Introduction
Postoperative cognitive dysfunction (POCD) is a type
of postoperative complication that occurs in the central nervous
system (CNS) (1,2). POCD is regarded as a mild
neurocognitive disorder without a formal definition (3,4) and
the diagnosis requires a validated neuropsychological test battery
(5). POCD occurs in the first few
weeks following surgery; it severely impairs cognitive function and
increases mortality in patients who are affected (6). POCD is now considered to be a public
health concern, which has a negative impact on the quality of life
of patients who are affected and causes a high burden for social
medical care (7). According to
data from the Ministry of Civil Affairs of China, in 2018, the
elderly population (>60 years old) reached 249.49 million,
accounting for 17.9% of the total population of China (8). Thus, POCD may become a major health
issue, due to lack of efficient diagnosis in the coming decades
(9). Isoflurane-inhaled anesthesia
was reported to be associated with the development of POCD by
influencing brain activity, yet the exact mechanism has not been
elucidated (10). Therefore, a
deeper understanding of the role that isoflurane plays in the
development of POCD is of great importance to discover novel
approaches to eliminate isoflurane-induced cognitive
impairment.
Increasing evidence has demonstrated that
neuroinflammation is critical in the pathogenesis of
neurodegenerative diseases, including Alzheimer's disease (AD),
Parkinson's disease and POCD (11,12).
Surgery and anesthesia have been reported to induce
neuroinflammatory responses and promote the secretion of
proinflammatory cytokines, such as interleukin (IL)-1β, IL-6 and
tumor necrosis factor-α, which further activate microglia to an
inflammatory phenotype (13,14).
Microglia, innate immune cells of the brain, serve a crucial role
in brain homeostasis. Activation of microglia is considered a
negative event and it is involved in the development of
neurocognitive disorders, including POCD (15,16).
Furthermore, exposure to isoflurane has been shown to impair the
spatial learning memory of aged mice, accompanied by activation of
microglia and neuroinflammation (17). Yet the precise mechanisms remain
unclear.
Inflammasomes are cytosolic protein complexes
assembled by pattern recognition receptors (PRRs),
pathogen-associated molecular patterns and danger-associated
molecular patterns (18). To date,
five inflammasomes have been discovered, among which the
PYRIN-containing Apaf1-like protein 1 (PYPAF1) inflammasome is the
most investigated (19). The
PYPAF1 inflammasome is comprised of a sensor protein PYPAF1,
apoptosis-associated speck-like protein containing a caspase
recruitment domain (ASC), aspartate-specific proteases and
pro-caspase-1. PYPAF1 interacts with ASC, induces the activation of
NF-κB and regulates inflammatory-associated processes (20). The PYPAF1 inflammasome activates
the protease caspase-1, promotes the secretion of IL-1β and IL-18,
and further induces neuroinflammation (21). Studies have shown that PYPAF1 may
contribute to neuroinflammation in numerous cognitive
impairment-related diseases (19),
and inhibition of PYPAF1 activation has been found to inhibit
inflammation in the brain (22);
however, to the best of our knowledge, its role in POCD has not
been elucidated.
In our preliminary studies, PYPAF1 was upregulated
in the hippocampus of POCD rats. As PYPAF1 is associated with
inflammation, it was hypothesized that PYPAF1 may have an important
role in the development of POCD. The aim of the present study was
to determine the possible mechanisms of POCD by examining PYPAF1
and inflammation, using a rat POCD model via isoflurane exposure.
In addition, the role that PYPAF1 plays in microglial activation
and neuroinflammation was investigated.
Materials and methods
Rat POCD model
A total of 138 healthy 20-month-old male Sprague
Dawley (SD) rats (weight, 500–520 g; Liaoning Changsheng
Biotechnology Co. Ltd.) were randomly divided into four groups:
Control; isoflurane; isoflurane + short hairpin (sh)RNA-PYPAF1; and
isoflurane + shRNA-negative control (NC). Rats were maintained in a
standard experimental rodent room under a 12-h light/dark circle,
at a temperature of 23±1°C and relative humidity of 50±1%, with
free access to water and food. The animals were adaptively fed for
1 week before establishment of the animal model. For the isoflurane
+ shRNA-PYPAF1 group, rats were injected with 5 µl lentivirus
containing PYPAF1-shRNA [108 transduction units (TU)/ml]
into the right lateral ventricle; for the isoflurane + shRNA-NC
group, rats were injected with 5 µl lentivirus carrying NC-shRNA
(108 TU/ml) into the right lateral ventricle. A total of
3 days after lentiviral injection, rats in the isoflurane,
isoflurane + shRNA-PYPAF1 and isoflurane + shRNA-NC groups were
maintained in a chamber prefilled with 2% isoflurane in 100% oxygen
for 4 h, rats in the control group were given 100% oxygen for 4 h
(gas flow rate, 2 l/min) (23). A
total of 18 rats in each group were randomly selected and
euthanized by intraperitoneal injection of 200 mg/kg sodium
pentobarbital on day 3, and six rats in each group were euthanized
on day 7 following isoflurane exposure, and the hippocampi were
collected. Hippocampi from 12 rats euthanized on day 3 and six rats
euthanized on day 7 were cryopreserved in liquid nitrogen for
molecular biological analysis. Hippocampi from six rats euthanized
on day 7 were fixed with 4% paraformaldehyde at 4°C overnight for
histological examinations. The other six rats in each group were
used for behavioral testing. Another 18 SD rats were randomly
divided into three groups (control, shRNA-PYPAF1 or shRNA-NC) for
the assessment of transfection efficiency. All animal experiments
were performed at the animal center at the First Hospital of Jilin
University (Changchun, China) following the guidelines for the care
and use of laboratory animals (24), and this study was approved by the
First Hospital of Jilin University.
Construction of PYPAF1 shRNA
lentiviral vectors
To generate PYPAF1-targeting shRNA expressing
plasmids, double-stranded oligonucleotides encoding PYPAF1 shRNA
were amplified in a system containing 5X oligo annealing buffer (1
mM EDTA, 50 mM NaCl, 10 mM Tris), sense and antisense primers
containing the PYPAF1 shRNA sequence (Sangon Biotech Co., Ltd.)
using the following reaction conditions: 95°C for 5 min, 90°C for 5
min, 85°C for 5 min and 80°C for 5 min, followed by overnight
annealing at room temperature. The primer sequences were as
follows: Forward, 5′-ccggcccGGCTATGTACTATCT
GCTAttcaagagaTAGCAGATAGTACATAGCCttttt-3′ and reverse,
5′-aattaaaaaGGCTATGTACTATCTGCTAtctcttgaa TAGCAGATAGTACATAGCCggg-3′.
After the Tet-pLKO-puro lentiviral vector (cat. no. 21915; Addgene,
Inc.) was linearized with FastDigest AgeI and FastDigest
EcoRI (cat. nos. FD1464 and FD0274; Thermo Fisher
Scientific, Inc.), the oligonucleotides encoding PYPAF1 shRNA were
inserted into the lentiviral vector with T4 DNA Ligase (cat. no.
2011A; Takara Biotechnology Co., Ltd.). The recombinant plasmids
were then transformed into Escherichia coli DH5α-competent
cells (cat. no. 9057; Takara Biotechnology Co., Ltd.). The plasmids
were extracted from the positive clones using a Plasmid Maxi
Preparation kit (cat. no. DP2802; BioTeke Corporation). A total of
24 h prior to transfection, 293T cells were plated into a 10-cm
plate at density of 6×106. Tet-pLKO-puro-PYPAF1 shRNA
lentiviral vectors (3 µg), packaging plasmid psPAX2 (5 µg; cat. no.
12260; Addgene, Inc.) and envelop plasmid pMD2.G (5 µg; cat. no.
12259; Addgene, Inc.) were co-transfected into 293T cells (Procell
Life Science & Technology Co., Ltd.) using
Lipofectamine® 3000 (cat. no. L3000-008; Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 48 h to perform viral
packaging. Lentivirus-containing supernatants were then centrifuged
at 4,000 × g at 4°C for 10 min, filtered through a 0.45-µm filter
and centrifuged at 4,000 × g at 4°C for 5 min. The lentivirus stock
was collected and stored at −80°C. The shRNA sequences were as
follows: PYPAF1 shRNA, sense,
5′-CCGGCCCGGCTATGTACTATCTGCTATTCAAGAGATAGCAGATAGTACATAGCCTTTTT-3′
and antisense,
5′-AATTAAAAAGGCTATGTACTATCTGCTATCTCTTGAATAGCAGATAGTACATAGCCGGG-3′;
and NC-shRNA, sense,
5′-CCGGCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT-3′
and antisense,
5′-AATTAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG-3′.
Reverse transcription-quantitative PCR
(RT-qPCR)
Hippocampal tissues were lysed and total RNA was
extracted using commercial RNAprep pure kit (Tiangen Biotech Co.,
Ltd.). Total RNA was subsequently reverse transcribed into cDNA
using oligo(dT)15, dNTP, M-MLV and RNase inhibitor
(Tiangen Biotech Co., Ltd.). The RT conditions were as follows:
25°C for 10 min, 42°C for 50 min and 80°C for 10 min. qPCR was
performed using 2X Power Taq PCR MasterMix (BioTeke Corporation)
and SYBR-Green (Beijing Solarbio Science and Technology Co., Ltd.)
on an Exicycler™ 96 RT-PCR system (Bioneer Corporation). The
thermocycling conditions were as follows: Initial denaturation at
94°C for 5 min, followed by 40 cycles at 94°C for 10 sec, 60°C for
20 sec and 72°C for 30 sec, and a final extension step at 72°C for
6 min. The data were analyzed using the 2−ΔΔCq method
(25). GAPDH was used as an
internal control. The primers used are listed as follows: PYPAF1,
forward, 5′-GCCTTGAAGAGGAGTGGATAG-3′ and reverse,
5′-TGGGTGTAGCGTCTGTTGAG-3′; IL-1β, forward,
5′-TTCAAATCTCACAGCAGCAT-3′ and reverse, 5′-CACGGGCAAGACATAGGTAG-3′;
IL-18, forward, 5′-GCAGTAATACGGAGCATAAA-3′ and reverse,
5′-ATCCTTCACAGATAGGGTCA-3′; and GAPDH, forward,
5′-ACGTTGACATCCGTAAAGAC-3′ and reverse,
5′-TAGGAGCCAGGGCAGTAA-3′.
Western blot analysis
Hippocampal tissues were lysed with RIPA buffer and
protein concentration was determined using a BCA protein
quantification kit (both purchased from Beijing Solarbio Science
and Technology Co., Ltd.). Proteins (40 µg) were separated by
SDS-PAGE using 8 and 14% gels and transferred to PVDF membranes
(EMD Millipore; Merck KGaA). After blocking with 5% skimmed milk
for 1 h at room temperature, PVDF membranes were incubated with
primary antibodies at 4°C overnight, followed by incubation with
secondary antibodies at 37°C for 1 h. Subsequently, the protein
bands were visualized using ECL reagent (Beijing Solarbio Science
and Technology Co., Ltd.) and semi-quantified using
Gel-Pro-Analyzer 4 (Media Cybernetics, Inc.). PYPAF1 antibody
(1:1,000; cat. no. A5652; ABclonal Biotech Co., Ltd.), ASC antibody
(1:1,000; cat. no. A1170; ABclonal Biotech Co., Ltd.), Bcl-2
antibody (1:2,000; cat. no. 12789-1-AP; Wuhan Sanying
Biotechnology), Bax antibody (1:5,000; cat. no. 50599-2-lg; Wuhan
Sanying Biotechnology), cleaved caspase-3 antibody (1:1,000; cat.
no. 9654; Cell Signaling Technology, Inc.) and GAPDH antibody
(1:10,000; cat. no. 60004-1-Ig; Wuhan Sanying Biotechnology) were
the primary antibodies used. The secondary antibodies used were
horseradish peroxidase (HRP)-conjugated goat anti-rabbit lgG
(1:3,000; cat. no. SE134; Beijing Solarbio Science and Technology
Co., Ltd.) and HRP-conjugated goat anti-mouse lgG (1:3,000; cat.
no. SE131; Beijing Solarbio Science and Technology Co., Ltd.).
Immunofluorescence assay
Hippocampal samples were embedded in paraffin and
sliced into 5-µm sections. The sections were dehydrated at 60°C in
an incubator for 2 h, deparaffinized and heated in citric
acid/sodium citrate solution at 95°C for 10 min. After blocking
with goat serum (cat. no. SL038; Beijing Solarbio Science and
Technology Co., Ltd.) for 15 min at room temperature, tissue
sections were washed and incubated with PYPAF1 antibody (1:200;
cat. no. NBP2-12446SS; Novus Biologicals, LLC) and ionized calcium
binding adapter molecule-1 (Iba-1) antibody (1:200; cat.no.
ab15690; Abcam) at 4°C overnight. Tissue sections were washed with
PBS three times, incubated with FITC-labeled goat anti-mouse IgG
(1:200; cat. no. A0568; Beyotime Institute of Biotechnology) and
Cy3 labeled goat anti-rabbit IgG (1:200; cat. no. A0516; Beyotime
Institute of Biotechnology) for 90 min at room temperature, and
incubated with DAPI at room temperature for 5 min (Beyotime
Institute of Biotechnology). Tissue sections were sealed with
anti-fluorescence quenching reagent (Beijing Solarbio Science and
Technology Co., Ltd.) and images were obtained using a fluorescence
microscope (×400, magnification).
TUNEL-NeuN staining
Hippocampal sections were deparaffinized and
incubated with 0.1% Triton X-100 for 8 min at room temperature
(Beyotime Institute of Biotechnology). After retrieval with citric
acid/sodium citrate solution at 95°C for 10 min, tissues were
incubated with TUNEL solution (enzyme solution:label solution, 1:9;
Roche Diagnostics) for 60 min at 37°C, then with NeuN antibody
(1:300; cat. no. ab104224; Abcam) at 4°C overnight. Samples were
then washed with PBS three times and incubated with Cy3 labeled
goat anti-mouse IgG (1:200; cat. no. A0521; Beyotime Institute of
Biotechnology) for 60 min at room temperature. Tissues were sealed
with anti-fluorescence quenching reagent (Beijing Solarbio Science
and Technology Co., Ltd.) and images were obtained using a
fluorescence microscope (×400, magnification). Three sections from
each sample and three fields of view in each section were randomly
selected for observation, and the average values were used as the
final data.
Morris water maze
The Morris water maze was performed 7 days after
isoflurane exposure to assess the spatial learning and memory
ability of rats. The water maze was composed of a circular tub
(diameter, 180 cm) and a colorless escape platform (diameter, 12
cm) submerged 2 cm below the water surface. Rats were placed into
the water from different quadrants up against the wall, and the
time it took to escape was recorded. If rats failed to identify the
platform within 120 sec, they were guided to the platform and
allowed to rest for 15 sec. Rats were subjected to the training
from different quadrants four times a day, for 5 days.
Subsequently, the platform was removed on the 6th day of the trial,
and the escape time, times of platform crossing and retention time
at the target quadrant were recorded.
ELISA
Rat hippocampal tissues were homogenized on ice in
saline to extract protein and protein concentration was quantified
using a BCA protein quantification kit (Beijing Solarbio Science
and Technology Co., Ltd.). The amount of IL-1β and IL-18 was
determined using a commercial IL-1β ELISA kit [cat. no.
70-EK301B/3-96; MultiSciences (Lianke) Biotech Co. Ltd.] and IL-18
ELISA kit (cat. no. SEA064Ra; Wuhan USCN Business Co., Ltd.),
according to the manufacturers' instructions.
Caspase-1 activity detection
Hippocampal tissue (10 mg) was lysed in 100 µl lysis
buffer and protein concentration was quantified using a Bradford
protein assay kit (Beyotime Institute of Biotechnology). Caspase-1
activity was determined by measuring the optical density values at
405 nm using a commercial caspase-1 activity assay kit (cat. no.
BC3810; Beijing Solarbio Science and Technology Co., Ltd.),
according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using GraphPad Prism v7 (GraphPad Software, Inc.). The
escape time in the Morris water maze was analyzed using two-way
mixed ANOVA followed by post hoc Tukey test. The remaining data,
including the escape time in each day, were compared using one-way
ANOVA followed by post hoc Tukey test. All experiments were
repeated at least three times and P<0.05 was considered to
indicate a statistically significant difference.
Results
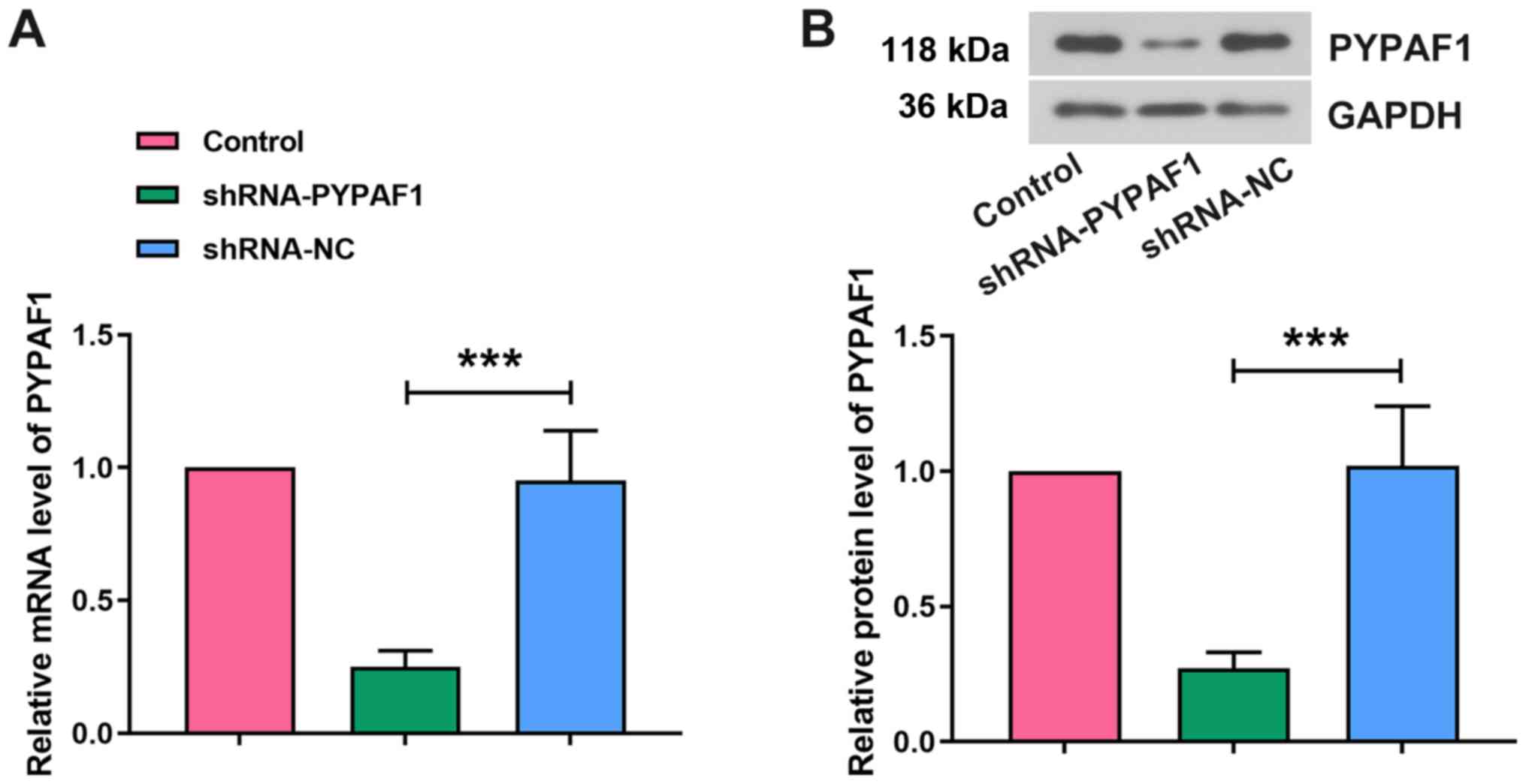
PYPAF1 expression is successfully
silenced by shRNA-PYPAF1
The expression of PYPAF1 in the hippocampus was
detected 3 days after lentiviral infection to confirm the
efficiency of RNA interference (RNAi). The mRNA expression levels
of PYPAF1 were significantly lower in the shRNA-PYPAF1 group
compared with in the shRNA-NC group (Fig. 1A). Western blot analysis revealed
similar results (Fig. 1B),
indicating that the expression of PYPAF1 was successfully knocked
down in the hippocampus of aged rats.
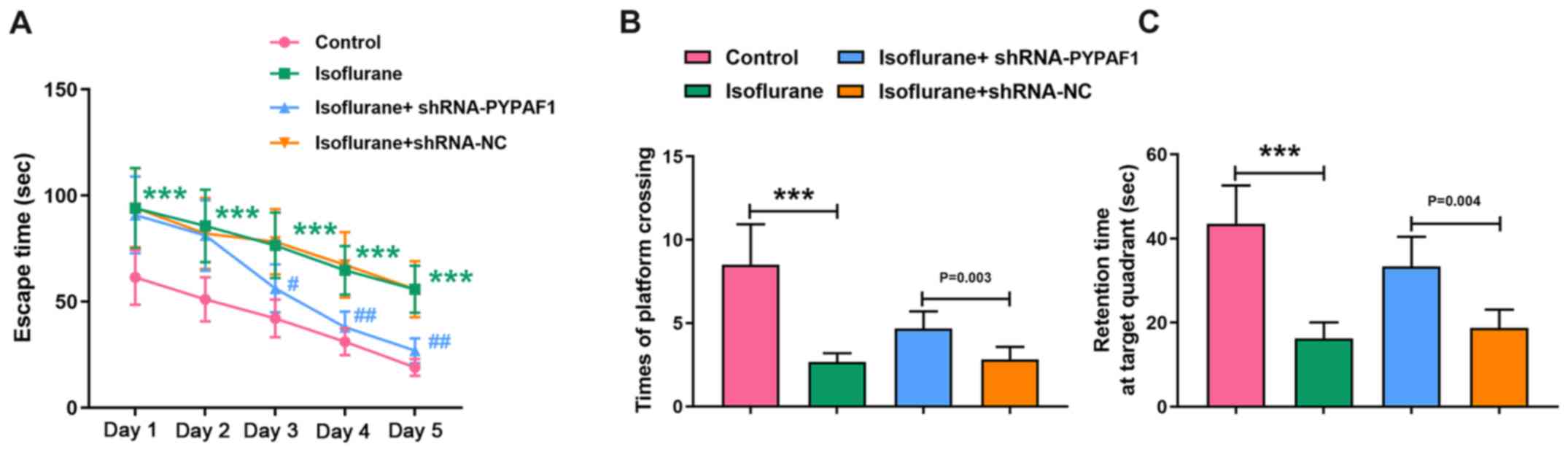
PYPAF1 silencing alleviates the
cognitive dysfunction induced by isoflurane in rats
The Morris water maze was performed to evaluate the
spatial learning ability of rats 7 days after isoflurane treatment.
Escape time of rats in the isoflurane group was significantly
increased compared with the control group throughout the experiment
(P<0.001). shRNA-NC did not apparently change the escape time of
isoflurane-exposed rats. However, PYPAF1-targeting shRNA markedly
shortened the escape time of isoflurane-exposed rats compared with
the isoflurane + shRNA-NC group from day 3 (P<0.05 at day 3;
P<0.01 at days 4 and 5; Fig.
2A). Times of platform crossing and retention time at the
target quadrant were recorded on day 6 of the Morris water maze
test. Rats subjected to isoflurane exposure had a lower number of
platform crossings compared with rats in the control group, while
PYPAF1 silencing increased the number of platform crossings
(Fig. 2B). Furthermore, rats
exposed to isoflurane spent less time at the target quadrant
compared with rats in the control group, while silencing of PYPAF1
increased the retention time (Fig.
2C).
PYPAF1 silencing inhibits the
activation of microglia
The expression levels of PYPAF1 and ASC were
determined in the hippocampus on days 3 and 7 following isoflurane
exposure. The mRNA expression levels of PYPAF1 were significantly
higher in rats exposed to isoflurane compared with the control
group, while PYPAF1 silencing using a lentivirus decreased the
levels of PYPAF1 on day 3. Similarly, significant associations were
observed on day 7 following isoflurane treatment (Fig. 3A). Isoflurane treatment
significantly increased the protein expression levels of PYPAF1 and
ASC on day 3, whereas PYPAF1 silencing partially abrogated the
increase and the trend was more notable on day 7 following
isoflurane exposure (Fig. 3B and
C). Furthermore, the immunofluorescence assay revealed that
isoflurane exposure increased the expression of Iba-1, which was
significantly alleviated by knockdown of PYPAF1 (Fig. 3D).
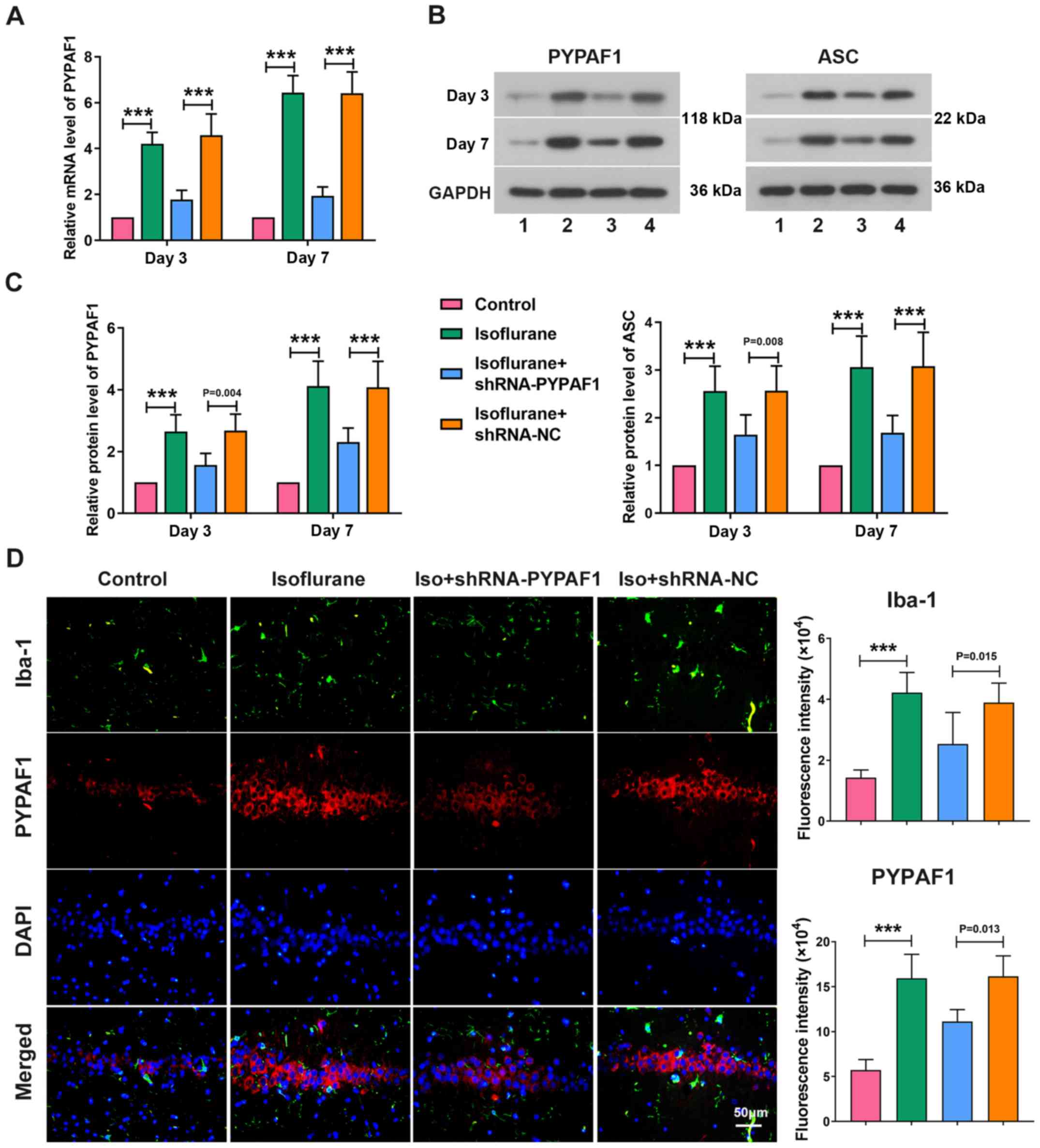 | Figure 3.PYPAF1 silencing inhibits the
activation of microglia. (A) mRNA levels of PYPAF1, and (B and C)
protein levels of PYPAF1 and ASC in the hippocampus were measured
using reverse transcription-quantitative PCR and western blot
analysis on days 3 and 7 following isoflurane treatment,
respectively. (B) Lane 1, control; lane 2, isoflurane; lane 3,
isoflurane + shRNA-PYPAF1; lane 4, isoflurane + shRNA-NC. (D)
Expression of PYPAF1 and Iba-1 in the hippocampus were determined
using immunofluorescence assay. ***P<0.001. ASC,
apoptosis-associated speck-like protein containing a caspase
recruitment domain; Iba-1, ionized calcium binding adapter
molecule-1; Iso, isoflurane; NC, negative control; PYPAF1,
PYRIN-containing Apaf1-like protein 1; shRNA, short hairpin
RNA. |
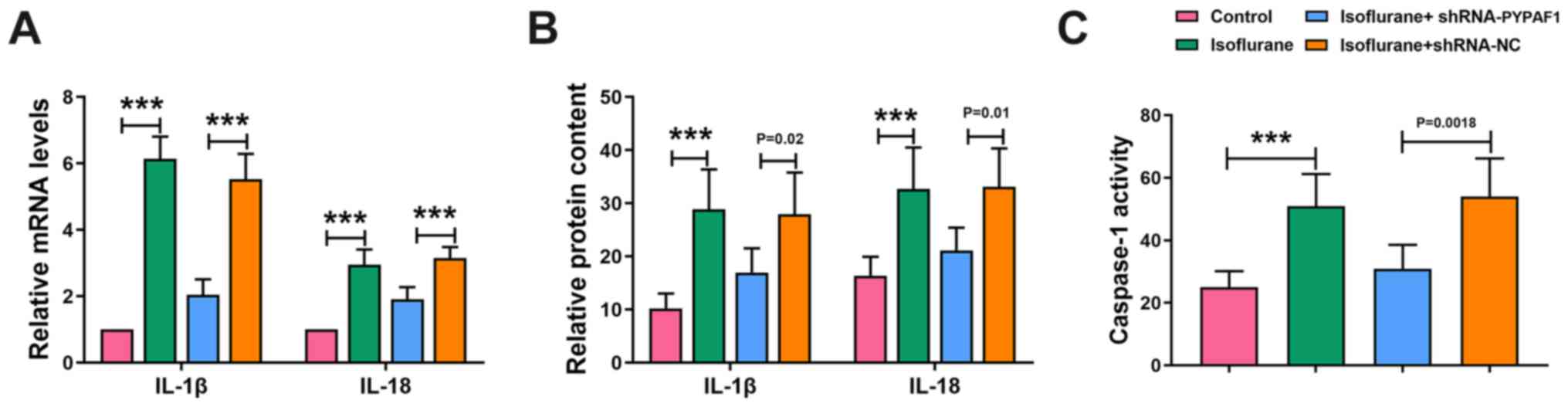
PYPAF1 silencing inhibits
neuroinflammation
RT-qPCR and ELISA were performed to determine the
levels of IL-1β and IL-18. The mRNA expression and concentration of
IL-1β and IL-18 in the hippocampus were increased following
isoflurane exposure, whereas PYPAF1 silencing decreased their
levels (Fig. 4A and B).
Furthermore, isoflurane treatment enhanced the activity of
caspase-1, which was partially abolished by silencing PYPAF1
(Fig. 4C). These results indicated
that silencing of PYPAF1 inhibited neuroinflammation in rats.
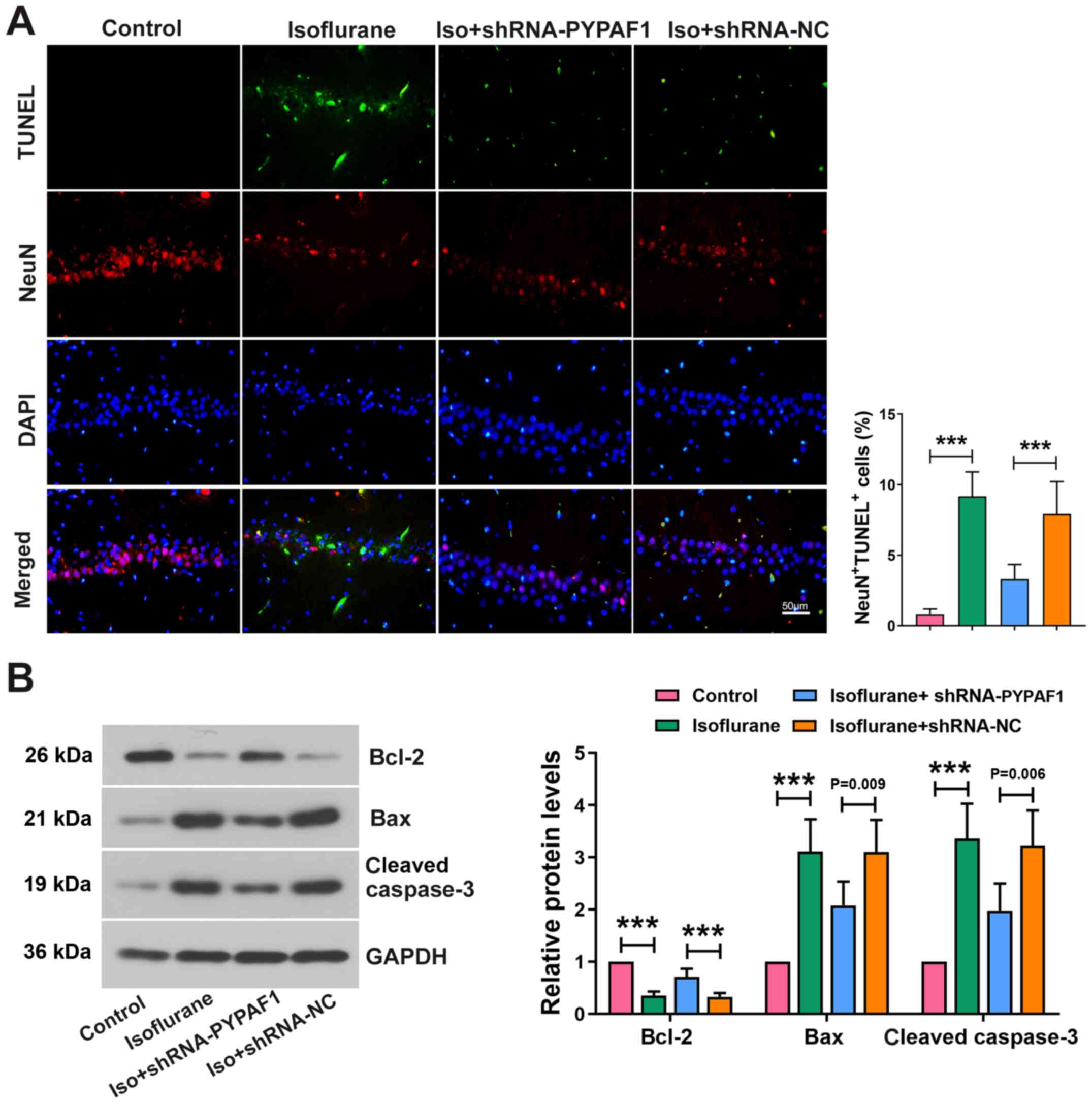
PYPAF1 silencing alleviates neuronal
apoptosis
TUNEL assay was performed to assess neuronal
apoptosis in the hippocampus. Apoptotic neurons were markedly
increased following isoflurane exposure, which was partially
reversed following PYPAF1 knockdown. Isoflurane treatment decreased
the expression of NeuN, whereas silencing PYPAF1 alleviated the
effects of isoflurane (Fig. 5A).
Furthermore, the protein expression levels of Bax and cleaved
caspase-3 were increased by isoflurane, and the elevation was
partially abolished by silencing of PYPAF1. The protein levels of
Bcl-2 revealed the opposite results (Fig. 5B).
Discussion
In the present study, isoflurane upregulated the
expression levels of PYPAF1 in the hippocampus of aged rats.
RNAi-mediated PYPAF1 knockdown significantly improved learning and
memory function of isoflurane-exposed aged rats. In addition,
PYPAF1 knockdown inhibited activation of microglia, production of
proinflammatory cytokines and neuronal apoptosis in the hippocampus
of isoflurane-exposed aged rats.
Isoflurane is a widely used inhalational anesthetic
agent owing to its low cost and relatively few side effects
(26). Inhaled anesthesia could
induce cerebral cognitive alterations in elderly patients (10,27).
Furthermore, a clinical trial involving elderly patients that were
subjected to laparoscopic cholecystectomy revealed that patients
who received isoflurane anesthesia displayed a significantly higher
incidence of POCD compared with those who received sevoflurane and
propofol (10,28). In the present study, the spatial
learning ability of rats exposed to isoflurane was markedly
reduced, which was accompanied by increased expression of PYPAF1
and ASC, whereas shRNA-mediated PYPAF1 knockdown markedly
alleviated the cognitive dysfunction of aged rats and decreased the
expression of ASC. The PYPAF1 inflammasome is abundantly expressed
in the CNS and activation of the PYPAF1 inflammasome is involved in
the pathogenesis of neurological diseases, including AD and
depression (29,30). PYPAF1 is the cytosolic sensor
molecule of the PYPAF1 inflammasome and abundant expression of
PYPAF1 is crucial for inflammasome formation (21). ASC activates the PYPAF1
inflammasome by recruiting the cysteine protease pro-caspase-1 via
its caspase recruitment domain (31). In the present study, shRNA-mediated
PYPAF1 knockdown hindered the assembling of the PYPAF1 inflammasome
and suppressed the expression of ASC. Furthermore, isoflurane
exposure led to the activation of caspase-1, which was also
inhibited following knockdown of PYPAF1. PYPAF1 silencing also
abolished isoflurane-induced elevated IL-1β and IL-18 at both the
mRNA and protein levels. These findings are consistent with
previous reports, which indicated that activation of the PYPAF1
inflammasome further activated inflammatory protease caspase-1, and
promoted the secretion of pro-inflammatory cytokines IL-1β and
IL-18 (21,32). Increased levels of IL-1β and IL-18
may trigger a series of signaling pathways and inflammatory
responses, which could lead to neuronal injury or apoptosis,
whereas knockdown of IL-1β in mice significantly alleviated
neuroinflammatory and memory dysfunction following surgery and
anesthesia (33,34). The results from the present study
indicated that the PYPAF1/ASC pathway may contribute to
isoflurane-induced POCD in aged rats.
Microglia, a primary effector cell that responds to
pathological challenges in the CNS, serves a crucial role in
neuroprotection and in the maintenance of hemostasis (35). Mild activation of microglia exerts
a protective effect on the CNS, whereas its over-activation results
in neuronal dysfunction (36,37).
Furthermore, microglia express numerous PRRs that are essential for
assembling the inflammasome (38).
In the present study, isoflurane exposure led to activation of
microglia in the hippocampus of aged rats, and PYPAF1 knockdown
inhibited the activation of microglia, as evidenced by the decrease
in the expression of Iba-1. It is well known that microglial
activation occurs in the early stage of neuroinflammation, which
leads to the production of proinflammatory cytokines and induces
cognitive dysfunction (39). In
addition, aging causes microglia to be more susceptible to
activation (40). The findings
from the present study indicated that PYPAF1 may have a critical
role in microglial activation following isoflurane exposure.
However, the present study has not revealed whether this inhibitory
effect on microglial activation is direct or indirect; therefore,
further investigation is required in future studies.
Neuronal function is the foundation of learning and
memory (41). In the present
study, isoflurane exposure induced neuronal apoptosis and decreased
NeuN expression in the rat hippocampus, whereas PYPAF1 silencing
alleviated neuronal apoptosis and elevated the levels of NeuN.
Isoflurane exposure elevated the expression of pro-apoptotic
factor, Bax, and cleaved caspase-3, and decreased the expression of
the anti-apoptotic factor, Bcl-2. Furthermore, silencing of PYPAF1
abrogated isoflurane-induced neuronal apoptosis. These results
suggested that PYPAF1 knockdown may inhibit neuronal apoptosis in
the hippocampus of aged rats exposed to isoflurane. However,
whether PYPAF1-targeting shRNA directly affects apoptotic pathways,
or inhibits apoptosis acts via its effect on inflammasome
inhibition, requires further investigation. The role that the
PYPAF1 inflammasome plays in the development of POCD has not been
well reported. Wang et al (42) revealed that isoflurane exposure
induced cognitive impairment and neuroinflammation, which are more
likely to occur in aged mice, and a caspase-1 inhibitor mitigated
cognitive dysfunction. In addition, knockdown of PYPAF1 in BV-2
cells inhibited PYPAF1 inflammasome activation and IL-1β production
(42). To the best of our
knowledge, the present study was the first to reveal that PYPAF1
knockdown protects aged rats from isoflurane-induced cognitive
impairment, which may be associated with the effect on inhibition
of microglial activation and neuronal apoptosis in the
hippocampus.
There are limitations to the present study. Firstly,
analysis of the hippocampus and proinflammatory factors were
examined 3 and 7 days following exposure to isoflurane. The changes
in PYPAF1 expression at earlier and later time points will be
observed in future studies. Secondly, the exact role of PYPAF1 in
different types of brain cells, such as microglia and neurons,
under the exposure of isoflurane was not determined, as these could
not be isolated and cultured from aged animals.
In conclusion, the findings from the present study
demonstrated that PYPAF1 knockdown alleviated isoflurane-induced
cognitive impairment in aged rats. Neuroinflammation and neuronal
apoptosis inhibition may contribute to this effect. Therefore, the
PYPAF1 inflammasome may serve as a potential therapeutic target for
the treatment of POCD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ designed the study. XZ, XF, FL and JQ contributed
to the acquisition, analysis and interpretation of data. XZ drafted
the article. YZ critically revised it for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed at the animal
center at the First Hospital of Jilin University following the
guidelines for the care and use of laboratory animals, and the
study was approved by the First Hospital of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hartholt KA, van der Cammen TJ and Klimek
M: Postoperative cognitive dysfunction in geriatric patients. Z
Gerontol Geriatr. 45:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rundshagen I: Postoperative cognitive
dysfunction. Dtsch Arztebl Int. 111:119–125. 2014.PubMed/NCBI
|
|
3
|
Rudolph JL, Marcantonio ER, Culley DJ,
Silverstein JH, Rasmussen LS, Crosby GJ and Inouye SK: Delirium is
associated with early postoperative cognitive dysfunction.
Anaesthesia. 63:941–947. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nadelson MR, Sanders RD and Avidan MS:
Perioperative cognitive trajectory in adults. Br J Anaesth.
112:440–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polunina AG, Golukhova EZ, Guekht AB,
Lefterova NP and Bokeria LA: Cognitive dysfunction after on-pump
operations: neuropsychological characteristics and optimal core
battery of tests. Stroke Res Treat. 2014:3028242014.PubMed/NCBI
|
|
6
|
Saczynski JS, Inouye SK, Kosar C, Tommet
D, Marcantonio ER, Fong T, Hshieh T, Vasunilashorn S, Metzger ED,
Schmitt E, et al: Cognitive and brain reserve and the risk of
postoperative delirium in older patients. Lancet Psychiatry.
1:437–443. 2014. View Article : Google Scholar
|
|
7
|
Krenk L, Rasmussen LS and Kehlet H: New
insights into the pathophysiology of postoperative cognitive
dysfunction. Acta Anaesthesiol Scand. 54:951–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ministry of Civil Affairs of the People's
Republic of China, . The social service development statistical
bulletin 2018. Feb
13–2020
|
|
9
|
Kincannon CL, He W and West LA: Demography
of aging in China and the United States and the economic well-being
of their older populations. J Cross Cult Gerontol. 20:243–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mandal PK, Schifilliti D, Mafrica F and
Fodale V: Inhaled anesthesia and cognitive performance. Drugs Today
(Barc). 45:47–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amor S and Woodroofe MN: Innate and
adaptive immune responses in neurodegeneration and repair.
Immunology. 141:287–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Zhang X, Dong H, Hu Y and Qian Y:
Bidirectional relationship of mast cells-neurovascular unit
communication in neuroinflammation and its involvement in POCD.
Behav Brain Res. 322:60–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu
Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, et al: The
inhalation anesthetic isoflurane increases levels of
proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging.
33:1364–1378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sierra A, Gottfried-Blackmore AC, McEwen
BS and Bulloch K: Microglia derived from aging mice exhibit an
altered inflammatory profile. Glia. 55:412–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hansen DV, Hanson JE and Sheng M:
Microglia in Alzheimer's disease. J Cell Biol. 217:459–472. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu LL, Ji MH, Zhang H and Yang JJ, Sun
XR, Tang H, Wang J, Liu WX and Yang JJ: NADPH oxidase 2-derived
reactive oxygen species in the hippocampus might contribute to
microglial activation in postoperative cognitive dysfunction in
aged mice. Brain Behav Immun. 51:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HL, Ma RH, Fang H, Xue ZG and Liao
QW: Impaired spatial learning memory after isoflurane anesthesia or
appendectomy in aged mice is associated with microglia activation.
J Cell Death. 8:9–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song L, Pei L, Yao S, Wu Y and Shang Y:
NLRP3 inflammasome in neurological diseases, from functions to
therapies. Front Cell Neurosci. 11:632017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manji GA, Wang L, Geddes BJ, Brown M,
Merriam S, Al-Garawi A, Mak S, Lora JM, Briskin M, Jurman M, et al:
PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with
ASC and regulates activation of NF-kappa B. J Biol Chem.
277:11570–11575. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Próchnicki T, Mangan MS and Latz E: Recent
insights into the molecular mechanisms of the NLRP3 inflammasome
activation. F1000Res. 5:F10002016. View Article : Google Scholar
|
|
22
|
Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu
J, Kang N and Wang X: Chrysophanol inhibits NALP3 inflammasome
activation and ameliorates cerebral ischemia/reperfusion in mice.
Mediators Inflamm. 2014:3705302014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Li X, Li F and An L: Berberine
alleviates postoperative cognitive dysfunction by suppressing
neuroinflammation in aged mice. Int Immunopharmacol. 38:426–433.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Academy of Sciences, . The
National Academies Collection: Reports funded by National
Institutes of Health. National Academies Press. (Washington, DC).
1975.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powell LM and Molyneux M: Should patients
be advised not to drive for 4 days after isoflurane anaesthesia?
Anaesthesia. 72:682–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahajan VA, Ni Chonghaile M, Bokhari SA,
Harte BH, Flynn NM and Laffey JG: Recovery of older patients
undergoing ambulatory anaesthesia with isoflurane or sevoflurane.
Eur J Anaesthesiol. 24:505–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng YJ, Wu QH and Zhang RQ: Effect of
propofol, sevoflurane, and isoflurane on postoperative cognitive
dysfunction following laparoscopic cholecystectomy in elderly
patients: A randomized controlled trial. J Clin Anesth. 38:165–171.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaufmann FN, Costa AP, Ghisleni G, Diaz
AP, Rodrigues AL, Peluffo H and Kaster MP: NLRP3
inflammasome-driven pathways in depression: Clinical and
preclinical findings. Brain Behav Immun. 64:367–383. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
White CS, Lawrence CB, Brough D and
Rivers-Auty J: Inflammasomes as therapeutic targets for Alzheimer's
disease. Brain Pathol. 27:223–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Proell M, Gerlic M, Mace PD, Reed JC and
Riedl SJ: The CARD plays a critical role in ASC foci formation and
inflammasome signalling. Biochem J. 449:613–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yatsiv I, Morganti-Kossmann MC, Perez D,
Dinarello CA, Novick D, Rubinstein M, Otto VI, Rancan M, Kossmann
T, Redaelli CA, et al: Elevated intracranial IL-18 in humans and
mice after traumatic brain injury and evidence of neuroprotective
effects of IL-18-binding protein after experimental closed head
injury. J Cereb Blood Flow Metab. 22:971–978. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS,
et al: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nayak D, Roth TL and McGavern DB:
Microglia development and function. Annu Rev Immunol. 32:367–402.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lalancette-Hébert M, Gowing G, Simard A,
Weng YC and Kriz J: Selective ablation of proliferating microglial
cells exacerbates ischemic injury in the brain. J Neurosci.
27:2596–2605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moss DW and Bates TE: Activation of murine
microglial cell lines by lipopolysaccharide and interferon-gamma
causes NO-mediated decreases in mitochondrial and cellular
function. Eur J Neurosci. 13:529–538. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghosh S, Castillo E, Frias ES and Swanson
RA: Bioenergetic regulation of microglia. Glia. 66:1200–1212. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernandes A, Miller-Fleming L and Pais TF:
Microglia and inflammation: Conspiracy, controversy or control?
Cell Mol Life Sci. 71:3969–3985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fougère B, Boulanger E, Nourhashémi F,
Guyonnet S and Cesari M: Chronic Inflammation: Accelerator of
Biological Aging. J Gerontol A Biol Sci Med Sci. 72:1218–1225.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kennedy MB: Synaptic signaling in learning
and memory. Cold Spring Harb Perspect Biol. 8:a0168242013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Z, Meng S, Cao L, Chen Y, Zuo Z and
Peng S: Critical role of NLRP3-caspase-1 pathway in age-dependent
isoflurane-induced microglial inflammatory response and cognitive
impairment. J Neuroinflammation. 15:1092018. View Article : Google Scholar : PubMed/NCBI
|