Introduction
Renal cell carcinoma (RCC) is a common cancer that
accounts for ~3% of all diagnosed human cases of cancer (1,2).
Surgery is the primary treatment strategy for localized RCC, but a
third of patients with localized RCC experience metastases after
nephrectomy (3). Chemotherapy is
often an ineffective treatment strategy for RCC (4,5).
Although cisplatin is an effective therapeutic agent in a number of
different types of cancer, RCC displays resistance to cisplatin
(6), with only 4–6% of patients
with RCC responding to chemotherapy alone (7). Therefore, improving the knowledge of
the molecular mechanisms underlying RCC chemoresistance and
identifying novel RCC therapeutic targets is required.
The dedicator of cytokinesis (DOCK) family is one of
the two members of the RAC guanine nucleotide exchange factor (GEF)
family, which contain the evolutionarily conserved lipid-binding
DOCK homology region-1 (DHR-1) domain and the catalytic DHR-2
domain (8). As a RAC-specific GEF,
DOCK1 regulates phagocytosis, myoblastic fusion, cell migration and
circular dorsal fold formation (9,10).
Abnormal expression and activity of DOCK1 is associated with the
malignant characteristics of multiple tumors (11–14).
Increasing evidence has indicated that DOCK1 regulates invasion and
metastasis by acting on downstream receptor tyrosine kinases
(15–17). Moreover, previous studies have
revealed that DOCK1 expression levels correlate with
chemoresistance in several types of cancer, such as bladder cancer
and non-small-cell lung carcinomas (12,18).
However, the function of DOCK1 during RCC is not completely
understood.
Considering the important role of DOCK1 during tumor
development (11), DOCK1-selective
inhibitors have been investigated. TBOPP
[1-[2-(3′-(trifluoromethyl)-(1,1′-biphenyl)-4-yl)-2-oxoethyl]-5-pyrrolidinylsulfonyl-2(1H)-pyridone]
is a cellular activity inhibitor that binds to the DHR-2 domain of
DOCK1 with high affinity, blocking its interaction with RAC family
small GTPase 1 (RAC1) to inhibit its GEF activity without
influencing the GEF activity of the diffuse B-cell lymphoma family,
including T cell lymphoma invasion and metastasis 1 and Trio Rho
guanine nucleotide exchange factor (19). TBOPP also displays an anti-invasion
effect (20). However, the role of
TBOPP during RCC has not been previously reported; therefore, the
present study aimed to investigate the role of DOCK1 and TBOPP
during RCC cisplatin resistance.
Materials and methods
Cell culture
The 786-O, ACHN, and Caki-1 cell lines were
purchased from the American Type Culture Collection. 786-O cells
were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.). Caki and ACHN cells were
maintained in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS and 1% penicillin-streptomycin solution (Gibco;
Thermo Fisher Scientific, Inc.). Cells were cultured at 37°C with
5% CO2. TBOPP was purchased from WuXi AppTec and
dissolved in DMSO before use. Cisplatin was purchased from
Sigma-Aldrich, Merck KGaA. For single agent treatment groups, renal
carcinoma cells were treated with 0, 0.039, 0.078, 0.156, 0.312,
0.625, 1.25, 2.5, 5, 10, 20 or 40 µM TBOPP for 48 h at 37°C, or 0,
0.625, 1.25, 2.5, 5 or 10 µM cisplatin for 48 h at 37°C. For the
combined treatment group, renal carcinoma cells were treated with
10 µM TBOPP and 0, 0.625, 1.25, 2.5, 5 or 10 µM cisplatin for 48 h
at 37°C.
Small interfering (si)RNA
transfection
The DOCK1-specific and negative control siRNAs were
designed by Shanghai GenePharma Co., Ltd. The sequences of the
DOCK1 siRNAs were as follows: sc-35207A sense,
GAGACAGAUUGGCUUUGAATT and antisense, UUCAAAGCCAAUCUGUCUCTT;
sc-35207B sense, GAGAGAACCAUAUAUACAATT and antisense,
UUGUAUAUAUGGUUCUCUCTT; and sc-35207C sense, CAGCAAACAUCAAGAGAUATT
and antisense, UAUCUCUUGAUGUUUGCUGTT. The sequences of the negative
control siRNA were as follows: Sense, UUCUCCGAACGUGUCACGUTT and
antisense, ACGUGACACGUUCGGAGAATT. Cells were transfected with 8 µM
DOCK1 siRNAs (the mix of three sequences) or negative control
siRNAs using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 24 h transfection, the cells were used for subsequent
experimentation.
Western blotting
Western blotting was performed according to a
previously described protocol (21). Following 24 h treated with TBOPP or
transfected with siRNA, the cells were collected, and total protein
was extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a bicinchoninic
acid assay kit (Applygen Technologies, Inc.). Equal amounts of
proteins (40 µg per lane) were separated via 10% SDS-PAGE and
transferred to a PVDF membrane. Subsequently, the membrane was
blocked with 5% non-fat milk in TBS and 0.1% Tween-20 buffer for 1
h at room temperature. The membrane was incubated overnight at 4°C
with primary antibodies targeted against: DOCK1 (dilution 1:1,000;
cat. no. ab97325; Abcam) and GAPDH (1:1,000; cat. no. ab9485;
Abcam). Following primary incubation, the membrane was incubated
with horseradish peroxidase-conjugated secondary antibodies
(dilution 1:500; cat. no. 7074; Cell Signaling Technology) at room
temperature for 2 h. Protein bands were visualized using a Pierce™
ECL kit according to the manufacturer's protocol (Thermo Fisher
Scientific, Inc.). Protein expression was semi-quantified using
ImageJ software (version 1.8.0; National Institutes of Health).
GAPDH was used as the loading control.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed using the CCK-8 assay
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's protocol. Briefly, RCC cells treated with TBOPP or
DOCK siRNA for 24 h were seeded (3×104 cells/well) into
96-well plates. Following treatment with cisplatin, 10 µl CCK-8
solution was added to each well and incubated for 2 h at room
temperature. The absorbance value of each well at a wavelength of
450 nm was measured using an MRX II microplate reader (Dynex
Technologies).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
The Click-iTEdU Imaging kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for the EdU assay according to
the manufacturer's protocol. Briefly, cells were seeded
(1×104 cells/well) into a 96-well plate. Following
treatment, cells were fixed with 4% formaldehyde for 30 min,
permeated with 0.5% Triton-X-100 for 10 min at room temperature and
washed with PBS. Subsequently, 100 µl EdU reagent was added to each
well for 30 min at room temperature and cells were incubated with
100 µl Hoechest 33342 for 10 min to label the nuclei. Cells were
observed using a fluorescence microscope at ×200 magnification.
Cell apoptosis determination by flow
cytometry
Early and late apoptosis was detected using a
FITC-conjugated Annexin-V and propidium iodide (PI) kit (BD
Biosciences), according to the manufacturer's protocol. Cells
(2-3×105) were washed twice with pre-chilled PBS and
resuspended in 100 µl binding buffer with 5 µl FITC-conjugated
Annexin-V for 30 min at room temperature in the dark. Subsequently,
cells were incubated with 100 µl PI for 5 min at room temperature
and then 400 µl binding buffer was added. Apoptotic cells were
analyzed using a BD Laser II flow cytometer (BD Biosciences) and
FlowJo software 7.6 (FlowJo LLC).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc.) and GraphPad Prism (version 7;
GraphPad Software, Inc.). Statistical differences between two
groups were analyzed using a Student's t-test. Statistical
differences among multiple groups were analyzed by one-way ANOVA
followed by Tukey's post hoc test. Data are presented as the mean ±
standard deviation of three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
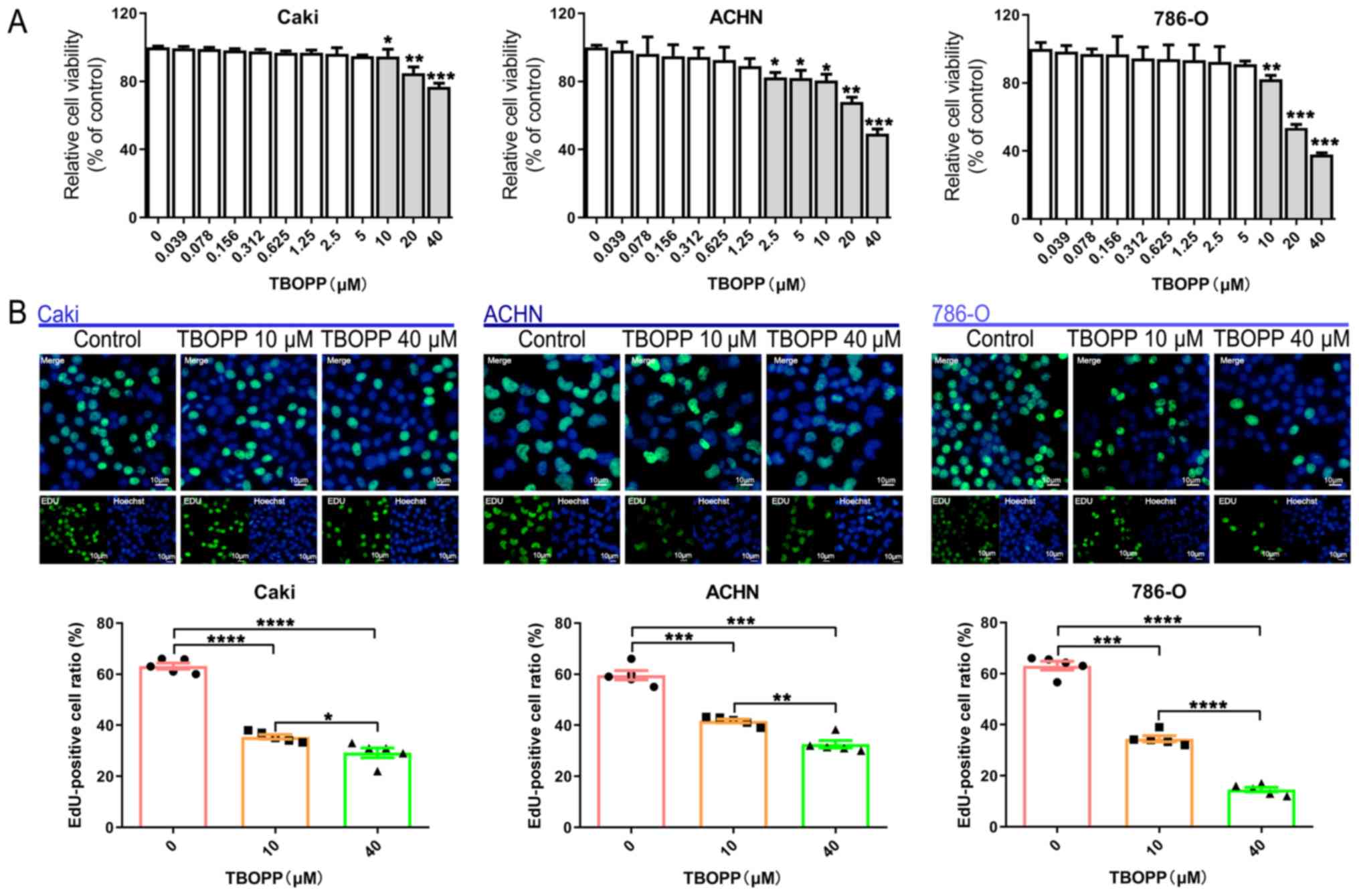
TBOPP decreases RCC cell proliferation
and viability
To investigate the function of TBOPP, RCC cells were
treated with different concentrations (0–40 µM) of TBOPP and cell
viability was assessed using a CCK-8 assay. TBOPP decreased RCC
cell viability from 10 µM in a concentration-dependent manner
(Fig. 1A). Subsequently, cells
treated with two concentrations of TBOPP (10 and 40 µM) were
assessed using the EdU assay. The results suggested that cell
proliferation was also significantly decreased in the TBOPP
treatment groups compared with the control group (Fig. 1B). The results demonstrated the
anti-RCC function of TBOPP; 10 µM TBOPP significantly reduced cell
viability in all 3 cell lines and also significantly decreased cell
proliferation in all 3 cell lines; thus, 10 µM TBOPP was used for
subsequent experiments.
TBOPP inhibits RCC cell cisplatin
resistance
The effect of TBOPP on cisplatin resistance in RCC
was investigated. TBOPP combined with cisplatin further reduced RCC
cell viability compared with cisplatin alone (Fig. 2A). The EdU assay suggested that
TBOPP significantly enhanced the antiproliferative activity of
cisplatin in RCC cells (Fig. 2B).
Moreover, TBOPP significantly increased cisplatin-induced RCC cell
apoptosis (Fig. 2C). Collectively,
the results demonstrated that TBOPP inhibited RCC cell cisplatin
resistance.
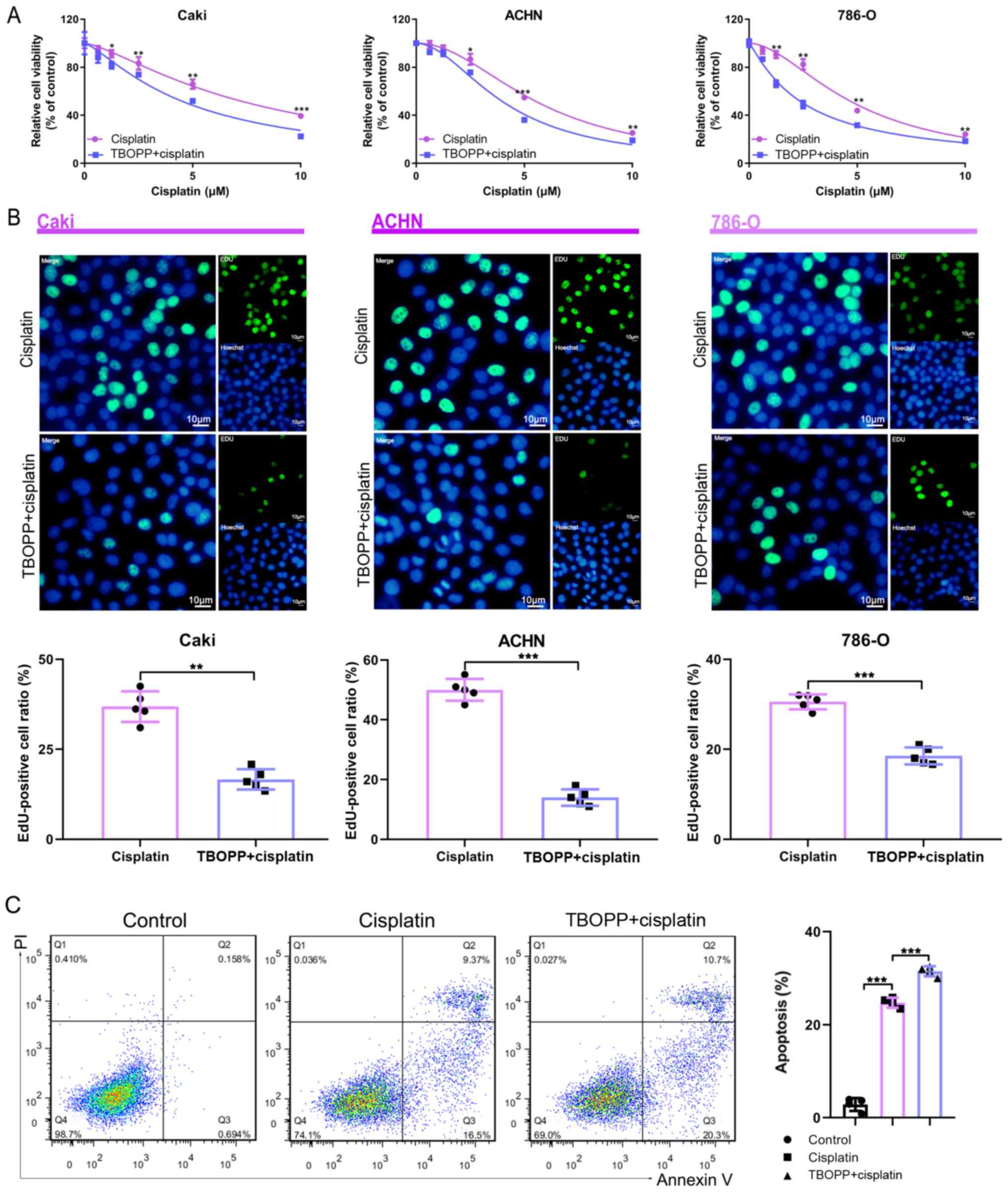 | Figure 2.TBOPP sensitizes RCC cells to
cisplatin. (A) RCC cell viability following treatment with
cisplatin alone or cisplatin plus 10 µM TBOPP, as determined by the
Cell Counting Kit-8 assay. *P<0.05, **P<0.01 and
***P<0.001 vs. the TBOPP + cisplatin group. (B) RCC cell
proliferation following treatment with cisplatin alone or cisplatin
plus 10 µM TBOPP, as determined by the EdU assay. Scale bar, 10 µm.
The rate of EdU-positive cells in the S phase is presented.
**P<0.01 and ***P<0.001, as indicated. (C) Flow cytometric
analysis of RCC cell apoptosis following treatment with cisplatin
alone or cisplatin plus 10 µM TBOPP. Cells in quadrants 2 and 3
were considered to be apoptotic. ***P<0.001, as indicated.
TBOPP,
1-[2-(3′-(trifluoromethyl)-(1,1′-biphenyl)-4-yl)-2-oxoethyl]-5-pyrrolidinylsulfonyl-2(1H)-pyridone;
RCC, renal cell carcinoma; EdU, 5-Ethynyl-2′-deoxyuridine; PI,
propidium iodide. |
DOCK1 knockdown sensitizes RCC cells
to cisplatin
Subsequently, the role of DOCK1 during RCC was
investigated. Firstly, the expression of DOCK1 in the three RCC
cell lines was measured. DOCK1 expression levels were highest in
the Caki cell line, followed by the ACHN cell line and the 786-O
cell line (Fig. 3A). The CCK-8
assay indicated that following treatment with cisplatin, the Caki
cell line displayed the highest cell viability among the three RCC
cell lines assessed (IC50 values: Caki, 7.804 µM; ACHIN,
5.105 µM; 786-O: 3.770 µM) (Fig.
3B). To further verify the role of DOCK1 during RCC, a DOCK1
siRNA was used to significantly knockdown DOCK1 expression, which
was confirmed by western blotting (Fig. 3C). The CCK-8 assay suggested that
DOCK1 knockdown significantly enhanced cisplatin-mediated
reductions in cell viability (Fig.
3D). Additionally, flow cytometry analysis of apoptosis
demonstrated that DOCK1 knockdown promoted cisplatin-induced
apoptosis (Fig. 3E). Furthermore,
the EdU assay indicated that following treatment with cisplatin,
the proliferation of DOCK1 siRNA-treated cells was significantly
lower compared with the control cells (Fig. 3F). The results suggest that DOCK1
promotes RCC cell cisplatin resistance.
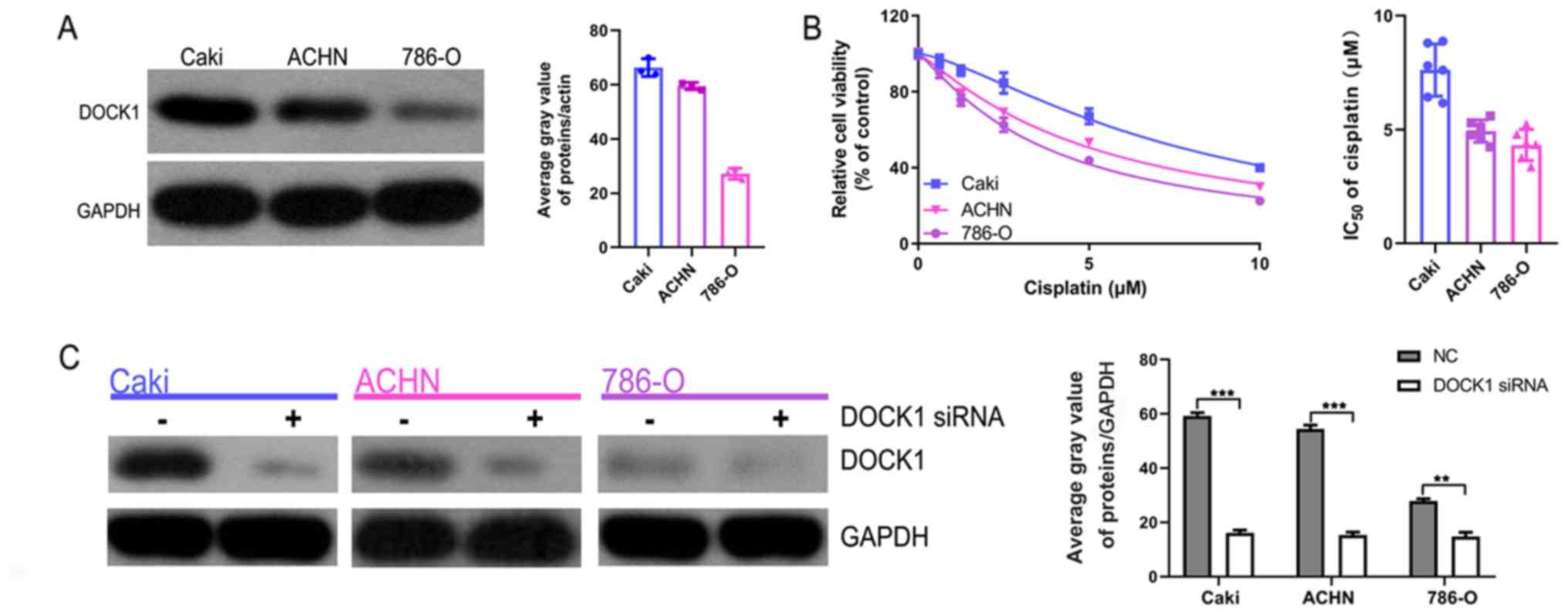 | Figure 3.DOCK1 knockdown enhances cisplatin
sensitivity during RCC. (A) DOCK1 expression levels in the three
RCC cell lines were determined by western blotting. (B) The CCK-8
assay was performed to determine cell viability and the
IC50 value of cisplatin in the three RCC cell lines. (C)
The transfection efficiency of DOCK1 siRNA (the mix of three
sequences) was assessed by western blotting. **P<0.01 and
***P<0.001, as indicated. (D) The CCK-8 assay was performed to
determine cell viability in the three RCC cell lines following
co-treatment with cisplatin and DOCK1 siRNA or negative control
siRNA. *P<0.05, **P<0.01 and ***P<0.001 vs. the cisplatin
group. (E) Flow cytometry analysis of RCC cell apoptosis following
co-treatment with cisplatin and DOCK1 siRNA or negative control
siRNA. **P<0.01 and ***P<0.001, as indicated. (F) RCC cell
proliferation was assessed by the EdU assay following co-treatment
with cisplatin and DOCK1 siRNA or negative control siRNA. Scale
bar, 10 µm. ***P<0.001 and ****P<0.0001, as indicated. DOCK1,
dedicator of cytokinesis 1; RCC, renal cell carcinoma; CCK-8, Cell
Counting Kit-8; siRNA, small interfering RNA; EdU,
5-Ethynyl-2′-deoxyuridine; NC, negative control; PI, propidium
iodide. |
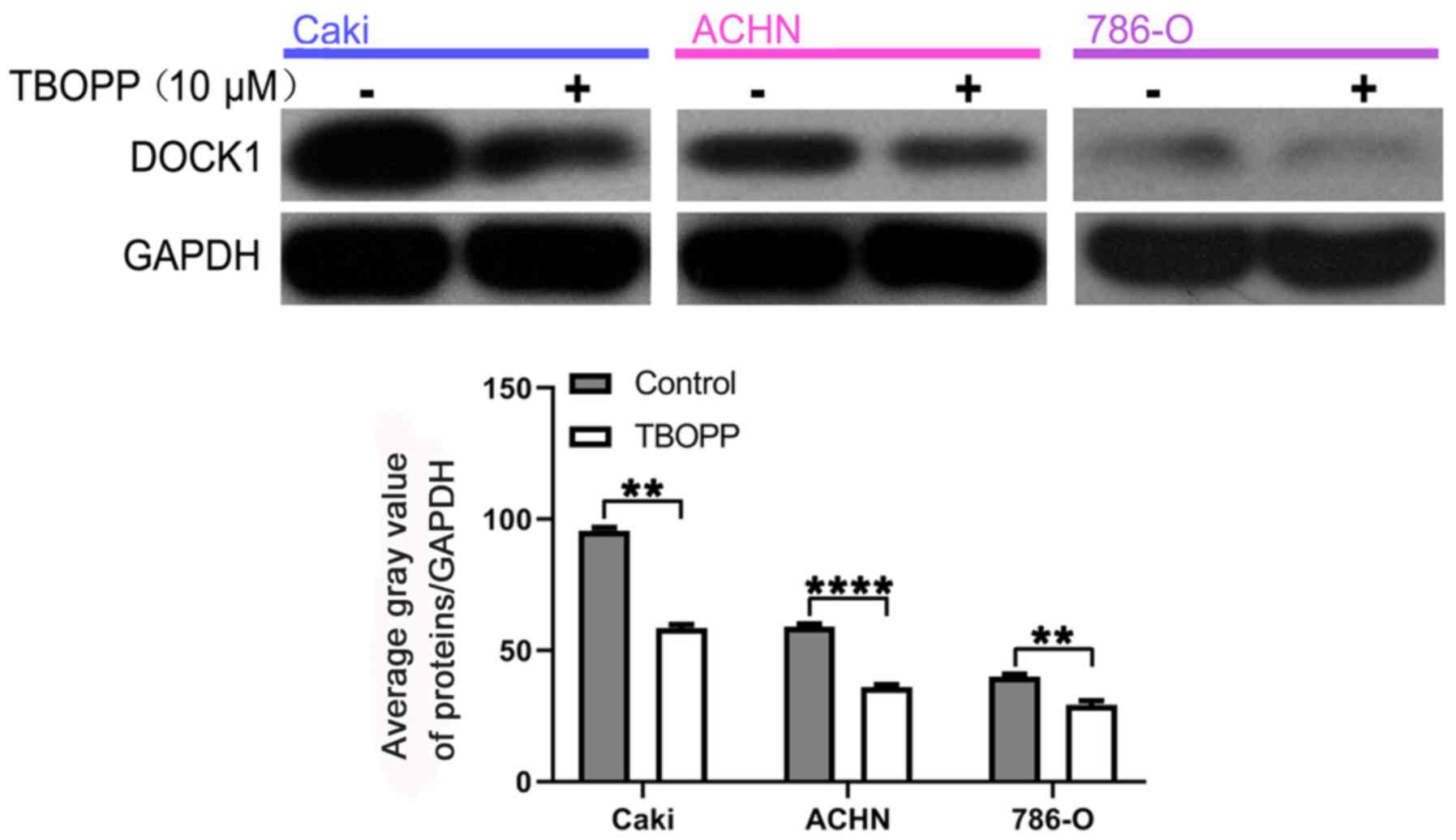
TBOPP function during RCC is mediated
by DOCK1
TBOPP is an inhibitor of DOCK1; therefore, to
explore the relationship between TBOPP and DOCK1 during RCC, the
inhibitory role of TBOPP on DOCK1 expression in RCC cells was
assessed by western blotting. TBOPP significantly decreased DOCK1
expression levels compared with the control group (Fig. 4). Subsequently, the cell viability
of cells co-treated with cisplatin and DOCK1 siRNA alone or DOCK1
siRNA plus TBOPP was assessed by the CCK-8 assay. Cell viability in
the DOCK1 siRNA group and the DOCK1 siRNA plus TBOPP group was not
significantly different (Fig. 5A),
which indicated that TBOPP-mediated cisplatin sensitivity did not
occur in the absence of DOCK1 expression. The relationship between
TBOPP and DOCK1 during RCC was further indicated by the results of
the flow cytometry analysis of cell apoptosis (Fig. 5B). Transfection efficiency of DOCK1
siRNA was confirmed by western blotting (Fig. 5C). The results demonstrated that
DOCK1 was involved in TBOPP-mediated cisplatin sensitivity.
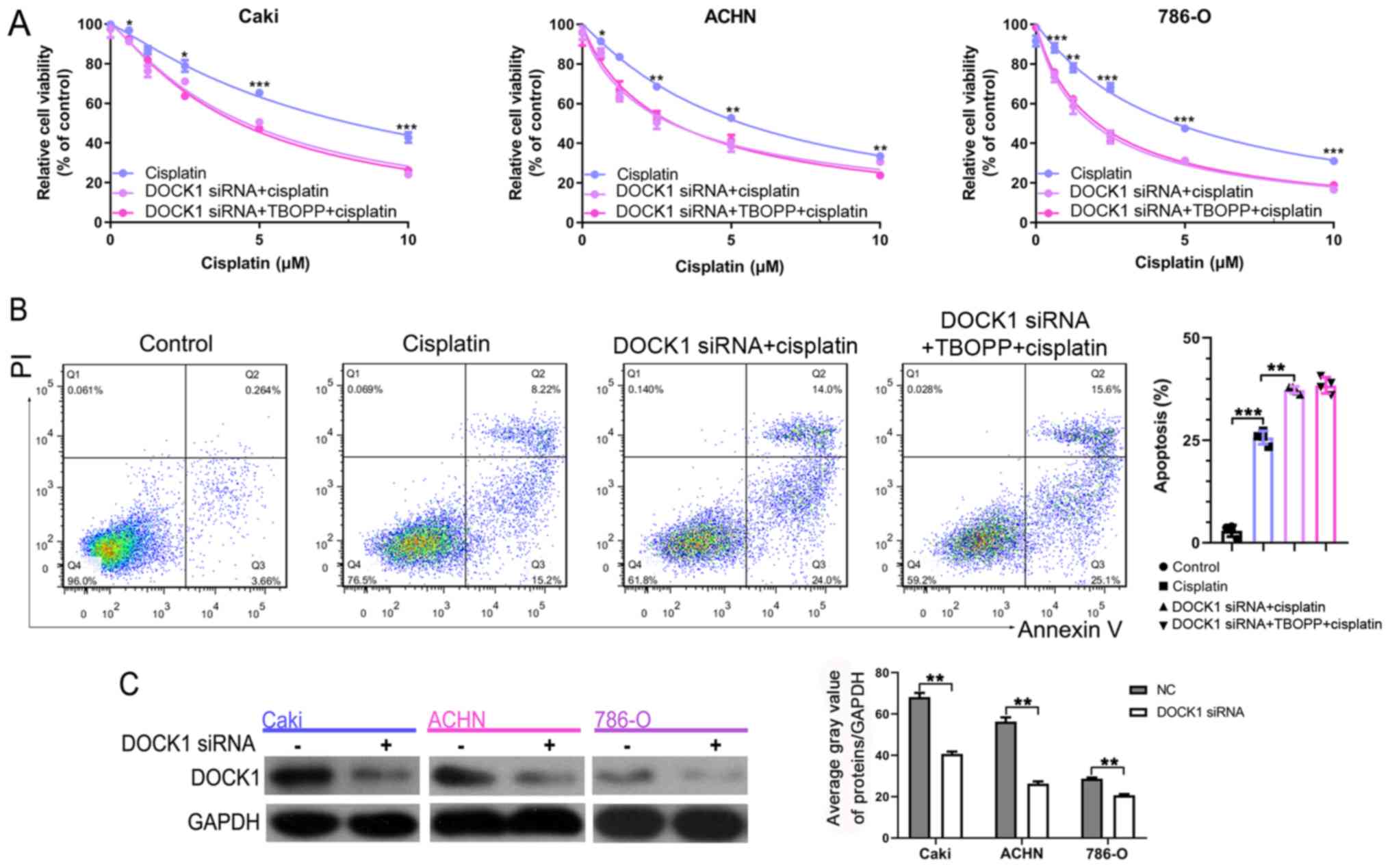 | Figure 5.TBOPP enhances cisplatin sensitivity
by inhibiting DOCK1. (A) RCC cell viability following co-treatment
with cisplatin and DOCK1 siRNA or DOCK1 siRNA plus 10 µM TBOPP.
*P<0.05, **P<0.01 and ***P<0.001 vs. TBOPP + cisplatin.
(B) Flow cytometry analysis of RCC cell apoptosis following
co-treatment with cisplatin and DOCK1 siRNA or DOCK1 siRNA plus 10
µM TBOPP. **P<0.01 and ***P<0.001, as indicated. (C) The
transfection efficiency of DOCK1 siRNA (the mix of three sequences)
was assessed by western blotting. **P<0.01, as indicated. TBOPP,
1-[2-(3′-(trifluoromethyl)-(1,1′-biphenyl)-4-yl)-2-oxoethyl]-5-pyrrolidinylsulfonyl-2(1H)-pyridone;
DOCK1, dedicator of cytokinesis 1; RCC, renal cell carcinoma;
siRNA, small interfering RNA; NC, negative control; PI, propidium
iodide. |
Discussion
In patients with RCC, infrequent responses due to
intrinsic and acquired chemoresistance significantly limit the
clinical use of chemotherapeutic agents (22). Therefore, identifying the
mechanisms underlying RCC chemoresistance and developing novel
therapeutic strategies to resensitize RCC to anticancer drugs is
important. The present study aimed to investigate the role of DOCK1
and its inhibitor TBOPP during RCC chemoresistance. The results
suggested that TBOPP and DOCK1 may serve as therapeutic targets to
alleviate chemoresistance in patients with RCC.
A previous study demonstrated the inhibitory role of
TBOPP against DOCK1, and TBOPP has been reported to display an
antitumor role in other types of cancer, such as colon cancer and
skin malignant melanoma (19,20).
The present study further demonstrated the inhibitory effect of
TBOPP on DOCK1, and displayed the role of TBOPP during RCC,
suggesting that TBOPP may serve as a chemotherapeutic sensitizer
for RCC.
The DOCK family comprises 11 members, which are
atypical Rho guanine nucleotide exchange factors (23). Previous studies have revealed the
essential roles of DOCK in several cellular processes, including
tumorigenesis (15,24). By activating RAC1, DOCK1 displays
key functions in cytoskeletal organization, myoblast fusion and
phagocytosis (25–27). A number of studies have suggested
that DOCK1 is related to tumorigenesis, tumor growth and invasion
in several types of cancer, such as glioblastoma and ovarian
cancer, implying that DOCK1 plays a vital role in human cancer
progression (28–30). Moreover, the biological role of
DOCK1 during bladder cancer suggested that downregulation of DOCK1
could sensitize bladder cancer cells to cisplatin by inhibiting the
epithelial-mesenchymal transition (12). In addition, DOCK inhibition by
enoxaparin could improve the antitumor and antimigration activity
of gefitinib during lung cancer (18). However, the role of DOCK1 during
RCC has not been previously reported. The results of the present
study suggested that DOCK1 expression levels were associated with
cisplatin resistance, which was alleviated by DOCK1 inhibition,
suggesting that DOCK1 was related to RCC cisplatin resistance.
Although the results indicated that TBOPP sensitized
RCC to cisplatin by inhibiting DOCK1, the present study had a
number of limitations. Firstly, the present study did investigate
the role of DOCK1 and TBOPP in normal kidney cells. Secondly, the
effect of TBOPP on the sensitivity of newer, more RCC specific
agents, including sorafenib or pazopanib, was not investigated.
Thirdly, Tajiri et al (19)
reported that DOCK1 expression is driven by oncogenic mutations of
RAS; however, the relationship between DOCK1 and RAS was not
explored in the present study.
In conclusion, the present study demonstrated that
DOCK1 was associated with RCC cisplatin resistance, which was
ameliorated by TBOPP. The results suggested that DOCK1 inhibition
may serve as a potential therapeutic strategy to alleviate RCC
chemoresistance, and TBOPP might serve as a chemotherapeutic
sensitizer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81602217).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and WC conceived the study. XZ, SX and SZ
performed the experiments. JM, YC and XL analyzed the data. WZ
participated in the design of the study and drafted the manuscript.
HN conceived the study and revised the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
DOCK1
|
dedicator of cytokinesis 1
|
|
TBOPP
|
1-[2-(3′-(trifluoromethyl)-
(1,1′-biphenyl)-4-yl)-2-oxoethyl]-5-pyrrolidinylsulfonyl-
2(1H)-pyridone
|
|
GEF
|
guanine nucleotide exchange factor
|
|
DHR-1
|
DOCK homology region-1
|
|
RAC1
|
RAC family small GTPase 1
|
References
|
1
|
Jonasch E, Gao J and Rathmell WK: Renal
cell carcinoma. BMJ. 349:g47972014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi SH, Klatte T, Usher-Smith J and
Stewart GD: Epidemiology and screening for renal cancer. World J
Urol. 36:1341–1353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Poppel H, Becker F, Cadeddu JA, Gill
IS, Janetschek G, Jewett MA, Laguna MP, Marberger M, Montorsi F,
Polascik TJ, et al: Treatment of localised renal cell carcinoma.
Eur Urol. 60:662–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vitale MG, Bracarda S, Cosmai L, Crocetti
E, Di Lorenzo G, Lapini A, Mandressi A, Martorana G, Masini C,
Montironi R, et al: Management of kidney cancer patients: 2018
guidelines of the Italian Medical Oncology Association (AIOM).
Tumori. 105 (4_suppl):S3–S12. 2019. View Article : Google Scholar
|
|
5
|
Sánchez-Gastaldo A, Kempf E, González Del
Alba A and Duran I: Systemic treatment of renal cell cancer: A
comprehensive review. Cancer Treat Rev. 60:77–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanji N and Yokoyama M: Treatment of
metastatic renal cell carcinoma and renal pelvic cancer. Clin Exp
Nephrol. 15:331–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yagoda A, Petrylak D and Thompson S:
Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin
North Am. 20:303–321. 1993.PubMed/NCBI
|
|
8
|
Laurin M and Côté JF: Insights into the
biological functions of Dock family guanine nucleotide exchange
factors. Genes Dev. 28:533–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morishita K, Anh Suong DN, Yoshida H and
Yamaguchi M: The drosophila DOCK family protein Sponge is required
for development of the air sac primordium. Exp Cell Res.
354:95–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanematsu F, Nishikimi A, Watanabe M,
Hongu T, Tanaka Y, Kanaho Y, Côté JF and Fukui Y: Phosphatidic
acid-dependent recruitment and function of the Rac activator DOCK1
during dorsal ruffle formation. J Biol Chem. 288:8092–8100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laurin M, Huber J, Pelletier A, Houalla T,
Park M, Fukui Y, Haibe-Kains B, Muller WJ and Côté JF: Rac-specific
guanine nucleotide exchange factor DOCK1 is a critical regulator of
HER2-mediated breast cancer metastasis. Proc Natl Acad Sci USA.
110:7434–7439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen DJ, Chen W, Jiang H, Yang H, Wang YC
and Chen JH: Downregulation of DOCK1 sensitizes bladder cancer
cells to cisplatin through preventing epithelial-mesenchymal
transition. Drug Des Devel Ther. 10:2845–2853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Li H, Yin C, Sun X, Zheng S,
Zhang C, Shi L, Liu Y and Lu S: Dock1 promotes the mesenchymal
transition of glioma and is modulated by MiR-31. Neuropathol Appl
Neurobiol. 43:419–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang G, Zhang J, Yang X, Zhang X, Yang S,
Wang J, Hu K, Shi J, Ke X and Fu L: High expression of dedicator of
cytokinesis 1 adversely influences the prognosis of acute myeloid
leukemia patients undergoing allogeneic hematopoietic stem cell
transplantation. Cancer Manag Res. 11:3053–3060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gadea G and Blangy A: Dock-family exchange
factors in cell migration and disease. Eur J Cell Biol. 93:466–477.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jarzynka MJ, Hu B, Hui KM, Bar-Joseph I,
Gu W, Hirose T, Haney LB, Ravichandran KS, Nishikawa R and Cheng
SY: ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange
factor, promote human glioma cell invasion. Cancer Res.
67:7203–7211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Y, Wang S and Zhang Y:
Downregulation of Dock1 and Elmo1 suppresses the migration and
invasion of triple-negative breast cancer epithelial cells through
the RhoA/Rac1 pathway. Oncol Lett. 16:3481–3488. 2018.PubMed/NCBI
|
|
18
|
Pan Y and Li X, Duan J, Yuan L, Fan S, Fan
J, Xiaokaiti Y, Yang H, Wang Y and Li X: Enoxaparin sensitizes
human non-small-cell lung carcinomas to gefitinib by inhibiting
DOCK1 expression, vimentin phosphorylation, and Akt activation. Mol
Pharmacol. 87:378–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tajiri H, Uruno T, Shirai T, Takaya D,
Matsunaga S, Setoyama D, Watanabe M, Kukimoto-Niino M, Oisaki K,
Ushijima M, et al: Targeting Ras-driven cancer cell survival and
invasion through selective inhibition of DOCK1. Cell Rep.
19:969–980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomino T, Tajiri H, Tatsuguchi T, Shirai
T, Oisaki K, Matsunaga S, Sanematsu F, Sakata D, Yoshizumi T,
Maehara Y, et al: DOCK1 inhibition suppresses cancer cell invasion
and macropinocytosis induced by self-activating Rac1P29S
mutation. Biochem Biophys Res Commun. 497:298–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Fang L, Zang Y, Ren J and Xu Z:
CIP2A promotes proliferation, invasion and chemoresistance to
cisplatin in renal cell carcinoma. J Cancer. 9:4029–4038. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molina AM, Motzer RJ and Heng DY: Systemic
treatment options for untreated patients with metastatic clear cell
renal cancer. Semin Oncol. 40:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Namekata K, Kimura A, Kawamura K, Harada C
and Harada T: Dock GEFs and their therapeutic potential:
Neuroprotection and axon regeneration. Prog Retin Eye Res. 43:1–16.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt S and Debant A: Function and
regulation of the Rho guanine nucleotide exchange factor Trio.
Small GTPases. 5:e297692014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laurin M, Dumouchel A, Fukui Y and Côté
JF: The Rac-specific exchange factors Dock1 and Dock5 are
dispensable for the establishment of the glomerular filtration
barrier in vivo. Small GTPases. 4:221–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laurin M, Fradet N, Blangy A, Hall A,
Vuori K and Côté JF: The atypical Rac activator Dock180 (Dock1)
regulates myoblast fusion in vivo. Proc Natl Acad Sci USA.
105:15446–15451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Albert ML, Kim JI and Birge RB:
Alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for
phagocytosis of apoptotic cells. Nat Cell Biol. 2:899–905. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Dai JM, Che YL, Gao YM, Peng HJ,
Liu B, Wang H and Linghu H: Elmo1 helps dock180 to regulate Rac1
activity and cell migration of ovarian cancer. Int J Gynecol
Cancer. 24:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Xiong X, Abdalla A, Alejo S, Zhu L,
Lu F and Sun H: HGF-induced formation of the MET-AXL-ELMO2-DOCK180
complex promotes RAC1 activation, receptor clustering, and cancer
cell migration and invasion. J Biol Chem. 293:15397–15418. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng H, Hu B, Jarzynka MJ, Li Y, Keezer S,
Johns TG, Tang CK, Hamilton RL, Vuori K, Nishikawa R, et al:
Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine
residue Y722 by Src family kinases mediates EGFRvIII-driven
glioblastoma tumorigenesis. Proc Natl Acad Sci USA. 109:3018–3023.
2012. View Article : Google Scholar : PubMed/NCBI
|