precision
The method precision was assessed using six
replicates/day of in-house QC samples at three concentrations
(intra-assay) and in three different days (inter-assay). A
coefficient of variation (CV) <15% for the intra-assay and
<20% for the inter-assay was accepted.
Accuracy
Spike recovery was evaluated via supplementing a
range of testosterone with different concentrations (10.00, 200.00
and 800.00 ng/dl) into two serum samples (mixed serum from female
healthy donors). The testosterone concentrations of the two serum
samples were 29.56 and 57.32 ng/dl, respectively. The recovery was
calculated as [(final concentration-initial concentration)/added
concentration] ×100%.
Sensitivity
The limit of quantification (LOQ) was determined
using five replicates as the lowest concentration, which generated
a signal-to-noise ratio (S/N) >10, with an accuracy between 80
and 120% of the true value, and CV <20%. The limit of detection
(LOD) represents the absolute limit of detection that produced an
S/N >3.
Linearity
The linear range of the present method was
determined using a 10-point calibration curve (testosterone,
1.00–1,000.00 ng/dl) measured in four replicates. The applicability
of sample dilution was assessed for samples with analyte
concentrations above the upper limit of the linear range. The
sample dilution experiments were performed with high QC samples
that were diluted with saline using dilution factors ranging from 1
to 10 (a total of 9-level dilutions). Each dilution level was
repeated in triplicate and the mean value was used for the
analysis. The dilution factors were applied to calculate the final
concentrations and the results were compared with the values
obtained from undiluted samples.
Specificity
Structural steroid analogues were added to the
samples to assess the co-elution. The absence of a peak with mass
transitions (289.2 to 97.0 for testosterone; 292.2–97.0 for T-D3)
at the retention times for testosterone and IS (2.40 min) confirmed
the absence of interference with the quantification of testosterone
in serum. The ratio of quantification ions to confirmation ions
(QI/CI) of testosterone was monitored in the samples and compared
to the QI/CI ratio of calibrator solutions to confirm that no
interfering compounds were present. Co-elution was considered when
the QI/CI ratio differed >20% (18,20).
ME
ME was evaluated in three matrices
(charcoal-stripped serum, male serum and female serum) and one set
of neat samples in methanol (matrix-free), according to previous
studies (21,22). A 7-point calibration curve ranging
from 10–1,000 ng/dl for testosterone was prepared in each matrix.
The mass spectrometry response (area count ratios of analyte over
IS) was compared in all three matrices with that of the neat
samples prepared in methanol. The sample ME was calculated using
the following formula: ME %=B/A ×100, where B corresponded with the
area count ratios of analyte to IS obtained from samples in matrix
and A corresponded with the area count ratios in matrix-free
samples.
Extraction efficiency
The extraction efficiency was assessed using low,
medium and high QC samples. In one set of QC samples, the IS
solution was added at the beginning of sample preparation (A),
while in another set of QC samples it was added at the end of the
sample preparation (B). The efficiency was calculated using the
following equation: Measured concertation (B)/measured
concentration (A) ×100.
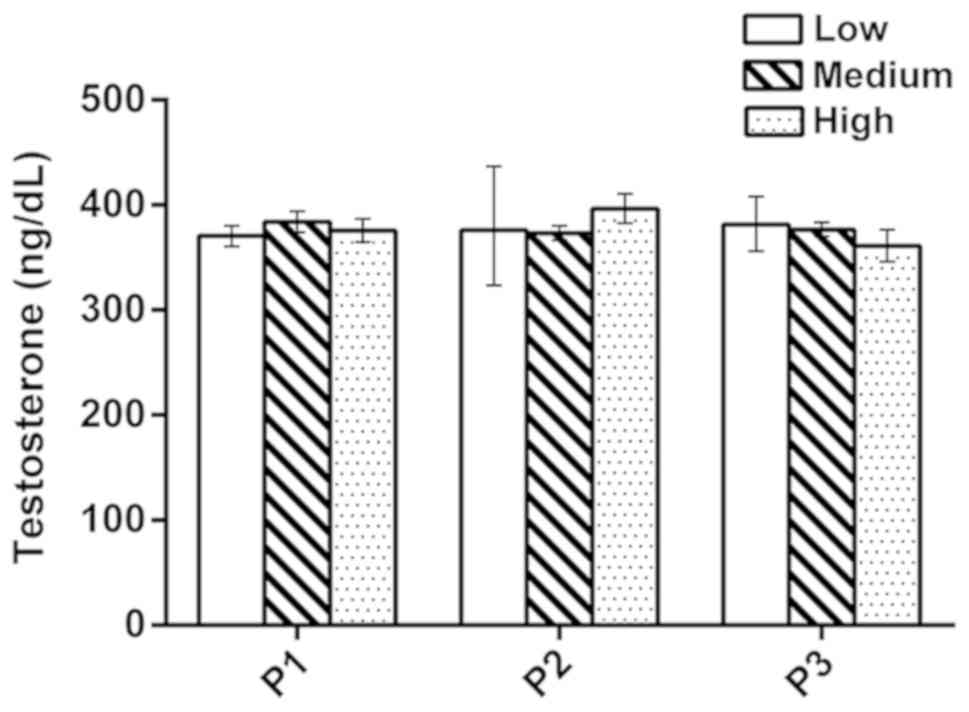
Stability of sample preparation
The stability of sample preparation was evaluated
via comparing the measurement values of the medium QC samples that
were obtained with the aforementioned method compared with those
obtained following modification of three principal sample
preparation parameters. The first parameter (P1) was the
equilibration time, during which the sera were incubated with IS to
achieve equilibration between free and protein-bound IS (incubation
durations tested: Low, 10 min; medium, 15 min; high, 20 min). The
second parameter (P2) was the time allowed for the removal of
testosterone from proteins (incubation durations tested: Low, 90
min; medium, 120 min; high, 150 min). The third parameter (P3) was
the buffer concentration used to remove testosterone from proteins
(buffer concentrations tested; Low, 0.3 mol/l; medium 0.5 mol/l;
high 0.7 mol/l).
Method applicability
The present method was used to analyze 30 samples
from female and male patients to evaluate the applicability of the
present method in the general population. The data are represented
by the median and the concentration range of the test results.
Results
Chromatographic characteristics
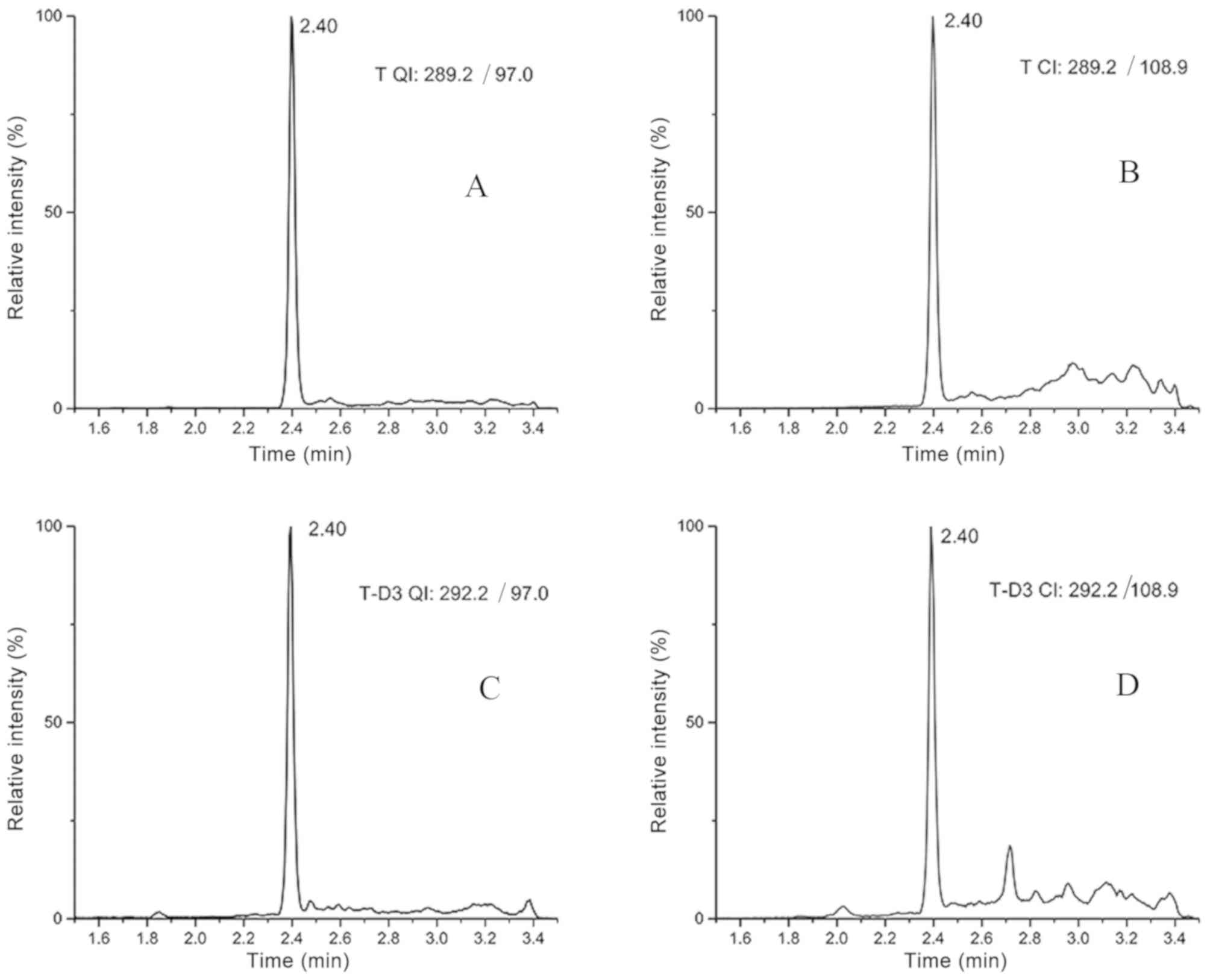
The chromatographic retention time of testosterone
and T-D3 was 2.40 min (Fig. 1),
while the total analysis time of each sample was 5 min.
Precision
Precision was estimated via measuring QC samples at
three concentrations in 3 days (six replicates/day). As presented
in Table II, the intra-assay CV
ranged from 1.40 to 2.77%, while the inter-assay CV was from 3.06
to 3.66%. The estimated intra-assay and inter-assay precision of
this UPLC-MS/MS was within the acceptance criteria (23).
 | Table II.Intra-assay and inter-assay
precision. |
Table II.
Intra-assay and inter-assay
precision.
|
| Intra-assay, n=6 | Inter-assay,
n=18 |
|---|
|
|
|
|
|---|
| Group | Mean | SD | CV, % | Mean | SD | CV, % |
|---|
| Low | 52.26 | 1.57 | 2.21 | 54.42 | 1.99 | 3.66 |
| Medium | 381.61 | 5.36 | 1.40 | 374.33 | 11.47 | 3.06 |
| High | 670.10 | 18.55 | 2.77 | 678.38 | 24.41 | 3.60 |
Accuracy
Testosterone (10.00, 200.00 and 800.00 ng/dl) was
added to the serum samples. The actual concentrations, which were
measured following addition of pure testosterone, were close to
female and male patient levels (1,24).
The recovery rates ranged from 94.32 to 108.60%, as presented in
Table III, and all were within
the acceptable range of 80–120% (19).
 | Table III.Recovery of testosterone added in
human serum samples. |
Table III.
Recovery of testosterone added in
human serum samples.
| Sample | Added, ng/dl | Measured,
ng/dl | Recovery, % |
|---|
| 1 | 0 |
29.56 | −a |
|
|
10.00 |
40.10 | 105.40 |
|
| 200.00 | 223.15 | 96.80 |
|
| 800.00 | 810.06 | 97.56 |
| 2 | 0 |
57.32 | −a |
|
|
10.00 |
68.18 | 108.60 |
|
| 200.00 | 245.96 | 94.32 |
|
| 800.00 | 822.68 | 95.67 |
Sensitivity
LOD was evaluated via considering the lowest
concentration at which the S/N was >3. LOQ was determined as the
concentration providing S/N ≥10 within CV <20%. In the current
method, LOD and LOQ were 0.50 and 1.00 ng/dl, respectively.
Linearity
For the evaluation of linearity, the ratio of the
analyte peak area to the IS peak area was plotted against
testosterone to generate the calibration curves. A calibration
curve (Y=0.0044X+0.0667) was generated with ten concentrations of
calibrators. The calibration curve was linear within the range of
1.00–1,000.00 ng/dl of testosterone (R2>0.999). The
weighted regression parameters from four replicate calibration
curves were consistent [mean slope, 0.0044; 95% confidence interval
(CI), 0.0043–0.0045; mean intercept, 0.0667; 95% CI,
0.0665–0.0669]. Dilutions of samples up to 10X with saline were
indicated to result in accurate measurements (Table SII). The accuracy of diluted
samples was 100.37% (95%CI: 99.30–101.44%). The high QC samples
were diluted to obtain accurate results, which was to support the
expansion of the measurement range. The measurement range could be
extended from 1.00 to 10,000.00 ng/dl.
Specificity
Fig. 1 depicts the
ion chromatograms that were observed for the quantitative and
confirmation ion chromatography of T-D3 and testosterone in serum
samples. The structural analogues of testosterone either did not
contain the same mass transitions that were used for quantification
or were chromatographically separated from testosterone with the
UPLC conditions described in the current study. Structural
analogues of testosterone include dihydrotestosterone, estradiol,
estriol, progesterone, estrone, cortisol and corticosterone
(2). Other potential interferences
can be detected using the QI/CI ratio. For the present method, the
QI/CI ratios in 30 single-donor serum samples were compared with
the neat calibrators. The mean QI/CI ratio of the calibrators was
1.15 (95% CI, 1.10–1.21) and mean ratio of the 30 samples was 1.21
(95% CI, 1.15–1.26). The QI/CI ratios of all 30 serum samples were
within ±20% of the mean QI/CI ratio of the calibrators, indicating
that the current method was not affected by interfering
compounds.
ME
The mean ME % determined in four different matrices
ranged from 99.7 to 102.2% (Table
IV). The slopes of all matrices and matrix-free curves were
similar (~0.004). The results of the present study indicated that
the current method was not affected by different matrices.
 | Table IV.Assessment of ME. |
Table IV.
Assessment of ME.
| Matrix | Slope |
R2-value | ME, % |
|---|
| Methanol
(matrix-free) | 0.0044 | 0.999 | −a |
| Charcoal | 0.0042 | 0.997 | 102.2 |
| Female serum | 0.0046 | 0.999 | 101.8 |
| Male serum | 0.0043 | 0.998 |
99.7 |
Extraction efficiency
The extraction efficiency of three different levels
of QC ranged from 85.02 to 93.29% (Table V).
 | Table V.Extraction efficiency of testosterone
in three levels of quality control. |
Table V.
Extraction efficiency of testosterone
in three levels of quality control.
| Samples | A, ng/dl | B, ng/dl | Extraction
efficiency, % |
|---|
| Low |
60.58 | 56.52 | 93.29 |
| Medium | 382.15 | 324.91 | 85.02 |
| High | 667.70 | 598.64 | 89.66 |
Stability of sample preparation
The medium QC samples were obtained using the
aforementioned method. Following alteration in the sample
preparation parameters, the measured values exhibited a good
consistency (Fig. 2). For value
assignment, three replicate preparations of the medium QC samples
were prepared in each independent run. All data were presented as
the mean ± standard deviation. The results indicated that the
current method was not affected by the alterations in the sample
processing parameters.
Method applicability
The minimum value and the maximum value were 12.45
and 874.47 ng/dl, respectively. Samples with wide range of
testosterone concentrations were detected via the aforementioned
method (Table VI).
 | Table VI.Detection of testosterone in serum
samples. |
Table VI.
Detection of testosterone in serum
samples.
| Samples | Age range,
years | n | Median, ng/dl | Range, ng/dl |
|---|
| Female | 20-58 | 15 |
26.04 | 12.45–56.80 |
| Male | 18-85 | 15 | 463.34 | 269.81–874.47 |
Discussion
In the current study, an ID-UPLC-MS/MS method was
developed to detect testosterone in human serum. The method used
isotope-labeled IS, two-step LLE and UPLC to provide the desirable
accuracy, specificity, precision and matrix-independent
measurements. The intra-assay CV of high, medium and low QC samples
were 2.77, 1.40 and 2.21%, respectively, while the inter-assay CV
were 3.60, 3.06 and 3.66%, respectively. The method precision was
indicated to be below the suggested maximum variation for total
testosterone measurements of 5.3% (23). Moreover, the current method
exhibited a good consistency between low and high concentrations of
testosterone. In addition, the present method required a simple
sample preparation and a small sample volume, therefore it may be
suitable for routine clinical practice.
The isotope-labeled IS compensates for the potential
sample loss during sample preparation. The IS exhibited the same
chromatographic properties as the corresponding analytes. The
sample preparation included two steps of LLE. The first LLE step
was to separate lipid and protein. The second LLE step was to
remove acid impurities, such as fatty acids and phospholipids.
These impurities are often the source of ion inhibition and ME
(25). Introducing the second LLE
step resulted in detection limits and S/N ratios that were more
consistent across individual samples and minimized the
contamination of the UPLC-MS/MS system, thereby prolonging the
column lifetime. The outstanding performance of the UPLC-MS/MS
system provided a support for analysis, a sample retention time of
2.40 min and an injection volume of 5 µl, which shortened the
analysis time compared with a previous study (17) and increased the possibility of
testing multiple replicates of the sample.
The extraction efficiency of the current method
ranged from 85.02 to 93.29%. If an equilibrium is reached, the
recovery of testosterone and the IS should be equal. Therefore, the
extraction efficiency was not indicated to affect the accuracy of
the method, as the quantification was based on the area ratio of
testosterone and IS. However, maximizing recovery during sample
preparation ensured an adequate signal strength during the
UPLC-MS/MS analysis. In addition, if sufficient analytes and IS are
not recovered, the extraction efficiency may affect the detection
limit of the method. The detection limit of the current method was
calculated to be 0.50 ng/dl.
The linear range of the present method was indicated
to be 1 to 1,000 ng/dl, and the extended measurement range was 1 to
10,000 ng/dl. The current method was revealed to quantify
testosterone over a wide concentration range that covers the low
concentrations observed in children, females and males. As
pediatric specimens were not collected and were not assessed for
the applicability of the method, additional studies are required in
the future to increase the credibility.
In conclusion, a reliable ID-UPLC-MS/MS method with
precision, specificity and sensitivity was developed for clinical
determination of total testosterone in human serum. The measurement
values that were obtained with the current method were indicated to
meet the performance criteria for accuracy in clinical mass
spectrometry, which are provided by CLSI (18) and the Hormone Standardization
Program of the Center for Disease Control (23). The present ID-UPLC-MS/MS method may
be used as a routine method in clinical laboratories to detect the
total serum testosterone concentration in humans.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by National Natural
Science Foundation of China (grant no. 81772259).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, BQ and DW were involved in the conception of the
study and guided the experiment. GS designed and drafted the
manuscript. GS and JX performed the experiments. LL, XL and YC
conducted data analysis. HL and SG were involved in revising the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Medical University (approval no.
TMUHMEC2017008) and was conducted in accordance with the principles
of the Declaration of Helsinki. Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhasin S, Brito JP, Cunningham GR, Hayes
FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC and
Yialamas MA: Testosterone therapy in men with hypogonadism: An
endocrine society clinical practice guideline. J Clin Endocrinol
Metab. 103:1715–1744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kushnir MM, Rockwood AL, Roberts WL,
Pattison EG, Bunker AM, Fitzgerald RL and Meikle AW: Performance
characteristics of a novel tandem mass spectrometry assay for serum
testosterone. Clin Chem. 52:120–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salonia A, Rastrelli G, Hackett G,
Seminara SB, Huhtaniemi IT, Rey RA, Hellstrom WJG, Palmert MR,
Corona G, Dohle GR, et al: Paediatric and adult-onset male
hypogonadism. Nat Rev Dis Primers. 5:382019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin KA, Anderson RR, Chang RJ, Ehrmann
DA, Lobo RA, Murad MH, Pugeat MM and Rosenfield RL: Evaluation and
treatment of hirsutism in premenopausal women: An endocrine society
clinical practice guideline. J Clin Endocrinol Metab.
103:1233–1257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
La'ulu SL, Kalp KJ and Straseski JA: How
low can you go? Analytical performance of five automated
testosterone immunoassays. Clin Biochem. 58:64–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui Y, She T, Zhao H, Li J, Li L, Gao W
and Li H: Competitive light-initiated chemiluminescent assay: Using
5-α-dihydrotestosterone-BSA as competitive antigen for quantitation
of total testosterone in human sera. Anal Bioanal Chem.
411:745–754. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taieb J, Mathian B, Millot F, Patricot MC,
Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C and Boudou P:
Testosterone measured by 10 immunoassays and by isotope-dilution
gas chromatography-mass spectrometry in sera from 116 men, women,
and children. Clin Chem. 49:1381–1395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosner W, Auchus RJ, Azziz R, Sluss PM and
Raff H: Position statement: Utility, limitations, and pitfalls in
measuring testosterone: An Endocrine Society position statement. J
Clin Endocrinol Metab. 92:405–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Catlin DH, Demers LM, Starcevic B
and Swerdloff RS: Measurement of total serum testosterone in adult
men: Comparison of current laboratory methods versus liquid
chromatography-tandem mass spectrometry. J Clin Endocrinol Metab.
89:534–543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kushnir MM, Rockwood AL and Bergquist J:
Liquid chromatography-tandem mass spectrometry applications in
endocrinology. Mass Spectrom Rev. 29:480–502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
International Organization for
Standardization, . In vitro diagnostic medical devices-measurement
of quantities in samples of biological origin-metrological
traceability of values assigned to calibrators and control
Materials. ISO 17511 (Geneva). ISO. 2003.
|
|
12
|
Stanczyk FZ and Clarke NJ: Advantages and
challenges of mass spectrometry assays for steroid hormones. J
Steroid Biochem Mol Biol. 121:491–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vesper HW, Bhasin S, Wang C, Tai SS, Dodge
LA, Singh RJ, Nelson J, Ohorodnik S, Clarke NJ, Salameh WA, et al:
Interlaboratory comparison study of serum total testosterone
[corrected] measurements performed by mass spectrometry methods.
Steroids. 74:498–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Star-Weinstock M, Williamson BL, Dey S,
Pillai S and Purkayastha S: LC-ESI-MS/MS analysis of testosterone
at sub-picogram levels using a novel derivatization reagent. Anal
Chem. 84:9310–9317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keski-Rahkonen P, Huhtinen K, Poutanen M
and Auriola S: Fast and sensitive liquid chromatography-mass
spectrometry assay for seven androgenic and progestagenic steroids
in human serum. J Steroid Biochem Mol Biol. 127:396–404. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan TF, Le J, Cui Y, Peng R, Wang ST and
Li Y: An LC-MS/MS analysis for seven sex hormones in serum. J Pharm
Biomed Anal. 162:34–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tai SS, Xu B, Welch MJ and Phinney KW:
Development and evaluation of a candidate reference measurement
procedure for the determination of testosterone in human serum
using isotope dilution liquid chromatography/tandem mass
spectrometry. Anal Bioanal Chem. 388:1087–1094. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lynch KL: CLSI C62-A: A New standard for
clinical mass spectrometry. Clin Chem. 62:24–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guidance on the verification of
quantitative measurement procedures used in the clinical chemistry.
GL037. CNAS. 2019.
|
|
20
|
Rodriguez M and Orescan DB: Confirmation
and quantitation of selected sulfonylurea, imidazolinone, and
sulfonamide herbicides in surface water using electrospray LC/MS.
Anal Chem. 70:2710–2717. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Botelho JC, Ribera A, Cooper HC and Vesper
HW: Evaluation of an isotope dilution HPLC tandem mass spectrometry
candidate reference measurement procedure for Total 17-β estradiol
in human serum. Anal Chem. 88:11123–11129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matuszewski BK, Constanzer ML and
Chavez-Eng CM: Strategies for the assessment of matrix effect in
quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem.
75:3019–3030. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun YM, Botelho JC, Chandler DW, Katayev
A, Roberts WL, Stanczyk FZ, Vesper HW, Nakamoto JM, Garibaldi L,
Clarke NJ and Fitzgerald RL: Performance criteria for testosterone
measurements based on biological variation in adult males:
Recommendations from the partnership for the accurate testing of
hormones. Clin Chem. 58:1703–1710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Legro RS, Schlaff WD, Diamond MP,
Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC,
Krawetz SA, Snyder PJ, et al: Total testosterone assays in women
with polycystic ovary syndrome: Precision and correlation with
hirsutism. J Clin Endocrinol Metab. 95:5305–5313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neville D, Houghton R and Garrett S:
Efficacy of plasma phospholipid removal during sample preparation
and subsequent retention under typical UHPLC conditions.
Bioanalysis. 4:795–807. 2012. View Article : Google Scholar : PubMed/NCBI
|